Abstract
The aim of this study was to examine the effect of ACS14, a hydrogen sulfide (H2S)-releasing derivative of aspirin (Asp), on Asp-induced gastric injury. Gastric hemorrhagic lesions were induced by intragastric administration of Asp (200 mg/kg, suspended in 0.5% carboxymethyl cellulose solutions) in a volume of 1 ml/100 g body weight. ACS14 (1, 5 or 10 mg/kg) was given 30 min before the Asp administration. The total area of gastric erosions, H2S concentration and oxidative stress in gastric tissues were measured three hours after administration of Asp. Treatment with Asp (200 mg/kg), but not ACS14 (430 mg/kg, at equimolar doses to 200 mg/kg Asp), for 3 h significantly increased gastric mucosal injury. The damage caused by Asp was reversed by ACS14 at 1–10 mg/kg in a concentration-dependent manner. ACS14 abrogated Asp-induced upregulation of COX-2 expression, but had no effect on the reduced PGE2 level. ACS14 reversed the decreased H2S concentrations and blood flow in the gastric tissue in Asp-treated rats. Moreover, ACS14 attenuated Asp-suppressed superoxide dismutase-1 (SOD-1) expression and GSH activity, suggesting that ACS14 may stimulate antioxidants in the gastric tissue. ACS14 also obviously inhibited Asp-induced upregulation of protein expression of oxidases including XOD, p47phox and p67phox. In conclusion, ACS14 protects Asp induced gastric mucosal injury by inhibiting oxidative stress in the gastric tissue.
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are among the most commonly prescribed drugs due to their high efficacy in reducing of pain, fever, inflammation and protection against stroke and myocardial infarction [1]. However, their clinical use is commonly associated with the occurrence of adverse effects at the level of digestive tract, ranging from dyspeptic symptoms, gastrointestinal erosions and peptic ulcers to more serious complications, such as overt bleeding or perforation [2]. Redox imbalances appear to play a major pathogenic role in aspirin (Asp) toxicity and gastropathy [3]. To overcome the adverse effects related to NSAID-induced gastrointestinal toxicity, different therapeutic strategies have been evaluated [4]–[6]. This may include reducing the risk of gastrointestinal damage induced by NSAIDs and enhancing the protective function of the gastric mucosa.
Hydrogen sulfide (H2S) is now well recognized to be an endogenous gaseous mediator. Like nitric oxide, another “gasotransmitter”, H2S also regulates various physiological functions [7], [8]. It was recently found that H2S produces strong anti-oxidative [9], anti-apoptotic [10] and anti-inflammatory effects [11] in different tissue injuries. H2S and H2S-releasing molecules are able to enhance intracellular antioxidant activities by means of several mechanisms, including stimulation of glutathione and induction of the antioxidant and tissue protective protein heme oxygenase-1 [12]–[14].
ACS14 is a H2S releasing compound, 2-acetyloxybenzoic acid 4-(3-thioxo-3H-1, 2-dithiol-5-yl) phenyl ester (ACS 14, S-aspirin) [15], [16]. The pharmacological profile of ACS14 was described recently [15]. It contains a dithiolethione moiety which gradually releases H2S for a sustained period [17]. In the present study, we therefore investigated the effect of ACS14 on Asp-induced gastric mucosal injury by examining whether ACS14 can prevent Asp-induced redox imbalances in rats.
Methods
Animals
Male Sprague-Dawley rats, 200–240 g, were obtained from the Animal Center of Xuzhou Medicine College (Xuzhou, China) and were housed at 22°C in a controlled environment with 12 h of artificial light per day. They were fasted for 20–24 h before the experiments but had free access to drinking water. All animal experiments were conducted in accordance with international ethical guidelines and the experimental protocols for using rats have been reviewed and approved by the Animal Ethics Committee at Xuzhou Medicine College.
Asp-induced Gastric Mucosal Injury and ACS14 Treatment
Gastric hemorrhagic lesions were induced by intragastric administration of Asp (200 mg/kg in 0.5% carboxymethyl cellulose solutions) in a volume of 1 ml/100 g body weight. To investigate the preventive effect of ACS14 on Asp-induced gastric mucosal injury, ACS14 synthesized as previously described [15] at doses of 1, 5 or 10 mg/kg (dissolved in DMSO) was injected intraperitoneally 30 min before the administration of Asp. Three hours after administration of Asp, the animals were killed by over-dose injection of pentobarbital sodium (60 mg/kg i.p.) and stomachs were harvested for other experiments.
Gastric Damage: Macroscopic Analysis
Both cardia and pylorus of stomach were ligated. 10 ml of 10% formaldehyde solution was injected into gastric cavity. The whole stomach was fixed in the same concentration of formaldehyde solution overnight. On the second day, the stomach was opened along the greater curvature, washed lightly and flattened on a piece of cardboard. The total number of gross mucosal lesions per stomach was counted and each lesion was scored according to the following scheme: grade1: petechial lesion, grade 2: lesion≤2 mm, grade 3: 2< lesion≤4 mm, grade 4: 4< lesion≤6mm and grade 5: lesion greater than 6 mm.
Measurement of H2S Concentration in Plasma and Gastric Tissue
The method for measurement of H2S concentration was described in our previous publications [18], [19]. Briefly, 75 µl plasma or gastric mucosal homogenates from each group were diluted in deionized water (final volume, 500 µl). H2S was trapped by addition of zinc acetate (1% w/v, 250 µl). Subsequently, N, N-dimethyl-p-phenylenediamine sulphate (20 µM; 133 µl) in 7.2 M HCl was added, followed by FeCl3 (30 µM; 133 µl) in 1.2 M HCl. Thereafter, trichloroacetic acid (10% w/v, 250 µl) was used to precipitate any protein that might be present in the culture media and upon centrifugation (10,000 g) absorbance (670 nm) of aliquots from the resulting supernatant (300 µl) was determined using a 96 well microplate reader [20].
Determination of PGE2 Levels
Tissue from each rat stomach was removed, weighed (approximately 0.1 g), and placed in a test tube containing 1 ml of 0.1 M phosphate buffer, pH 7.4, 1 mM EDTA, and 10 µM indomethacin. The tissue was homogenized and centrifuged for 20 min at 1,000 g at 4°C. Prostaglandin E2 (PGE2) content in supernatant was determined by an enzyme immunoassay kit following the protocol described by the manufacturer (Biovol Technologies, China). Results are expressed as picograms of PGE2 per milligram of protein. Proteins were determined by using the bicinchoninic acid (BCA) kit (Beyotime Institute of Biotechnology, China).
