An anatomic lobectomy is the most common surgery in general thoracic surgery, and it is called a pneumonectomy if the scope of resection is expanded and a segmentectomy if this scope is reduced. A thoracoscopic lobectomy is undoubtedly a representative surgery of the application of thoracoscopic technology in thoracic surgery, showing that the thoracoscopic technology can meet the diagnostic and treatment needs for lung disease, which leads to a greatly expanded scope of its application. This chapter will describe the thoracoscopic anatomic lobectomy, segmentectomy, and pneumonectomy. Before we introduce the surgery, we will first take a brief look at the anatomy of the lung (Figures 1,2).
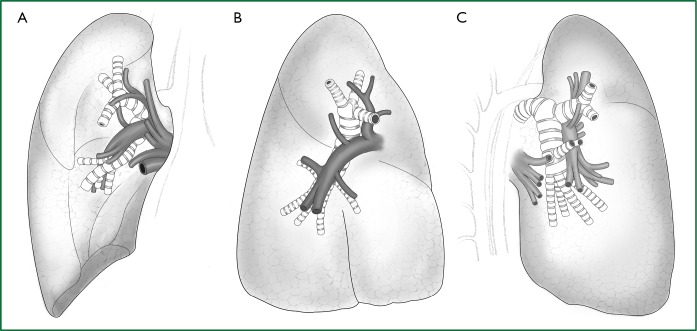
Figure 1.
Anatomy of the right lung. (A) Frontal view of the right pulmonary lobe; (B) lateral view of the right pulmonary lobe; and (C) lateral-rear view of the right pulmonary lobe.
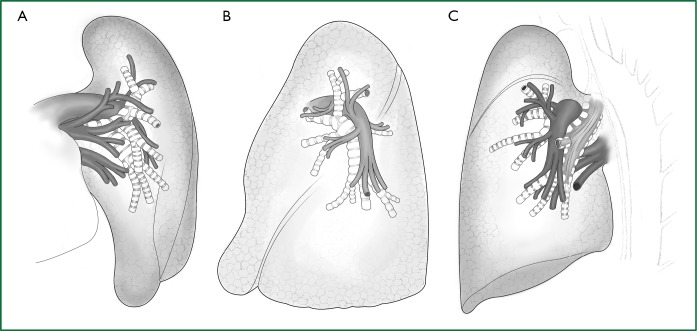
Figure 2.
Anatomy of the left lung. (A) Frontal view of the left pulmonary lobe; (B) lateral view of the left pulmonary lobe; and (C) lateral-rear view of the left pulmonary lobe.
The pulmonary parenchyma is composed of the bronchial tree, the alveolar tissue, and the pulmonary artery and vein. The right horizontal fissure and an oblique fissure divide the right lung into the upper, middle, and lower lobes. There is only an oblique fissure to divide the left lung into the upper and lower lobes. A considerable number of people have underdeveloped or even undeveloped fissures, resulting in covered hilar vessels that cannot be fully exposed.
The right upper pulmonary vein is located in front of the right pulmonary artery trunk, and the right upper pulmonary artery is composed of the apical, anterior, and posterior ascending branches. The first two branches are located in front of the hilum, and the third one is located at the posterior segment of the horizontal fissure. The right lower pulmonary artery is located at the corresponding position of the posterior ascending branch in the horizontal fissure and is divided into the dorsal segment and the basilar segment. The right lower pulmonary vein is located at the lower pole of the hilum. The right middle pulmonary artery is divided into the inner and outer branches at the middle of the horizontal fissure. All of the blood vessels described above can be clearly viewed in front of the hilum or at the midaxillary line with an endoscopic lens.
The left upper pulmonary vein is located in front of the left hilum, and the left lower pulmonary vein is located at the lower pole of the left hilum. The left upper pulmonary artery is located at the upper pole and behind the hilum and is divided into the apicoanterior and apicoposterior segments. The former is in front of the left upper pulmonary bronchus, and the latter is behind the left upper pulmonary bronchus, while the lingual segment is in the middle of the fissure. The left lower pulmonary artery is divided into the dorsal segment in the back of the oblique fissure and the basilar segment in the front-middle of the oblique fissure. All of the above blood vessels, except for the apicoposterior segment, can be viewed in the front-middle part of the hilum with an endoscopic lens.
Thoracoscopic lobectomy
Introduction
An anatomic lobectomy was first reported by Blades and Kent in 1940 for the surgical treatment of bronchiectasis. After 40 years of development, it has become the best described and most common surgical procedure for the treatment of lung cancer. In 1992, Lewis et al. first reported a thoracoscopic lobectomy. At that time, a lobectomy was achieved by the approach using a linear cutting stapler to directly cut and staple the en bloc hilar root along with the lobe of the lung. Since 1995, Rodney J. Landreneau from the USA has reported on a thoracoscopic anatomic lobectomy. After years of hard work, an unadulterated thoracoscopic lobectomy is technically feasible, and a video-assisted thoracoscopic surgery with minimal incision (hybrid VATS) can basically replace the traditional thoracotomy. During a video-assisted thoracoscopic surgery with minimal incision, surgeons can look at part of the surgical field directly, and often, the surgical procedures are similar to those of the traditional surgery. This paper focuses on the unadulterated thoracoscopic lobectomy.
The indications for surgery
Indications
(I) benign lesions of the lung, such as bronchiectasis, destroyed lung, lung cysts, tuberculosis, or lung hemangioma;
(II) stage I lung cancer;
(III) some stage II lung cancers, if the tumor size is 3-5 cm; no invasion of central bronchus as indicated by fiberoptic bronchoscopy; and hilar lymphadenectasis shown by computed topography (CT) imaging less than 1.5 cm in size.
Contraindications
(I) patients with a poor general condition, or those who cannot tolerate the one-lung ventilation anesthesia;
(II) lung cancer of stage II or beyond;
(III) patients with a coagulation dysfunction;
(IV) a massive fungoma in the lung.
Some of these contraindications are not absolute, for instance, a large tumor volume is a contraindication for unadulterated thoracoscopic lobectomy, but it is not a contraindication for a video-assisted thoracoscopic surgery with minimal incision.
The preoperative preparation
The preparation for the patient is basically the same as for a traditional thoracic surgery. For the thoracoscopic surgery, the preparation includes assembling the following: a complete set of thoracoscopic video equipment, imaging and recording devices, endoscope, thoracoscopic puncture casing, electric surgical knife, the endoscopic cutting stapler, suction apparatus, small thoracotomy device, and different types of clamps.
For a thoracoscopic pulmonary resection, a 10-mm 30° thoracoscope and three-chip video system are preferred. The 0° thoracoscope is easier for beginners to learn, but the views of some angles are not as good as with the 30° thoracoscope.
The necessary surgical equipment can be categorized as common surgical equipment and endoscopic surgical equipment, which are shown in Tables 1 and 2.
Table 1. Common surgical equipment.
| Scalpel and blade | Ring forceps |
| Towel forceps | Right-angle forceps |
| Peanut | Suction device |
| Rib spreader, small | Long needle holder |
| Vessel clamp | Electric surgical knife |
| Tissue scissors | Suture |
| Long tissue scissors | Vascular blocking clamp |
Table 2. Endoscopic surgical equipment.
| Trocar: 10 mm and 5 mm | Thoracoscope: 0° or 30° |
| Endoscopic biopsy forceps | Endoscopic grasping forceps |
| Endoscopic vascular clamp | Endoscopic clip applier |
| Endoscopic cutting stapler | Endoscopic scissors |
| Knot pusher | Endoscopic sector retractor: 3- or 5-leaf |
| Endoscopic needle holder | Ultrasonic scalpel: non-essential |
| Electric hook |
Among this surgical equipment, the most useful tools are the endoscopic cutting stapler, electric hook, and knot pusher (Figure 3). The currently used endoscopic electrocoagulation hook is the same as that used in laparoscopic surgery, with the disadvantage of a long hook that affects the operational precision. Accordingly, the surgeons have designed a different electrocoagulation hook (Figure 4) with a shortened hook body, which can be controlled like a pen by hand. It has a different length and can be configured for use with a conventional electric knife handle, resulting in precise and flexible operation.
Figure 3.
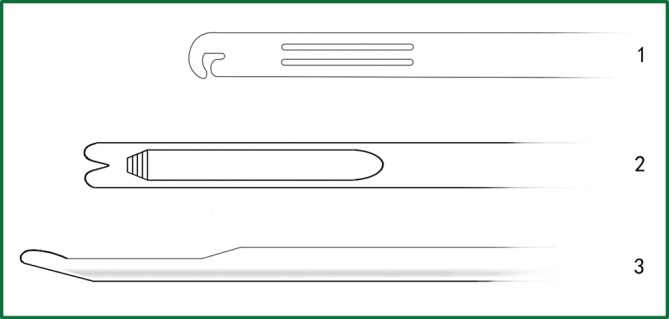
The different designs of knot pushers. 1. The knot pusher with a “C”-shaped head; 2. the knot pusher with a “Y”-shaped head; and 3. the lateral tube of a knot pusher.
Figure 4.
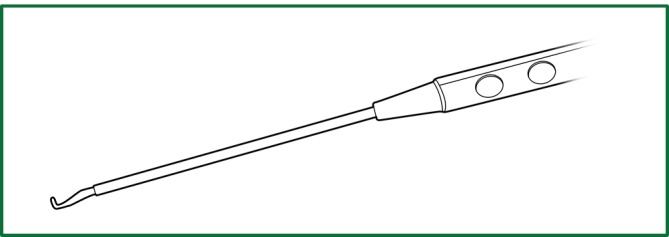
The appearance of the electric hook designed by Jianxing He.
Anesthesia
A double-lumen tube intubation combined with intravenous anesthesia is applied. If possible, fiberoptic bronchoscopy is used to guide the positioning to ensure good one-lung ventilation.
Position
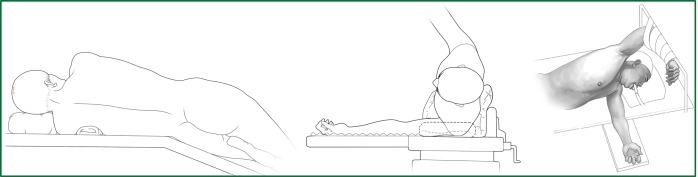
Patients usually lie on the side of the healthy lung (Figure 5). The waist bridge is elevated so that the intercostal space is expanded as much as possible to facilitate the operation. Moreover, the surgical operating table can be rotated to facilitate the operation during the surgery. In addition, the patient is positioned close to the surgeon to make it more comfortable for the surgeon to operate.
Figure 5.
Surgical position.
The surgical procedures
A lobectomy includes several steps, as described below.
Incisions
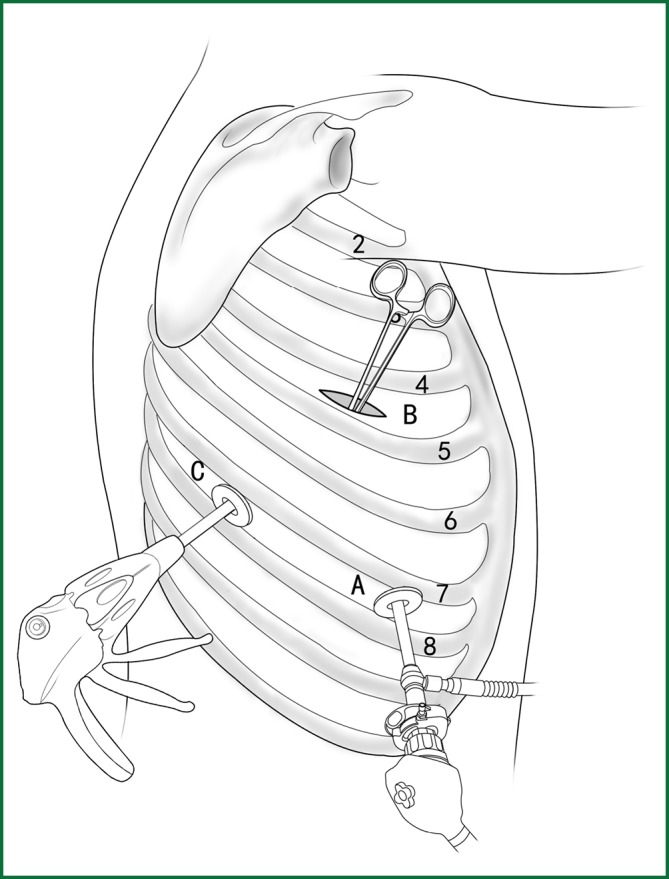
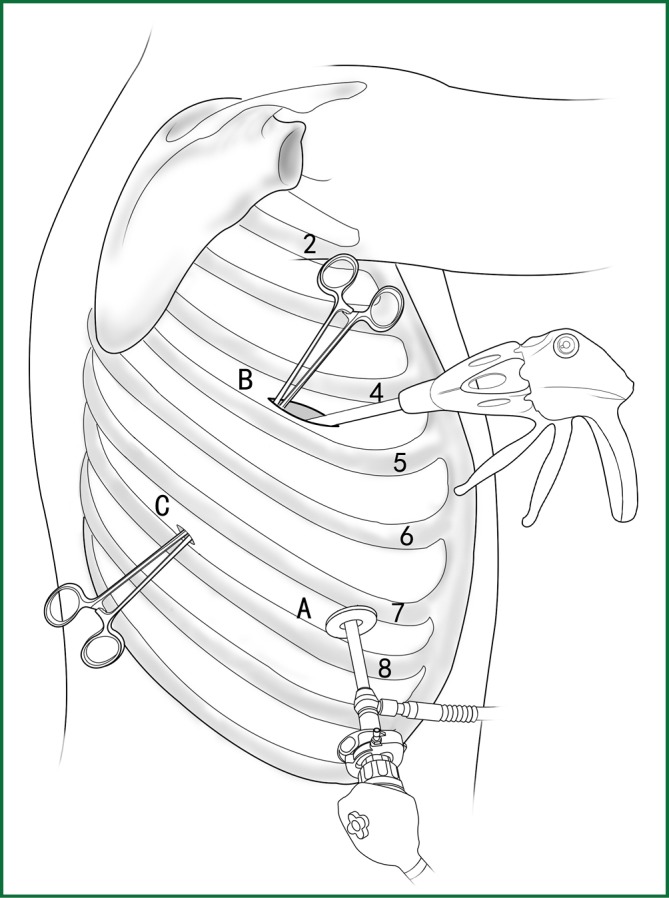
Usually three incisions are made during the surgery (Figure 6).
