Abstract
Background: There are variable observations in published literature regarding smoking status and stage of lung cancer (LC) with positive, negative and no associations being reported. In particular, data regarding the association of quantified smoking status (QSS) with non-small cell lung cancer (NSCLC) stage at the time of diagnosis is limited. In India, bidi - the hand rolled form of tobacco wrapped in the dried tendu leaf - is the most common smoking product. The current study was conducted to assess stage differences, if any, based upon QSS, among newly diagnosed LC patients.
Methods
A systematic review of English literature was performed for previous publications that had assessed NSCLC stage differences in relation to QSS. Collected data on demographic and disease characteristics of 654 LC patients presenting to the authors’ institute was also analyzed. Smoking index (SI) was used for QSS and was defined as number of bidis and cigarettes smoked per day multiplied by years smoked. Patients were categorized as never-smokers [Group I, n=151]; light/moderate smokers (SI=1-300) [Group II, n=202] and heavy smokers (SI ≥301) [Group III, n=301]. Multivariate logistic regression analysis (LRA) was performed to derive adjusted odds ratios (ORs) and 95% confidence intervals (CIs).
Results
Among the 520 NSCLC patients, mean [standard deviation (SD)] age in groups I, II and III was 54.5 (12.5), 58.6 (9.9) and 61.2 (9.4) years respectively (P<0.001). Percentage of males in the three groups was 48.1%, 88.0%, and 97.9% (P<0.001). Age and gender differences between groups I, II and III were also significant among 134 small cell lung cancer patients with mean (SD) ages of 44.0 (10.6), 55.7 (10.3) and 58.9 (9.3) years (P<0.001) and percentage of males being 50.0%, 90.4% and 95.5% respectively (P<0.001). Among NSCLC patients, distribution in groups I, II and III respectively of squamous (28.1%, 50.0% and 57.9%) and non-squamous histologies (59.3%, 37.3% and 27.2%) differed significantly (P<0.001). Stage distribution observed for NSCLC patients in groups I, II and III respectively was as follows: stages I-IIIA (8.1%, 19.3 and 18.7%), stage IIIB (24.4%, 34.7% and 42.1%) and stage IV (67.4%, 46.0% and 39.1%). The difference was statistically significant (P<0.001). Differences remained significant (P<0.001) for presence of extrathoracic disease [ETD] (41.5%, 28.0% and 16.6%). On multivariate LRA, SI ≥301 was the only variable that was independently associated with both advanced stage (IIIB-IV) [OR=0.25 (95% CI=0.11-0.61)] and ETD [OR=0.29 (95% CI=0.16-0.53)] at presentation.
Conclusions
Among newly diagnosed NSCLC patients in North India, significant differences exist, based upon SI, for disease stage. Heavy smoking was independently associated with lower odds of having advanced stage as well as with lower odds of having ETD at the time of diagnosis. This observation of the current study however requires confirmation by larger prospective studies.
KEY WORDS : Non-small cell lung cancer, stage, smoking index, systematic review, extrathoracic disease
Background
Tobacco smoking remains the most important risk factor for development of lung cancer (LC). There are variable observations in published literature regarding smoking status and stage of lung cancer (LC) with positive, negative and no associations being reported (1-3). There is also paucity of data from South Asia in relation to the association between quantified smoking status (QSS) and LC stage at the time of diagnosis.
Prior work by our group on the assessment of the current clinico-epidemiological profile of LC patients has shown that no significant differences exist in the demographical, histological or smoking profiles compared to those seen three decades earlier. However, statistically significant differences were observed between current/ex-smokers and never-smokers in relation to non-small cell LC (NSCLC) stage (4). We, therefore, postulated that smoking status could be associated with disease stage among newly diagnosed NSCLC patients in a quantitative manner. The current study was conducted to assess the relationship between QSS and NSCLC stage at presentation.
Methods
Study population
Consecutive newly diagnosed patients, with cytologically or histopathologically proven LC, who were initiated on treatment at the Lung Cancer Clinic of the authors’ institute, a tertiary level referral health care facility in North India, between January 2008 to June 2011 comprised the study population. Data regarding demographic characteristics [including age, gender and performance status (PS)], histological type, stage of disease and details of smoking status was collected for all patients.
Quantification of smoking
Quantification of smoking was done using the smoking index (SI). As previously published by the authors, SI was defined as the number of bidis/cigarettes smoked per day multiplied by the number of years smoked (5,6). The concept of using SI for quantification of smoke exposure is based on this fact that bidi - the hand rolled form of tobacco wrapped in the dried tendu leaf - is the most common smoking product in India (7). Moreover, the number of bidis in a given pack is variable in contrast to cigarettes since the former is a cottage industry with much less standardization in its manufacturing process. It has been shown in previous studies that bidis and cigarettes are associated with similar risks in relation to lung cancer and that for calculating time-intensity tobacco smoke exposure, one bidi should be considered to be equivalent to one cigarette (8-10). Based upon SI, patients were categorized into the following groups:
-
Never-smokers
IIa. Light smokers [SI=1-100]
-
IIb. Moderate smokers [SI=101-300]
Heavy smokers [SI≥301]
Staging
In order to maintain uniformity, staging for non-small cell cancer (NSCLC) cases was done using the 6th edition of the TNM classification based on tumor size and extension (T), lymph nodal involvement (N), and presence of distant metastasis (M) (11,12). Small cell lung cancer (SCLC) was staged as either limited (disease restricted to one hemithorax, with or without regional lymph node metastases and/or ipsilateral pleural effusion) or extensive (13).
