Abstract
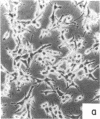
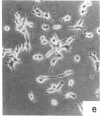
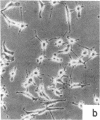
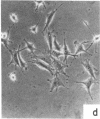
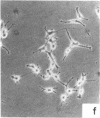
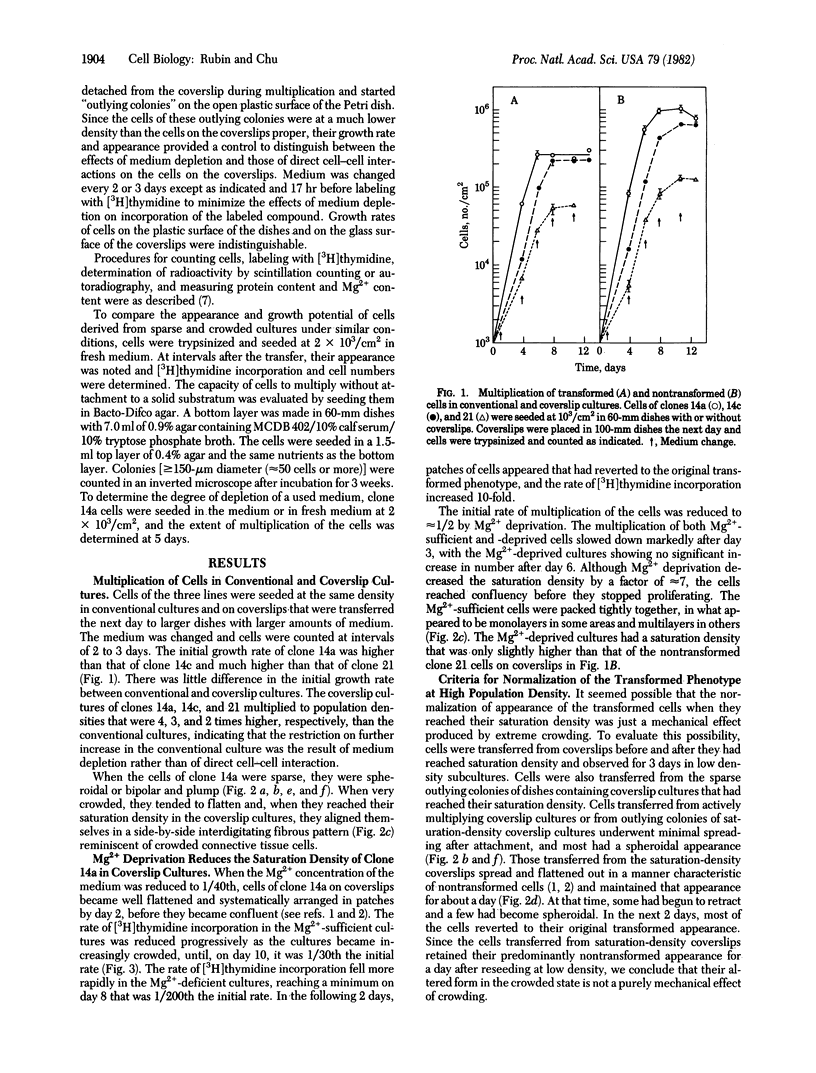
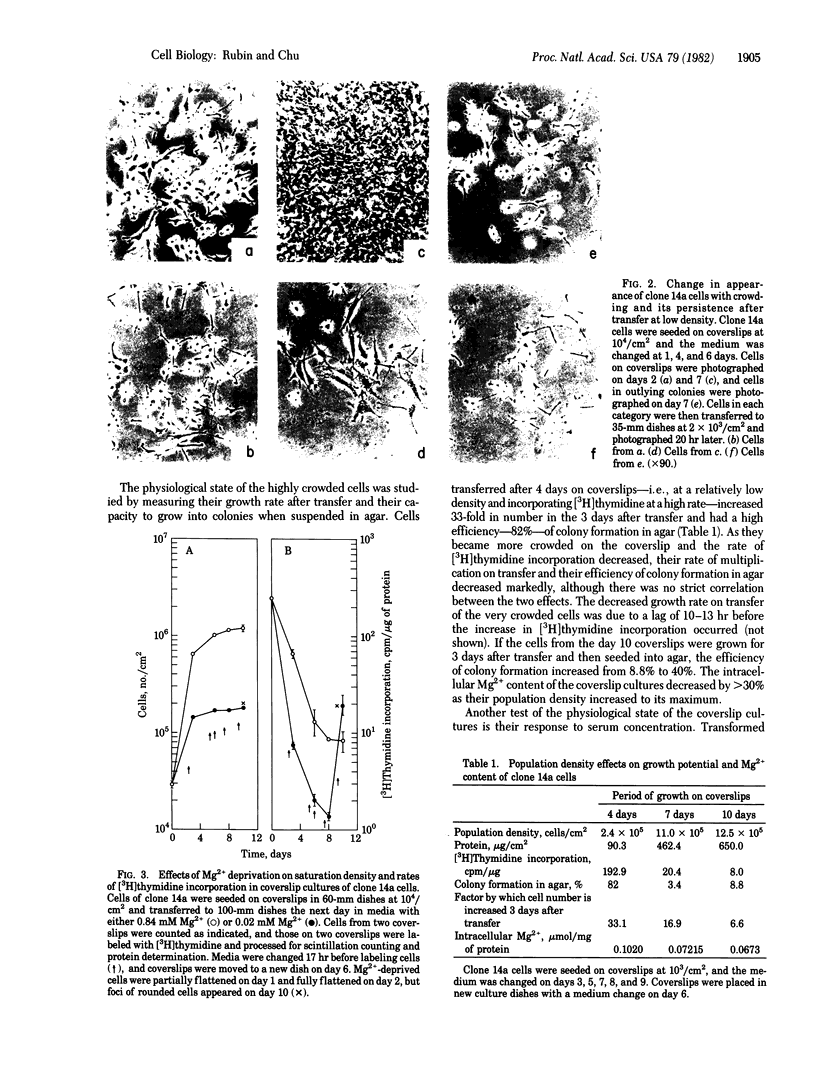
Nontransformed and moderately and highly transformed BALB/c 3T3 cells maintained on small coverslips in a large volume of medium multiplied to 2, 3, and 4 times higher population density, respectively, than they did in conventional cultures. Deprivation of Mg2+ caused highly transformed cells on coverslips to assume the appearance of nontransformed cells, decrease their rate of multiplication, and stop further growth at a much lower saturation density than the same cells in physiological Mg2+. The latter cells reached a saturation density of 10(6)/cm2 and their rate of DNA synthesis decreased progressively with increased crowding. At saturation density, cells in physiological Mg2+ took on an appearance and arrangement similar to normal fibroblasts. They developed a high requirement for serum to initiate DNA synthesis. When transferred at low density, they flattened out on a plastic surface and maintained the appearance of nontransformed cells for approximately 1 day. Onset of DNA synthesis and multiplication in the transferred cells was delayed for periods characteristic of quiescent nontransformed cells stimulated by fresh medium or transfer. Cells from crowded coverslips were approximately 1/10th as efficient at colony formation when suspended in agar as cells from uncrowded coverslips. They also had a significantly lower Mg2+ content. The crowded cells returned to their transformed morphological and growth behavior 2 to 3 days after transfer at low density. We conclude that a very high degree of crowding causes highly transformed cells to revert to the phenotype of nontransformed cells. Other treatments such as deprivation of Mg2+ or inorganic orthophosphate can achieve similar results. It appears that a balanced reduction in rates of metabolism and multiplication can restore the normal phenotype to transformed cells, implying that they differ only quantitatively from nontransformed cells. The putative role of Mg2+ in the regulation of multiplication and in transformation of animal cells is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartholomew J. C., Yokota H., Ross P. Effect of serum on the growth of Balb oT3 A31 mouse fibroblasts and an SV40-transformed derivative. J Cell Physiol. 1976 Jul;88(3):277–286. doi: 10.1002/jcp.1040880303. [DOI] [PubMed] [Google Scholar]
- Jetten A. M., Jetten M. E., Shapiro S. S., Poon J. P. Characterization of the action of retinoids on mouse fibroblast cell lines. Exp Cell Res. 1979 Mar 15;119(2):289–299. doi: 10.1016/0014-4827(79)90356-2. [DOI] [PubMed] [Google Scholar]