Figure 6.
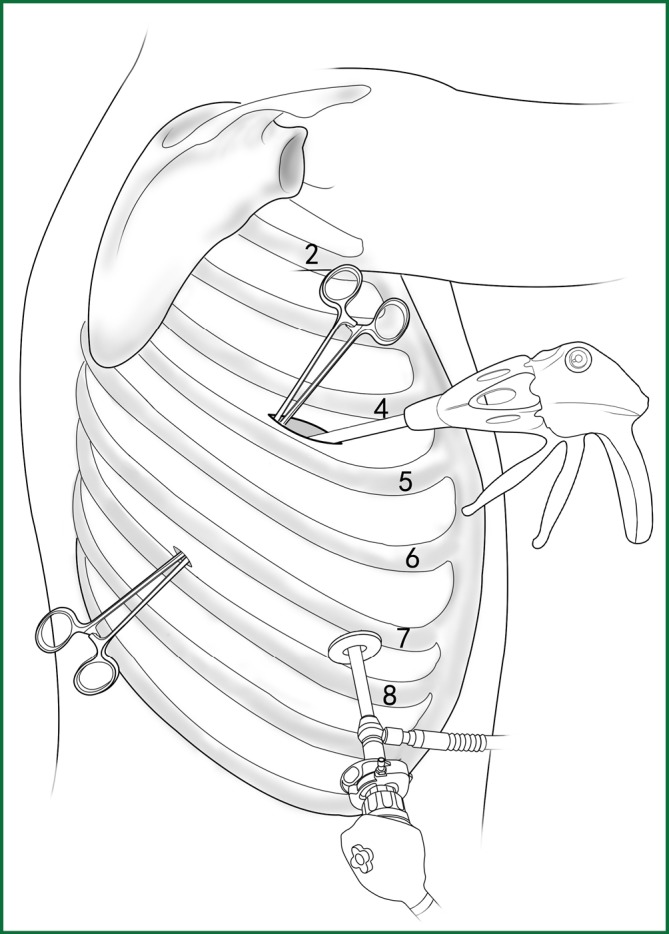
The distribution of incisions in a VATS lobectomy.
The incision for observation is 1.2 cm in length and is located at the midaxillary line or anterior-axillary line of the 7th or 8th intercostal space. From this position, the video-assisted thoracoscope can access the sectional image of the thoracic cavity to explore whether there are surgical contraindications within the entire surgical field.
The incision for the main operation is generally located between the anterior-axillary line and mid-nipple line at the 4th or 5th intercostal space. The length of this incision differs with different minimally invasive styles. The length for c-VATS (complete-VATS) is between 3-5 cm, with an average of 3.5 cm, while the length for a-VATS(assisted-VATS) is between 5-10 cm, with an average of 7.5 cm. The length of the incision for the main operation depends on the size of the resected lobe, the degree of pleural adhesions, the degree of the fissure development and the operational skills of the surgeon. The location of this incision is determined by the video-assisted thoracoscopy so that the incision can be aimed at the interlobar fissure of the resected lobe, making it more convenient to handle the hilum. The center of the incision for the main operation differs due to the habit of the surgeon, and it could be close to the anterior-axillary line, the midaxillary line, or the posterior-axillary line. Because the intercostal space is wider in the anterior chest, it is convenient to insert equipment in and take equipment out of the thoracic cavity and to remove the specimen if the incision is close to this area. Additionally, this incision location can minimize the separation of the trapezius muscle. For female patients, the location of the skin incision can be beneath the breast folds for a better appearance, and the thoracic cavity can be accessed after softly stretching the skin and the soft tissue with the incision protector. It should be emphasized that the use of a rigid rib distractor should be avoided as much as possible.
A 1.2-cm incision for the auxiliary operation is made between the posterior-axillary line and the scapular line of the same intercostal space as used for the incision for observation. Some surgeons choose the first intercostal space under the scapular line to make a 1.2-cm incision for the auxiliary operation. Alternatively, a 2-cm incision is made at the anterior-axillary line of the 5th intercostal space, and a 2-cm incision is made at the posterior-axillary line of the 6th intercostal space. Then, an auxiliary incision is made at the same location as mentioned above.
The distance between the incisions should be maximized and located far from the lesion, which is conducive to the operation. The three incisions should form a triangle and point to the lesion from different directions. Sometimes, a 4th or 5th incision is made according to the needs of the operation.
In general, a lobectomy includes several basic steps as follows: the handling of the fissure, vascular system, and bronchus. The basic sequences of various pulmonary resections are shown in Table 3. However, the actual sequence of operation often depends on the intraoperative condition, unexpected events, and the surgeon’s judgment. For example, the vein can be separated before the difficult-to-handle artery.
Table 3. The operational sequence of various VATS lobectomies.
| Left upper lobectomy | Left lower lobectomy |
| Fissure | Fissure |
| Lingual segment of the left upper pulmonary artery | Dorsal segment of the left lower pulmonary artery |
| Anterior segment of the left upper pulmonary artery | Basilar segment of the left lower pulmonary artery |
| Left upper pulmonary vein | Left lower pulmonary vein |
| Apicoposterior segment of the left upper pulmonary artery | Left lower lobar bronchus |
| Left upper lobar bronchus | |
| Left pneumonectomy | |
| Left lower pulmonary vein | |
| Left upper pulmonary vein | |
| Left pulmonary artery | |
| Left main bronchus | |
| Right upper lobectomy | Right middle lobectomy |
| Fissure | Fissure |
| Recurrent branch of the right upper pulmonary artery | Right middle pulmonary artery |
| Right upper pulmonary vein | Middle-lobe branch of the right upper pulmonary vein |
| Apicoanterior branch of the right upper pulmonary artery | Right middle lobar bronchus |
| Right upper lobar bronchus | |
| Right lower lobectomy | Right pneumonectomy |
| Fissure | Right lower pulmonary vein |
| Dorsal segment of the right lower pulmonary artery | Right upper pulmonary vein |
| Basilar segment of the right lower pulmonary artery | Right pulmonary artery |
| Right lower pulmonary vein | Right main bronchus |
| Right lower lobar bronchus |
The following is a description of the handling of the fissure, vascular system, and bronchus.
Handling of the fissure
Some of the pulmonary arterial vessels in the lobe pass along the fissure. The purpose of the handling of the fissure is to clarify the directions of the pulmonary artery branches in the lobe to be resected and to separate the fused lung tissue between the adjacent lobes.
For patients with a well-developed fissure, the separation can be performed with an electric surgical knife, ultrasonic scalpel, or even scissors. Blunt and sharp techniques are combined for the separation. The pleura and the adhesions are loosened to prepare for the hilar dissection.
For patients with a partially or completely fused fissure, there are usually two approaches for a lobectomy. For the first approach, an artificial fissure is created to expose all of the blood vessels and bronchi in the lobe, which is followed by the lobectomy. For the second approach, the blood vessels and bronchi are handled before the fissure, which is the so-called retrograde lobectomy or one-way lobectomy.
The key of the first approach is to free a channel for the creation of an artificial fissure within the lung parenchyma. It is currently the most commonly used method and is the focus of the following explanation. The following anatomical diagrams aid in the understanding of how to create a channel (Figures 7,8).
Figure 7.
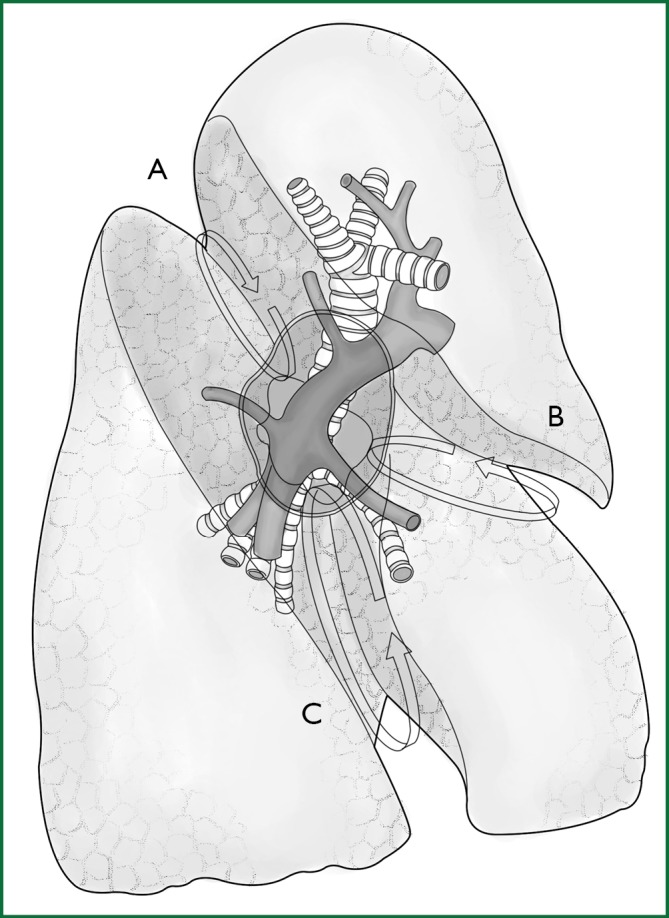
The fissures of the right lobe. (A) The upper part of the right oblique fissure. The arrow crosses the gap between the recurrent branch of the right upper pulmonary artery and the dorsal segment of the right lower pulmonary artery; (B) The horizontal fissure. The arrow crosses the gap above the right middle pulmonary artery; and (C) The lower part of the right oblique fissure. The arrow crosses the gap between the right middle pulmonary artery and the basilar segment of the right lower pulmonary artery.
Figure 8.
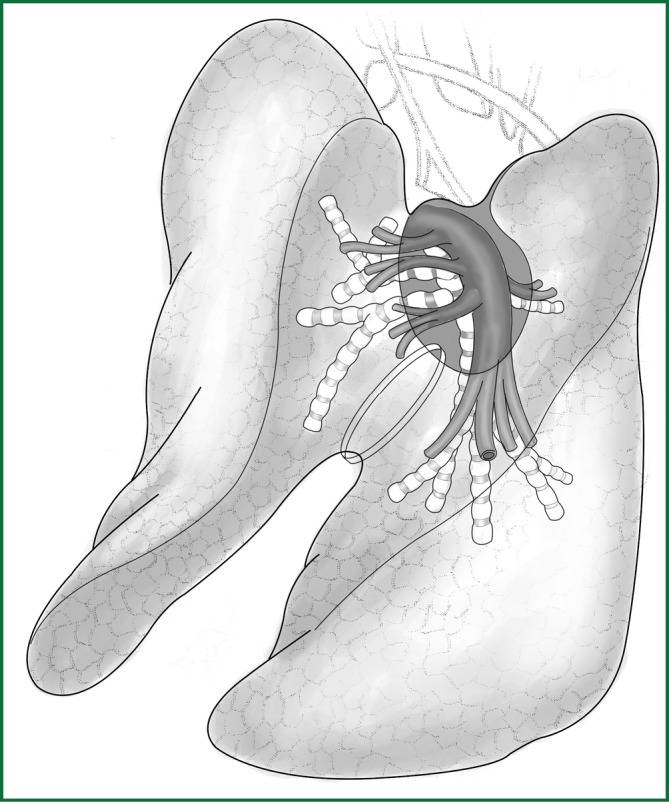
The fissures of the left lobe. The lower part of the left oblique fissure. The arrow crosses the gap between the lingual segment of the left upper pulmonary artery and the basilar segment of the left lower pulmonary artery.
