Abstract
Background
Increasing evidence indicates that the rapid component of delayed rectifier potassium current (IKr) is modulated by α- and β-adrenergic stimulation. However, the role and mechanism regulating IKr through β2-adrenoreceptor (β-AR) stimulation in heart failure (HF) are unclear.
Methodology/Principal Findings
In the present study, we investigated the effects of fenoterol, a highly selective β2-AR agonist, on IKr in left ventricular myocytes obtained from control and guinea pigs with HF induced by descending aortic banding. IKr was measured by using whole cell patch clamp technique. In control myocytes, superfusion of fenoterol (10 µM) caused a 17% decrease in IKr. In HF myocytes, the same concentration of fenoterol produced a significantly greater decrease (33%) in IKr. These effects were not modified by the incubation of myocytes with CGP-20712A, a β1-AR antagonist, but were abolished by pretreatment of myocytes with ICI-118551, a β2-AR antagonist. An inhibitory cAMP analog, Rp-cAMPS and PKA inhibitor significantly attenuated fenoterol-induced inhibition of IKr in HF myocytes. Moreover, fenoterol markedly prolonged action potential durations at 90% (APD90) repolarization in HF ventricular myocytes.
Conclusions
The results indicate that inhibition of IKr induced by β2-AR stimulation is increased in HF. The inhibitory effect is likely to be mediated through a cAMP/PKA pathway in HF ventricular myocytes.
Introduction
Heart failure (HF) is associated with significant mortality, with nearly 50% of deaths occurring suddenly, primarily from ventricular tachycardia to ventricular fibrillation [1]. Sympathetic nerve activity is increased in HF [2]. It is widely accepted that the cardiac response to catecholamines is mediated primarily by β-adrenoreceptors (β-ARs). Arrhythmogenesis in HF is enhanced by β-adrenergic stimulation [3].
The human ether-a-go-go-related gene (hERG or KCNH2) [4] encodes the α subunit of the channel underlying IKr [5], which is crucial for the repolarization of cardiac action potentials (AP). IKr is modulated by catecholamines. There is increasing evidence that hERG/IKr channels are modulated by various G protein-coupled receptors including α- and β-ARs, which act through the intracellular signaling modulators cAMP, protein kinase A (PKA), and protein kinase C (PKC) [6], [7], [8], [9]. Stimulation of β-AR by 10 µM isoprenaline decreased IKr tail currents in guinea pig ventricular myocytes [7]. Similarly, PKA-mediated phosphorylation of expressed hERG channels significantly decreased hERG currents [10]. However, recent studies have revealed that IKr tail currents are enhanced by 100 nM isoprenaline in canine ventricular myocytes [11]. It is well known that β1-AR is downregulated and β2-AR is relatively preserved in HF. In addition, experimental studies have demonstrated that canine ventricular response to β2-agonists is increased in tachypacing failure [12]. The responsiveness of cardiac L-type calcium current to β2-AR stimulation is increased in rats with HF induced by ligation of the coronary artery [13]. However, the role and mechanism of β2-AR activation in IKr in HF have not been previously assessed. Accordingly, the present study was designed to examine the role and possible mechanisms of β2-AR activation in IKr in left ventricular (LV) myocytes from HF guinea pigs using the whole cell patch clamp technique.
Results
Validity of the Guinea Pig Model
Following 12 weeks of thoracic aortic banding, the ejection fraction (EF) and fractional shortening (FS) of the heart of guinea pigs were significantly decreased; LV end-diastolic diameter, LV end-systolic diameter, the QT interval, and corrected QT interval were significantly increased while there were no changes in heart rate (Table 1). Cell capacitance of HF LV myocytes (n = 35 cells; 20 animals) and control LV myocytes (n = 35 cells; 16 animals) was 163±7 and 121±3 pF, respectively (P<0.001). Cell capacitance was increased in HF LV myocytes. All guinea pigs with HF developed ascites and pleural effusion. Thus, the HF model of guinea pigs induced by pressure overload is valid.
Table 1. Echocardiographic and electrocardiographic data of control and heart failure guinea pigs.
| Group | CTL(n = 10) | HF(n = 10) |
| EF (%) | 80.97±0.28 | 67.88±2.66** |
| FS (%) | 44.47±0.27 | 34.01±1.95** |
| LVEDd (mm) | 6.25±0.91 | 7.75±1.32* |
| LVESd (mm) | 3.51±0.61 | 5.28±0.48** |
| HR(beats/min) | 243±11 | 260±12 |
| QT(ms) | 142.44±5.73 | 167.98±9.37* |
| QTC(ms) | 284.43±10.76 | 327.48±8.55* |
Values are mean±SEM. Control (CTL), sham-operated guinea pigs; EF, ejection fraction; FS, fractional shortening; LVEDd, left ventricle end-diastolic diameter; LVESd, left ventricle end-systolic diameter; HR, heart rate; QT, interval; QTc, corrected QT interval; n, number of guinea pigs. *p<0.05, **p<0.01 vs. CTL.
HF Reduces Ikr Tail Current Density
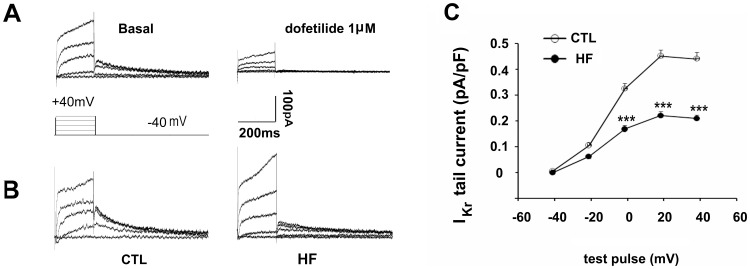
Ikr tail currents of guinea pig LV myocyte were completely abolished by the specific Ikr blocker, dofetilide (1 µm) (Figure 1A), indicating that Ikr tail currents were measured free of contamination by other currents under the given experimental conditions. The amplitude of IKr tail currents in HF myocytes was smaller than that of sham-operated controls (Figure 1B). Figure 1C shows the current–voltage (I–V) relationship of IKr tail current density. The IKr tail current density in HF myocytes was significantly lower than that in control myocytes, from 0 mV to positive potentials (at +40 mV, 0.21±0.01 pA/pF, n = 33, vs. 0.43±0.03 pA/pF, n = 24; p<0.001).