Histological classification
For all cases, the cytological and/or histolopathological examination of aspirated/biopsied tissue specimen(s) was done and the diagnosis of lung cancer established at the authors’ institute. The cases were classified on the basis of morphology into different types using the WHO classification of lung tumors (14) as:
-
Non-small cell carcinoma (NSCLC)
Squamous cell carcinoma
Adenocarcinoma
Large cell carcinoma
Undifferentiated
Small cell carcinoma (SCLC)
Miscellaneous
Statistical analysis
Descriptive data is presented as mean [standard deviation (SD)], median [inter-quartile range (IQR)] and as percentages. Numerical and categorical data were compared between groups using one way analysis of variance (ANOVA) and chi-square test respectively. Logistic regression analysis was performed to derive odds ratios (ORs) and 95% confidence intervals (CIs). Initially, the variables were analyzed using univariate analysis to derive crude ORs, and if found significant (P<0.10) these variables were then entered in a multivariate model to derive adjusted ORs and 95% CIs. Variables that were considered clinically relevant, even if they were not found to be significant on univariate analysis, were included in the multivariate model. Two logistic regression models were used for NSCLC stage and for presence of extra-thoracic disease in NSCLC. In one model, the histology variable was dichotomous [squamous and non-squamous] with non-squamous group consisting of adenocarcinoma (ADC), large cell carcinoma (LCC) and undifferentiated NSCLC (NSCLC-Undiff). In the other model, the histology variable was trichotomous [squamous, ADC plus LCC and NSCLC-Undiff]. All analyses were performed using the statistical software SPSS version 11.0 (SPSS Inc., Chicago, IL, USA).
Systematic literature review
A systematic review of English literature was also performed for all previous publications that had assessed NSCLC stage differences in relation to QSS. This was done by a PUBMED (National Library of Medicine, Bethesda, MD) search of all relevant articles published in the English language from 1975 (first citation result) to August 2011. Details of literature search and data extraction are provided in Supplementary Appendix.
Results
A total of 674 patients were initiated on treatment during the study period. Of these, 20 patients had non-bronchogenic tumors and were excluded from analysis. The demographic profile of the 654 patients who comprised the study population is represented in Table 1. The male to female ratio was 5:1 while that of current/ex-smokers to never-smokers was 3.3:1.
Table 1. Demographical, histology and disease stage profile of 654 patients who comprised the study population.
| Age [years; mean (SD)] | 58.1 (10.8) |
|---|---|
| Gender | |
| • Males | 545 (83.3%) |
| • Females | 109 (16.7%) |
| Smoking Status | |
| • Never-smokers | 151 (23.1%) |
| • Current/ex-smokers | 503 (76.9%) |
| Karnofsky performance status* | |
| • 100 | 251 (39.2%) |
| • 90 | 141 (22.0%) |
| • 80 | 110 (17.2%) |
| • ≤70 | 138 (21.6%) |
| Histology | |
| • Squamous cell | 249 (38.1%) |
| • Adenocarcinoma | 180 (27.5%) |
| • Large cell carcinoma | 20 (3.1%) |
| • NSCLC-Undiff | 71 (10.9%) |
| • Small cell | 134 (20.5%) |
| Extrathoracic disease | 174 (26.6%) |
| Non-small cell lung cancer | (n=520) |
| • T group | |
| ➢ T1 | 23 (4.4%) |
| ➢ T2 | 52 (10.0%) |
| ➢ T3 | 156 (30.0%) |
| ➢ T4 | 289 (55.6%) |
| • N group | |
| ➢ N0 | 81 (15.6%) |
| ➢ N1 | 65 (12.5%) |
| ➢ N2 | 222 (42.7%) |
| ➢ N3 | 152 (29.2%) |
| • M Stage | |
| ➢ M0 | 268 (51.5%) |
| ➢ M1 | 252 (48.5%) |
| • Stage | |
| ➢ I-II | 16 (3.1%) |
| ➢ IIIA | 68 (13.1%) |
| ➢ IIIB | 184 (35.4%) |
| ➢ IV | 252 (48.5%) |
| Small cell lung cancer | (n=134) |
| • Stage | |
| ➢ Limited disease | 60 (44.8%) |
| ➢ Extensive disease | 74 (55.2%) |
Data is presented as number (percentage) unless specified otherwise; *Details available for 640 patients; SD = Standard deviation, NSCLC-Undiff = Undifferentiated NSCLC.
Description of smoking patterns
Majority of current/ex-smokers were exclusively bidi smokers (n=286, 56.9%). Exclusive cigarette smoking was seen in 65 (12.9%) patients while other traditional Indian smoking products like ‘hookah’ were used exclusively by 16 (3.2%) current/ex-smokers. Combined bidi and cigarette smoking was seen in 136 (27.0%) current/ex-smokers. The median (IQR) smoking index was 435 [240-750], 400 [250-700] and 400 [250-600] for exclusive bidi smokers, exclusive cigarette smokers and combined bidi-cigarette smokers respectively. The distribution of exclusive bidi smokers, exclusive cigarette smokers and combined bidi-cigarette smokers did not differ between Groups II and III. Exclusive bidi smokers constituted 56.5% (n=105) and 60.1% (n=181) of patients in Groups II and III respectively while exclusive cigarette smokers constituted 14.0% (n=26) and 13.0% (n=39) respectively. The pattern of combined bidi-cigarette smoking was observed in 29.6% (n=55) and 26.9% (n=81) of patients in Groups II and III respectively. Time since quitting was available for 159 patients who had quit smoking prior to diagnosis. The median (IQR) time of quitting smoking was 4.0 months [range, 1.5-24.0 months].
The distribution of patient population into groups based upon SI was as follows: never-smokers [Group I, n=151]; light smokers (SI=1-100) [Group IIa, n=59]; moderate smokers (SI=101-300) [Group IIb, n=143] and heavy smokers (SI≥301) [Group III, n=301]. For purpose of analysis, Groups IIa and IIb were merged into Group II.
