Abstract
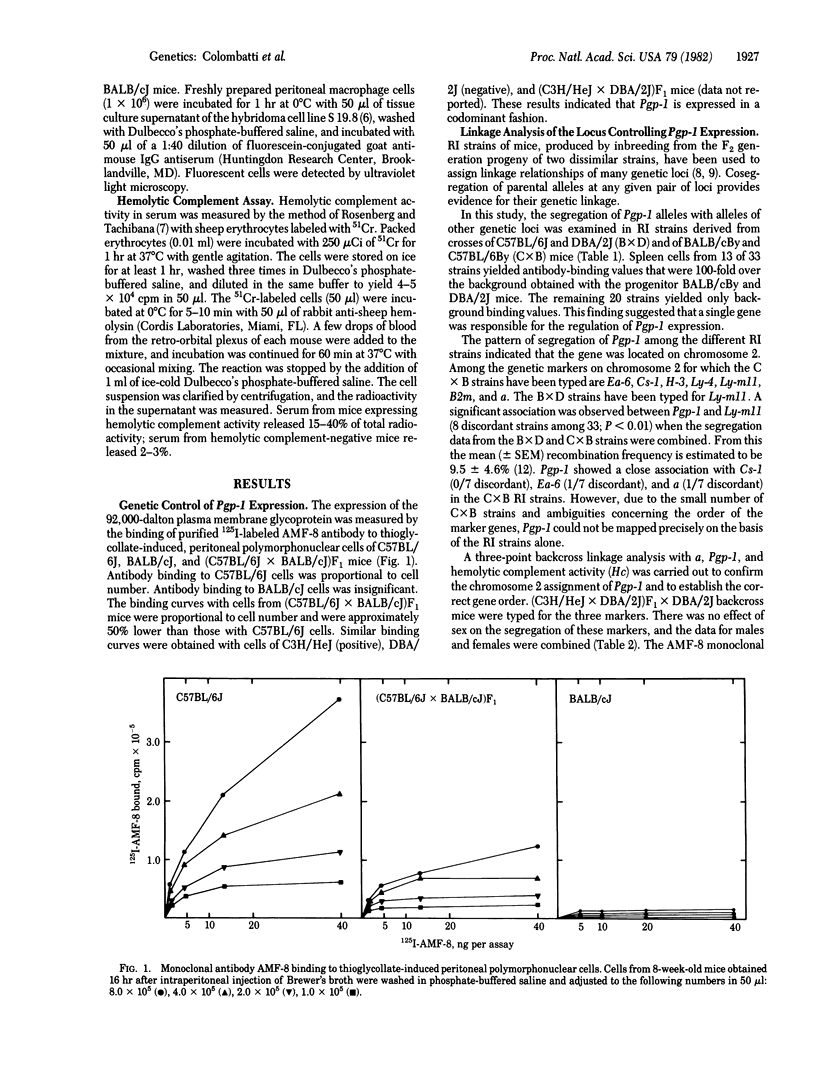
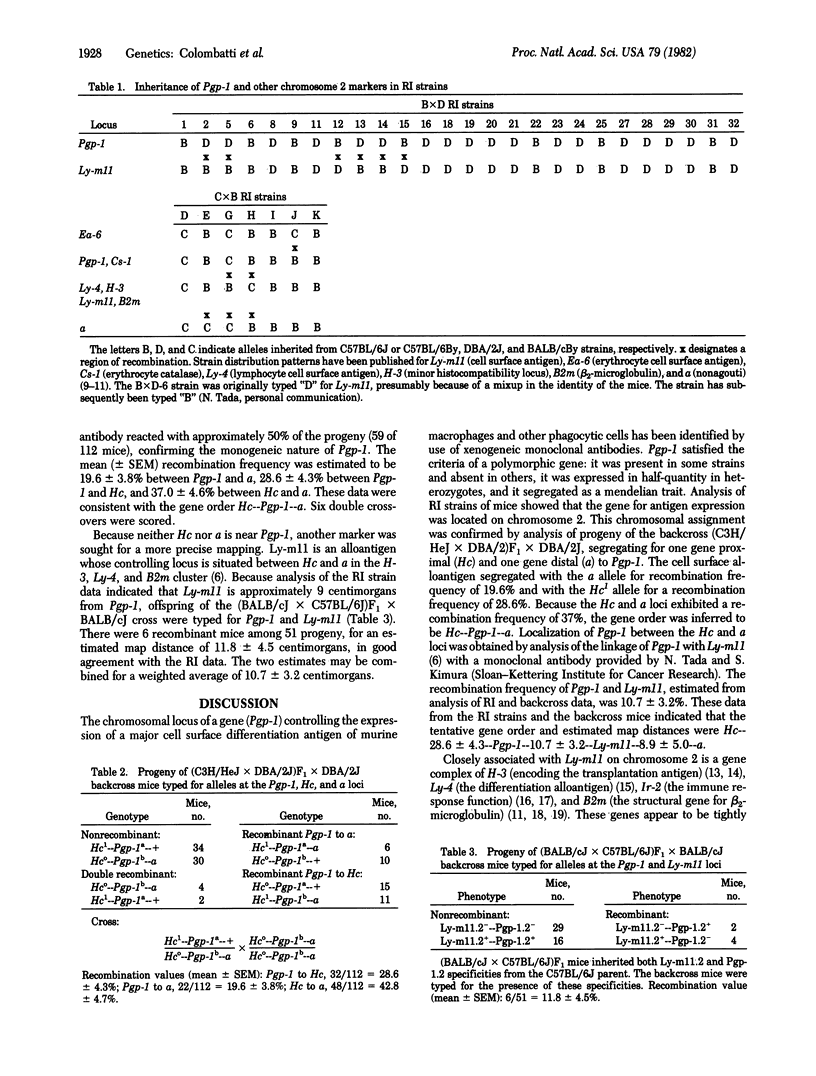
A gene controlling the expression of a polymorphic 92,000-dalton glycoprotein of mouse macrophages and granulocytes has been identified. This glycoprotein was previously shown to be the major iodinated, trypsin-sensitive component of the murine phagocyte cell surface. The gene has been provisionally designated Pgp-1 for phagocyte glycoprotein 1. Expression of the glycoprotein was measured by monoclonal antibody binding to a polymorphic antigenic determinant. Antibody binding to cells of positive strains of mice was proportional to cell number, whereas binding to cells of negative strains was insignificant. The concentration of the antigen in cells of heterozygous mice was approximately 50% of that in homozygous mice. Thirteen of 33 recombinant inbred strains of mice were positive, with binding values 100-fold over background, suggesting that a single gene controlled expression of the antigen. Segregation of the antigen correlated with markers on chromosome 2. The segregation of Pgp-1, with nonagouti coat color (a) and hemolytic complement (Hc) activity among progeny of (C3H/HeJ x DBA/2J)F1 x DBA/2J mice confirmed the single gene control and the chromosomal assignment. Another gene on chromosome 2, Ly-m11, was also typed by using (BALB/cJ x C57BL/6J)F1 x C57BL/6J mice. The data from both of these crosses indicated the following gene order: Hc--Pgp-1--Ly-m11--a.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey D. W. Recombinant-inbred strains. An aid to finding identity, linkage, and function of histocompatibility and other genes. Transplantation. 1971 Mar;11(3):325–327. doi: 10.1097/00007890-197103000-00013. [DOI] [PubMed] [Google Scholar]
- Benacerraf B. Role of MHC gene products in immune regulation. Science. 1981 Jun 12;212(4500):1229–1238. doi: 10.1126/science.6165083. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S., Thomson J. N., Pearse B. M. Coated pits act as molecular filters. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4156–4159. doi: 10.1073/pnas.77.7.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Gasser D. L. Genetic control of the immune response in mice. I. Segregation data and localization to the fifth linkage group of a gene affecting antibody production. J Immunol. 1969 Jul;103(1):66–70. [PubMed] [Google Scholar]
- Goding J. W. Evidence for linkage of murine beta 2-microglobulin to H-3 and Ly-4. J Immunol. 1981 Apr;126(4):1644–1646. [PubMed] [Google Scholar]