Figure 1. Changes of IKr in heart failure (HF).
(A) Recording of Ikr tail current in a representative left ventricle (LV) myocyte before (left) and 10 min after exposure to dofetilide (1 µM) (right). (B) Representative tail traces of IKr in LV myocytes isolated from control (CTL, left) and HF guinea pigs (right). (C) The average current-voltage relationship of IKr plotted for control (n = 24 cells, 8 hearts) and HF (n = 33 cells, 10 hearts) myocytes (***p<0.001, HF vs. CTL). Test pulses were applied at various voltages from −40 to +40 mV (step width 20 mV, step duration 200 ms) before returning to −40 mV for tail current recording.
Ikr Inhibition Induced by β2-AR Stimulation in HF Ventricular Myocytes
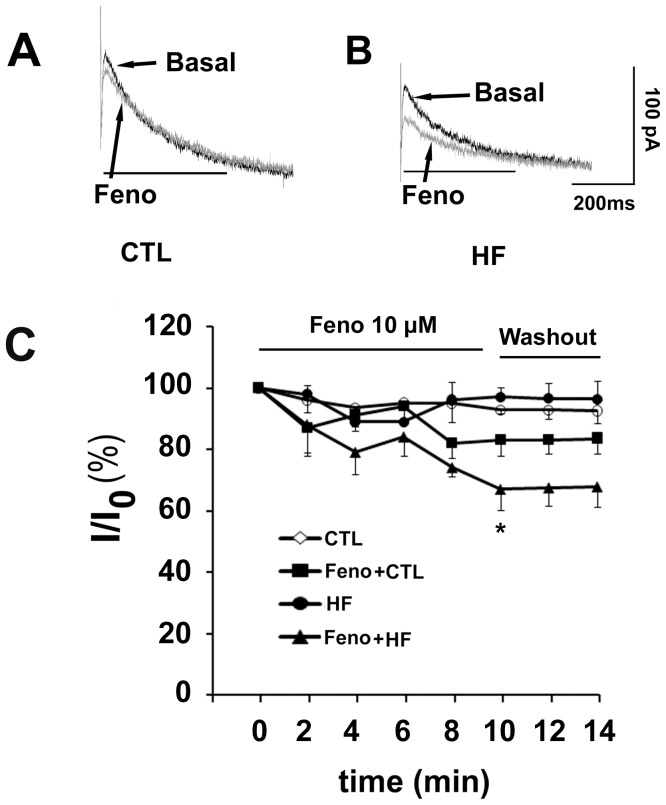
In control myocytes, the selective β2-AR agonist fenoterol (10 µM) decreased Ikr tail currents by 17±5% (n = 6, vs. control myocytes in the absence of fenoterol, p = 0.11; Figure 2A and 2C). In contrast, fenoterol (10 µM) decreased Ikr tail currents by 33±7% in HF myocytes (n = 6, vs. HF myocytes in the absence of fenoterol, p<0.05; Figure 2B and 2C). The reduction of IKr tail current occurred within 2–4 min and reached saturation about 10 min after addition of fenoterol (10 µM) into the bath. After washout of fenoterol, Ikr tail current reached 83.5±4.5% and 67.8±6.7% of the control and HF value, respectively. The inhibitory effect of fenoterol was almost not reversible (Figure 2C).
Figure 2. Effects of fenoterol (Feno) on Ikr.
(A and B) Superimposed tail current traces of Ikr recorded before and 10 min after application of 10 µM Feno in a control myocyte and a heart failure (HF) myocyte, respectively. (C) Time-dependence of current reduction by the β2-AR agonist fenoterol in control (n = 6 cells, 3 hearts) and HF myocytes (n = 6 cells, 4 hearts, *p<0.05, Feno+HF vs. HF). Current amplitudes were measured at the voltage of +40 mV.
Effects of β1- and β2-AR Blockade on Fenoterol-induced Decrease in Ikr
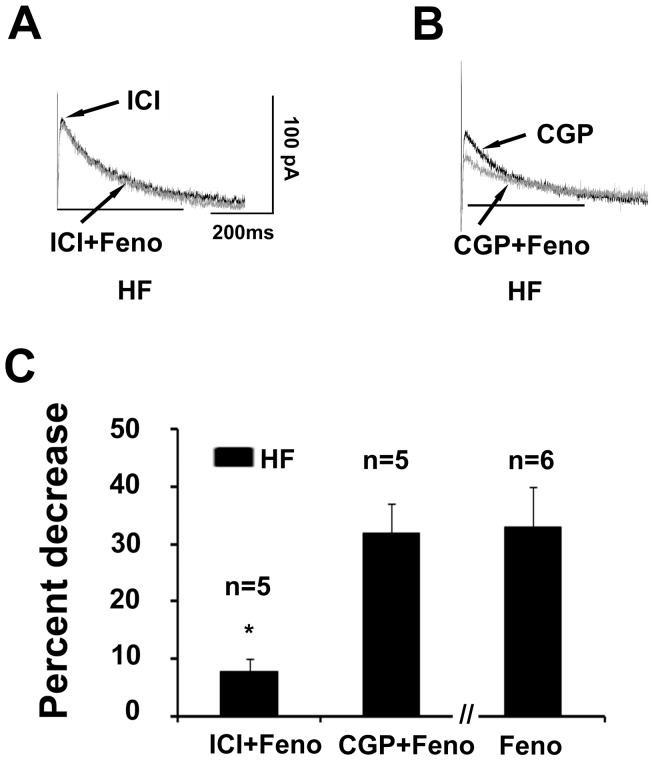
We used the selective β2-AR antagonist ICI118551 to examine whether the IKr response to fenoterol was mediated through β2-AR in HF myocytes. When 10 µM ICI118551 was applied along with fenoterol, it almost totally prevented fenoterol-induced inhibition of IKr (fenoterol alone, 33±7% decrease, n = 6; fenoterol plus ICI118551, 8±3% decrease, n = 5; p<0.05; Figure 3A and 3C). However, in five HF myocytes, fenoterol decreased IKr by 32±5% in the presence of CGP20712A, a selective β1-AR antagonist (Figure 3B and 3C). Under these conditions, the present results supported the role of the β2-AR subtype in the modulation of IKr in HF myocytes.