Group comparisons in non-small cell lung cancer
Significant differences were observed among the three groups (Table 2) in relation to age and gender distribution but not for baseline Karnofsky PS. Distribution of histological types in groups I, II and III respectively was significantly different (Table 2). In group I, adenocarcinoma (56.3%) was the commonest histology whereas in groups II and III, it was squamous cell (50.0% and 57.9% respectively). Statistically significant differences (P<0.001) were also observed among the three groups (Table 2) in relation to stage of disease at presentation. Highest percentage of stage IV disease (67.4%) was seen in group I. Group differences were statistically significant (P<0.001) when assessed for presence of extrathoracic disease [ETD] also - 41.5%, 28.0% and 16.6% in groups I, II and III respectively. The tumor (T) and nodal (N) stage distribution did not differ among groups.
Table 2. Demographical, histology and disease stage profile among non-small cell lung cancer patients grouped on the basis of smoking index (SI).
| Total | Group I never-smokers |
Group II smoking index=1-300 |
Group III smoking index≥301 | P value | ||
|---|---|---|---|---|---|---|
| Number of cases | 520 | 135 | 150 | 235 | ||
| Age [years Mean (SD)] | 58.7 (10.8) | 54.5 (12.5) | 58.6 (9.9) | 61.2 (9.4) | <0.001 | |
| Males | 427 (82.1%) | 65 (48.1%) | 132 (88.0%) | 230 (97.9%) | <0.001 | |
| Karnofsky PS** | 0.442 | |||||
| • 100 | 202 (39.9%) | 52 (40.0%) | 54 (37.0%) | 96 (41.7%) | ||
| • 90 | 114 (22.5%) | 32 (24.6%) | 32 (21.9%) | 50 (21.7%) | ||
| • 80 | 79 (15.6%) | 13 (10.0%) | 27 (18.5%) | 39 (17.0%) | ||
| • ≤70 | 111 (21.9%) | 33 (25.4%) | 33 (22.6%) | 45 (19.6%) | ||
| Histology | <0.001 | |||||
| • Squamous cell | 249 (47.9%) | 38 (28.1%) | 75 (50.0%) | 136 (57.9%) | ||
| • ADC and large cell | 200 (38.4%) | 80 (59.3%) | 56 (37.3%) | 64 (27.2%) | ||
| • NSCLC-Undiff | 71 (13.7%) | 17 (12.6%) | 19 (12.7%) | 35 (14.9%) | ||
| Stage descriptors | ||||||
| • T group | 0.081 | |||||
| ➢ T1-T2 | 75 (14.4%) | 25 (18.5%) | 26 (17.3%) | 24 (10.2%) | ||
| ➢ T3 | 156 (30.0%) | 34 (25.2%) | 41 (27.3%) | 81 (34.5%) | ||
| ➢ T4 | 289 (55.6%) | 76 (56.3%) | 83 (55.3%) | 130 (55.3%) | ||
| • N group | 0.494 | |||||
| ➢ N0-N1 | 146 (28.1%) | 41 (30.4%) | 42 (28.0%) | 63 (26.8%) | ||
| ➢ N2 | 222 (42.7%) | 49 (36.3%) | 65 (43.3%) | 108 (46.0%) | ||
| ➢ N3 | 152 (29.2%) | 45 (33.3%) | 43 (28.7%) | 64 (27.2%) | ||
| • M group | <0.001 | |||||
| ➢ M0 | 268 (51.5%) | 44 (32.6%) | 81 (54.0%) | 143 (60.9%) | ||
| ➢ M1 | 252 (48.5%) | 91 (67.4%) | 69 (46.0%) | 092 (39.1%) | ||
| • Stage | <0.001 | |||||
| ➢ I-IIIA | 84 (16.2%) | 11 (8.1%) | 29 (19.3%) | 44 (18.7%) | ||
| ➢ IIIB | 184 (35.4%) | 33 (24.4%) | 52 (34.7%) | 99 (42.1%) | ||
| ➢ IV | 252 (48.5%) | 91 (67.4%) | 69 (46.0%) | 92 (39.1%) | ||
| • Extrathoracic disease | 137 (26.3%) | 56 (41.5%) | 42 (28.0%) | 39 (16.6%) | <0.001 | |
Data is presented as number (percentage) unless specified otherwise; **Details available for 506 patients; SD = Standard deviation, PS Performance Status, ADC = Adenocarcinoma, NSCLC = Non-small cell lung cancer, NSCLC-Undiff = Undifferentiated NSCLC.
Group comparisons in small cell lung cancer
Among the 134 patients with small cell lung cancer (SCLC), the number of patients in Groups I, II and III was 16, 52 and 66 respectively. Significant differences were observed between the three groups in relation to age and gender distribution among SCLC patients, as was observed in the case of NSCLC. The mean (SD) age of 44.0 (10.6) years observed in group I was significantly lower (P<0.001) than that observed in group II [55.7 (10.3) years] and group III [58.9 (9.3) years]. Similarly, the percentage of males in group I was 50.0% while it was 90.4% and 95.5% in groups II and III respectively (P<0.001). However unlike NSCLC, the groups in SCLC were similar in terms of disease stage. Extensive disease was observed in 56.3%, 59.6% and 51.5% of patients in groups I, II and III (P=0.677) while ETD was seen in 37.5%, 30.8% and 22.7% (P=0.401). Karnofsky PS was similar in the three groups.
Logistic regression analysis in non-small cell lung cancer
On univariate as well as multivariate logistic regression analysis (LRA) (Table 3), both smoking categories (SI=1-300 and SI ≥301) were observed to be associated with lower odds of having advanced NSCLC (stages IIIB-IV) at presentation as compared to never-smokers. Age and histology (dichotomous variable) did not show any association on either univariate or multivariate LRA. Female gender showed lower odds for advanced NSCLC at diagnosis on multivariate LRA. On analysis for factors independently associated with presence of ETD at the time of diagnosis (Table 3), it was only the group of heavy smokers (SI ≥301) that had significantly lower odds as compared to never-smokers on multivariate LRA. Non-squamous histology was also observed to have significantly higher odds as compared to squamous histology for presence of ETD on both univariate and multivariate LRA.
