Abstract
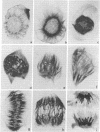
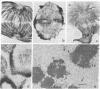
A procedure is presented for the immunocytochemical visualization of microtubules in interphase and mitotic cells of Haemanthus endosperm. It includes preservation of microtubules (MTs) with glutaraldehyde and uses colloidal gold, coated with secondary antibodies, in a novel indirect-light microscopic technique: the immuno-gold staining method. This immunocytochemical stain allows us to follow the changes in distribution of MTs during mitosis with greater precision and specificity than allowed by other light microscopic techniques. Many aspects of MT arrangements, as reported from ultrastructural studies, are corroborated and extended. This demonstrates the reliability of the technique. In addition, a number of significant observations were made. These concern (i) the presence of a network of MTs in interphase cells, (ii) the transformation of this network into a spindle-like cage of MTs (the clear zone) surrounding the nucleus during prophase, (iii) the drastic rearrangement of MT distribution during prometaphase, (iv) new evidence for the formation of aster-like arrays of polar MTs during anaphase, and (v) the development of the phragmoplast.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bajer A. S., Molè-Bajer J. Asters, poles, and transport properties within spindlelike microtubule arrays. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):263–283. doi: 10.1101/sqb.1982.046.01.029. [DOI] [PubMed] [Google Scholar]
- De Mey J., Moeremans M., Geuens G., Nuydens R., De Brabander M. High resolution light and electron microscopic localization of tubulin with the IGS (immuno gold staining) method. Cell Biol Int Rep. 1981 Sep;5(9):889–899. doi: 10.1016/0309-1651(81)90204-6. [DOI] [PubMed] [Google Scholar]
- Euteneuer U., McIntosh J. R. Polarity of some motility-related microtubules. Proc Natl Acad Sci U S A. 1981 Jan;78(1):372–376. doi: 10.1073/pnas.78.1.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Seib E., Herth W., Osborn M., Weber K. Reaction of the anastral mitotic apparatus of endosperm cells of the plant Leucojum aestivum with antibodies to tubulin from porcine brain as revealed by immunofluorescence microscopy. Cell Biol Int Rep. 1977 Jan;1(1):75–83. doi: 10.1016/0309-1651(77)90013-3. [DOI] [PubMed] [Google Scholar]
- Fuller G. M., Brinkley B. R., Boughter J. M. Immunofluorescence of mitotic spindles by using monospecific antibody against bovine brain tubulin. Science. 1975 Mar 14;187(4180):948–950. doi: 10.1126/science.1096300. [DOI] [PubMed] [Google Scholar]
- Geoghegan W. D., Ackerman G. A. Adsorption of horseradish peroxidase, ovomucoid and anti-immunoglobulin to colloidal gold for the indirect detection of concanavalin A, wheat germ agglutinin and goat anti-human immunoglobulin G on cell surfaces at the electron microscopic level: a new method, theory and application. J Histochem Cytochem. 1977 Nov;25(11):1187–1200. doi: 10.1177/25.11.21217. [DOI] [PubMed] [Google Scholar]
- Hepler P. K., Jackson W. T. Microtubules and early stages of cell-plate formation in the endosperm of Haemanthus katherinae Baker. J Cell Biol. 1968 Aug;38(2):437–446. doi: 10.1083/jcb.38.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horisberger M., Rosset J., Bauer H. Colloidal gold granules as markers for cell surface receptors in the scanning electron microscope. Experientia. 1975 Oct 15;31(10):1147–1149. doi: 10.1007/BF02326761. [DOI] [PubMed] [Google Scholar]
- Howard R. J., Aist J. R. Hyphal tip cell ultrastructure of the fungus Fusarium: improved preservation by freeze-substitution. J Ultrastruct Res. 1979 Mar;66(3):224–234. doi: 10.1016/s0022-5320(79)90120-5. [DOI] [PubMed] [Google Scholar]
- INOUE S., BAJER A. Birefringence in endosperm mitosis. Chromosoma. 1961;12:48–63. doi: 10.1007/BF00328913. [DOI] [PubMed] [Google Scholar]
- Inoué S., Fuseler J., Salmon E. D., Ellis G. W. Functional organization of mitotic microtubules. Physical chemistry of the in vivo equilibrium system. Biophys J. 1975 Jul;15(7):725–744. doi: 10.1016/S0006-3495(75)85850-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoué S., Sato H. Cell motility by labile association of molecules. The nature of mitotic spindle fibers and their role in chromosome movement. J Gen Physiol. 1967 Jul;50(6 Suppl):259–292. [PMC free article] [PubMed] [Google Scholar]
- Mazia D., Paweletz N., Sluder G., Finze E. M. Cooperation of kinetochores and pole in the establishment of monopolar mitotic apparatus. Proc Natl Acad Sci U S A. 1981 Jan;78(1):377–381. doi: 10.1073/pnas.78.1.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett-Heaps J. D., McDonald K. L., Tippit D. H. Cell division in the pennate diatom Diatoma vulgare. Protoplasma. 1975;86(1-3):205–242. doi: 10.1007/BF01275633. [DOI] [PubMed] [Google Scholar]
- Weber K., Pollack R., Bibring T. Antibody against tuberlin: the specific visualization of cytoplasmic microtubules in tissue culture cells. Proc Natl Acad Sci U S A. 1975 Feb;72(2):459–463. doi: 10.1073/pnas.72.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick S. M., Seagull R. W., Osborn M., Weber K., Gunning B. E. Immunofluorescence microscopy of organized microtubule arrays in structurally stabilized meristematic plant cells. J Cell Biol. 1981 Jun;89(3):685–690. doi: 10.1083/jcb.89.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]