Figure 3. β2-AR mediates the inhibition of Ikr by fenoterol (Feno) in heart failure (HF) myocytes.
(A and B) Superimposed tail current traces of Ikr recorded before and 10 min after application of 10 µM fenoterol in the presence of the selective β2-AR antagonist ICI118551 (ICI, 10 µM) and β1-AR antagonist CGP20712A (CGP, 10 µM) in a HF myocyte, respectively. (C) Summarized data for percent decrease in the amplitude of IKr tail current evoked by ICI plus fenoterol, CGP plus fenoterol, and fenoterol alone (n = 5 and 6 cells, 3 hearts, *P<0.05, Feno+ICI versus Feno). Current amplitudes were measured at the voltage of +40 mV.
β-AR mRNA and Protein Expression in HF
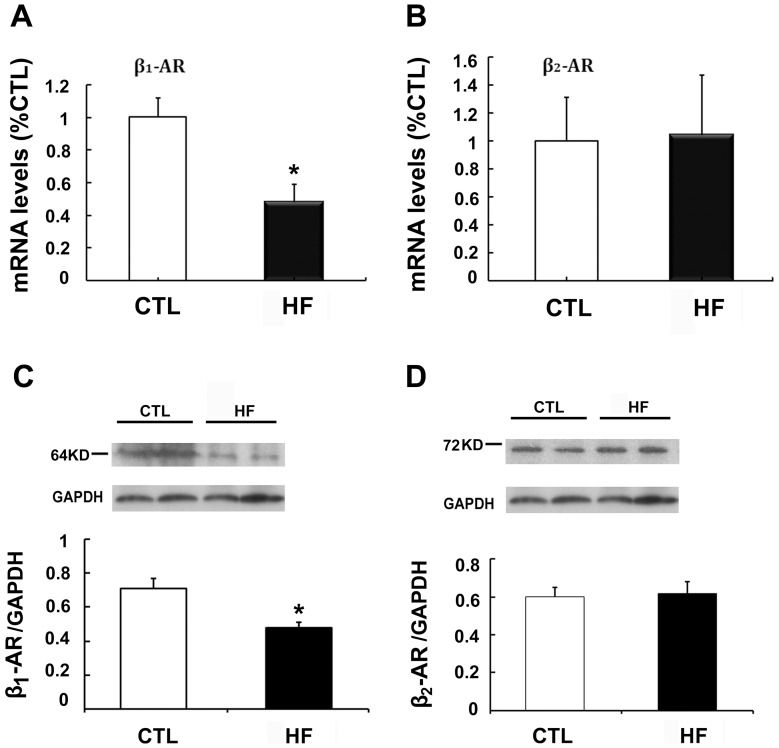
Figure 4A and B show that β1-AR mRNA levels were reduced 52% in HF guinea pigs compared with controls (n = 5, p<0.05), whereas β2-AR mRNA levels remained unchanged. β1-AR protein showed a significant decline, as illustrated in Figure 4C. β2-AR protein remained unchanged (Figure 4D).
Figure 4. β1-and β2-AR mRNA and protein expression in heart failure (HF).
(A) β1-AR mRNA level (n = 5, *p<0.05, HF vs.control, CTL). (B) β2-AR mRNA level (n = 5). (C) Representative protein bands of β1-AR in control and HF samples (upper); β1-AR protein expression (bottom, n = 5, *p<0.05, HF vs. CTL). (D) Representative protein bands of β2-AR in control and HF samples (upper); β2-AR protein expression (bottom).