Table 3. Logistic regression analysis for factors associated with presentation of non-small cell lung cancer patients in advanced stage (IIIB-IV) and with extrathoracic disease.
| Advanced stage |
Extrathoracic disease |
|||||
|---|---|---|---|---|---|---|
| Univariate OR (95% CI) |
Multivariate OR (95% CI) |
Univariate OR (95% CI) |
Multivariate OR (95% CI) |
|||
| Gender | ||||||
| Male | 1.00 | 1.00 | 1.00 | 1.00 | ||
| Female | 1.00 (0.55-1.84) | 0.46 (0.21-0.99)* | 1.61 (0.99-2.60) | 0.74 (0.41-1.34) | ||
| Age | 1.00 (0.97-1.02) | 1.00 (0.98-1.03) | 0.99 (0.97-1.01) | 1.00 (0.98-1.02) | ||
| Histological type | ||||||
| Squamous | 1.00 | 1.00 | 1.00 | 1.00 | ||
| Non-squamous | 1.31 (0.82-2.10) | 1.14 (0.70-1.85) | 2.78 (1.83-4.22)** | 2.31 (1.50-3.57)** | ||
| Smoking Status | ||||||
| Never-smoker | 1.00 | 1.00 | ||||
| Current/ex-smoker | 0.38 (0.20-0.74)** | 0.38 (0.25-0.57)** | ||||
| Smoking Group | ||||||
| Never-smoker | 1.00 | 1.00 | 1.00 | 1.00 | ||
| SI=1-300 | 0.37 (0.18-0.77)** | 0.27 (0.12-0.62)** | 0.55 (0.34-0.90)* | 0.57 (0.33-1.01) | ||
| SI ≥301 | 0.39 (0.19-0.77)** | 0.25 (0.11-0.61)** | 0.28 (0.17-0.46)** | 0.29 (0.16-0.53)** | ||
OR=Odds Ratio, CI=Confidence Intervals, SI=Smoking Index; **P≤0.01; *P≤0.05.
When analyses for advanced stage and ETD in NSCLC were carried out with the LRA model wherein the histology variable was trichotomous, the results were similar to the model with a dichotomous variable. ADC plus LCC as a group and NSCLC-Undiff group had similar ORs as were obtained with the non-squamous NSCLC group.
Subgroup analyses in non-small cell lung cancer
Subgroup analyses based upon histology and gender were carried out in an attempt to assess whether the observed differences in the entire NSCLC population were also seen in more homogenous cohorts.
Group comparisons based upon SI for both squamous and non-squamous NSCLC are depicted in Table 4. Differences in distribution of gender, age, disease stage and ETD were observed here also although for squamous histology, stage differences were not statistically significant. For analysis restricted to squamous histology and after multivariate LRA, both SI=1-300 [OR=0.34 (95% CI=0.10-1.12; P=0.08)] as well as SI≥301 [OR=0.33 (95% CI=0.10-1.14; P=0.08)] were associated with lower odds of presenting with advanced disease, although this did not reach statistical significance. However, gender showed a significant association [OR=0.26 (95% CI=0.09-0.74; P=0.01)] in this analysis. In relation to presentation with ETD among squamous histology, both SI=1-300 [OR=0.28 (95% CI=0.11-0.73; P=0.01)] and SI≥301 [OR=0.17 (95% CI=0.07-0.45); P<0.001] had lower odds as compared to never-smokers on multivariate LRA. No other variables showed significant associations for ETD.
Table 4. Demographical and disease stage profile among squamous and non-squamous NSCLC patients grouped on the basis of smoking index (SI).
| Squamous NSCLC |
Non-Squamous NSCLC |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group I NS | Group II SI=1-300 |
Group III SI ≥301 |
P value | Group I NS | Group II SI=1-300 |
Group III SI ≥301 | P value | ||
| Number of cases | 38 | 75 | 136 | 97 | 75 | 99 | |||
| Age (years) Mean (SD) | 55.8 (12.3) | 58.1 (10.7) | 60.9 (8.4) | 0.009 | 54.0 (12.6) | 59.0 (9.1) | 61.6 (10.6) | <0.001 | |
| Males | 23 (60.5%) | 66 (88.0%) | 133 (97.8%) | <0.001 | 42 (43.3%) | 66 (88.0%) | 97 (98.0%) | <0.001 | |
| Stage descriptors | |||||||||
| • T group | 0.433 | 0.143 | |||||||
| ➢ T1-T2 | 7 (18.4%) | 8 (10.7%) | 11 (8.1%) | 18 (18.6%) | 18 (24.0%) | 13 (13.1%) | |||
| ➢ T3 | 9 (23.7%) | 20 (26.7%) | 42 (30.9%) | 25 (25.8%) | 21 (28.0%) | 39 (39.4%) | |||
| ➢ T4 | 22 (57.9%) | 47 (62.7%) | 83 (61.0%) | 54 (55.7%) | 36 (48.0%) | 47 (47.5%) | |||
| • N group | 0.997 | 0.283 | |||||||
| ➢ N0-N1 | 10 (26.3%) | 19 (25.3%) | 34 (25.0%) | 31 (32.0%) | 23 (30.7%) | 29 (29.3%) | |||
| ➢ N2 | 18 (47.4%) | 35 (46.7%) | 62 (45.6%) | 31 (32.0%) | 30 (40.0%) | 46 (46.5%) | |||
| ➢ N3 | 10 (26.3%) | 21 (28.0%) | 40 (29.4%) | 35 (36.0%) | 22 (29.3%) | 24 (24.2%) | |||
| • M group | 0.034 | 0.002 | |||||||
| ➢ M0 | 17 (44.7%) | 50 (66.7%) | 91 (66.9%) | 27 (27.8%) | 31 (41.3%) | 52 (52.5%) | |||
| ➢ M1 | 21 (55.3%) | 25 (33.3%) | 45 (33.1%) | 70 (72.2%) | 44 (58.7%) | 47 (47.5%) | |||
| • Stage | 0.120 | 0.005 | |||||||
| ➢ I-IIIA | 5 (13.2%) | 16 (21.3%) | 24 (17.6%) | 6 (6.2%) | 13 (17.3%) | 20 (20.2%) | |||
| ➢ IIIB | 12 (31.6%) | 34 (45.3%) | 67 (49.3%) | 21 (21.6%) | 18 (24.0%) | 32 (32.3%) | |||
| ➢ IV | 21 (55.3%) | 25 (33.3%) | 45 (33.1%) | 70 (72.2%) | 44 (58.7%) | 47 (47.5%) | |||
| • ETD | 14 (36.8%) | 12 (16.0%) | 15 (11.0%) | 0.001 | 42 (43.3%) | 30 (40.0%) | 24 (24.2%) | 0.013 | |
Data is presented as number (percentage) unless specified otherwise; SD=Standard deviation, ETD=Extrathoracic disease, SI=Smoking index, NS=Never-smokers, NSCLC=Non-small cell lung cancer.
