Abstract
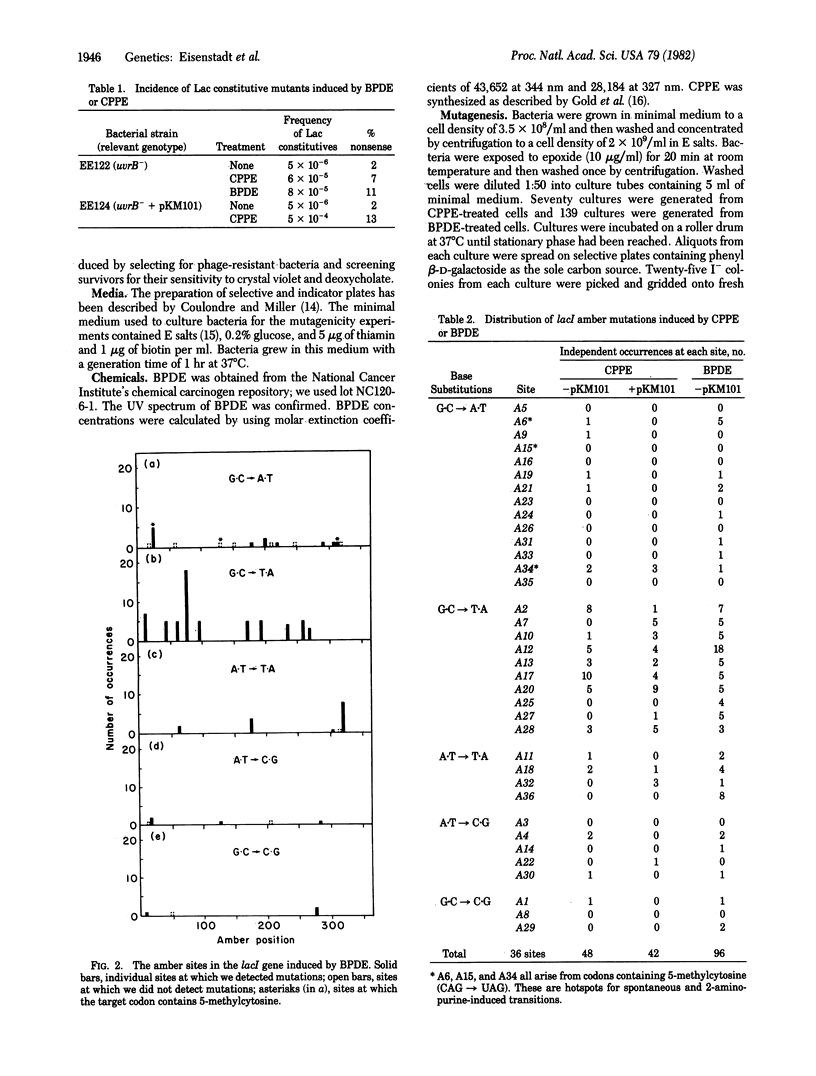
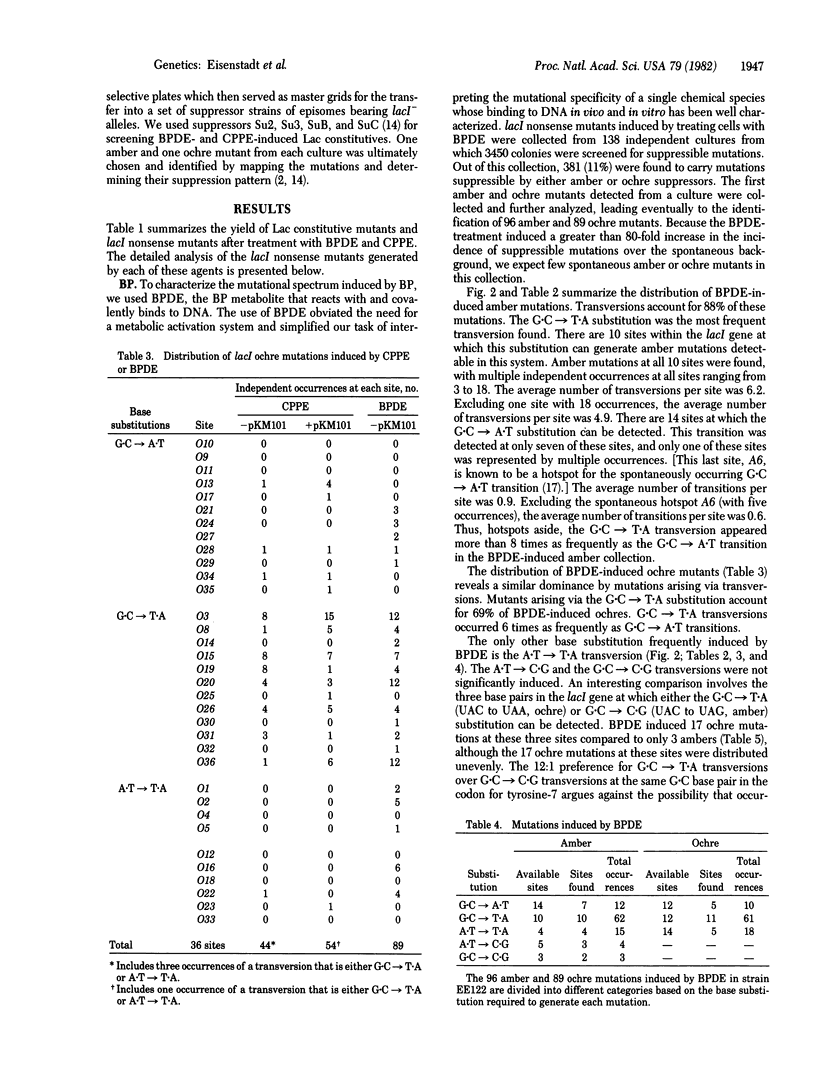
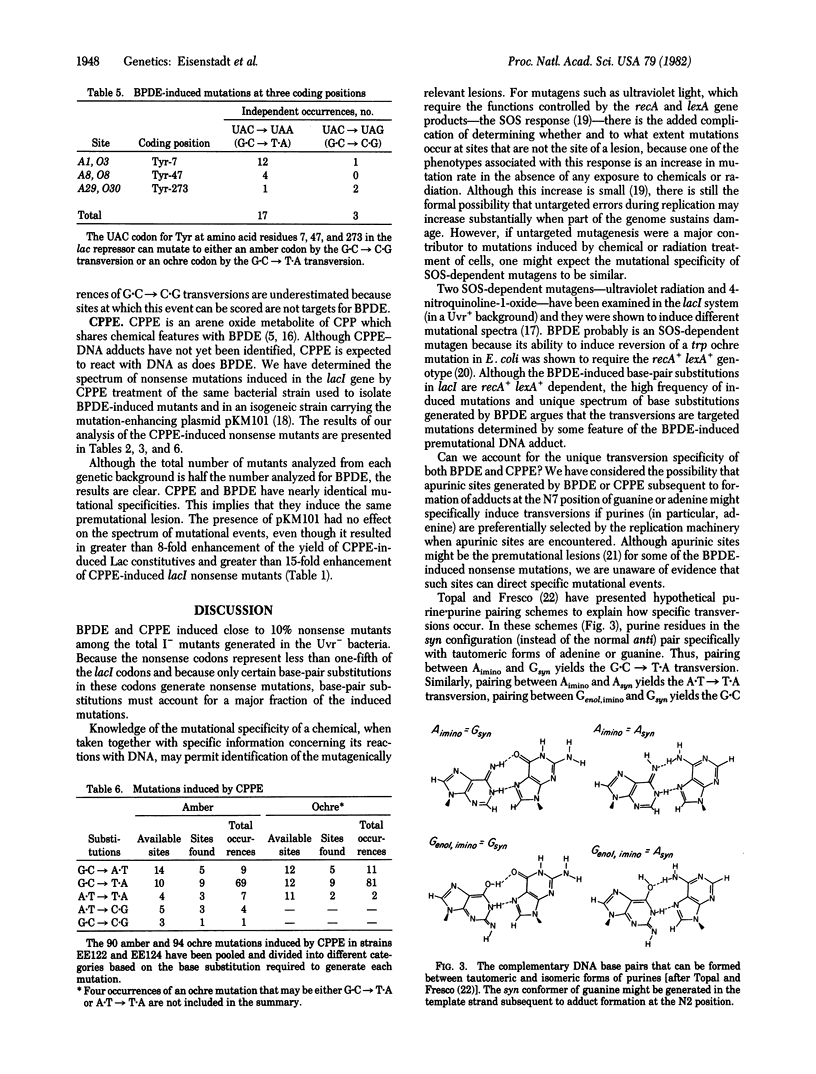
We have determined the spectrum of base-pair substitution mutations induced in the lacI gene of a uvrB- strain of Escherichia coli by two polycyclic aromatic hydrocarbons--(+/-)7 alpha,8 beta-dihydroxy-9 beta,10 beta-epoxy-7,8,9,10 tetrahydrobenzo[a]pyrene (BPDE), and 3,4-epoxycylopenta[cd]pyrene (CPPE). Approximately 10% of all lacI mutations induced by either BPDE or CPPE are nonsense mutations, suggesting that base-pair substitutions are a large fraction of the mutational events induced by these agents in the uvrB- bacteria. Both carcinogens specifically induced the G . C leads to T . A and, to a lesser extent, the A . T leads to T . A transversions. One possible mechanism for transversion induction at G . C sites by BPDE might involve carcinogen binding to the exocyclic amino group of guanine in the template strand followed by a rotation of the modified base around its glycosylic bond from the anti to the syn conformation. This could allow specific pairing of modified bases with an imino tautomer of adenine.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Durston W. E., Yamasaki E., Lee F. D. Carcinogens are mutagens: a simple test system combining liver homogenates for activation and bacteria for detection. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2281–2285. doi: 10.1073/pnas.70.8.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROOKES P., LAWLEY P. D. EVIDENCE FOR THE BINDING OF POLYNUCLEAR AROMATIC HYDROCARBONS TO THE NUCLEIC ACIDS OF MOUSE SKIN: RELATION BETWEEN CARCINOGENIC POWER OF HYDROCARBONS AND THEIR BINDING TO DEOXYRIBONUCLEIC ACID. Nature. 1964 May 23;202:781–784. doi: 10.1038/202781a0. [DOI] [PubMed] [Google Scholar]