Taking the right lobe as an example, the specific operational procedures to free the channel for an artificial fissure in the lung parenchyma are described as follows:
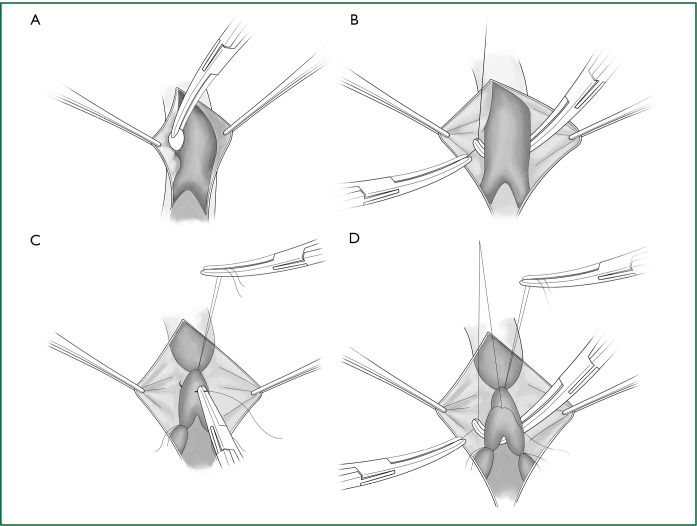
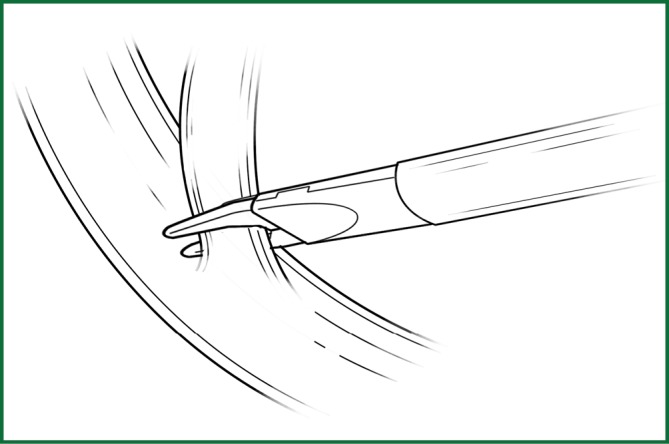
(I) Incision of the mediastinal pleura
Technical highlights: the location of the pleural incision is close to the edge of the lung (Figure 9), resulting in less bleeding. The “pick” technique with an electric hook can safely cut open the mediastinal pleura. The scope of the pleural incision surrounds the lobe and is slightly over the fissure.
Figure 9.
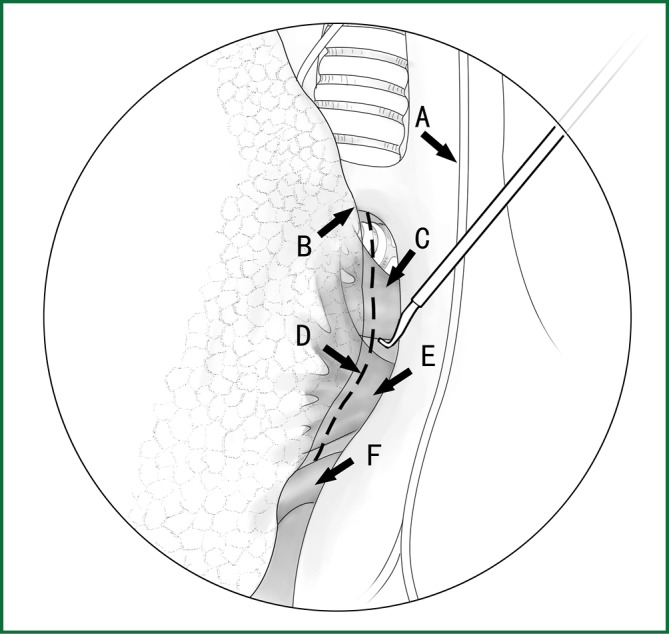
The incision of the mediastinal pleura. (A) Phrenic nerve; (B) lung edge; (C) pulmonary artery; (D) cutting line; (E) the upper pulmonary vein; and (F) the lower pulmonary vein.
The loosening of the lower pulmonary ligament actually also involves cutting open the mediastinal pleura. The lower pulmonary ligament is formed with the convergence of the front and rear pleura. It is relatively thick and contains the small blood vessels and lymphatic vessels. When loosening the lower pulmonary ligament, the incision location should also be close to the lung edge, and it can be cut after clamping with the titanium clip (Figures 10,11). Both the electric hook and the ultrasonic scalpel are safe and convenient incision tools.
Figure 10.
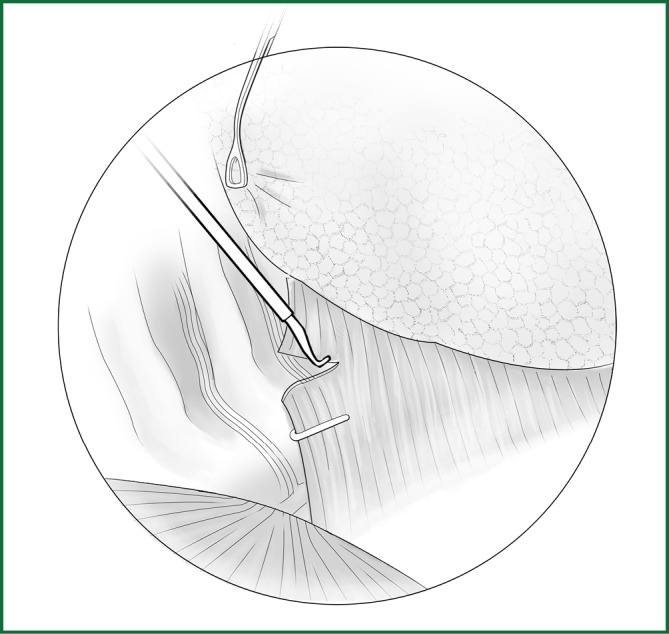
Incision of the lower pulmonary ligament.
Figure 11.
The location of the various devices when loosening the lower pulmonary ligament.
Incision A is used for thoracoscopic viewing, and the ring forceps is inserted through incision B to lift the lower lobe upward to tighten the lower pulmonary ligament. The electric hook is inserted through incision C to reach and loosen the lower pulmonary ligament.
(II) Using the location of the lobar vein to determine the location of the fissure
On the anterior mediastinal surface of the lobe, the location of the lobar vein can be used to locate the fissure (Figure 12). The oblique fissure is located between the lower pulmonary vein and the upper pulmonary vein. The horizontal fissure is located between the upper-lobe branch and the middle-lobe branch of the upper pulmonary vein.
Figure 12.
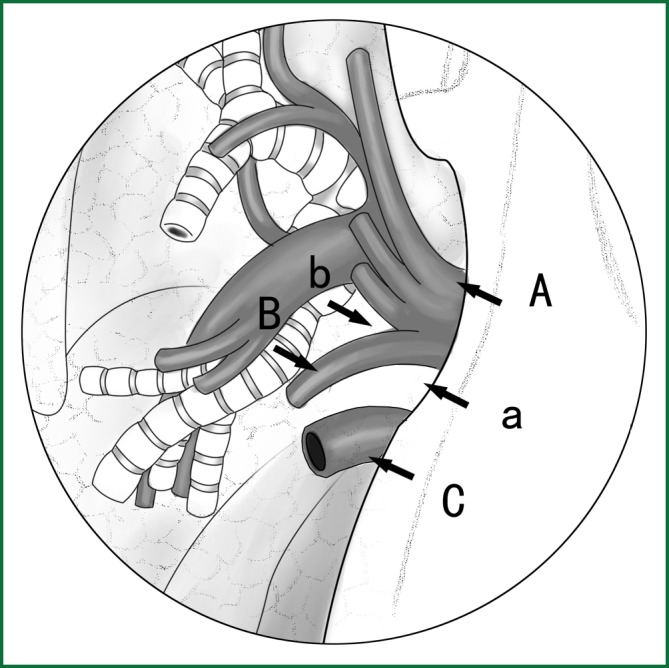
Locating the fissure using the location of the lobar veins. A. The lower pulmonary vein; B. the middle-lobe branch of the upper pulmonary vein; and C. the upper pulmonary vein. a. the gap for the cutting of the oblique fissure and b. the gap for the cutting of the horizontal fissure.
On the posterior mediastinal surface of the lobe, the location of the lobar bronchus can be used to locate the fissure (Figure 13). The oblique fissure is located between the right upper lobar bronchus and the middle bronchus.
Figure 13.
Locating the fissure using the location of the lobar bronchus. The arrow shows the gap between the right upper lobar bronchus and the middle bronchus.
(III) Separating a channel for the creation of the fissure in the pulmonary arteriovenous surface-lung parenchyma with a right-angle clamp or small head ring forceps
Taking the right lobe as an example, the starting and the ending points of the channel for the creation of the artificial fissure are shown in Table 4.
Table 4. The starting and ending points of the channel for the creation of an artificial fissure in the right lobe.
| The location of the fissure to be created | Starting point | Ending point |
|---|---|---|
| The upper part of the right oblique fissure |
The gap between the recurrent branch of the right upper pulmonary artery and the dorsal segment of the right lower pulmonary artery |
The gap between the right upper lobar bronchus and the middle lobar bronchus |
| The horizontal fissure |
The gap between the right middle pulmonary artery and the basilar segment of the right lower pulmonary artery (upper) |
The gap between the upper-lobe branch and the middle-lobe branch of the right upper pulmonary vein |
| The lower part of the right oblique fissure |
The gap between the right middle pulmonary artery and the basilar segment of the right lower pulmonary artery (lower) |
The gap between the middle-lobe branch of the right upper pulmonary vein and the right lower pulmonary vein |
(IV) The fissure is cut via the channel with an automatic linear cutting stapler
If the fissure is poorly developed and the tissue adjacent to the fissure is essentially fused, a cutting stapler can be used to cut open some tissue at the location of the fissure. After getting close to the pulmonary vein, the cutting stapler can be guided by a guiding tube via the previously separated channel and then triggered to completely cut open the fissure (Figures 14,15). The body of the guiding tube has an enlarged section, and the maximum diameter is the same as that of the tail of guiding tube. Therefore, as long as the body of the tube can pass through, the endoscopic cutting stapler that is guided by the tail will also be able to safely pass through.
Figure 14.
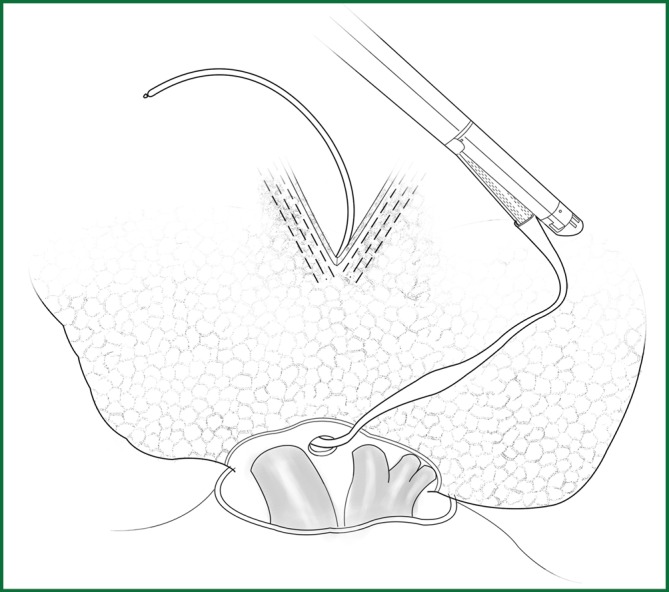
The fissure is cut with the guiding tube.
Figure 15.
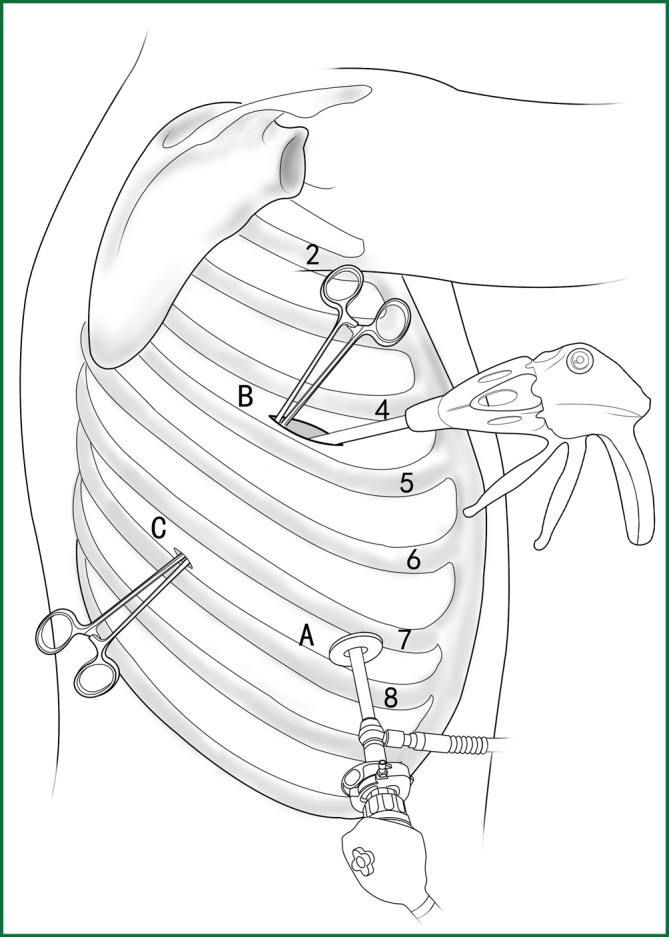
The locations of the various devices when cutting the fissure.