Measurement of Malondialdehyde (MDA) Levels and Glutathione (GSH) Activity in Gastric Tissue
Approximately 0.5 g of gastric tissue from individual rats was homogenized in 4.5 ml physiological saline and the supernatants were obtained by centrifugation at 2,000 g for 10 min. The protein concentration in the gastric mucosal homogenates was measured by using the bicinchoninic acid (BCA) kit (Beyotime Institute of Biotechnology, China). MDA levels and GSH activity in gastric tissue supernatants were measured using the enzyme-specific activity detection kits (Nanjing Jiancheng Bioengineering Co., China), according to the manufacturer’s instructions.
Determination of Gastric Blood Flow
Rats were anesthetized with pentobarbital sodium (60 mg/kg i.p.) and operated along the mid-line of abdomen to expose the stomach. Laser Doppler blood flow meter and miniature surface probes (moorVMS, UK) were used to record the blood flow. The acquired signal was converted to blood perfusion unit (BPU) and recorded with a computer. The curve was analyzed with moorVMS v1.0 software. Only stable signals were included and calculated. Blood flow was recorded for three times (15 s for each time) in each rat.
Western Blot Analysis
Gastric samples were lysed in buffer. The protein concentration of each lysate was determined using the BCA kit according to the manufacture's protocol. 7.5%, 10% or 12.5% SDS-polyacrylamide gels were used depending on the molecular weight of the measured proteins. After electrophoresis, the polyvinylidene fluoride (PVDF) membranes were washed in Tris-buffered saline containing 0.1% Tween-20 (TBST) for 1 h, and then incubated with the relevant antibody at 4°C overnight. All antibodies (anti-SOD1 antibody, anti-XOD antibody, anti-COX2 antibody, anti-phosphorylated p22phox antibody, anti-p47phox antibody, anti-p67phox antibody and anti-gp91phox antibody) were purchased from Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA. Membranes were washed three times in TBST buffer (10 mmol/l Tris, pH 7.5; 150 mmol/l NaCl, 0.05% Tween-20), followed by incubation with secondary antibody. The NBT/BCIP western blot analysis system according to the manufacturer's protocol was used for detection the protein signals. The results are the average of four independent experiments.
Statistical Analysis
All data were presented as mean ± SEM. Statistical significance was assessed with one-way analysis of variance (ANOVA) followed by a post hoc (Bonferroni) test for multiple group comparison. Differences with p-value less than 0.05 were considered statistically significant.
Results
Effect of ACS14 and Asp on Gastric Mucosa
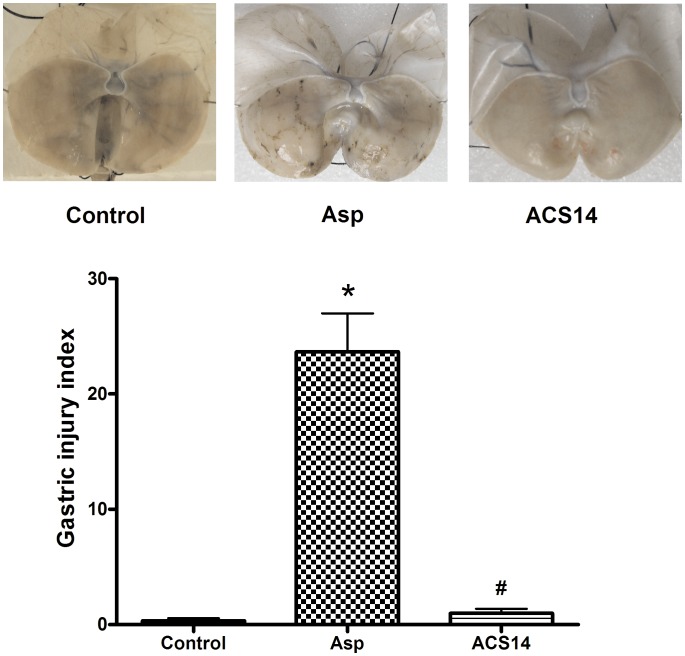
We first compared the effect of ACS14 and Asp on gastric mucosa. We found that intragastric administration of ACS14 at 430 mg/kg (at equimolar doses to 200 mg/kg Asp) did not cause any damage in the gastric mucosa. However, Asp at 200 mg/kg induced severe mucosal damage. These data suggest that H2S released from ACS14 may protect against Asp-induced gastric damage (Fig. 1).
Figure 1. Effect of Asp at 200 mg/kg and ACS14 (430mg/kg) on the morphology of gastric mucosa in rats.
ACS14 or Asp was administered to rats 3 h by intragastric administration. Representative photographs (A) and group data (B) showing that Asp, but not ACS14, induced significant gastric mucosal injury. Data are presented as means ± SE. n = 6. * P<0.05 compared with control; # P<0.05 compared with Asp.