- Lindgren A., Westermark B. Serum requirement and density dependent inhibition of human malignant glioma cells in culture. Exp Cell Res. 1977 Feb;104(2):293–299. doi: 10.1016/0014-4827(77)90094-5. [DOI] [PubMed] [Google Scholar]
- Matsuhisa T., Mori Y., Tamura H. Phenotypic reversion of SV40-transformed 3T3 cells by dimethylsulfoxide. Cell Biol Int Rep. 1981 Feb;5(2):179–186. doi: 10.1016/0309-1651(81)90026-6. [DOI] [PubMed] [Google Scholar]
- Mintz B., Illmensee K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3585–3589. doi: 10.1073/pnas.72.9.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., Willingham M. Cellular transformation and the 'morphologic phenotype' of transformed cells. Nature. 1978 Aug 17;274(5672):645–650. doi: 10.1038/274645a0. [DOI] [PubMed] [Google Scholar]
- Rubin A. H., Terasaki M., Sanui H. Magnesium reverses inhibitory effects of calcium deprivation on coordinate response of 3T3 cells to serum. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4379–4383. doi: 10.1073/pnas.75.9.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H. Central role for magnesium in coordinate control of metabolism and growth in animal cells. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3551–3555. doi: 10.1073/pnas.72.9.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H. Growth regulation, reverse transformation, and adaptability of 3T3 cells in decreased Mg2+ concentration. Proc Natl Acad Sci U S A. 1981 Jan;78(1):328–332. doi: 10.1073/pnas.78.1.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H., Vidair C., Sanui H. Restoration of normal appearance, growth behavior, and calcium content to transformed 3T3 cells by magnesium deprivation. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2350–2354. doi: 10.1073/pnas.78.4.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOKER M. REGULATION OF GROWTH AND ORIENTATION IN HAMSTER CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Oct;24:165–174. doi: 10.1016/0042-6822(64)90099-6. [DOI] [PubMed] [Google Scholar]
- Sanui H., Rubin H. Correlated effects of external magnesium on cation content and DNA synthesis in culture chicken embryo fibroblasts. J Cell Physiol. 1977 Jul;92(1):23–31. doi: 10.1002/jcp.1040920104. [DOI] [PubMed] [Google Scholar]
- Shipley G. D., Ham R. G. Improved medium and culture conditions for clonal growth with minimal serum protein and for enhanced serum-free survival of Swiss 3T3 cells. In Vitro. 1981 Aug;17(8):656–670. doi: 10.1007/BF02628401. [DOI] [PubMed] [Google Scholar]
- Stoker M. G., Shearer M., O'Neill C. Growth inhibition of polyoma-transformed cells by contact with static normal fibroblasts. J Cell Sci. 1966 Sep;1(3):297–310. doi: 10.1242/jcs.1.3.297. [DOI] [PubMed] [Google Scholar]