Incision A is used for thoracoscopic viewing, the ring forceps is inserted through incision B to lift the lobe upward, and another ring forceps pulls the lobe downward via incision C to tighten the fissure. The endoscopic cutting stapler cuts the fissure via incision B.
The separated lung tissue can be sutured mechanically or manually. A continuous back and forth stitching with 3-0 prolene thread is used to suture the cut edges of the lung tissue, which requires a relatively small operating space with the same reliable result (Figure 16).
Figure 16.
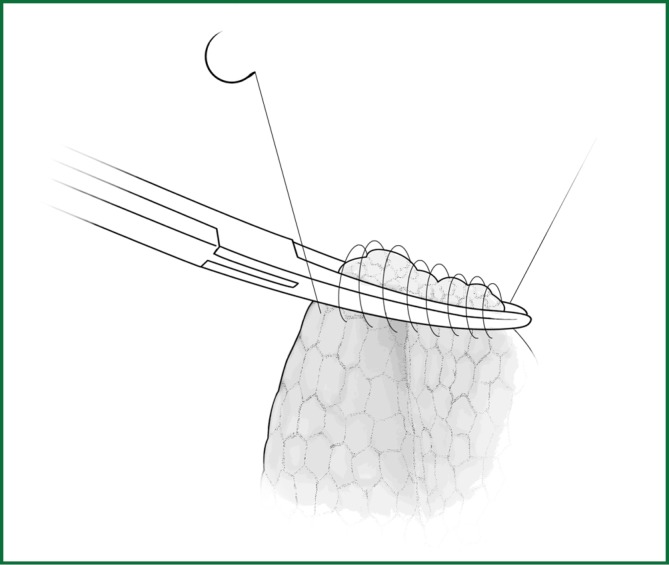
The edges of lung tissue are sutured with a continuous back and forth stitching using prolene thread.
Freeing and ligation of the hilar vessels
The approach for freeing the pulmonary vessels is similar to that of the traditional surgery. After the blood vessels are freed, the following steps are used:
(i) ligation;
(ii) suture;
(iii) clamping with a vascular clip;
(iv) the use of a cutting stapler or stapler.
(I) Ligation of blood vessels
The method for a silk-thread ligation of blood vessels with a knot pusher involves tying a knot outside the thoracic cavity, which is then pushed into the thoracic cavity using a knot pusher (Figure 17). This is a convenient and economical method in thoracoscopic surgery. During the operation, the following can be performed to facilitate the ligation:
Figure 17.
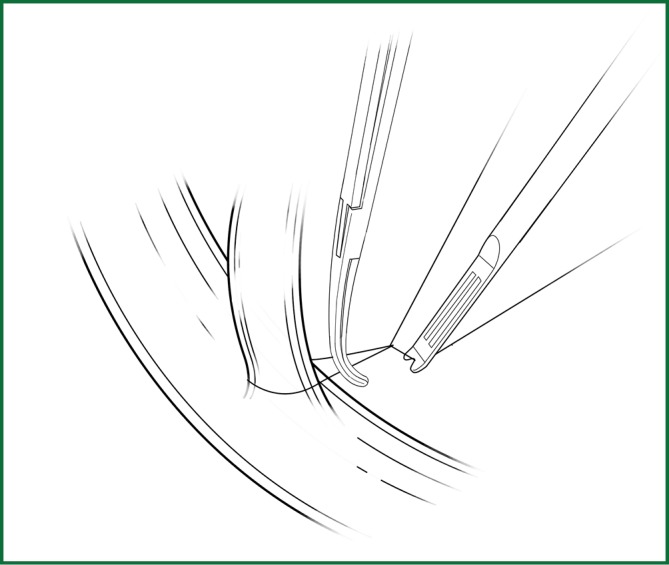
The silk ligation of blood vessels using the knot pusher.
• The lobar tissue is slightly lifted up, leading to a certain degree of tension in the blood vessels; therefore, the ligature can be slipped downward to the beginning part of the blood vessel.
• The ligature is pressed downward by a right-angle clamp so that the ligation is close to the beginning part of the blood vessel.
• The ligature is pushed down by the knot pusher. It should be noted that the knot pusher should push the thread, not the knot directly.
(II) Suture of blood vessels
Thread is used to suture the pulmonary vessels. If bleeding occurs while freeing the vascular tissue or if the freed blood vessels are thick, suturing is often required. The technique is the same as in the traditional surgery. A knot is tied outside the thoracic cavity and then pushed into the thoracic cavity with a knot pusher (Figure 18). This method is economical and reliable, and it is often used for thoracoscope-assisted surgery with a small incision.
Figure 18.
Suture of blood vessels.
(III) Clamping blood vessels with a clip
The clamping of blood vessels by a vessel clamp is suitable for thoracoscopic surgery (Figure 19), especially for low-pressure blood vessels. The method is simple and quick. The postoperative width of the blood vessels is slightly increased. Therefore, when applying the vessel clamp, clamping should be applied only after a complete control of the blood vessels is confirmed.
Figure 19.
Clamping blood vessels with a titanium clip.
(IV) Handling the blood vessels using a cutting stapler
This approach is applicable for thick and large blood vessels, such as pulmonary veins and the pulmonary artery trunk (Figure 20).
Figure 20.
Ligating the blood vessels using the cutting stapler. Up: direct ligation of the blood vessel and Down: ligation of the blood vessel under the guidance of the pediatric urethral catheter.
When handling the blood vessels with a cutting stapler, the cutting stapler typically only needs to be triggered after it passes through the gap between the blood vessel and its surrounding tissues. If the gap between the blood vessel and its surrounding tissues is small, it can pass through the gap under the guidance of a guiding tube before the triggering, which is safer. The specialized guiding tube is currently available, as described above. In our clinical practice, a pediatric Foley catheter with removed lateral branch is used as the guiding tube with the same satisfactory result.
Before triggering, it is important to verify that the correct staples are installed. To prevent uncontrollable bleeding in the case of a stapler failure, a thread ligation can be performed first to ligate the proximal end of the blood vessels.
(V) Freeing and severing of the bronchus
After the hilar vessels are ligated, the bronchus has already been freed, but the peribronchial fibrous tissue and lymphoid tissue should also be removed. It is best to use electrocautery to sever the peribronchial tissues to clarify the direction of the bronchus and to determine whether there is adhesion with other tissues. The bronchial artery or lymphatic vessels can be clamped using a titanium clip or ligated with thread first. The bronchus is sutured and severed by an endoscopic linear cutting stapler, or it is severed after the bronchus is stapled using an endoscopic linear stapler (Figure 21). An endoscopic linear cutting stapler is an ideal device to sever the bronchus, resulting in reliable stapling. An linear stapler has the same staple result as an endoscopic linear cutting stapler, but its “T”-shape head is large, which sometimes increases the difficulty of the operation.
Figure 21.
Severing of the bronchus using the cutting stapler.
Detailed illustrations of various lobectomies are presented below.
The right upper lobectomy
(I) Surgical procedures
(i) Cut open the mediastinal pleura and fissure
After the right phrenic nerve near the mediastinal pleura is located in front of the hilum, the mediastinal pleura is longitudinally cut behind the phrenic nerve (Figures 22,23). The cutting range of the mediastinal pleura starts from the area beneath the arch of the azygos vein at the top, turns along the posterior wall of the right main bronchus towards the rear of the hilum, and ends at the level of the right middle lobe bronchus. All bleeding is stopped with electric coagulation.
Figure 22.
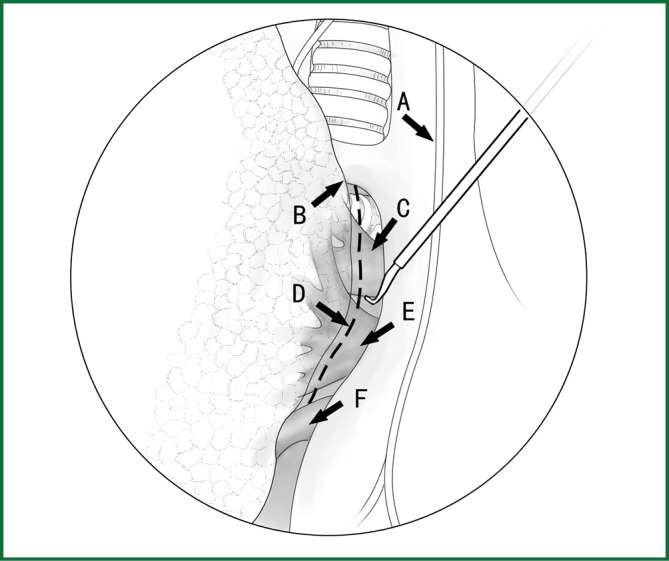
Cut open the mediastinal pleura of the hilum. (A) Phrenic nerve; (B) lung edge; (C) pulmonary artery; (D) incision line; (E) upper pulmonary vein; and (F) lower pulmonary vein.
Figure 23.
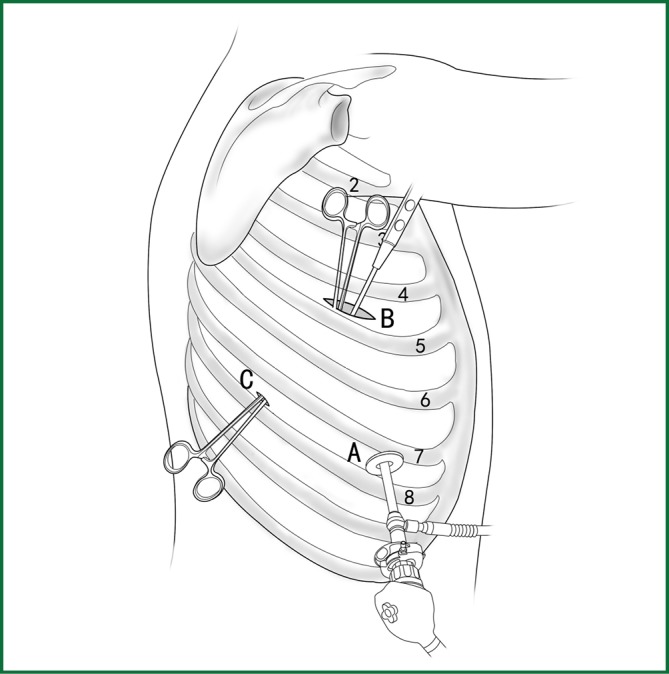
The location of the various devices. Incision A is used for thoracoscopic viewing; the ring forceps is used to lift and pull the lobe via incisions B and C; the mediastinal pleura is cut by the electric hook via incision B.
The fissure is cut according to the method described above.
(ii) Handling of the right upper pulmonary vein
The cut mediastinal pleura is gently pushed toward the lung with the "peanut" to expose the upper pulmonary vein. The sharp technique is used to free the pulmonary vein from the lung, which was performed horizontally underneath the sheath until the converging point of the right middle pulmonary vein and right upper pulmonary vein is reached (Figure 24). The vein is ligated with a small right-angle clamp and 7# thread, and the proximal end of the vein is sutured with No. 4 thread or ligated using a stapler.
Figure 24.
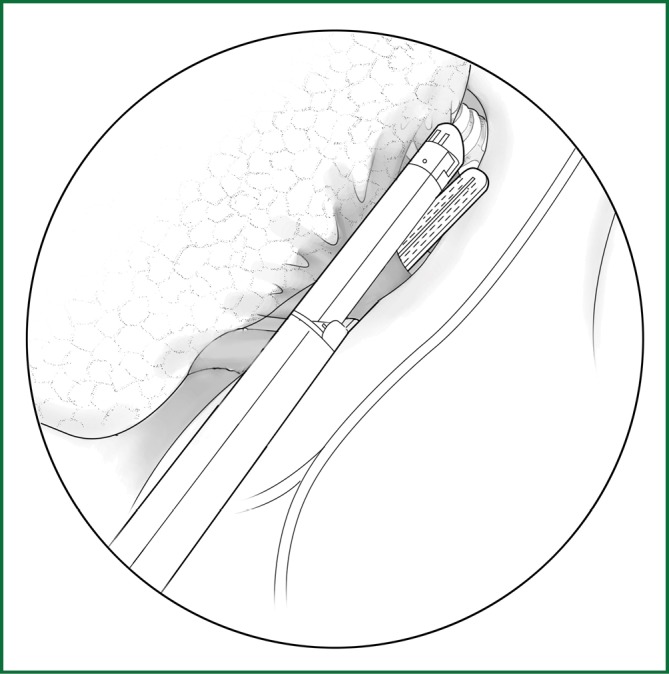
Ligating the right upper pulmonary vein using an endoscopic cutting stapler.