Effect of ACS14 and NaHS on Asp-induced Gastric Mucosal Injury
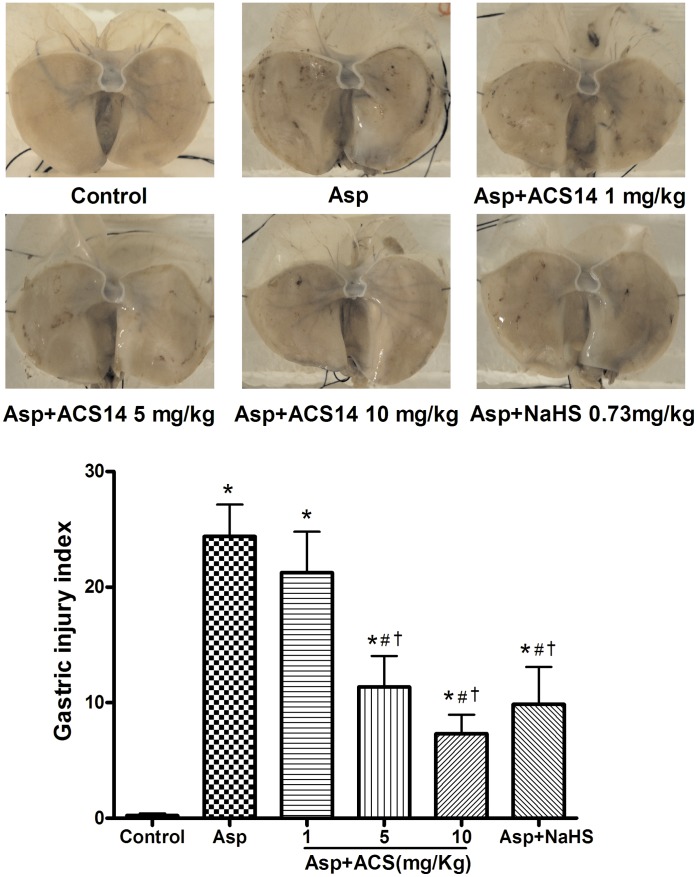
Treatment of rats with Asp (200 mg/kg) for 3 h significantly increased gastric mucosal injury. As shown in Fig. 2, Asp induced the appearance of multiple visible gastric petechial erosions. The size of erosions ranged from 2 to 10 mm in length and about 1 mm in width. This damage was reversed by ACS14 at 1–10 mg/kg in a concentration-dependent manner. The significant effect was observed when ACS14 was at 5–10 mg/kg. NaHS (an H2S donor) at 0.73 mg/kg (produced H2S approximately equivalent to that caused by ACS14 at 5 mg/kg) also decreased the gastric mucosal injury induced by Asp to a similar extent caused by ACS14 at 5 mg/kg. These data imply that ACS14 may protect gastric mucosa against Asp-induced mucosal injury via releasing H2S.
Figure 2. Effect of ACS14 on Asp-induced gastric mucosal injury in rats.
ACS14 (1, 5 or 10 mg/kg) was given (i.p.) 30 min before intragastric administration of Asp (200 mg/kg). Representative photographs (A) and group data (B) showing ACS14 significantly attenuated Asp-induced gastric mucosal injury. Data are presented as means ± SE. n = 8. *P<0.05 compared with control; #P<0.05 compared with Asp; †P<0.05 compared with Asp+ACS14 1 mg/kg.
ACS14 and NaHS Increased H2S Concentrations in Plasma and Gastric Tissue
Rats treated with Asp didn’t affect the H2S concentrations in plasma. Treatment with ACS14 (5 and 10 mg/kg) and NaHS (0.73 mg/kg) significantly increased the H2S concentrations in plasma (Fig. 3A). Interestingly, Asp treatment significantly decreased the local H2S concentration in gastric tissue. This effect was reversed by ACS14 pretreatment at 10 mg/kg. (Fig. 3B).
Figure 3. Effect of ACS14 on H2S concentration and basal gastric mucosal blood flow in rats.
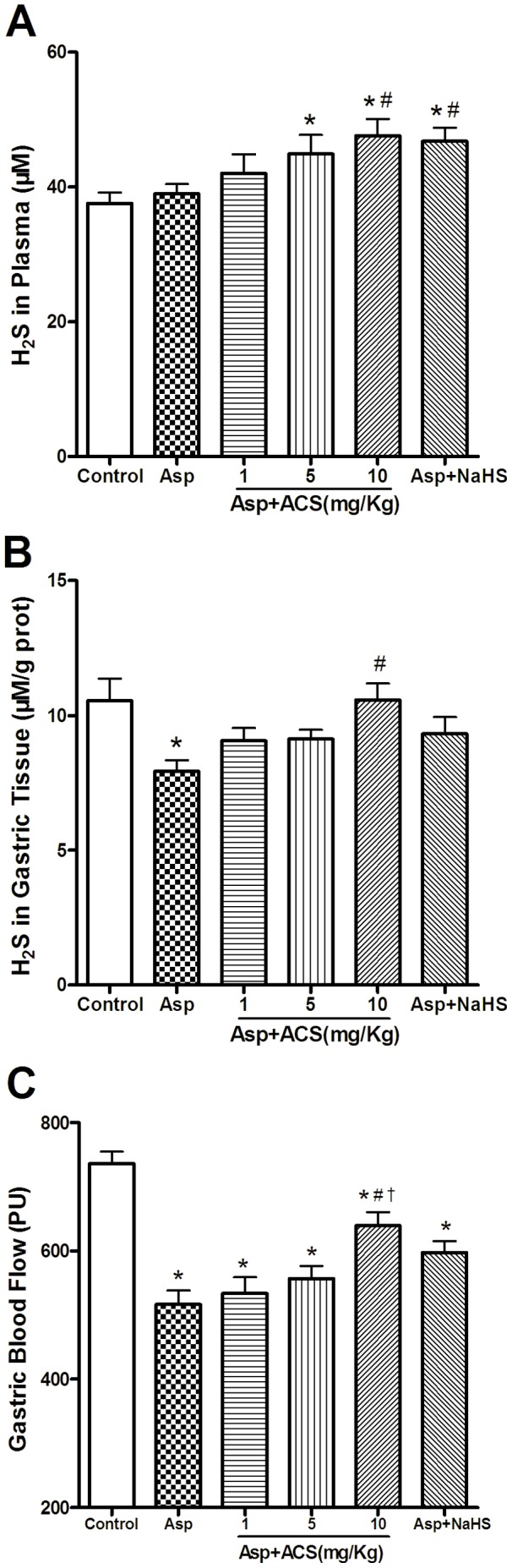
A & B. ACS14 increased H2S concentrations in both blood plasma (A) and gastric tissues (B). n = 7. C. ACS14 increased basal gastric mucosal blood flow in rats. n = 8. Data are the means±SE. *P<0.05 compared with control; #P<0.05 compared with Asp; †P<0.05 compared with Asp+ACS14 1 mg/kg.
Effect of ACS14 and NaHS on Gastric Blood Flow
Since H2S may dilate blood vessel, we therefore examined the effect of ACS14 on gastric blood flow. As shown in Fig. 3C, Asp significantly decreased gastric blood flow. This effect was reversed by ACS14 at 10 mg/kg. ACS14 at 1 mg/kg, 5 mg/kg and NaHS at 0.73 mg/kg failed to significantly change the gastric blood flow.
Effects of ACS14 on COX-2 Expression and PGE2 Content in Gastric Tissue
Cyclooxygenase 2 (COX-2) is an inducible enzyme that participates in inflammation by producing prostanoids including PGE2. COX-2 expression was significantly increased in the gastric tissue after treatment with Asp (Fig. 4A). Pretreatment with ACS14 at 5–10 mg/kg and NaHS at 0.73 mg/kg reversed the up-regulated expression of COX-2, suggesting that the protective effects may be mediated by suppression of COX-2 expression. However, the PGE2 level was markedly reduced in rats treated with Asp (200 mg/kg). This effect was not rescued by treatment with either ACS14 or NaHS (Fig. 4B).