IKr Inhibition Induced by β2-AR Stimulation Involves the cAMP/PKA Pathway
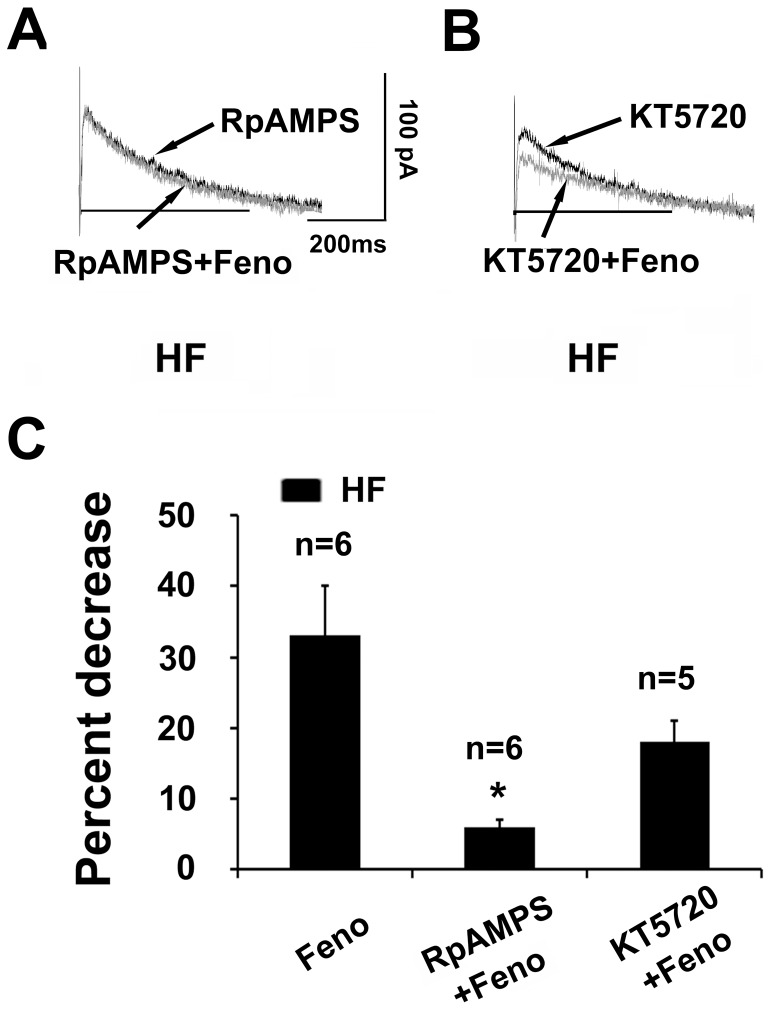
It is known that the hERG K+ channel can be modulated by cAMP and PKA. β2-AR is coupled to the stimulatory Gs protein, which leads to the activation of adenylyl cyclase and production of cAMP. To examine whether cAMP activation mediates the IKr response to β2-AR stimulation, we investigated the effect of inhibitory cAMP analog Rp-cAMPS on the stimulatory action of fenoterol. As shown in Figure 5A and 5C, with intracellular application of Rp-cAMPS (100 µM), fenoterol–induced IKr decrease was almost fully prevented in HF myocytes (fenoterol alone, 33±7% decrease, n = 6; Rp-cAMPS, 6±1% decrease, n = 6; p<0.05). These results suggest that IKr inhibition induced by β2-AR stimulation involves cAMP activation.
Figure 5. Effects of cAMP inhibition and PKA inhibition on Ikr response to β2-AR stimulation.
(A) Superimposed tail current traces of Ikr recorded before and 10 min after application of 10 µM fenoterol (Feno) in the presence of RpCAMPS (100 µM) in pipette solution in a heart failure (HF) myocyte. (B) Superimposed tail current traces of Ikr recorded before and 10 min after application of 10 µM Feno in the presence of PKA inhibitor, KT5720 (2.5 µM) in a HF myocyte. (C) Summarized data for percent decrease in the amplitude of IKr tail current evoked by Feno alone, RpCAMPS plus fenoterol, and KT5720 plus Feno in HF myocytes (n = 6, 6, and 5 cells, 3 hearts, *p<0.05, RpCAMPS+ Feno vs. Feno). Current amplitudes were measured at the voltage of +40 mV.
We further examined the effect of PKA inhibition on the inhibitory effects of fenoterol. As illustrated in Figure 5B and 5C, fenoterol-induced Ikr decrease was largely abolished by pretreatment of HF myocytes with KT5720 (fenoterol alone, 33±7% decrease, n = 6; fenoterol plus KT5720, 18±4% decrease, n = 5; p = 0.051), supporting an involvement of PKA activation.
Action Potential Duration (APD) is Prolonged by β2-AR Stimulation in HF Ventricular Myoctyes
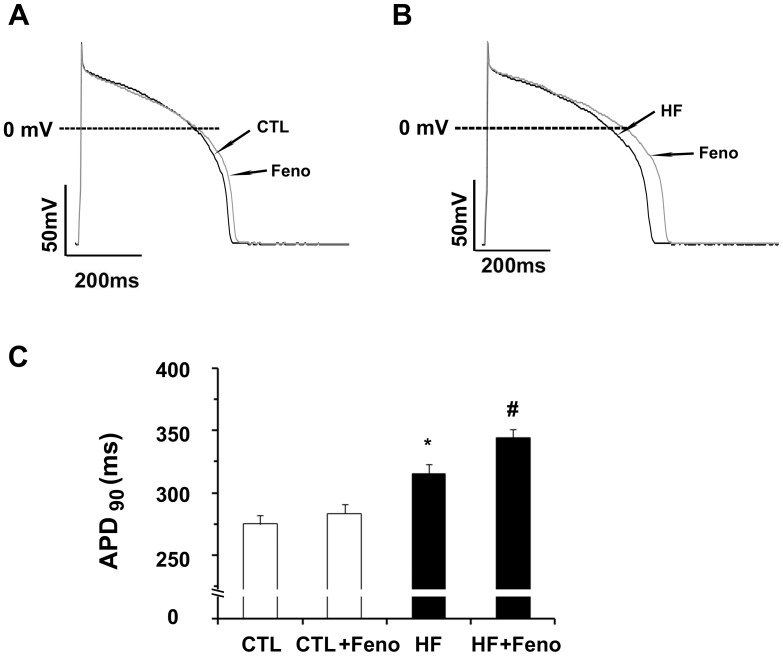
Because action potential duration (APD) is dependent on the balance between depolarizing inward and repolarizing outward currents, an alteration in the amplitude of major repolarizing current such as IKr would lead to substantial changes in the repolarization process. We therefore examined the effects of fenoterol on AP in LV myocytes. Figure 6A and 6B shows the superimposed traces of AP recorded before and during exposure to fenoterol (10 µM) in control and HF myocytes. A summary of corresponding data is shown in Figure 6C. APD at 90% repolarization (APD90) was significantly prolonged in HF myocytes compared with control (315.1±8.1 ms, n = 8, vs. 274.8±7.3 ms, n = 9; p<0.01). Fenoterol caused significant prolongation of APD90 in HF myocytes (9.5%, 344.6±6.5 vs. 315.1±8.1ms, n = 8; p<0.05), whereas no significant prolongation of APD90 was observed in control myocytes (3%, 283.6±7.7 vs. 274.8±7.3 ms, n = 9). Fenoterol did not influence the AP amplitude and resting potentials in control and HF ventricular myocytes.