(iii) Handling of the pulmonary artery
The right upper lobe is pulled down and backward to expose the upper front edge of the hilum. The azygos vein is pushed upward along the cut mediastinal pleura so that the entry of the right pulmonary artery trunk from the lung parenchyma can be seen in a front-up to out-down direction through the gap between the posterior wall of the upper vena cava and the azygos vein. Along the front-up edge of the pulmonary artery, the arterial sheath is cut open, and the lung parenchyma is stripped for approximately 1 cm towards the distal end of the artery along the sheath, which then exposes the branching of the apicoanterior artery from the right pulmonary artery trunk. When a sufficient length of artery is freed, it can be ligated and severed with a conventional method or an endoscopic cutting stapler (Figure 25).
Figure 25.
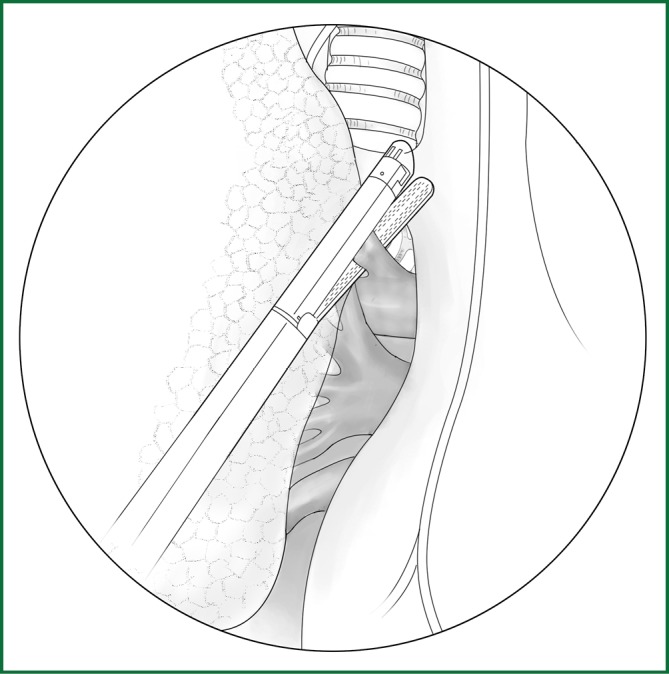
The apicoanterior branch of the right upper pulmonary artery is severed with an endoscopic cutting stapler.
The upper lobe parenchyma is dissected upward along the distal bronchial wall. Meanwhile, the fibrous tissues and lymph nodes on the surface of the middle pulmonary artery are removed to expose the posterior ascending branch of the upper pulmonary artery. It is severed after a conventional ligation with thread or clamping with a vascular clip (Figure 26). The detailed procedures are provided in the above descriptions.
Figure 26.
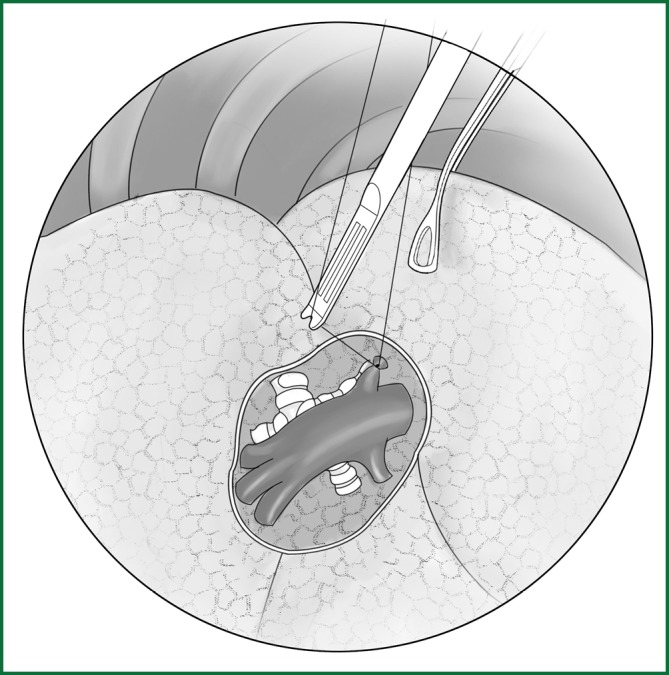
The ascending branch of the right upper pulmonary artery is ligated with a knot pusher.
The typical locations of various devices when the blood vessels are severed with an endoscopic cutting stapler are shown in Figure 27.
Figure 27.
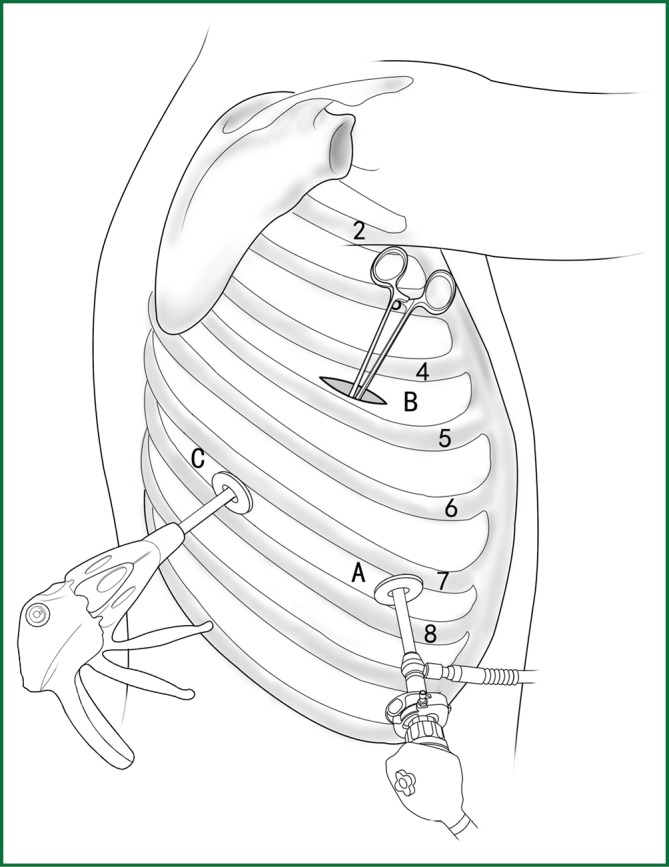
The location of various devices when the blood vessel is severed using an endoscopic cutting stapler.
The ring forceps is used to lift the lobe via incision B. Incision A is used for thoracoscopic viewing, and the blood vessel is severed by an endoscopic cutting stapler via incision C. The locations of the thoracoscope and endoscopic cutting stapler are interchangeable, as long as the endoscopic cutting stapler can conveniently pass through the gap between the blood vessels and tissue during the surgery.
(iv) Handling of the upper lobar bronchus
The lobe is pulled forward and downward, and the lung is separated along the right main bronchus toward the distal end to expose the right upper lobar bronchus. The lung is pulled forward to expose the rear edge of the hilum, and then the mediastinal pleura is lifted. The lung is separated downward along the membrane of the right main bronchus until the junction of the upper lobar bronchus and the middle lobar bronchus is exposed, and the accompanying bronchial artery is ligated and severed (Figure 28). The bronchus is sutured using an endoscopic cutting stapler 0.5 cm away from the root of the upper lobe. After disinfection, the stump can be reinforced by a continuous suture with absorbable sutures in case of necessary (Figure 29).
Figure 28.
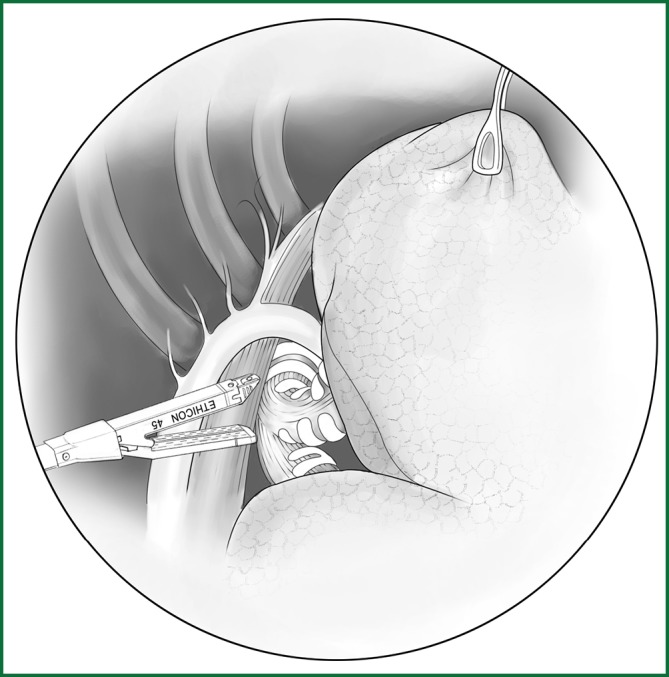
Sever the right upper lobar bronchus.
Figure 29.
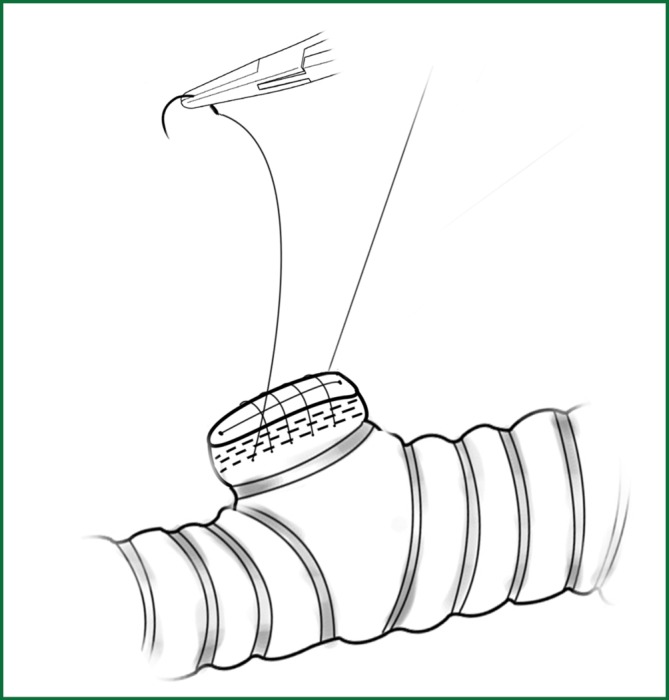
The reinforcement of the bronchus stump with a continuous suture.
(v) Remove the lobe and loosen the pulmonary ligament
The typical locations of the various devices while the right upper lobar bronchus is severed using an endoscopic cutting stapler are shown in Figure 30.
Figure 30.
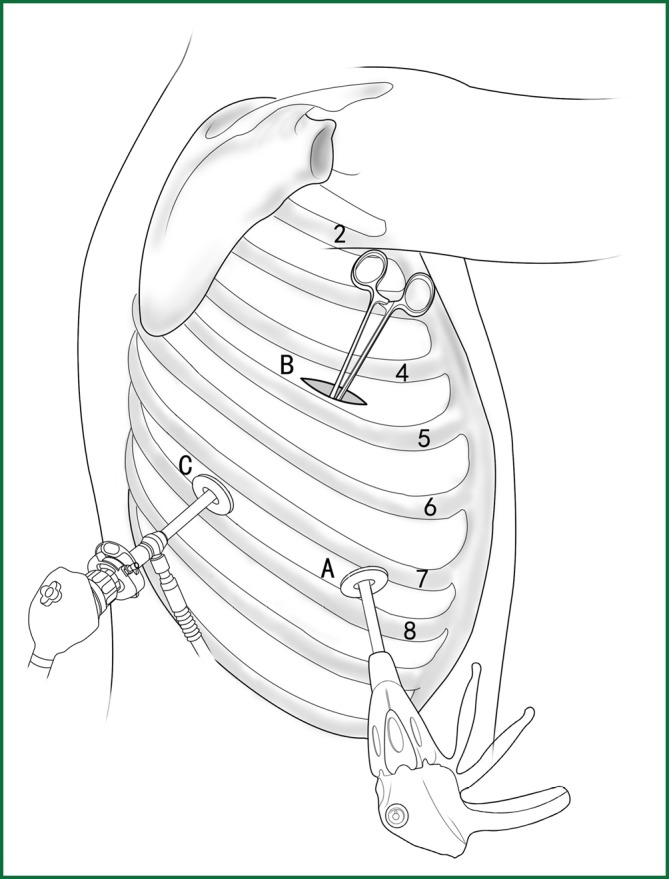
The location of various devices while severing the right upper lobar bronchus.
Incision A is used for thoracoscopic viewing. The ring forceps is used to lift the lobe upward via incision B, allowing the endoscopic cutting stapler to be as close as possible to the starting point of the bronchus. An endoscopic cutting stapler is used to sever the blood vessels of the right upper lobar bronchus via incision C. The locations of the thoracoscope and the endoscopic cutting stapler are interchangeable.
The right middle lobectomy
(I) Surgical procedures
(i) Cut open the mediastinal pleura and fissure
After the right phrenic nerve near the mediastinal pleura is located in front of the hilum, the mediastinal pleura is longitudinally cut open behind the phrenic nerve. The cutting range of the mediastinal pleura spans from the horizontal fissure at the top to the oblique fissure at the bottom. The detailed procedures can be found in the description for the right upper lobectomy. The fissure is cut according to the method described above.