Figure 4. Effect of ACS14 on COX-2 expression (A) and PGE2 content (B) in gastric tissue.
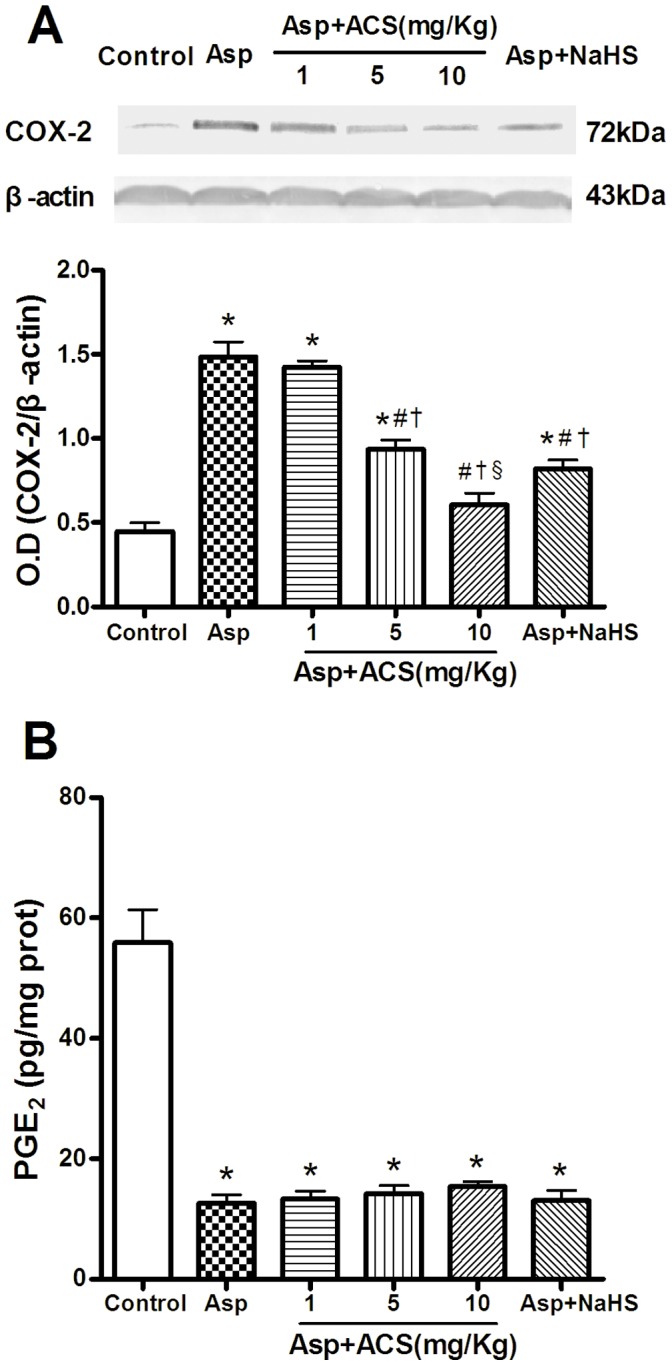
A. Representative Western blots and group data showing that ACS14 reversed Asp-upregulated COX-2 expression. n = 4. B. Both ACS14 and NaHS failed to change Asp-suppressed PGE2 level in gastric tissue. n = 7. Data are the means ± SE.*P<0.05 compared with control; #P<0.05 compared with Asp; †P<0.05 compared with Asp+ACS14 1 mg/kg; §P<0.05 compared with Asp+ACS14 5 mg/kg.
ACS14 and NaHS Decreased Asp-induced Gastric Oxidative Stress
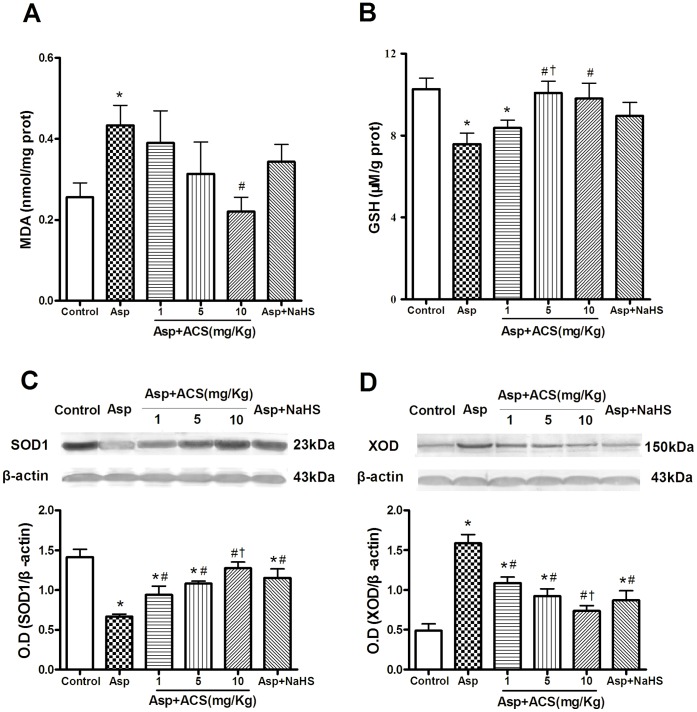
It is well known that Asp-induced gastric injury is caused by oxidative stress [3], [21]. We therefore examined the levels of gastric MDA, one of the markers of free radical species-related injury. As expected, the levels of gastric MDA were significantly elevated in Asp group of rats, as compared with the control group (Fig. 5A, P<0.05). Pretreatment with ACS14 at 10 mg/kg obviously reduced the elevated MDA level. Treatment with NaHS (0.73 mg/kg) showed a trend of decreasing gastric MDA when compared with Asp alone group, but no significant difference was found. The results suggest that ACS14 appeared to be a potent antioxidant regulator to attenuate the Asp-induced gastric injury in rats.
Figure 5. Effect of ACS14 on levels of MDA (A) and GSH (B) and protein expressions of SOD1 (C) and XOD (D) of gastric tissues in Asp-treated rats.