Figure 6. Effects of fenoterol (Feno) on action potential duration (APD).
(A and B) Superimposed action potentials recorded before and 10 min after exposure to 10 µM Feno in a control and a heart failure (HF) myoctye of guinea pigs, respectively. (C) Summarized data for changes in APD at 90% of repolarization (APD90) by exposure to Feno in control (n = 8 cells, 3 hearts) and HF myocytes (n = 9 cells, 4 hearts, *p<0.01, HF vs. control; #p<0.05, HF +fenoterol vs. HF).
Discussion
The present study demonstrates, for the first time, that increased response to β2-AR stimulation produces an inhibitory effect on IKr in ventricular myocytes of guinea pigs with descending aortic banding-induced heart failure, and this effect is induced by the activation of the cAMP/PKA pathway.
Inhibitory Effect of β2-AR Activation on IKr in HF Ventricular Myocytes
The hERG channel (Kv11.1) underlies the rapid component of delayed rectifier potassium current (Ikr) and is critical for the kinetics of cardiac AP repolarization [5], [14]. There is increasing evidence that hERG/IKr channels are modulated by various G protein-coupled receptors including α- and β-ARs, acting through the intracellular signaling modulators cAMP, PKA, and PKC [6], [7], [8], [9], [11]. It has been increasingly recognized that β1-and β2-AR coexist in the heart. In large mammal hearts, β2-AR may account for approximately 40% of β-AR in total. The reported ratio of β1-/β2-AR was 85/15 in guinea pig hearts [15]. Consistent with previous observations [16], in our HF guinea pigs, β1-AR was downregulated; however, β2-AR was unaltered. The increase of β2-AR density has been demonstrated in the LV of dogs with pacing-induced HF [17]. It has been demonstrated that in diseased ventricles, β2-AR stimulation exhibits a heightened response [12], [13]. Our results demonstrate, for the first time, that the responsiveness of IKr to β2-AR stimulation is enhanced in HF guinea pigs ventricular myocytes. The effect of selective β2-AR agonist fenoterol (10 µM) on IKr was far less effective in control myocytes; whereas, IKr was significantly inhibited by 10 µM fenoterol in HF myocytes. The reduction of IKr tail current occurred within 2–4 min and reached saturation about 10 min after addition of fenoterol into the bath. After washout of fenoterol, the inhibitory effect on IKr tail current was almost irreversible. In order to identify the β2-AR specific effect, we used salbutamol (10 µM), another highly selective β2-AR agonist, and achieved similar results (data not shown). Moreover, the inhibitory effect of fenoterol was almost completely abolished by β2-AR antagonist, but not by the β1-AR antagonist. Therefore, our findings indicate that the IKr inhibition induced by fenoterol is due to β2-AR activation.
Heart failure induced by pressure overload is a non-ischemic heart failure, which exhibits clinical and pathological features typical of hypertension-induced heart failure. Consistent with previous observations, myocytes from these hearts were enlarged. This model of heart failure is widely applied in the study of cardiac cellular electrophysiology. Sympathetic nervous system is activated in response to exercise and emotional stress, which results in increased levels of local and circulating catecholamines. Plasma norepinephrine levels of ∼ 3 µM and peak exercise norepinephrine levels of ∼ 15 µM have been detected in chronic HF in humans [18], giving our present findings utilizing 10 µM fenoterol a clinical relevance.
cAMP/PKA Involvement in the Inhibitory Effect of β2-AR Activation on IKr
Stimulation of different G protein-coupled receptors, including α1- and β-ARs, regulates IKr through intracellular messengers and provides a link between autonomic stimulation and cardiac repolarization [19]. Stimulation of β2-AR in the heart has classically been known to result in Gs-mediated stimulation of adenylyl cyclase (AC), which leads to increased cellular cAMP, thus activating PKA [20]. It has been demonstrated in the Xenopus laevis oocyte expression system that PKA can reduce hERG currents via direct phosphorylation of all four putative PKA consensus sites [10]. In the present study, we demonstrated that fenoterol-induced inhibition of IKr was fully prevented by intracellular application of Rp-cAMPS, an inhibitory cAMP analog, suggesting that this effect may be mediated through a cAMP-dependent mechanism in HF ventricular myocytes. Furthermore, fenoterol-induced inhibition of IKr was partly attenuated by PKA inhibitor, implicating the involvement of PKA activation. The dual regulation of IKr by cAMP and PKA phosphorylation has been previously demonstrated [21]. hERG current may be decreased by cAMP-dependent activation of PKA; however, a putative direct binding of cAMP to the channel causes opposite effects. The net effect of dual hERG current regulation by PKA and cAMP is current reduction. A recent study has shown that β2-AR redistribution in HF can change cAMP compartmentation [22]. Our results do not exclude the possibility of direct binding of cAMP to the channel.