(ii) Handling of the middle pulmonary vein
The upper pulmonary vein, which converges between the upper and the lower branches, is dissected, and the lower branch is the middle pulmonary vein. A complete separation of the horizontal fissure helps to clarify the boundary between the upper and the middle veins. The middle vein is freed and handled by the method described above (Figure 31). The usual locations of the various devices while the middle vein is severed using an endoscopic cutting stapler are shown in Figure 32.
Figure 31.
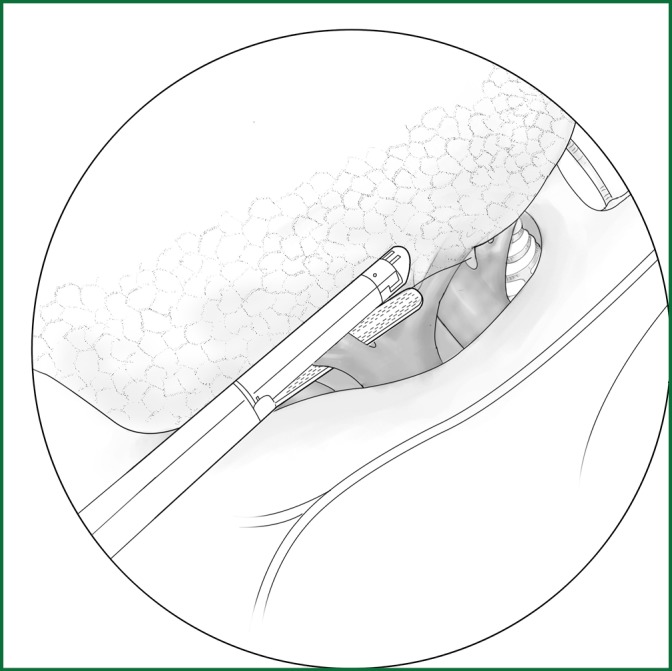
Severing of the middle vein using an endoscopic cutting stapler.
Figure 32.
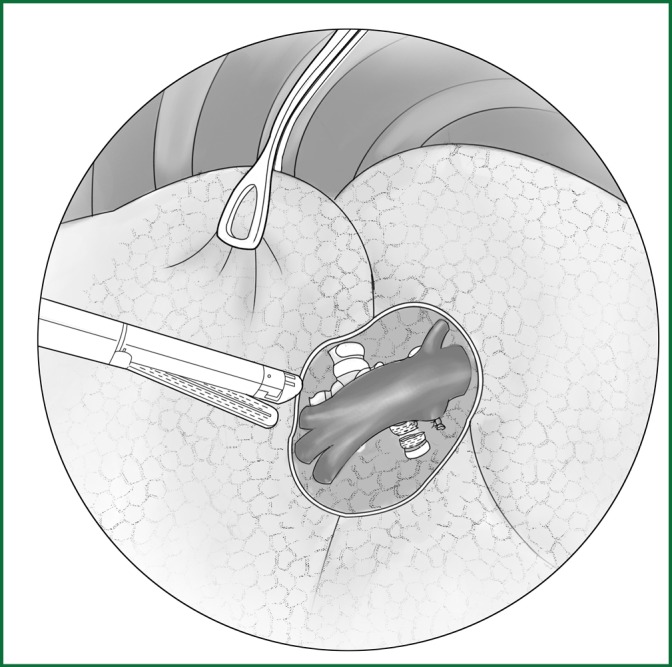
The location of the various devices.
Incision A is used for thoracoscopic viewing, the ring forceps is used to lift the lobe via incision B, and the middle vein is severed using an endoscopic cutting stapler via incision C. The locations of the thoracoscope and the endoscopic cutting stapler are interchangeable.
(iii) Handling of the middle pulmonary artery
The interlobar arterial sheath is cut open at the middle of the oblique fissure, and the horizontal artery branching from the anterior wall of interlobar artery is the middle artery. It can be severed after ligation (Figure 33) or severed using an endoscopic cutting stapler.
Figure 33.

Ligation of the right middle pulmonary artery.
(iv) Handling of the middle lobar bronchus
After the ligation of the middle artery, the proximal end of the middle artery is gently lifted to expose the middle lobar bronchus. While the assistant lifts the middle lobe with a ring forceps, the surgeon pushes the interlobar artery trunk backward to separate the interlobar artery from the middle lobar bronchus. After the lymph nodes at the root of the middle lobar bronchus are removed, the middle lobar bronchus is completely freed. It is severed using an endoscopic cutting stapler (Figure 34).
Figure 34.
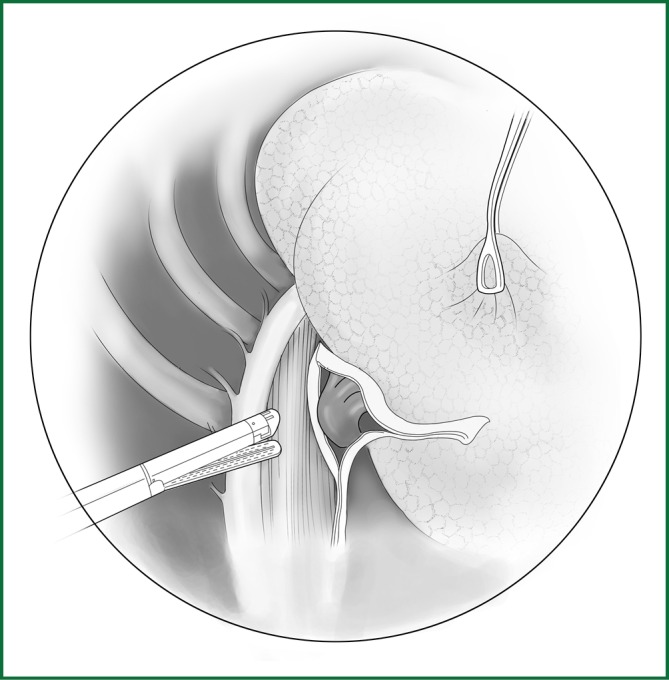
Severing of the middle lobar bronchus using an endoscopic cutting stapler.
The right lower lobectomy
(I) Surgical procedures
(i) Cut open the mediastinal pleura and fissure
The mediastinal pleura is longitudinally cut open behind the phrenic nerve. The cutting range of the mediastinal pleura starts from the horizontal oblique fissure and reaches the level of the middle lobar bronchus intermedius on the upper posterior side. The fissure is cut according to the method described above.
(ii) Handling of the right lower pulmonary vein
The right lower lobe is pulled forward, and the lower lobar ligament is loosened. After the lower pulmonary vein is located at the upper end of the lower pulmonary ligament behind the hilum, the surface of the mediastinal pleura is cut open, and the fibrous connective tissues on the vein surface are stripped so that the upper and lower edges of the vein are completely freed. After the right lower pulmonary vein is freed, it is severed using an endoscopic cutting stapler, or it is severed using an endoscopic cutting stapler after a thread ligation of the end proximal to the heart (Figures 35,36).
Figure 35.
Severing of the right lower pulmonary vein using the endoscopic cutting stapler.
Figure 36.
The location of various devices. Incision A is used for thoracoscopic viewing; the ring forceps is used to lift and pull the lobe via incisions B and C; and the endoscopic cutting stapler is used to sever the right lower pulmonary vein via incision B.
(iii) Handling of the right lower pulmonary artery
After the right upper lobe and the right lower lobe are pulled upward and downward, respectively, the interlobar arterial sheath is cut open at the junction of the middle of the oblique fissure and the horizontal fissure. The dorsal segmental artery branches into the terminal branches of the interlobar artery, the anterior inner segmental, and the posterior outer segmental arteries. The lower pulmonary artery is freed and severed using an endoscopic cutting stapler (Figure 37).
Figure 37.
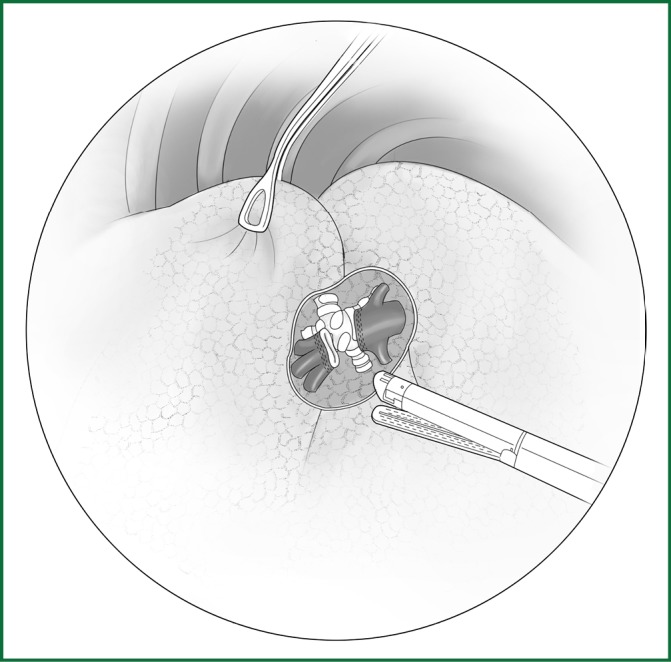
(upper right). Severing of the right lower pulmonary artery.
(iv) Handling of the lower lobar bronchus
After the handling of the pulmonary artery is completed, the lower lobar bronchus below is naturally exposed. The anatomical relationship of the middle and the lower lobar bronchus is similar to that of the artery. The handling is similar to the aforementioned approach (Figure 38).
Figure 38.
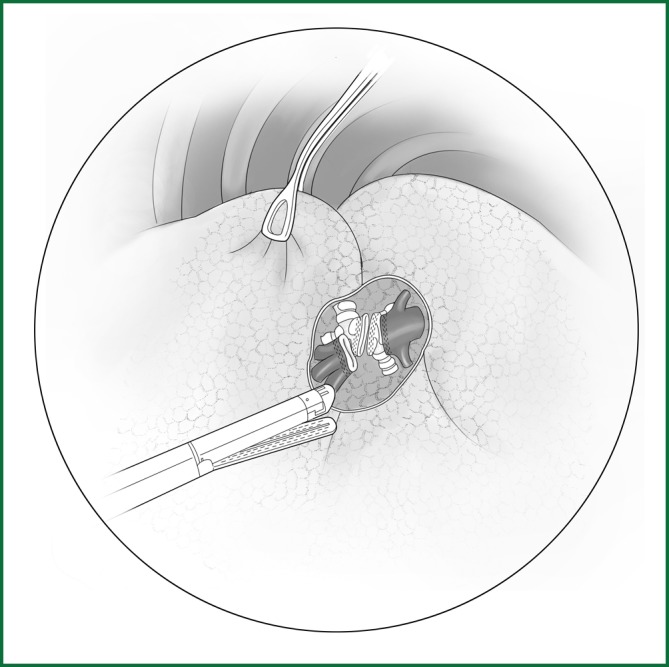
(left). Severing of the right lower lobar bronchus.
The locations of the various devices during the above procedures are shown in Figure 39.
Figure 39.
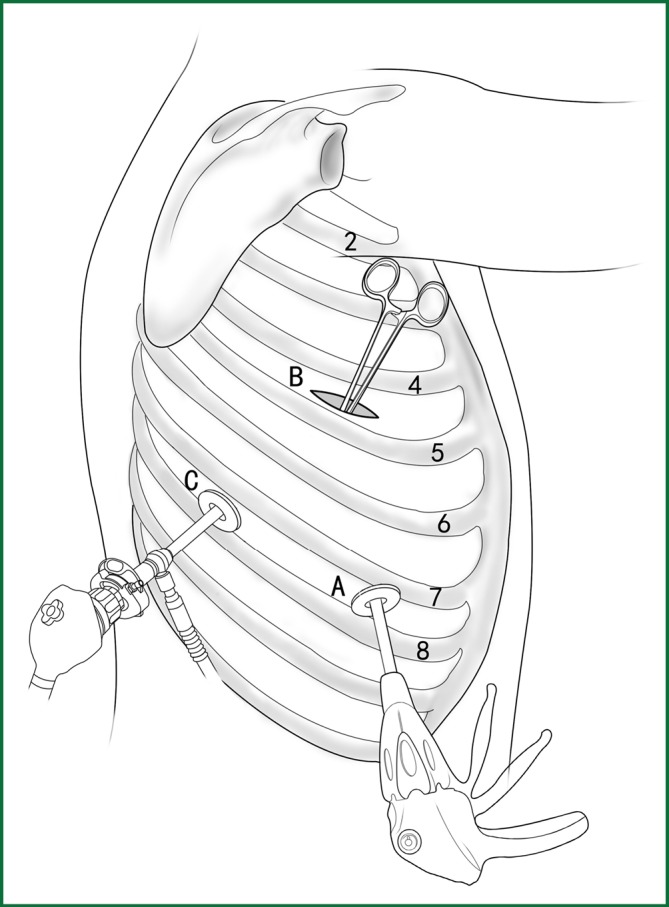
The locations of the various devices.
Incision A is used for the thoracoscopic viewing, and the ring forceps is used to lift the lobe via incision B. An endoscopic cutting stapler is used to sever the right lower pulmonary vein via incision C. According to the actual situation during the surgery, the locations of the endoscopic cutting stapler and the thoracoscope are interchangeable between incisions A and C.