When analysis was carried out for non-squamous histology as a unified group, after multivariate LRA, both SI=1-300 [OR=0.30 (95% CI=0.10-0.96; P=0.04)] and SI≥301 [OR=0.25 (95% CI=0.08-0.81; P=0.02)] were again associated with lower odds of presenting with advanced disease. No other variables showed any significant associations. In relation to ETD among non-squamous histology group, only SI≥301 [OR=0.38 (95% CI=0.18-0.79; P=0.01)] had significantly lower odds compared to never-smokers. Smoking group SI=1-300 and other variables did not show any significant associations for ETD.
When analysis was restricted to male subjects, statistically significant differences in relation to age, histology, disease stage and presence of ETD were observed (Table 5). Both SI=1-300 [OR=0.32 (95% CI=0.11-0.99; P=0.047)] as well as SI≥301 [OR=0.27 (95% CI=0.09-0.80; P=0.02)] had lower odds on multivariate LRA for presentation in advanced disease. Histology and other variables did not show significant associations. For presentation with ETD among males, it was only heaving smoking category that had lower odds as compared to never-smokers after multivariate LRA [OR for SI≥301=0.35 (95% CI=0.18-0.65; P=0.001)]. Non-squamous histology [OR=2.43 (95% CI=1.51-3.91; P<0.001)] also showed significant association for ETD.
Table 5. Demographical, histology and disease stage profile among male non-small cell lung cancer patients grouped on the basis of smoking index (SI).
| Group I never-smokers |
Group II smoking index =1-300 | Group III smoking index≥301 |
P value | ||
|---|---|---|---|---|---|
| Number of cases | 65 | 132 | 230 | ||
| Age (years) Mean (SD) | 56.3 (11.9) | 58.9 (10.1) | 61.3 (9.5) | 0.001 | |
| Histology | 23 (35.4%) | 66 (50.0%) | 133 (57.8%) | 0.008 | |
| • Squamous cell | 32 (49.2%) | 49 (37.1%) | 62 (27.0%) | ||
| • ADC and large cell | 10 (15.4%) | 17 (12.9%) | 35 (15.2%) | ||
| • NSCLC-Undiff | 9 (23.7%) | 20 (26.7%) | 42 (30.9%) | ||
| Stage descriptors | |||||
| • T group | 0.026 | ||||
| ➢ T1-T2 | 12 (18.5%) | 24 (18.2%) | 23 (10.0%) | ||
| ➢ T3 | 12 (18.5%) | 35 (26.5%) | 80 (34.8%) | ||
| ➢ T4 | 41 (63.0%) | 73 (55.3%) | 127 (55.2%) | ||
| • N group | 0.383 | ||||
| ➢ N0-N1 | 23 (35.4%) | 31 (23.5%) | 61 (26.5%) | ||
| ➢ N2 | 23 (35.4%) | 61 (46.2%) | 107 (46.5%) | ||
| ➢ N3 | 19 (29.2%) | 40 (30.3%) | 62 (27.0%) | ||
| • M group | 0.001 | ||||
| ➢ M0 | 23 (35.4%) | 68 (51.5%) | 139 (60.4%) | ||
| ➢ M1 | 42 (64.6%) | 64 (48.5%) | 91 (39.6%) | ||
| • Stage | 0.006 | ||||
| ➢ I-IIIA | 4 (6.2%) | 22 (16.7%) | 43 (18.7%) | ||
| ➢ IIIB | 19 (29.2%) | 46 (34.8%) | 96 (41.7%) | ||
| ➢ IV | 42 (64.6%) | 64 (48.5%) | 91 (39.6%) | ||
| • Extrathoracic disease | 27 (41.5%) | 39 (29.5%) | 39 (17.0%) | <0.001 | |
Data is presented as number (percentage) unless specified otherwise; SD = Standard deviation, ADC = Adenocarcinoma, NSCLC = Non-small cell lung cancer, NSCLC-Undiff = Undifferentiated NSCLC.