Data are presented as means±SE. n = 4–8. *P<0.05 compared with control; #P<0.05 compared with Asp; †P<0.05 compared with Asp+ACS14 1 mg/kg.
GSH is the most important antioxidant. As shown in Fig. 5B, Asp markedly suppressed intracellular GSH production from 10.27±0.54 µmol/g protein to 7.59±1.54 µmol/g protein. This is consistent with the previous findings [22]. Pretreatment with ACS14 at 5 and 10 mg/kg significantly increased the gastric GSH level.
SOD-1 is one of three superoxide dismutases responsible for destroying free superoxide radicals in the body. As shown in Fig. 5C, Asp treatment significantly decreased the expression of SOD-1 in the gastric tissue. Similarly, pretreatment with ACS14 and NaHS reversed the down-regulation of SOD-1 expression induced by Asp.
Xanthine oxidase (XOD) is an oxidase which produces reactive oxygen species. XOD expression in gastric tissue was significantly increased by Asp treatment (Fig. 5D). Treatment with ACS14 and NaHS suppressed Asp-induced upregulation of XOD expression.
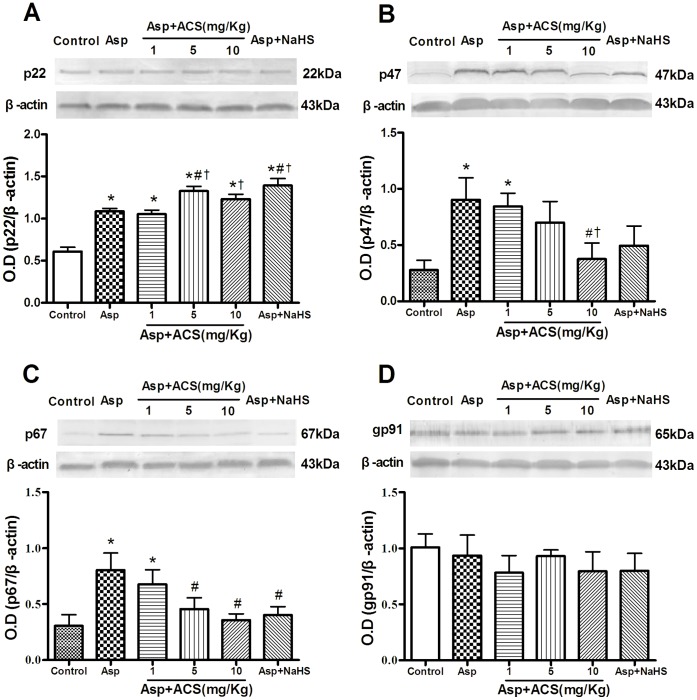
The NADPH oxidase is a membrane-bound enzyme complex. It generates superoxide by transferring electrons from NADPH to molecular oxygen to produce the superoxide. NADPH comprises p22phox, gp91phox, p40phox, p47phox, p67phox, and the small GTP-binding protein Rac [23]–[25]. As shown in Fig. 6, Asp significantly upregulated the protein expression of p22phox (Fig. 6A), p47phox (Fig. 6B) and p67phox (Fig. 6C), pretreatment with ACS14 and NaHS significantly attenuated the expression of p47phox and p67phox, but enhanced the expression of p22phox. However, all the three drugs, Asp, ACS14 and NaHS had no significant effect on gp91phox (Fig. 6D).
Figure 6. Effect of ACS14 on protein expressions of p22phox, p47phox, p67phox and gp91phox protein expressions of gastric tissue in Asp-treated rats.
Data are presented as means ± SE. n = 4. *P<0.05 compared with control; #P<0.05 compared with Asp; †P<0.05 compared with Asp+ACS14 1 mg/kg.
Discussion
Asp is widely used as an anti-inflammatory and analgesic drug. However, Asp at therapeutic dose for pain-relief often induces gastrointestinal adverse effects including gastric ulcer and bleeding. At low doses, Asp is also used to prevent cardiovascular and cerebrovascular disease [26], [27]. The recent clinical studies showed that even at low dose to prevent cardiovascular disease. Asp also induces gastroduodenal complications [28]–[31]. The annoying adverse effect may largely limit the clinical uses of NSAIDs. Therefore, development of new salicylate drugs which may not produce gastrointestinal toxicity is necessary and urgent.
H2S is increasingly being recognized as a fundamental signaling molecule, and many H2S-releasing compounds were developed in recent years [15], [32]–[34], such as H2S-releasing naproxen and H2S-releasing Asp. It has recently been found that NaHS significantly attenuated the gastric damage caused by Asp [31]. This prompted us to investigate whether H2S releasing Asp can still produce gastric injury. ACS14 is a developed H2S-releasing Asp. We found in the present study that ASC14 at the same dose as Asp failed to produce gastric injury, suggesting that H2S released from ACS14 may protect stomach against Asp-induced injury.
We then moved on to study whether ACS14 at low doses can prevent harmful effect of Asp in stomach. We found that ACS14 at 1–10 mg/kg reversed Asp induced gastric damage in a concentration-dependent manner. ACS14 is known to release H2S in vitro and in vivo [15], [35], [36]. We also found in the present study that ACS14 (at 5 and 10 mg/kg) significantly increased the H2S concentrations in plasma. These data confirmed that the beneficial effect was from the released H2S from ACS14.
COX is the rate-limiting enzyme to regulate the synthesis of prostaglandins by conversion of arachidonic acid to PGH2, the common precursor of bioactive prostaglandins. Two distinct COX isoforms were reported. COX-1 is responsible for constitutive prostaglandin formation, whereas COX-2 is usually induced in response to stress [37]. It was reported that Asp can rapidly up-regulate COX-2 expression in the stomach [38]–[40]. We found in the present study that ACS14 at 1–10 mg/kg reversed the up-regulated expression of COX-2, in a dose-dependent manner. This is consistent with a previous study that H2S significantly attenuated Asp-induced upregulation of COX-2 mRNA level [39].
Endogenous PGE2 derived from COX-2 is closely related to the recovery of gastric mucosal injury [41], [42] and plays an important role for the maintenance of gastric mucosal integrity by preventing exogenous injury to the stomach and accelerating gastric mucosal healing [43]. It was found in the present study that Asp markedly decreased PGE2 production. We therefore proposed that the upregulated COX-2 produce level was secondary to a compensatory response to inhibition of COX-2 activity and gastrin PG synthesis [38]. However, we found that neither ACS14 nor NaHS reversed Asp-impaired PGE2 production. Our data suggest that the protective effects of ACS14 and NaHS were not mediated by PGE2.