The left upper lobectomy
(I) Surgical procedures
(i) Cut open the mediastinal pleura and fissure (Figure 40)
Figure 40.

Incision of the mediastinal pleura. The dotted line indicated by the arrow is the cutting line along the pulmonary edge.
Unlike the right lung, the left aortic arch replaces the azygos vein as the upper boundary of the left hilum, and the descending aorta in the back forms the rear boundary of the hilum. In front of the hilum, the mediastinal pleura is cut upward and backward from the location of the phrenic nerve. The incision passes underneath the aortic arch and around the upper hilar edge and then goes downward in front of the descending aorta. Attention is needed to not to damage the phrenic nerve, the recurrent laryngeal nerve, or the vagus nerve. The fissure is cut, as described above.
(ii) Handling of the left upper pulmonary vein
The handling of the left upper pulmonary vein is simpler than the right vein because there is no need to be concerned about a middle pulmonary vein. The upper pulmonary lobe is pulled backward to locate the upper pulmonary vein behind the phrenic nerve. The pulmonary vein sheath is not as clear as the pulmonary artery, but the wall of the vein is tougher than that of the pulmonary artery; therefore, the dissection can be carried out without much concern. Once the affiliated branches of the upper pulmonary vein are exposed, a sufficient length of vein is obtained. The posterior wall is bluntly freed with a small right-angle forceps. The posterior wall is also right next to the middle of the left pulmonary artery trunk; therefore, attention is needed about the depth and proper limit during the procedure. A small right-angle clamp is used to guide the 7# thread, and the upper pulmonary vein is severed after the ligation or severed using the cutting stapler (Figure 41). All of the subsequent steps are the same as those for the procedures for the right upper lobe. The locations of the various devices during the above procedures are shown in Figure 42.
Figure 41.
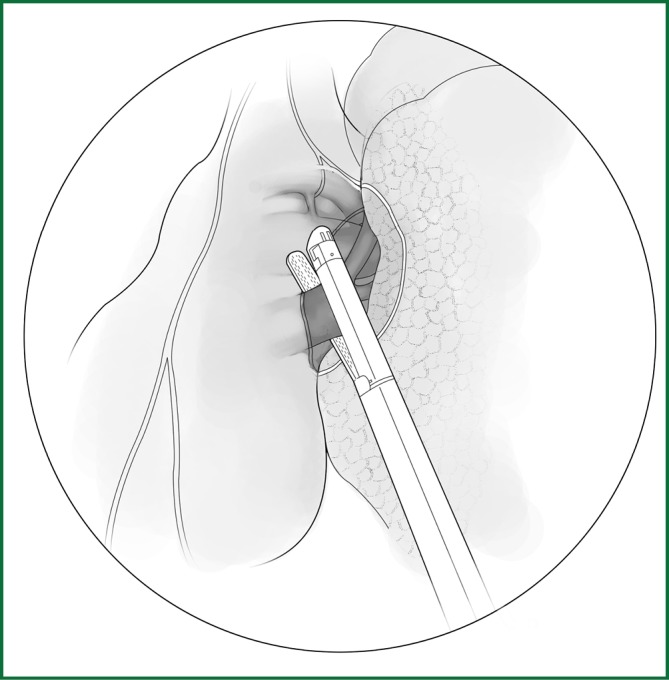
Severing of the left upper pulmonary vein using an endoscopic cutting stapler.
Figure 42.
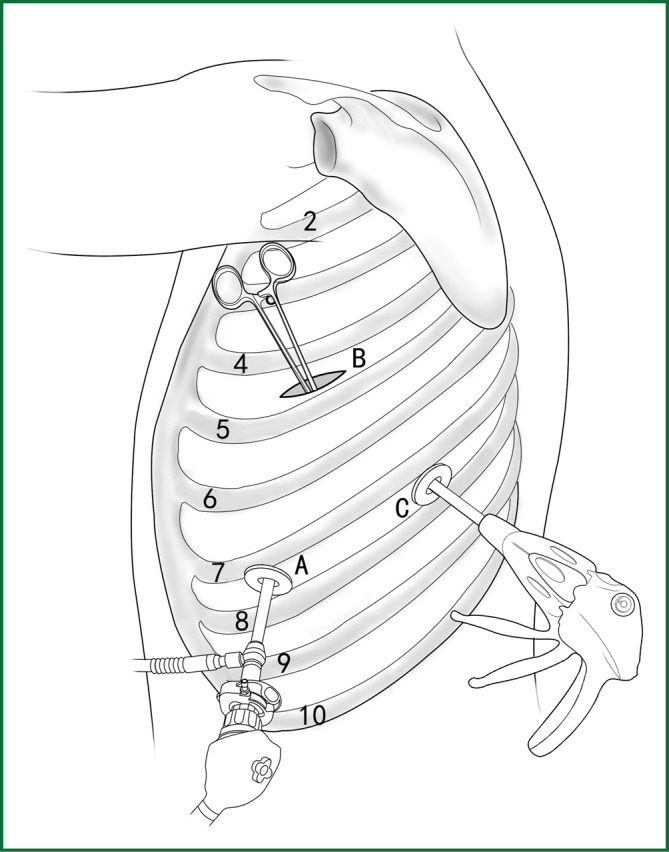
The location of the various devices.
Incision A is used for the thoracoscopic viewing, and the ring forceps is used to lift the lobe via incision B. An endoscopic cutting stapler is used to sever the left upper pulmonary vein via incision C. According to the actual situation during the surgery, the location of endoscopic cutting stapler and the thoracoscope are interchangeable between incisions A and C.
(iii) Handling of the pulmonary artery
After the upper and the lower lobes are pushed away from each other, the pulmonary artery sheath is cut open at the middle of the fissure. The sheath is longitudinally cut open along the fissure. At the leading edge of the interlobar artery in the front-middle 1/3 of the oblique fissure, 1-2 arterial branches that supply the upper lobe can be located, which is the lingual segmental artery. It is severed after a conventional ligation or using an endoscopic cutting stapler (Figure 43).
Figure 43.
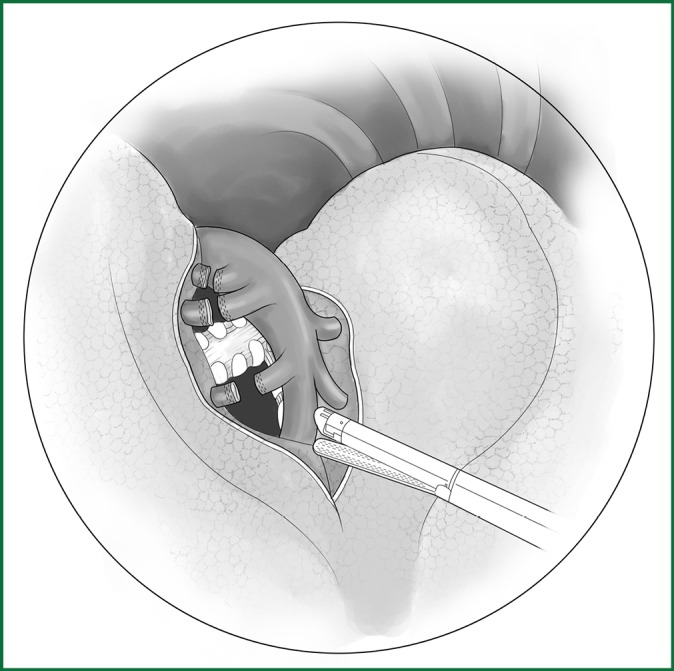
Severing of each branch of the upper left pulmonary artery using an endoscopic cutting stapler.
After the entire interlobar artery is well exposed, 1-2 branches of the posterior segmental artery and 1-2 branches of the anterior segmental artery are carefully freed and exposed along the upper edge of the interlobar artery and toward the heart. All of these branches are ligated with a conventional ligation. After the root of the apicoanterior segmental artery is vaguely exposed, the upper lobe is gently lifted forward and upward to expose the posterior upper part of the hilum, which is followed by a conventional ligation with a pulmonary right-angle clamp-guided thread or a severing with an endoscopic cutting stapler.
(iv) Handling of the bronchus
The left upper lobar bronchus is located at the middle of the oblique fissure, the leading edge of the interlobar artery, and the lingual segmental artery, and the deep part of posterior segmental upper artery can usually be exposed only after a ligation of the lingual segmental and posterior segmental arteries. After the lingual segmental and posterior segmental arteries are severed, the upper lobe is pulled forward and upward to expose the left upper lobar bronchus. It is routinely severed using an endoscopic cutting stapler (Figure 44).
Figure 44.
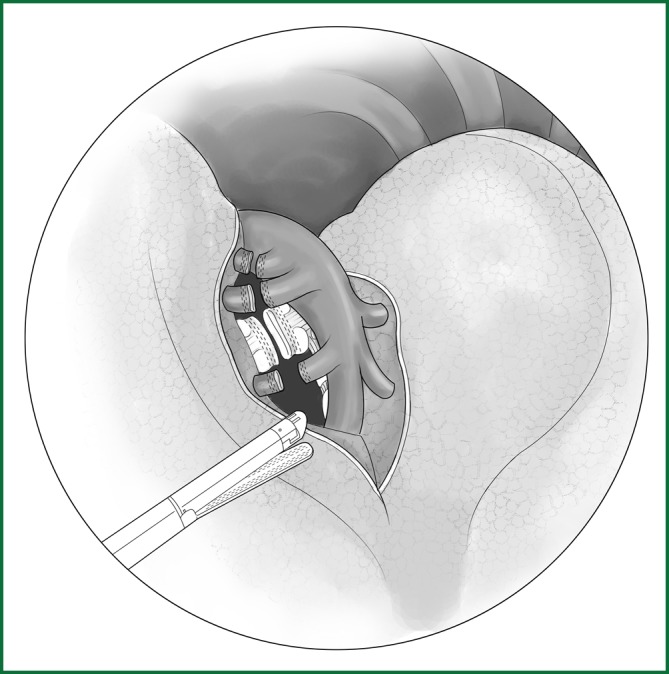
Severing of the upper left lobar bronchus using an endoscopic cutting stapler.
(v) Loosening the left lower pulmonary ligament
The left lower lobectomy
(I) Surgical procedures
(i) Cut open the mediastinal pleura and fissure
The procedure for cutting the mediastinal pleura is basically the same as in the left upper lobectomy, except that the range is the mediastinal pleura below the oblique fissure and around the hilum. The specific procedures for the fissure dissection are similar to the above description.
(ii) Severing of the left lower pulmonary vein
After the left lower pulmonary ligament is loosened, the left lower pulmonary vein is freed and severed using an endoscopic cutting stapler (Figure 45). It can also be ligated with thread.
Figure 45.
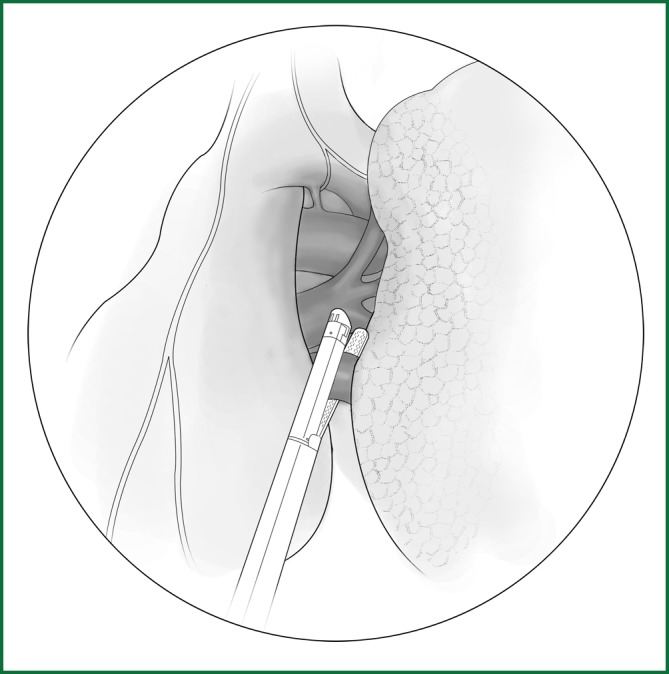
After the left lower pulmonary vein is freed, it is severed using an endoscopic cutting stapler.
(iii) Handling of the left lower pulmonary artery
The pulmonary arteries of the left lower lobe are handled along the fissure. After the interlobar artery is exposed in the middle of the fissure, the artery sheath is cut and dissected downward. The last artery that branches from the leading edge of the interlobar artery and towards the upper lobe is the lower lingual artery. The distal part of the basilar segmental artery is ligated. After the interlobar arterial sheath is cut upward, the artery that is above the lingual segmental artery and branches backward from the lingual segmental artery at the corresponding location of the upper lingual segmental artery is the dorsal segment, which is handled with the conventional approach (Figure 46).
Figure 46.