Cardiac β2-AR can couple with both Gs and Gi proteins. The complexity of the β2-AR signaling pathway, including functional compartmentalization of signaling mediated by Gi, phosphatidylinositol 3-kinase, and mitogen-activated protein kinases (MAPKs), has been documented [20], [23]. Recently, Li et al. has reported that one or more A-kinase anchoring proteins (AKAPs) targets PKA to HERG channels and may contribute to the acute regulation of IKr by cAMP [24]. AKAPs are a structurally diverse group of proteins that lack primary structure sequence homology but share the function of localizing PKA to subcellular structures, substrates, and oftentimes with other members of the signaling pathway [25]. β-adrenergic signaling is maintained by the localization of PKA and phosphodiesterases to subcellular microdomains. In addition, in the rat brain, β2-ARs are found to be associated directly with Cav1.2 in a macromolecular signaling complex [26]. Indeed, the precise mechanism for β2-adrenergic stimulation-induced inhibition of IKr for HF myocytes remains to be further elucidated. For example, it remains unclear whether Gi pathway or a β2-AR-macromolecular signaling complex mediates the inhibition of IKr via β2-AR in HF ventricular myocytes.
APD Prolongation by β2-AR Stimulation
One of the most characteristic electrophysiological remodeling in failing heart is APD prolongation, which is believed to mainly result from the downregulation of repolarizing outward potassium currents, including Ito (transient outward K+ current ), IKs and IKr in heart failure [27], [28]. In the present study, we found that QT interval was increased in failing guinea pigs and APD was prolonged in failing ventricular myocytes. Fenoterol caused significant prolongation of APD90 in failing ventricular myocytes, whereas no significant prolongation of APD90 was observed in control myocytes. The present study provides evidence that fenoterol-induced inhibition of IKr may result in delay in cardiac repolarization via stimulation of β2-AR in failing ventricular myocytes. Because the IKr is crucial for the repolarization of cardiac AP, inhibition of IKr induced by stimulation of β2-AR in failing ventricular myocytes may in part contribute to the delay in cardiac repolarization.Ventricular myocytes from the guinea pig heart possess a time- and voltage-independent Cl− current induced by β-adrenoceptor activation [29], [30]. Activation of this current will result in outward current during the plateau phase of AP, shortening APD [31]. One limitation of the present study is that the AP experiments were performed without considering cAMP modulated Cl− current. Therefore, the effect of fenoterol on cardiac repolarization should be studied without ignoring other currents in the future.
In conclusion, the inhibitory effect of IKr induced by β2-AR stimulation is increased in HF. The effect is likely to be mediated through a cAMP/PKA pathway mechanism in HF ventricular myocytes. This regulatory pathway of the β2-adrenergic system on Ikr current provides a possible relationship between stress and life-threatening arrhythmias in HF.
Materials and Methods
All experiments were performed in accordance with animal care protocols approved by the Nanjing Medical University Institutional Animal Care and Use Committee.
Heart Failure Model in Guinea Pigs
Heart failure was induced in adult male guinea pigs (250 to 300 g) by subtotal descending thoracic aortic banding. After sodium pentobarbital anesthesia (25 mg/kg, i.p.), the descending thoracic aorta was exposed sterilely through an incision in the left third intercostal space. A uniform degree of constriction around the descending thoracic aorta was produced by tightening a 2-0 surgical silk ligature around an 18-gauge needle. The needle was then withdrawn from the ligature, and the chest incision was closed. Sham animals underwent the same operation, with the exception that the aorta was not banded. Sham-operated animals were used as control. Aortic-banded animals and sham animals were housed and fed under identical conditions and were used 12 weeks after surgery. To monitor the progress of HF during the observation period, two-dimensional echocardiography (Sonos 5500, Hewlett Packard, Andover, MA, USA) was carried out periodically. ECGs and two-dimensional echocardiography were obtained under anesthesia (ketamine hydrochloride; 30 mg/kg, i.p.). The QTc was calculated by Bazett’s formula where QTc = QT/√RR. Following 12 weeks of aortic banding, guinea pigs showed signs of HF, including ventricular dilation, decreased ejection fraction (EF) and fractional shortening (FS), ascites, and pleural effusions.
Myocyte Isolation and Electrophysiological Recordings
Single left ventricular (LV) myocytes were enzymatically dissociated from the heart of guinea-pigs (550–600 g) as described previously [6] with minor modification. Cardiomyocytes were transferred to a recording chamber that was continuously perfused with a bath solution. Pipettes had resistances of 3–6 MΩ after filling with the pipette solution. Following seal formation, access developed within 2–5 min and series resistance was compensated up to 80%. Whole-cell patch-clamp recordings were performed with an EPC-9 amplifier (HEKA, Germany). Flow rate through the chamber was maintained at 2–3 ml/min. Ikr was determined using the following test pulse protocol: after a holding potential of −40 mV, test pulses were applied at various voltages from −40 to +40 mV (step width 20 mV, step duration 200 ms) prior to returning to −40 mV for tail current recording [6], [7]. IKr current was recorded at 37±0.5°C. Substance effects were investigated on tail currents after the test pulse was applied at +40 mV. Measurements were repeated every two min.
For AP recording, extracellular solution (in mmol/L) contained NaCl 137, KCl 5.4, MgCl2 1.0, CaCl2 1.8, glucose 10, and HEPES 10; pH was adjusted to 7.4 with NaOH. Pipette solution (in mmol/L) contained potassium aspartate 120, KCl 20, MgCl2 2.0, HEPES 10, EGTA 10, Na2ATP 10; pH was adjusted to 7.2 with KOH. APs were evoked using whole-cell current-clamp mode by suprathreshold current pulse of 5 ms duration at the frequency of 1 Hz. APD was measured at 90% repolarization (APD90). APs were generated with the same amplifier and recorded at 37±0.5°C.
Solutions and Drugs
Preparation of the solution was performed as described previously by Wang et al. [6]. Calcium currents were blocked by the addition of 10 µM nifedipine in the bath solution, and 10 µM chromanol was used to block Iks. Na2-ATP, EGTA, creatine phosphate, L-glutamic acid, HEPES, taurine, bovine serum albumin (BSA), nifedipine, isoproterenol, xamoterol, fenoterol, CGP20712A, ICI118551, and Rp-cAMPS were purchased from Sigma (St. Louis, MO), collagenase II from Invitrogen (Carlsbad, CA), and KT5720 from Merck KGaA (Darmstadt, Germany). Dofetilide, a specific blocker of Ikr or hERG, was kindly provided by Pfizer Inc (NY, USA). All other reagents were obtained from Amresco (OH, USA).