Oxidative stress is associated with increased production of oxidizing species or a significant decrease in the capability of antioxidant defenses [44]. H2S scavenges oxygen-derived free radicals [9], [45]–[49], which mediates the protective effects of NaHS against the toxicity of H2O2 in cells in vitro and also the ischemia-reperfusion-induced gastric mucosal damage in rats in vivo [47], [50]. We found in the present study that ACS14 significantly reduced Asp-induced elevation of MDA, one of the markers of free radical species-related injury. Glutathione is the major cellular antioxidant and plays an important role in antioxidative stress by H2S [9], [35], [51]–[53]. H2S protects neurons from oxidative stress by increasing the levels of GSH [9], [51], [54]. We found in the present study that ACS14 significantly increased the gastric GSH level. In addition, ACS14 also reversed Asp-reduced protein expression of SOD, which is responsible for converting superoxide radicals to molecular oxygen and hydrogen peroxide within cytoplasm and mitochondria [55]. Our data suggest that ACS14 may protect the gastric mucosa against Asp-induced damage via upregulation of antioxidants level.
We also examined the expressions of redox enzymes, NADPH oxidase. NADPH oxidase is a multicomponent enzyme that comprises p22phox, gp91phox, p40phox, p47phox, p67phox, and the small GTP-binding protein Rac [23]–[25], [56]. We found in the present study that Asp significantly upregulated the protein expression levels of 22phox, p47phox and p67phox, but not that of gp91. These data suggest that Asp may activate NADPH oxidase by stimulating some subunits of the complex. ACS14 at 10 mg/kg obviously attenuated Asp-induced upregulation of p47phox and p67phox subunit expression and therefore protected gastric tissue. Although p22phox expression was further increased by ACS14 and NaHS, which didn’t influence the protective role on Asp-induced gastric injury. This is consistent with the previous findings that NaHS can inhibit NADPH oxidase expression and concomitant O2.− formation [25], [57]–[59].
XOD catalyzes the conversion reactions of hypoxanthine to xanthine and xanthine to uric acid, the last reaction in the purine catabolism, with byproduct of toxic superoxide radical. In this regard, it is a key enzyme between purine and free radical metabolism [60]. It was reported that XOD is an endogenous source of ROS and reactive nitrogen species (RNS) that can induce oxidative stress and inflect tissue injury [61]. Our findings showed that Asp significant increased XOD protein level in gastric tissue and this effect was reversed by ACS14. Taken together, our data clearly demonstrated that ACS14 may protect gastric mucosa by suppression of oxidative stress.
We also investigated the effect of ACS14 on the gastric blood flow. It was found that ACS14 obviously increased Asp-reduced gastric blood flow. This may further contribute to its anti-oxidant effect as sufficient blood flow and oxygen supply may wash-out/inhibit Asp-induced O2.− production in gastric tissue. The mechanism underlying the ACS14-increased gastric blood flow may involve opening of KATP channels. This is supported by a previous study which showed that systemically application of exogenous H2S increased gastric mucosal blood flow by activation of KATP channels [50].
In conclusion, we demonstrated in the present study ACS14, an H2S releasing Asp, protects gastric mucosa against Asp induced injury via inhibition of oxidative stress and increasing blood flow locally.
Funding Statement
This study was supported by research grants from China National Science Fund in Jiangsu Province (BK2009088) and National Natural Science Foundation of China (30873055). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Konturek PC, Celinski K, Slomka M, Cichoz-Lach H, Burnat G, et al. (2008) Melatonin and its precursor L-tryptophan prevent acute gastric mucosal damage induced by aspirin in humans. J Physiol Pharmacol 59 Suppl 2: 67–75. [PubMed] [Google Scholar]
- 2. Blandizzi C, Tuccori M, Colucci R, Fornai M, Antonioli L, et al. (2009) Role of coxibs in the strategies for gastrointestinal protection in patients requiring chronic non-steroidal anti-inflammatory therapy. Pharmacol Res 59: 90–100. [DOI] [PubMed] [Google Scholar]
- 3. Becker JC, Domschke W, Pohle T (2004) Current approaches to prevent NSAID-induced gastropathy–COX selectivity and beyond. Br J Clin Pharmacol 58: 587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Silverstein FE, Graham DY, Senior JR, Davies HW, Struthers BJ, et al. (1995) Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal anti-inflammatory drugs. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 123: 241–249. [DOI] [PubMed] [Google Scholar]
- 5. Naesdal J, Brown K (2006) NSAID-associated adverse effects and acid control aids to prevent them: a review of current treatment options. Drug Saf 29: 119–132. [DOI] [PubMed] [Google Scholar]
- 6. Blandizzi C, Tuccori M, Colucci R, Gori G, Fornai M, et al. (2008) Clinical efficacy of esomeprazole in the prevention and healing of gastrointestinal toxicity associated with NSAIDs in elderly patients. Drugs Aging 25: 197–208. [DOI] [PubMed] [Google Scholar]
- 7. Wang R (2002) Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J 16: 1792–1798. [DOI] [PubMed] [Google Scholar]
- 8. Yang G, Wu L, Jiang B, Yang W, Qi J, et al. (2008) H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science 322: 587–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lu M, Hu LF, Hu G, Bian JS (2008) Hydrogen sulfide protects astrocytes against H(2)O(2)-induced neural injury via enhancing glutamate uptake. Free Radic Biol Med 45: 1705–1713. [DOI] [PubMed] [Google Scholar]