- Goding J. W., Walker I. D. Allelic forms of beta 2-microglobulin in the mouse. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7395–7399. doi: 10.1073/pnas.77.12.7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff R. J., Polinsky S. L., Snell G. D. Histocompatibility genes of mice. X. Additional non-H-2 typing. Transplantation. 1971 Jan;11(1):56–62. doi: 10.1097/00007890-197101000-00008. [DOI] [PubMed] [Google Scholar]
- Graff R. J., Snell G. D. Histocompatibility typing of inbred mice for known non-H-2 alleles. Transplant Proc. 1969 Mar;1(1):362–364. [PubMed] [Google Scholar]
- Hughes E. N., August J. T. Characterization of plasma membrane proteins identified by monoclonal antibodies. J Biol Chem. 1981 Jan 25;256(2):664–671. [PubMed] [Google Scholar]
- Hughes E. N., Mengod G., August J. T. Murine cell surface glycoproteins. Characterization of a major component of 80,000 daltons as a polymorphic differentiation antigen of mesenchymal cells. J Biol Chem. 1981 Jul 10;256(13):7023–7027. [PubMed] [Google Scholar]
- Hämmerling G. J., Hämmerling U., Flaherty L. Qat-4 and Qat-5, new murine T-cell antigens governed by the Tla region and identified by monoclonal antibodies. J Exp Med. 1979 Jul 1;150(1):108–116. doi: 10.1084/jem.150.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura K., Hutton J. J., Boyse E. A., Old L. J. Genetic linkage relationships of loci specifying differentiation alloantigens in the mouse. Transplantation. 1972 Mar;13(3):239–243. doi: 10.1097/00007890-197203000-00007. [DOI] [PubMed] [Google Scholar]
- Lerner E. A., Matis L. A., Janeway C. A., Jr, Jones P. P., Schwartz R. H., Murphy D. B. Monoclonal antibody against an Ir gene product? J Exp Med. 1980 Oct 1;152(4):1085–1101. doi: 10.1084/jem.152.4.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D. W., Williams A. F. The kinetics of antibody binding to membrane antigens in solution and at the cell surface. Biochem J. 1980 Apr 1;187(1):1–20. doi: 10.1042/bj1870001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie I. F., Potter T. Murine lymphocyte surface antigens. Adv Immunol. 1979;27:179–338. doi: 10.1016/s0065-2776(08)60263-1. [DOI] [PubMed] [Google Scholar]
- Mellman I. S., Unkeless J. C. Purificaton of a functional mouse Fc receptor through the use of a monoclonal antibody. J Exp Med. 1980 Oct 1;152(4):1048–1069. doi: 10.1084/jem.152.4.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengod G., Hughes E. N., August J. T. Murine cell surface glycoproteins: immunochemical analysis of a major differentiation alloantigen of phagocytic cells. Arch Biochem Biophys. 1981 Jul;209(2):718–722. doi: 10.1016/0003-9861(81)90335-0. [DOI] [PubMed] [Google Scholar]