- Coulondre C., Miller J. H. Genetic studies of the lac repressor. III. Additional correlation of mutational sites with specific amino acid residues. J Mol Biol. 1977 Dec 15;117(3):525–567. doi: 10.1016/0022-2836(77)90056-0. [DOI] [PubMed] [Google Scholar]
- Coulondre C., Miller J. H. Genetic studies of the lac repressor. IV. Mutagenic specificity in the lacI gene of Escherichia coli. J Mol Biol. 1977 Dec 15;117(3):577–606. doi: 10.1016/0022-2836(77)90059-6. [DOI] [PubMed] [Google Scholar]
- Eisenstadt E., Gold A. Cyclopenta[c,d]pyrene: a highly mutagenic polycyclic aromatic hydrocarbon. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1667–1669. doi: 10.1073/pnas.75.4.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fersht A. R., Knill-Jones J. W. DNA polymerase accuracy and spontaneous mutation rates: frequencies of purine.purine, purine.pyrimidine, and pyrimidine.pyrimidine mismatches during DNA replication. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4251–4255. doi: 10.1073/pnas.78.7.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamper H. B., Tung A. S., Straub K., Bartholomew J. C., Calvin M. DNA strand scission by benzo[a]pyrene diol epoxides. Science. 1977 Aug 12;197(4304):671–674. doi: 10.1126/science.877583. [DOI] [PubMed] [Google Scholar]
- Huberman E., Aspiras L., Heidelberger C., Grover P. L., Sims P. Mutagenicity to mammalian cells of epoxides and other derivatives of polycyclic hydrocarbons. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3195–3199. doi: 10.1073/pnas.68.12.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanovic V., Weinstein I. B. Genetic factors in Escherichia coli that affect cell killing and mutagenesis induced by benzo(a)pyrene-7,8-dihydrodiol 9,10-oxide. Cancer Res. 1980 Oct;40(10):3508–3511. [PubMed] [Google Scholar]