- 10. Hu LF, Lu M, Wu ZY, Wong PT, Bian JS (2009) Hydrogen sulfide inhibits rotenone-induced apoptosis via preservation of mitochondrial function. Mol Pharmacol 75: 27–34. [DOI] [PubMed] [Google Scholar]
- 11. Hu LF, Wong PT, Moore PK, Bian JS (2007) Hydrogen sulfide attenuates lipopolysaccharide-induced inflammation by inhibition of p38 mitogen-activated protein kinase in microglia. J Neurochem 100: 1121–1128. [DOI] [PubMed] [Google Scholar]
- 12. Qingyou Z, Junbao D, Weijin Z, Hui Y, Chaoshu T, et al. (2004) Impact of hydrogen sulfide on carbon monoxide/heme oxygenase pathway in the pathogenesis of hypoxic pulmonary hypertension. Biochem Biophys Res Commun 317: 30–37. [DOI] [PubMed] [Google Scholar]
- 13. Erdmann K, Cheung BW, Immenschuh S, Schroder H (2008) Heme oxygenase-1 is a novel target and antioxidant mediator of S-adenosylmethionine. Biochem Biophys Res Commun 368: 937–941. [DOI] [PubMed] [Google Scholar]
- 14. Szabo C (2007) Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov 6: 917–935. [DOI] [PubMed] [Google Scholar]
- 15. Sparatore A, Perrino E, Tazzari V, Giustarini D, Rossi R, et al. (2009) Pharmacological profile of a novel H(2)S-releasing aspirin. Free Radic Biol Med 46: 586–592. [DOI] [PubMed] [Google Scholar]
- 16. Rossoni G, Manfredi B, Tazzari V, Sparatore A, Trivulzio S, et al. (2010) Activity of a new hydrogen sulfide-releasing aspirin (ACS14) on pathological cardiovascular alterations induced by glutathione depletion in rats. Eur J Pharmacol 648: 139–145. [DOI] [PubMed] [Google Scholar]
- 17. Li L, Rossoni G, Sparatore A, Lee LC, Del Soldato P, et al. (2007) Anti-inflammatory and gastrointestinal effects of a novel diclofenac derivative. Free Radic Biol Med 42: 706–719. [DOI] [PubMed] [Google Scholar]
- 18. Bian JS, Yong QC, Pan TT, Feng ZN, Ali MY, et al. (2006) Role of hydrogen sulfide in the cardioprotection caused by ischemic preconditioning in the rat heart and cardiac myocytes. J Pharmacol Exp Ther 316: 670–678. [DOI] [PubMed] [Google Scholar]
- 19. Hu LF, Lu M, Tiong CX, Dawe GS, Hu G, et al. (2010) Neuroprotective effects of hydrogen sulfide on Parkinson's disease rat models. Aging Cell 9: 135–146. [DOI] [PubMed] [Google Scholar]
- 20. Pan TT, Feng ZN, Lee SW, Moore PK, Bian JS (2006) Endogenous hydrogen sulfide contributes to the cardioprotection by metabolic inhibition preconditioning in the rat ventricular myocytes. J Mol Cell Cardiol 40: 119–130. [DOI] [PubMed] [Google Scholar]
- 21. Pohle T, Brzozowski T, Becker JC, Van der Voort IR, Markmann A, et al. (2001) Role of reactive oxygen metabolites in aspirin-induced gastric damage in humans: gastroprotection by vitamin C. Aliment Pharmacol Ther. 15: 677–687. [DOI] [PubMed] [Google Scholar]
- 22. Giustarini D, Del Soldato P, Sparatore A, Rossi R (2010) Modulation of thiol homeostasis induced by H2S-releasing aspirin. Free Radic Biol Med 48: 1263–1272. [DOI] [PubMed] [Google Scholar]
- 23. Vulcano M, Dusi S, Lissandrini D, Badolato R, Mazzi P, et al. (2004) Toll receptor-mediated regulation of NADPH oxidase in human dendritic cells. J Immunol 173: 5749–5756. [DOI] [PubMed] [Google Scholar]
- 24. Li JM, Shah AM (2001) Differential NADPH- versus NADH-dependent superoxide production by phagocyte-type endothelial cell NADPH oxidase. Cardiovasc Res 52: 477–486. [DOI] [PubMed] [Google Scholar]
- 25. Muzaffar S, Jeremy JY, Sparatore A, Del Soldato P, Angelini GD, et al. (2008) H2S-donating sildenafil (ACS6) inhibits superoxide formation and gp91phox expression in arterial endothelial cells: role of protein kinases A and G. Br J Pharmacol. 155: 984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Patrono C, Garcia Rodriguez LA, Landolfi R, Baigent C (2005) Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med 353: 2373–2383. [DOI] [PubMed] [Google Scholar]
- 27. Suzuki T, Yoshida N, Nakabe N, Isozaki Y, Kajikawa H, et al. (2008) Prophylactic effect of rebamipide on aspirin-induced gastric lesions and disruption of tight junctional protein zonula occludens-1 distribution. J Pharmacol Sci 106: 469–477. [DOI] [PubMed] [Google Scholar]
- 28. Sorensen HT, Mellemkjaer L, Blot WJ, Nielsen GL, Steffensen FH, et al. (2000) Risk of upper gastrointestinal bleeding associated with use of low-dose aspirin. Am J Gastroenterol 95: 2218–2224. [DOI] [PubMed] [Google Scholar]
- 29. Derry S, Loke YK (2000) Risk of gastrointestinal haemorrhage with long term use of aspirin: meta-analysis. BMJ 321: 1183–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Garcia Rodriguez LA, Hernandez-Diaz S, de Abajo FJ (2001) Association between aspirin and upper gastrointestinal complications: systematic review of epidemiologic studies. Br J Clin Pharmacol 52: 563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fiorucci S, Antonelli E, Distrutti E, Rizzo G, Mencarelli A, et al. (2005) Inhibition of hydrogen sulfide generation contributes to gastric injury caused by anti-inflammatory nonsteroidal drugs. Gastroenterology 129: 1210–1224. [DOI] [PubMed] [Google Scholar]
- 32. Wallace JL, Caliendo G, Santagada V, Cirino G, Fiorucci S (2007) Gastrointestinal safety and anti-inflammatory effects of a hydrogen sulfide-releasing diclofenac derivative in the rat. Gastroenterology 132: 261–271. [DOI] [PubMed] [Google Scholar]
- 33. Wallace JL (2007) Building a better aspirin: gaseous solutions to a century-old problem. Br J Pharmacol 152: 421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wallace JL, Caliendo G, Santagada V, Cirino G (2010) Markedly reduced toxicity of a hydrogen sulphide-releasing derivative of naproxen (ATB-346). Br J Pharmacol 159: 1236–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Osborne NN, Ji D, Abdul Majid AS, Fawcett RJ, Sparatore A, et al. (2010) ACS67, a hydrogen sulfide-releasing derivative of latanoprost acid, attenuates retinal ischemia and oxidative stress to RGC-5 cells in culture. Invest Ophthalmol Vis Sci 51: 284–294. [DOI] [PubMed] [Google Scholar]