- Michaelson J. Genetic polymorphism of beta 2-microglobulin (B2m) maps to the H-3 region of chromosome 2. Immunogenetics. 1981;13(1-2):167–171. doi: 10.1007/BF00524613. [DOI] [PubMed] [Google Scholar]
- ROSENBERG L. T., TACHIBANA D. K. Activity of mouse complement. J Immunol. 1962 Dec;89:861–867. [PubMed] [Google Scholar]
- Silverstein S. C., Steinman R. M., Cohn Z. A. Endocytosis. Annu Rev Biochem. 1977;46:669–722. doi: 10.1146/annurev.bi.46.070177.003321. [DOI] [PubMed] [Google Scholar]
- Snell G. D., Cherry M., McKenzie I. F., Bailey D. W. Ly-4, a new locus determining a lymphocyte cell-surface alloantigen in mice. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1108–1111. doi: 10.1073/pnas.70.4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Monoclonal antibody analysis of complex biological systems. Combination of cell hybridization and immunoadsorbents in a novel cascade procedure and its application to the macrophage cell surface. J Biol Chem. 1981 Apr 25;256(8):3833–3839. [PubMed] [Google Scholar]
- Springer T., Galfré G., Secher D. S., Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- Sunderland C. A., McMaster W. R., Williams A. F. Purification with monoclonal antibody of a predominant leukocyte-common antigen and glycoprotein from rat thymocytes. Eur J Immunol. 1979 Feb;9(2):155–159. doi: 10.1002/eji.1830090212. [DOI] [PubMed] [Google Scholar]
- Tada N., Kimura S., Hatzfeld A., Hämmerling U. Ly-m11: the H-3 region of mouse chromosome 2 controls a new surface alloantigen. Immunogenetics. 1980;11(5):441–449. doi: 10.1007/BF01567813. [DOI] [PubMed] [Google Scholar]
- Taylor B. A., Bailey D. W., Cherry M., Riblet R., Weigert M. Genes for immunoglobulin heavy chain and serum prealbumin protein are linked in mouse. Nature. 1975 Aug 21;256(5519):644–646. doi: 10.1038/256644a0. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S. Interspecies spleen-myeloma hybrid producing monoclonal antibodies against mouse lymphocyte surface glycoprotein, T200. J Exp Med. 1978 Jul 1;148(1):313–323. doi: 10.1084/jem.148.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkeless J. C. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979 Sep 19;150(3):580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman S. H., Douglas S. D. Dynamics of the macrophage plasma membrane. Annu Rev Microbiol. 1979;33:267–307. doi: 10.1146/annurev.mi.33.100179.001411. [DOI] [PubMed] [Google Scholar]