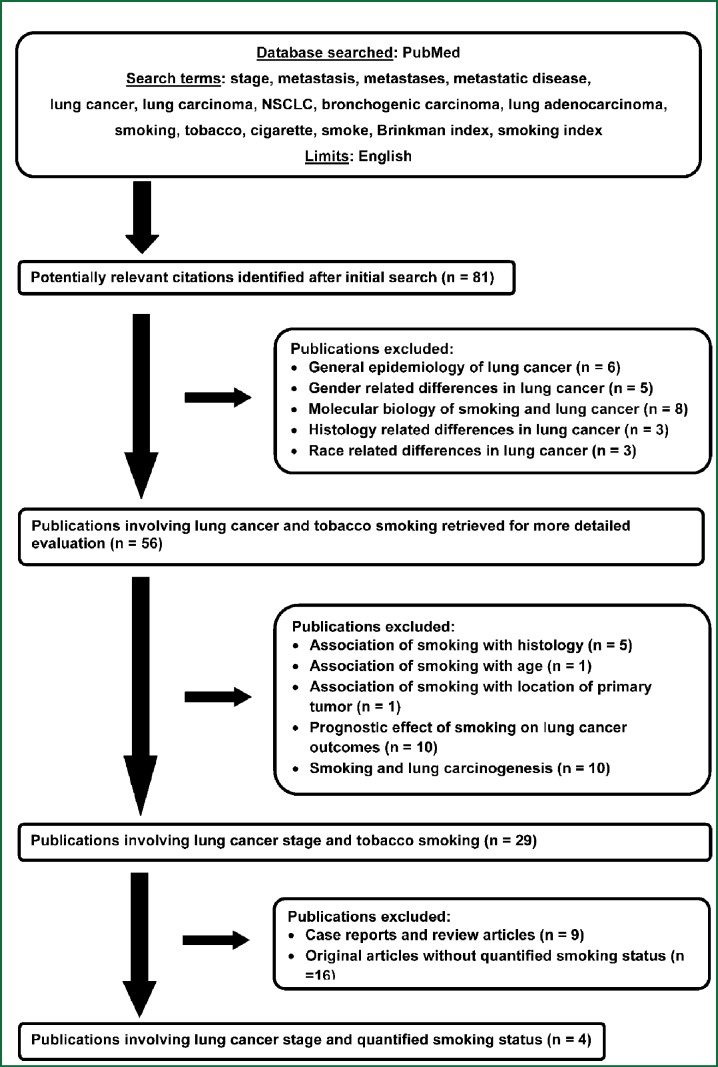
Results of literature review
The results of the literature search and the selection process for this systematic review are depicted in Figure 1. Overall, only four publications were identified wherein the association of NSCLC stage with QSS had been assessed (15-18). The results of the current study and those observed in previously published studies are summarized in Table 6.
Figure 1.
Flow diagram showing the selection process for this systematic review.
Table 6. Comparison of studies identified by a systematic literature review that reported stage distribution in relation to quantified smoking status .
| Study (Year) Ref No |
No of cases, country, time period |
% Smokers, Quantified smoking based groups |
NSCLC stage profile, Group differences^ in NSCLC stage |
Other baseline group differences^ (Gender, Age, Histology) |
|
| Holli K, et al. (1999) (15) |
290; Finland 1983-87 |
100%; Lifetime Cig: L (<500), M (500-800), H (>800) |
Stages I-IV No diff in T or N or M status Stage groups N.A. |
Males max (99%) in H, min (72%) in L. No diff in mean age. SqCC (63%) & SCLC (26%) max in H, ADC (17%) max in L. | |
| Maeshima AM, et al. (2008) (16) |
236; Japan 1984-90 |
58.9%; BI: NS, Sm 1-500, Sm >500 |
Stages I-IV No diff in T or N status No diff in stage I vs. II-IV distribution |
Gender distribution N.A., Age distribution N.A., Series consisted of surgically resected adenocarcinoma. | |
| Guo NL, et al. (2009) (17) |
327; USA N.A. |
100%; Sm <61 PYI, Sm >61 PYI |
Stages I-III TNM status N.A. Stage I more in Sm <61 PYI (76.4% vs. 64.7%); Stage II more in Sm>61 PYI (20.6% vs. 8.4%). |
Age ≥60 years more in Sm >61 PYI (77.9% vs. 64.4%) Males more in Sm >61 PYI (68.4% vs. 42.9%). SqCC (61.0%) more in Sm >61 PYI, ADC (83.2%) more in Sm <61 PYI. | |
| Janjigian YY, et al. (2010) (18) |
2010; USA 2003-06 |
83.5%; NS, Sm <15 PY, Sm >15 PY |
Stages IIIB-IV TNM status N.A. No diff in stage group |
Males max (55.6%) in Sm >15 PY, min (34.4%) in NS. Median age max (65 yrs) in Sm >15 PY, min (59 yrs) in NS. SqCC (12%) max in Sm >15 PY, ADC (69%) max in NS. | |
| Current study | 520; India 2008-11 |
74.0%, NS, L/M (SI 1-300), H (SI>300) |
Stages I-IV No diff in T or N status M1 min (39.1%) in H, max (67.4%) in NS. IIIB max (42.1%) in H, min (24.4%) in NS. ETD min (16.6%) in H, max (41.5%) in NS. |
Males max 97.9% in H, min (48.1%) in NS. Mean age max (61.0 yrs) in H, min (54.5 yrs) in NS. SqCC (57.9%) max in H, ADC (59.3%) max in NS. | |
ETD=Extrathoracic disease, BI=Brinkman index, SI=Smoking index, NSCLC=Non-small cell lung cancer, Cig=Cigarettes, Sm=Current/Ex-Smoker, NS=Never-Smokers, PY=Pack Years, PYI=Pack Years Index, H=Heavy, M=Medium, L=Light, max=maximum, min=minimum, resp=respectively, N.A.=Data Not Available, SqCC=Squamous cell carcinoma, SCLC=Small cell lung cancer, ADC=Adenocarcinoma; ^ Statistically significant.