- 36.Osborne NN, Ji D, Majid AS, Sodato PD, Sparatore A (2012) Glutamate oxidative injury to RGC-5 cells in culture is necrostatin sensitive and blunted by a hydrogen sulfide (H(2)S)-releasing derivative of aspirin (ACS14). Neurochem Int. [DOI] [PubMed]
- 37. Hu LF, Pan TT, Neo KL, Yong QC, Bian JS (2008) Cyclooxygenase-2 mediates the delayed cardioprotection induced by hydrogen sulfide preconditioning in isolated rat cardiomyocytes. Pflugers Arch 455: 971–978. [DOI] [PubMed] [Google Scholar]
- 38. D'Argenio G, Mazzone G, Tuccillo C, Grandone I, Gravina AG, et al. (2008) Apple polyphenol extracts prevent aspirin-induced damage to the rat gastric mucosa. Br J Nutr 100: 1228–1236. [DOI] [PubMed] [Google Scholar]
- 39. Fiorucci S, de Lima OM Jr, Mencarelli A, Palazzetti B, Distrutti E, et al. (2002) Cyclooxygenase-2-derived lipoxin A4 increases gastric resistance to aspirin-induced damage. Gastroenterology 123: 1598–1606. [DOI] [PubMed] [Google Scholar]
- 40. Davies NM, Sharkey KA, Asfaha S, Macnaughton WK, Wallace JL (1997) Aspirin causes rapid up-regulation of cyclo-oxygenase-2 expression in the stomach of rats. Aliment Pharmacol Ther 11: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 41. Brzozowski T, Konturek PC, Konturek SJ, Pajdo R, Schuppan D, et al. (2000) Involvement of cyclooxygenase (COX)-2 products in acceleration of ulcer healing by gastrin and hepatocyte growth factor. J Physiol Pharmacol 51: 751–773. [PubMed] [Google Scholar]
- 42. Mizuno H, Sakamoto C, Matsuda K, Wada K, Uchida T, et al. (1997) Induction of cyclooxygenase 2 in gastric mucosal lesions and its inhibition by the specific antagonist delays healing in mice. Gastroenterology 112: 387–397. [DOI] [PubMed] [Google Scholar]
- 43. Hatazawa R, Tanaka A, Tanigami M, Amagase K, Kato S, et al. (2007) Cyclooxygenase-2/prostaglandin E2 accelerates the healing of gastric ulcers via EP4 receptors. Am J Physiol Gastrointest Liver Physiol 293: G788–797. [DOI] [PubMed] [Google Scholar]
- 44. Schafer FQ, Buettner GR (2001) Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med 30: 1191–1212. [DOI] [PubMed] [Google Scholar]
- 45. Geng B, Chang L, Pan C, Qi Y, Zhao J, et al. (2004) Endogenous hydrogen sulfide regulation of myocardial injury induced by isoproterenol. Biochem Biophys Res Commun 318: 756–763. [DOI] [PubMed] [Google Scholar]
- 46. Devai I, Delaune RD (2002) Effectiveness of selected chemicals for controlling emission of malodorous sulfur gases in sewage sludge. Environ Technol 23: 319–329. [DOI] [PubMed] [Google Scholar]
- 47.Kimura H (2010) Hydrogen sulfide: its production, release and functions. Amino Acids. [DOI] [PubMed]
- 48. Goubern M, Andriamihaja M, Nubel T, Blachier F, Bouillaud F (2007) Sulfide, the first inorganic substrate for human cells. FASEB J 21: 1699–1706. [DOI] [PubMed] [Google Scholar]
- 49. Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, et al. (2007) Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci U S A 104: 15560–15565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yonezawa D, Sekiguchi F, Miyamoto M, Taniguchi E, Honjo M, et al. (2007) A protective role of hydrogen sulfide against oxidative stress in rat gastric mucosal epithelium. Toxicology 241: 11–18. [DOI] [PubMed] [Google Scholar]
- 51. Kimura Y, Kimura H (2004) Hydrogen sulfide protects neurons from oxidative stress. FASEB J 18: 1165–1167. [DOI] [PubMed] [Google Scholar]
- 52. Qu K, Lee SW, Bian JS, Low CM, Wong PT (2008) Hydrogen sulfide: neurochemistry and neurobiology. Neurochem Int 52: 155–165. [DOI] [PubMed] [Google Scholar]
- 53. Kimura Y, Goto Y, Kimura H (2010) Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid Redox Signal 12: 1–13. [DOI] [PubMed] [Google Scholar]
- 54. Kimura Y, Dargusch R, Schubert D, Kimura H (2006) Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxid Redox Signal 8: 661–670. [DOI] [PubMed] [Google Scholar]
- 55. Vacek TP, Gillespie W, Tyagi N, Vacek JC, Tyagi SC (2010) Hydrogen sulfide protects against vascular remodeling from endothelial damage. Amino Acids 39: 1161–1169. [DOI] [PubMed] [Google Scholar]
- 56. Brandes RP, Schroder K (2008) Differential vascular functions of Nox family NADPH oxidases. Curr Opin Lipidol 19: 513–518. [DOI] [PubMed] [Google Scholar]
- 57. Muzaffar S, Shukla N, Bond M, Newby AC, Angelini GD, et al. (2008) Exogenous hydrogen sulfide inhibits superoxide formation, NOX-1 expression and Rac1 activity in human vascular smooth muscle cells. J Vasc Res 45: 521–528. [DOI] [PubMed] [Google Scholar]
- 58. Shukla N, Rossoni G, Hotston M, Sparatore A, Del Soldato P, et al. (2009) Effect of hydrogen sulphide-donating sildenafil (ACS6) on erectile function and oxidative stress in rabbit isolated corpus cavernosum and in hypertensive rats. BJU Int 103: 1522–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sen U, Givvimani S, Abe OA, Lederer ED, Tyagi SC (2011) Cystathionine beta-synthase and cystathionine gamma-lyase double gene transfer ameliorate homocysteine-mediated mesangial inflammation through hydrogen sulfide generation. Am J Physiol Cell Physiol 300: C155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Metwally NS, Ali SA, Mohamed AM, Khaled HM, Ahmed SA (2011) Levels of certain tumor markers as differential factors between bilharzial and non-biharzial bladder cancer among Egyptian patients. Cancer Cell Int 11: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Harrison R (2002) Structure and function of xanthine oxidoreductase: where are we now? Free Radic Biol Med 33: 774–797. [DOI] [PubMed] [Google Scholar]