For stock solutions, chromanol and KT5720 were dissolved in dimethyl sulfoxide (DMSO) at a final concentration of 1 mM and stored at −20°C until use. Final concentration of DMSO was less than 0.5% in the bath, which exerted no effect on the currents measured.
Real Time RT-PCR Analysis
RNA was extracted from 100 mg of LV tissue using the Trizol reagent (Invitrogen) according to the manufacturer’s protocol. RNA concentration was determined spectrophotometrically. Reverse transcription of 2 µg RNA was performed in a 20-µl reaction mixture using an RT kit (Takara Biotech). The GAPDH housekeeping gene was used as a reference for loading control. The primers for genes examined are shown in Table 2. Optimized PCR cycle parameters included 2 min at 95°C, 10 sec at 95°C followed by 10 sec at 95°C, 10 sec at 57°C or 55°C, and 45 sec at 72°C for 40 cycles. Standard curves were determined for PCR efficiency. Quantitative real-time (RT) polymerase chain reaction PCR) was performed using the Applied Biosystems 7500 Real-time PCR System (Applied Biosystems, Foster City, CA, USA) with SYBR Green I (Invitrogen). Relative mRNA expression was determined using the 2−△Ct method.
Table 2. Gene-specific primer sequences used for real-time PCR analysis in this study.
| Gene | Forward primersequence | Reverse primersequence | GenBank |
| ADRB1 | TTCTATGTGCCCCTGTGCAT | GTGAACACGCCCATGATGAT | EU332753.1 |
| ADRB2 | CATCGTCAACATTGTGCACG | TGCCCCTGGTGACAAACAT | AJ459814.1 |
| GAPDH | ACCACAGTCCATGCCATCAC | TCCACCACCCTGTTGCTGTA |
ADRB1 and ADRB2 refer to β1-AR and β2-AR.
Western Blot Analysis
Membrane fractions were prepared as previously described [32]. Protein samples were separated by 10% SDS-PAGE. The separated proteins were transferred by electrophoresis to PVDF membranes. Membranes were blocked for two h with Tris-Tween buffered saline (TTBS) solution containing 5% nonfat dried milk and incubated overnight with primary antibody against β1-AR and β2-AR (both from Santa Cruz Biotechnology, Santa Cruz, CA). After washing, blots were incubated for one h with appropriate peroxidase-conjugated goat anti-rabbit IgG secondary antibody (Santa Cruz Biotechnology). Staining was determined with chemiluminescence and quantified using image acquisition and analysis software. All data were expressed relative to GAPDH staining for the same samples on the same gels.
Data Analysis
The data were acquired using Pulse +Pulsefit V 8.53 and were expressed as mean ± SEM. Western blot band intensities were expressed after background subtraction as optical density units (ODUs) normalized against GAPDH signal intensity for the same sample. Data comparisons were made using Student’s t-test (paired or unpaired). Differences were considered statistically significant if p<0.05.
Acknowledgments
We would like to thank Dr. Cui Bo for his prootreading of this thesis.
Funding Statement
This work was supported by the National Nature Science Foundation of China (81170162) http://www.nsfc.gov.cn/Portal0/default166.htm) and University Nature Science Foundation of Jiangsu Province (09KJB320008).The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, et al. (2010) Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation 121: e46–e215. [DOI] [PubMed] [Google Scholar]
- 2. Floras JS (2009) Sympathetic nervous system activation in human heart failure: clinical implications of an updated model. J Am Coll Cardiol 54: 375–385. [DOI] [PubMed] [Google Scholar]
- 3. Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM (2001) Arrhythmogenesis and contractile dysfunction in heart failure: Roles of sodium-calcium exchange, inward rectifier potassium current, and residual beta-adrenergic responsiveness. Circ Res 88: 1159–1167. [DOI] [PubMed] [Google Scholar]
- 4. Warmke JW, Ganetzky B (1994) A family of potassium channel genes related to eag in Drosophila and mammals. Proc Natl Acad Sci U S A 91: 3438–3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sanguinetti MC, Jiang C, Curran ME, Keating MT (1995) A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell 81: 299–307. [DOI] [PubMed] [Google Scholar]
- 6. Wang S, Xu DJ, Cai JB, Huang YZ, Zou JG, et al. (2009) Rapid component I(Kr) of cardiac delayed rectifier potassium currents in guinea-pig is inhibited by alpha(1)-adrenoreceptor activation via protein kinase A and protein kinase C-dependent pathways. Eur J Pharmacol 608: 1–6. [DOI] [PubMed] [Google Scholar]
- 7. Karle CA, Zitron E, Zhang W, Kathofer S, Schoels W, et al. (2002) Rapid component I(Kr) of the guinea-pig cardiac delayed rectifier K(+) current is inhibited by beta(1)-adrenoreceptor activation, via cAMP/protein kinase A-dependent pathways. Cardiovasc Res 53: 355–362. [DOI] [PubMed] [Google Scholar]
- 8. Thomas D, Kiehn J, Katus HA, Karle CA (2004) Adrenergic regulation of the rapid component of the cardiac delayed rectifier potassium current, I(Kr), and the underlying hERG ion channel. Basic Res Cardiol 99: 279–287. [DOI] [PubMed] [Google Scholar]