- Jeffrey A. M., Grzeskowiak K., Weinstein I. B., Nakanishi K., Roller P., Harvey R. G. Benzo(a)pyrene-7,8-dihydrodiol 9,10-oxide adenosine and deoxyadenosine adducts: structure and stereochemistry. Science. 1979 Dec 14;206(4424):1309–1311. doi: 10.1126/science.316186. [DOI] [PubMed] [Google Scholar]
- Jeffrey A. M., Weinstein I. B., Jennette K. W., Grzeskowiak K., Nakanishi K., Harvey R. G., Autrup H., Harris C. Structures of benzo(a)pyrene--nucleic acid adducts formed in human and bovine bronchial explants. Nature. 1977 Sep 22;269(5626):348–350. doi: 10.1038/269348a0. [DOI] [PubMed] [Google Scholar]
- Kadlubar F. F. A transversion mutation hypothesis for chemical carcinogenesis by N2-substitution of guanine in DNA. Chem Biol Interact. 1980 Sep;31(3):255–263. doi: 10.1016/0009-2797(80)90014-9. [DOI] [PubMed] [Google Scholar]
- McCann J., Choi E., Yamasaki E., Ames B. N. Detection of carcinogens as mutagens in the Salmonella/microsome test: assay of 300 chemicals. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5135–5139. doi: 10.1073/pnas.72.12.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann J., Spingarn N. E., Kobori J., Ames B. N. Detection of carcinogens as mutagens: bacterial tester strains with R factor plasmids. Proc Natl Acad Sci U S A. 1975 Mar;72(3):979–983. doi: 10.1073/pnas.72.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan T., Straub K., Calvin M. Benzo[alpha]pyrene diol epoxide covalently binds to deoxyguanosine and deoxyadenosine in DNA. Nature. 1977 Oct 20;269(5630):725–727. doi: 10.1038/269725a0. [DOI] [PubMed] [Google Scholar]