Discussion
The current study was conducted to assess whether any differences, based on QSS, exist among newly diagnosed NSCLC patients in relation to disease stage. Not only were significant differences seen between never-smokers, light/moderate smokers and heavy smokers, it was also observed that smoking index (SI) was inversely and independently associated with presence of advanced NSCLC at diagnosis. Presence of ETD at diagnosis was also an important factor that differed among the three groups. However, in this case, it was only heavy smokers, and not light/moderate smokers, who had significantly lower odds as compared to never-smokers. The association of heavy smoking (SI≥301) with lower odds of advanced disease as well as with lower odds of ETD amongst NSCLC patients was also consistently observed during subgroup analyses by histology and gender.
In the current study, one of the possible reasons for the NSCLC stage differences that were observed among groups is that current/ex-smokers and in particular heavy smokers could be presenting earlier to health care facilities. Tobacco smoking is an important risk factor for the development of chronic bronchitis as well as chronic obstructive pulmonary disease (COPD) - the differences between the two being largely based on absence or presence respectively of an obstructive defect on spirometry. Therefore patients in groups II and III could have been symptomatic much more or earlier than those in group I leading to the diagnosis of LC being made at an earlier stage. Another likely reason is the inherent differences in the biological characteristic of tumors in these groups (19). This is possibly distinct from their histological subtype since on multivariate LRA, both non-squamous histology group and heavy smoking showed independent associations with presence of ETD. The third reason is that smoking, in addition to its etiological role, has an influence in LC detection. This has been termed ‘detection bias’ namely the tendency for clinicians to pursue the diagnosis of LC preferentially in the presence of history of smoking (20). Its relevance was more for patients without an anatomic or symptomatic presentation suggestive of LC and in whom smoking history acted as a stimulus for diagnostic workup of LC.
The main strengths of the current study include assessment of the association of NSCLC stage and of ETD with smoking exposure using a quantified method. As evident from the systematic literature review, only four previously published studies had analysed data based upon QSS. None of the earlier studies had looked at ETD separately. In addition, this study had the second largest patient population and is also the only one from south Asia. Moreover, two of these studies comprised primarily of early stage (surgically resected) cases (16,17), one had exclusively ADC histology (16) and two studies did not have a never-smoking group (15,17).
In accordance with results from our recent study on LC epidemiology, we also found significant differences among the groups for age, gender and histological distribution (4). Highest mean age, percentage of males and of squamous histology was observed in the heavy smoking group in our study as well as in other studies (15,17,18). As regards to the gender difference, it is well known that in India, smoking is more prevalent among males as compared to females. In a large multi-centric study conducted previously by us that involved a sample size of 73,605 subjects aged 15 years of age and above, the prevalence of ever-smokers among males and females was 28.5% and 2.1% respectively (7). At the same time, it is worth mentioning here that given the predominance of males in the current study, we cannot exclude the presence of a gender related referral bias. Inequality for women exists in relation to many aspects of life in India including health seeking behaviour and health care utilization (21). It is possible that like other illnesses, lung cancer in this geographical area is underreported and undertreated among women. The association of tobacco smoking with development of LC is stronger for squamous histology than for ADC (22). Consequently, squamous cell carcinoma and ADC are more common among males and females respectively (23). It has been shown previously by us that the mean age at the time of diagnosis of LC is almost a decade lower than what is reported in the developed countries (24,25). However, reasons for the age difference between the groups remain unidentified and none of the other studies in this systematic review had addressed it either.
There were certain limitations of the current study. One of the important ones was that we did not include the symptom profile of patients at presentation nor was spirometry performed for them at that time period. Therefore, it is difficult to say with certainty that early presentation of heavy smokers was indeed related to symptoms attributable to smoking including the presence of COPD/chronic bronchitis. Secondly, we also did not assess for environmental tobacco smoke (ETS) exposure (passive smoking) or other environmental/occupational exposures that have been associated with the occurrence of LC. In our previously published studies, it was observed that for women, ETS exposure and indoor air pollution (from use of biomass cooking fuels) were important risk factors since the association of LC with smoking was not as strong as it was for men (8,26). It is possible that these factors may have contributed, to some extent, towards the observed group differences. Lastly, we used a time-intensity product for quantification of tobacco smoke exposure rather than time alone. In one study, smoking at a lower intensity for a longer duration was found to be more deleterious than smoking at a higher intensity for a shorter duration (27).
An important issue for consideration is the implication of the results of the current study and whether these would hold true for other populations or regions of the world or not. In India, rather than cigarette, bidi - the hand rolled form of tobacco wrapped in the dried tendu leaf - is the most common smoking product (7). It has been shown previously by us that bidi smoking is associated with the same risk of developing LC, as is cigarette smoking (8). Another case control study also found that both bidi and cigarette smokers had similar odds ratios for development of lung cancer (9). In a recent Indian study, on comparing breath carbon monoxide levels among bidi and cigarette smokers, it was found that the harmful effects of bidi smoking were no less than those from cigarette smoking (10). The authors recommended that one bidi should be considered to be equivalent to one cigarette for calculating time-intensity (pack-years) of smoking. We therefore believe that the results of this study may be valid in other regions of the world especially the developing and under-developed countries which share several similarities with India in terms of socio-economic and healthcare issues and wherein bidis or other non-standardized forms of tobacco smoking are prevalent.
In summary, among newly diagnosed LC patients in North India, significant differences exist between never-smokers, light/moderate smokers and heavy smokers in relation to stage of NSCLC at presentation. Heavy smoking has an independent and inverse association with advanced stage NSCLC and with ETD at diagnosis. The current study was neither aimed at nor does it negate the unequivocal evidence regarding the harmful effects of tobacco smoking on human health including its role as a proven risk factor for the occurrence of lung cancer. However, it is important that further studies are conducted to ascertain reasons for the observed associations between tobacco smoking and disease stage.