- 9. Zankov DP, Yoshida H, Tsuji K, Toyoda F, Ding WG, et al. (2009) Adrenergic regulation of the rapid component of delayed rectifier K+ current: implications for arrhythmogenesis in LQT2 patients. Heart Rhythm 6: 1038–1046. [DOI] [PubMed] [Google Scholar]
- 10. Thomas D, Zhang W, Karle CA, Kathofer S, Schols W, et al. (1999) Deletion of protein kinase A phosphorylation sites in the HERG potassium channel inhibits activation shift by protein kinase A. J Biol Chem. 274: 27457–27462. [DOI] [PubMed] [Google Scholar]
- 11. Harmati G, Banyasz T, Barandi L, Szentandrassy N, Horvath B, et al. (2011) Effects of beta-adrenoceptor stimulation on delayed rectifier K(+) currents in canine ventricular cardiomyocytes. Br J Pharmacol 162: 890–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Altschuld RA, Starling RC, Hamlin RL, Billman GE, Hensley J, et al. (1995) Response of failing canine and human heart cells to beta 2-adrenergic stimulation. Circulation 92: 1612–1618. [DOI] [PubMed] [Google Scholar]
- 13. Zhang ZS, Cheng HJ, Ukai T, Tachibana H, Cheng CP (2001) Enhanced cardiac L-type calcium current response to beta2-adrenergic stimulation in heart failure. J Pharmacol Exp Ther 298: 188–196. [PubMed] [Google Scholar]
- 14. Tamargo J, Caballero R, Gomez R, Valenzuela C, Delpon E (2004) Pharmacology of cardiac potassium channels. Cardiovasc Res 62: 9–33. [DOI] [PubMed] [Google Scholar]
- 15. Voss HP, Shukrula S, Wu TS, Donnell D, Bast A (1994) A functional beta-2 adrenoceptor-mediated chronotropic response in isolated guinea pig heart tissue: selectivity of the potent beta-2 adrenoceptor agonist TA 2005. J Pharmacol Exp Ther 271: 386–389. [PubMed] [Google Scholar]
- 16. Bristow MR, Ginsburg R, Umans V, Fowler M, Minobe W, et al. (1986) Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ Res 59: 297–309. [DOI] [PubMed] [Google Scholar]
- 17. Kiuchi K, Shannon RP, Komamura K, Cohen DJ, Bianchi C, et al. (1993) Myocardial beta-adrenergic receptor function during the development of pacing-induced heart failure. J Clin Invest 91: 907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vittorio TJ, Zolty R, Kasper ME, Khandwalla RM, Hirsh DS, et al. (2008) Differential effects of carvedilol and metoprolol succinate on plasma norepinephrine release and peak exercise heart rate in subjects with chronic heart failure. J Cardiovasc Pharmacol Ther 13: 51–57. [DOI] [PubMed] [Google Scholar]
- 19. Thomas D, Karle CA, Kiehn J (2006) The cardiac hERG/IKr potassium channel as pharmacological target: structure, function, regulation, and clinical applications. Curr Pharm Des 12: 2271–2283. [DOI] [PubMed] [Google Scholar]
- 20. Xiang Y, Kobilka BK (2003) Myocyte adrenoceptor signaling pathways. Science 300: 1530–1532. [DOI] [PubMed] [Google Scholar]
- 21. Cui J, Melman Y, Palma E, Fishman GI, McDonald TV (2000) Cyclic AMP regulates the HERG K(+) channel by dual pathways. Curr Biol 10: 671–674. [DOI] [PubMed] [Google Scholar]
- 22. Nikolaev VO, Moshkov A, Lyon AR, Miragoli M, Novak P, et al. (2010) Beta2-adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science 327: 1653–1657. [DOI] [PubMed] [Google Scholar]
- 23. Steinberg SF (2004) beta(2)-Adrenergic receptor signaling complexes in cardiomyocyte caveolae/lipid rafts. J Mol Cell Cardiol 37: 407–415. [DOI] [PubMed] [Google Scholar]
- 24. Li Y, Sroubek J, Krishnan Y, McDonald TV (2008) A-kinase anchoring protein targeting of protein kinase A and regulation of HERG channels. J Membr Biol 223: 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smith FD, Langeberg LK, Scott JD (2006) The where’s and when’s of kinase anchoring. Trends Biochem Sci 31: 316–323. [DOI] [PubMed] [Google Scholar]
- 26. Davare MA, Avdonin V, Hall DD, Peden EM, Burette A, et al. (2001) A beta2 adrenergic receptor signaling complex assembled with the Ca2+ channel Cav1.2. Science 293: 98–101. [DOI] [PubMed] [Google Scholar]
- 27. Nattel S, Maguy A, Le Bouter S, Yeh YH (2007) Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev 87: 425–456. [DOI] [PubMed] [Google Scholar]
- 28. Tomaselli GF, Marban E (1999) Electrophysiological remodeling in hypertrophy and heart failure. Cardiovasc Res 42: 270–283. [DOI] [PubMed] [Google Scholar]
- 29. Pelzer S, You Y, Shuba YM, Pelzer DJ (1997) Beta-adrenoceptor-coupled Gs protein facilitates the activation of cAMP-dependent cardiac Cl- current. Am J Physiol 273: H2539–2548. [DOI] [PubMed] [Google Scholar]
- 30. James AF, Tominaga T, Okada Y, Tominaga M (1996) Distribution of cAMP-activated chloride current and CFTR mRNA in the guinea pig heart. Circ Res 79: 201–207. [DOI] [PubMed] [Google Scholar]
- 31. Duan D (2009) Phenomics of cardiac chloride channels: the systematic study of chloride channel function in the heart. J Physiol 587: 2163–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zicha S, Moss I, Allen B, Varro A, Papp J, et al. (2003) Molecular basis of species-specific expression of repolarizing K+ currents in the heart. Am J Physiol Heart Circ Physiol 285: H1641–1649. [DOI] [PubMed] [Google Scholar]