- Osborne M. R., Harvey R. G., Brookes P. The reaction of trans-7,8-dihydroxy-anti-9,10-epoxy-7,8,9,10-tetrahydrobenzo(a)pyrene with DNA involves attack at the N7-position of guanine moieties. Chem Biol Interact. 1978 Jan;20(1):123–130. doi: 10.1016/0009-2797(78)90087-x. [DOI] [PubMed] [Google Scholar]
- Schaaper R. M., Loeb L. A. Depurination causes mutations in SOS-induced cells. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1773–1777. doi: 10.1073/pnas.78.3.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha N. K., Haimes M. D. Molecular mechanisms of substitution mutagenesis. An experimental test of the Watson-Crick and topal-fresco models of base mispairings. J Biol Chem. 1981 Oct 25;256(20):10671–10683. [PubMed] [Google Scholar]
- Topal M. D., Fresco J. R. Complementary base pairing and the origin of substitution mutations. Nature. 1976 Sep 23;263(5575):285–289. doi: 10.1038/263285a0. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Weinstein I. B., Jeffrey A. M., Jennette K. W., Blobstein S. H., Harvey R. G., Harris C., Autrup H., Kasai H., Nakanishi K. Benzo(a)pyrene diol epoxides as intermediates in nucleic acid binding in vitro and in vivo. Science. 1976 Aug 13;193(4253):592–595. doi: 10.1126/science.959820. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood A. W., Levin W., Chang R. L., Huang M. T., Ryan D. E., Thomas P. E., Lehr R. E., Kumar S., Koreeda M., Akagi H. Mutagenicity and tumor-initiating activity of cyclopenta(c,d)pyrene and structurally related compounds. Cancer Res. 1980 Mar;40(3):642–649. [PubMed] [Google Scholar]