Acknowledgements
The authors wish to thank Dr. Arjun Srinivasan and Dr. Karan Madan (Senior Residents, Department of Pulmonary Medicine, PGIMER) for their assistance in data collection.
Disclosure: The authors declare no conflict of interest.
References
- 1.Subramanian J, Velcheti V, Gao F, et al. Presentation and stage-specific outcomes of lifelong never-smokers with non-small cell lung cancer (NSCLC). J Thorac Oncol 2007;2:827-30 [DOI] [PubMed] [Google Scholar]
- 2.Nordquist LT, Simon GR, Cantor A, et al. Improved Survival in Never-Smokers vs Current Smokers With Primary Adenocarcinoma of the Lung. Chest 2004;126:347-51 [DOI] [PubMed] [Google Scholar]
- 3.Toh CK, Gao F, Lim WT, et al. Never-Smokers With Lung Cancer: Epidemiologic Evidence of a Distinct Disease Entity. J Clin Oncol 2006; 24:2245-51 [DOI] [PubMed] [Google Scholar]
- 4.Singh N, Aggarwal AN, Gupta D, et al. Unchanging clinico-epidemiological profile of lung cancer in North India over three decades. Cancer Epidemiol 2010;34:101-4 [DOI] [PubMed] [Google Scholar]
- 5.Jindal SK, Malik SK, Dhand R, et al. Bronchogenic carcinoma in Northern India. Thorax 1982;37:343-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh N, Aggarwal AN, Gupta D, et al. Prevalence of low body mass index among newly diagnosed lung cancer patients in North India and its association with smoking status. Thoracic Cancer 2011;2:27-31 [DOI] [PubMed] [Google Scholar]
- 7.Jindal SK, Aggarwal AN, Chaudhry K, et al. Tobacco smoking in India: prevalence, quit-rates and respiratory morbidity. Indian J Chest Dis Allied Sci 2006;48:37-42 [PubMed] [Google Scholar]
- 8.Gupta D, Boffetta P, Gaborieau V, et al. Risk factors of lung cancer in Chandigarh, India. Indian J Med Res 2001;113:142-50 [PubMed] [Google Scholar]
- 9.Prasad R, Ahuja RC, Singhal S, et al. A case-control study of bidi smoking and bronchogenic carcinoma. Ann Thorac Med 2010;5:238-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar R, Prakash S, Kuswah AS, et al. Breath Carbon Monoxide Concentration in Cigarette and Bidi Smokers in India. Indian J Chest Dis Allied Sci 2010;52:19-24 [PubMed] [Google Scholar]
- 11.Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest 1997;111:1710-7 [DOI] [PubMed] [Google Scholar]
- 12.Sobin LH, Wittekind C. TNM Classification of Malignant Tumours. 6th Edition. Hoboken, New Jersey: John Wiley & Sons, 2002. [Google Scholar]
- 13.Stahel RA, Ginsberg R, Havemann K, et al. Staging and prognostic factors in small cell lung cancer: a consensus report. Lung Cancer 1989;5:119-26 [Google Scholar]
- 14.Beasley MB, Brambilla E, Travis WD. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol 2005;40:90-7 [DOI] [PubMed] [Google Scholar]
- 15.Holli K, Visakorpi T, Hakama M.Smoking and survival from lung cancer. Acta Oncol 1999;38:989-92 [DOI] [PubMed] [Google Scholar]
- 16.Maeshima AM, Tochigi N, Tsuta K, et al. Histological evaluation of the effect of smoking on peripheral small adenocarcinomas of the lung. J Thorac Oncol 2008;3:698-703 [DOI] [PubMed] [Google Scholar]
- 17.Guo NL, Tosun K, Horn K. Impact and interactions between smoking and traditional prognostic factors in lung cancer progression. Lung Cancer 2009;66:386-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janjigian YY, McDonnell K, Kris MG, et al. Pack-years of cigarette smoking as a prognostic factor in patients with stage IIIB/IV nonsmall cell lung cancer. Cancer 2010;116:670-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudin CM, Avila-Tang E, Harris CC, et al. Lung cancer in never smokers: molecular profiles and therapeutic implications. Clin Cancer Res 2009;15:5646-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wells CK, Peduzzi PN, Feinstein AR. Presenting manifestations, cigarette smoking, and detection bias in age at diagnosis of lung cancer. Ann Epidemiol 2001;11:239-47 [DOI] [PubMed] [Google Scholar]
- 21.Balarajan Y, Selvaraj S, Subramanian SV. Health care and equity in India. Lancet 2011;377:505-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khuder SA. Effect of cigarette smoking on major histological types of lung cancer: a meta-analysis. Lung Cancer 2001;31:139-48 [DOI] [PubMed] [Google Scholar]
- 23.Wynder EL, Muscat JE. The changing epidemiology of smoking and lung cancer histology. Environ Health Perspect 1995;103:143-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jindal SK, Behera D. Clinical spectrum of primary lung cancer: review of Chandigarh experience of 10 years. Lung India 1990;8:94-8 [Google Scholar]
- 25.Behera D, Balamugesh T.Lung cancer in India. Indian J Chest Dis Allied Sci 2004;46:269-81 [PubMed] [Google Scholar]
- 26.Rapiti E, Jindal SK, Gupta D, et al. Passive smoking and lung cancer in Chandigarh, India. Lung Cancer 1999;23:183-9 [DOI] [PubMed] [Google Scholar]
- 27.Lubin JH, Caporaso NE. Cigarette smoking and lung cancer: modeling total exposure and intensity. Cancer Epidemiol Biomarkers Prev 2006;15:517-23 [DOI] [PubMed] [Google Scholar]