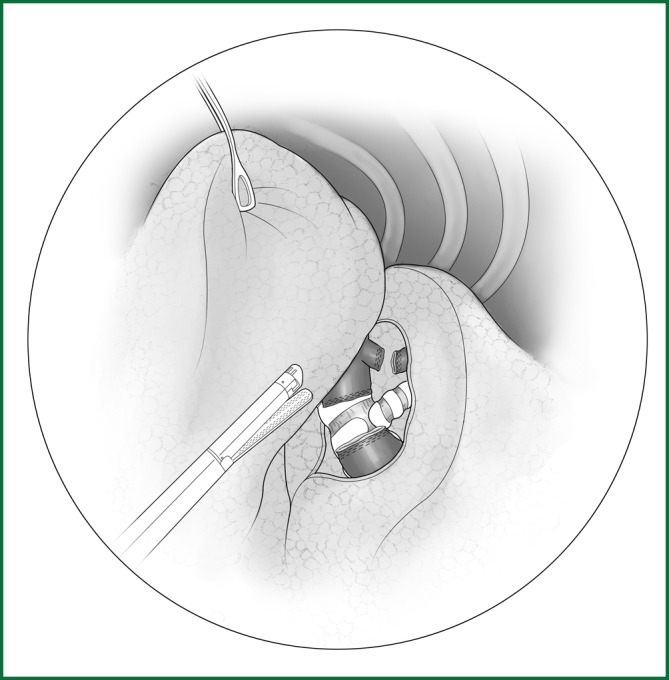
Severing of the left lower pulmonary artery trunk using an endoscopic cutting stapler.
(iv) Handling of the lower lobar bronchus
After the lower lobe artery is handled, the lingual segment of the upper lobe is gently lifted, and the interlobar artery is gently pushed away. The left lower lobar bronchus lies below and is handled with the conventional approach (Figure 47).
Figure 47.

Severing of the left lower lobar bronchus using an endoscopic cutting stapler.
Precautions
The middle of the right pulmonary artery trunk lies beneath the right upper pulmonary vein, and the uppermost affiliated branch of the upper pulmonary vein happens to cross the right pulmonary artery and the starting part of the apicoanterior segmental artery. Therefore, attention is needed to free the blood vessels in this area so that the upper edge and the first affiliated branch of the upper pulmonary vein are separated from the pulmonary artery that lies behind. After a small right-angle clamp is inserted at the back of the pulmonary vein, a gradual, blunt separation is performed along the gap between the upper pulmonary vein and the right pulmonary artery. During the separation, the clamp should stay close to the posterior wall of the upper pulmonary vein, as it is easy to damage the pulmonary artery behind it if the clamp is inserted too deeply. Because the posterior wall of the right upper pulmonary vein is next to the right pulmonary artery, the needle cannot go too deep during the suture; instead, the needle needs to stay close to the blood vessel. A suture that is too deep may mistakenly suture the anterior wall of the middle of the pulmonary artery trunk. When the upper pulmonary lobe is being removed, a tear resulting in bleeding from the pulmonary artery may occur. During the right upper lobectomy, the initial ligation and severing of the uppermost branch of the upper pulmonary veins will facilitate the exposure of the apicoanterior segmental artery.
There is generally one apicoanterior segmental artery in the right upper lobe, and it is thick and large. If the first artery is small, the existence of a second artery should be considered. In addition, the posterior ascending artery can also be handled before the severing of the upper lobe bronchus. When handling the posterior ascending artery, the upper and lower lobes are pushed away. Additionally, the interlobar artery is cut open slightly behind the junction of the horizontal fissure and the oblique fissure. The posterior ascending artery can be located above the starting point of the dorsal segmental artery along the upper edge of the interlobar artery.
The anatomical characteristics of the right lower lobe include the right middle lobar bronchus and the dorsal segment of bronchus branching forward and backward, respectively, at almost the same level, and the lower lobar bronchus "trunk" being short, which is extremely important to consider to prevent operational errors in the right middle and lower lobectomies.
If a tumor in the dorsal segment and the outer basilar segment of the lower lobe is too large, it is sometimes difficult to access the hilum from behind. The lower pulmonary vein can be handled via the lower pulmonary ligament. The procedures are as follows: the lower lobe is pulled towards the head; the pulmonary ligament is ligated in bundles, and the lymph nodes at the lower pulmonary ligament are removed; and the lower pulmonary vein can be exposed near the upper end of the ligament. If this still does not create a safe distance, the parietal pericardium can be cut in an arc shape along the root of the lower pulmonary vein, and an additional safe length about 1.5 cm is obtained using the intrapericardial segment.
The distance between the dorsal segmental artery and the middle pulmonary artery is short on the interlobar artery trunk. During the actual operation, it is recommended that the dorsal segmental artery be handled before the ligation of the lower basilar segmental arteries. During the ligation of the basilar artery, it is best to first verify the middle pulmonary artery and not to rashly ligate any horizontal vessel. During the ligation of the dorsal segmental artery, whether the blood vessel enters the upper lobe or not should be verified. In the case of “two” dorsal segmental arteries, it is necessary to determine whether there is any posterior ascending branch of the upper dorsal segmental artery.
For some patients, the convergences of various venous branches are close to each other, and the branches are scattered, resulting in a short pulmonary vein trunk and an insufficiently safe distance. In this case, it is more appropriate to ligate the branches individually. For central lung cancer, if the pulmonary vein trunk is partially invaded, the intrapericardial handling procedure should be adopted. The expand of the incision is generally needed. The pericardium is cut at the location where the pericardial pulmonary vein returns, and the incision is extended to 2-3 cm along the upper surface of the pulmonary vein. A finger is inserted to probe and verify that the intrapericardial segment of the pulmonary vein is not invaded before the pericardium is completely cut open along the pericardial polyline to fully expose the intrapericardial pulmonary vein. If necessary, the atrium can be partially resected. During the intrapericardial probing, it should be noted whether there is a tumor thrombus in the pulmonary vein. If a tumor thrombus in the pulmonary vein is suspected, an atrial clamp should be applied proximal to the thrombus to block its shedding path, and the thrombus should be removed after the vein is cut open, with the vein then being severed. After verifying that there is no thrombus debris at the proximal end, a 4-0 non-invasive suture is used for a continuous suture.
There are many variations in the anatomy of the left upper pulmonary artery, with large differences in the number of branches of various segments, which range from 3 to 7-8. Only after all arteries are ligated can it be confirmed that all segments of the left upper pulmonary artery have been handled. The first branch of the left pulmonary artery is the apicoanterior segmental artery. It is thick and short and forms an acute angle to the left pulmonary artery trunk, and it can easily be torn at the root by improper pulling that causes bleeding. The handling of the left upper lobar artery can start from the hilum and move towards the distal end to search for and handle the branches one by one. It can also be handled from the fissure and towards the proximal end. Special attention is needed while pulling the upper lobe to prevent the tearing of the apicoanterior segmental artery.
The upper left lobar bronchus is extremely short, often shorter than 1.0 cm. For a solid ligation, the lingual segmental and the apical segmental bronchi can be severed. The angle between the lingual segmental and the apicoanterior segmental bronchi is often large. They have to be verified before severing to prevent a missed ligation of the lingual segmental bronchi.
All of the possible unexpected events that can occur during a lobectomy by a traditional thoracotomy may happen in a thoracoscopic or thoracoscopic-assisted small incision lobectomy. When an unexpected event occurs, the handling principles are the same as the traditional thoracotomy. Because the incision is small and the space is small, the two-dimensional anatomical images are not as realistic as the three-dimensional ones, increasing the difficulty of handling. The most common unexpected event during surgery is bleeding. If intraoperative bleeding occurs while freeing the blood vessels, the bleeding site is first pressed with gauze that is placed into the thoracic cavity using an ring forceps, and the surrounding clots are removed by suction. After the bleeding blood vessel is identified, the handling method of ligation, suture, titanium clip clamping, or suture with 4-0 prolene thread is decided on. If the bleeding is difficult to stop under the endoscope, the incision should be extended after compression hemostasis to perform hemostasis under direct vision. If the bleeding is only on the fissure surface, it can be sutured with thread or prolene thread under the endoscope. A mechanical suture is extensively used in thoracoscopic surgery; therefore, precautions must be taken to prevent a mechanical failure. The correct staple should be selected according to the thickness of the tissue in the target area. For thick and large blood vessels, such as the pulmonary vein, if a cutting stapler is used for severing, a successful approach is to first ligate the proximal end of the blood vessels with thread after freeing the blood vessels, which is then followed by a mechanical suture or other methods.
While handling the blood vessels, the following unexpected events might occur: erroneous ligation of the middle pulmonary artery, missed ligation of the dorsal segmental artery of the lower pulmonary artery, or accidental damaging of the posterior segmental arteries of the upper pulmonary artery. For the erroneous ligation of the middle pulmonary artery, an additional middle lobectomy has to be performed. If the ligation of the dorsal segmental artery of the lower pulmonary artery is missed, there will be resistance while removing the lower lobe. In this case, the specimen should not be forcibly removed. Instead, it should be carefully examined and removed after the ligation and severing of the dorsal segmental artery. The posterior segmental artery of the upper pulmonary artery is small, and there are a large number of communicating branches of the apicoposterior segment; therefore, ischemic necrosis does not occur in the posterior segment of the upper lobe. In this case, handling of the bleeding is sufficient, and other remedial procedures are not needed.
The indications for intraoperative conversion to operation under direct vision include the following:
(I) an intraoperative bleeding that is difficult to control;
(II) Discovery that lymph nodes have broken through the capsule or invaded the adjacent vessels or that pleural metastasis has occurred (needed only in some patients);
(III) the tight adhesion at the hilum renders normal dissection impossible;
(IV) the fissure is not developed and cannot be cut under the endoscope, or the adhesion of the chest wall renders separation impossible under the endoscope;
(V) an estimate of long endoscopic operation time, resulting in a significantly prolonged time for surgery and anesthesia.
Thoracoscopic segmentectomy
Introduction
Each lung segment has a separate group of bronchi, arteries, and segmental veins shared with the adjacent segments. A resection based on their anatomy will not damage other lung segments. Therefore, for certain lesions that are restricted to one lung segment, especially benign lesions, a segmentectomy may be considered. The advantage of a lung segmentectomy is that it preserves as much normal lung tissue as possible, with only a small loss of lung function, while its disadvantage is the high technical requirement. A lung segmentectomy is most common in the lingual segment of the left upper lobe and the dorsal segment of the right lower lobe.
The lung segmentectomy is introduced below with a lung segmentectomy of the lingual segment of the left upper lobe as an example (Figure 48). The preoperative preparation, anesthesia, and patient’s position during the lung segmentectomy are similar as in the description of the lobectomy.
Figure 48.
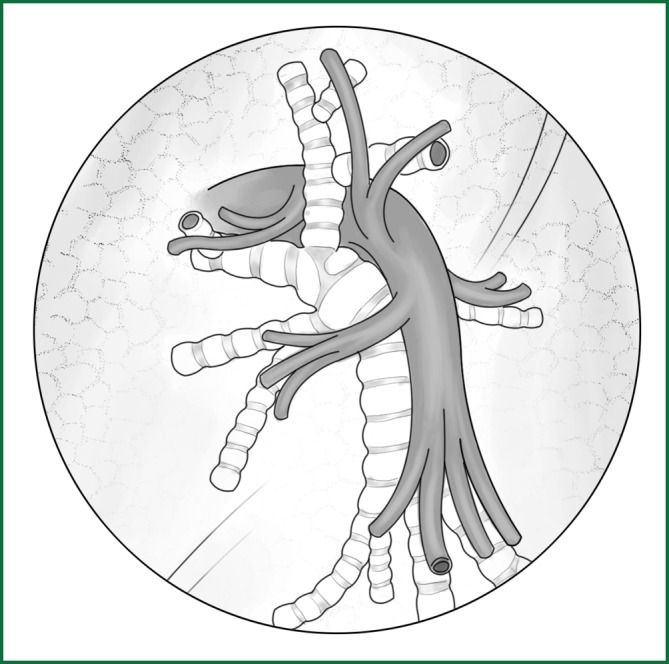
Anatomical schematic of the bronchus and arteries of the lingual segment.
Incision
A three-incision method is usually used to complete the VATS lung segmentectomy.
The 1st incision is used for observation. It is 1.2 cm in length and is located at the left midaxillary line or anterior-axillary line of the 6th or 7th intercostal space.
The 2nd incision is used for the main operation. It is generally located between the anterior-axillary line and mid-nipple line at the 4th or 5th intercostal space. It is approximately 3-5 cm in length. The length of the incision for the main operation depends on the size of the resected lobe, the degree of pleural adhesions, the degree of the fissure development, and the operational skills of the surgeon.
The 3rd incision is used for the auxiliary operation. It is located between the posterior-axillary line and the scapular line of the 6th or 7th intercostal space and should be as close as possible to the intercostal space of the observation incision. The incision is 5 mm-1.2 cm in length. The incision location is also selected under a thoracoscope.
Surgical procedures
Cut the left oblique fissure
The left lower lobe is pulled downward and backward, and the upper lobe is pulled forward and upward to expose and cut the oblique fissure of the left lung. According to the level of fissure development, the dissection can be carried out using a cutting stapler (Figure 49), electric hook, or ultrasonic scalpel.
Figure 49.
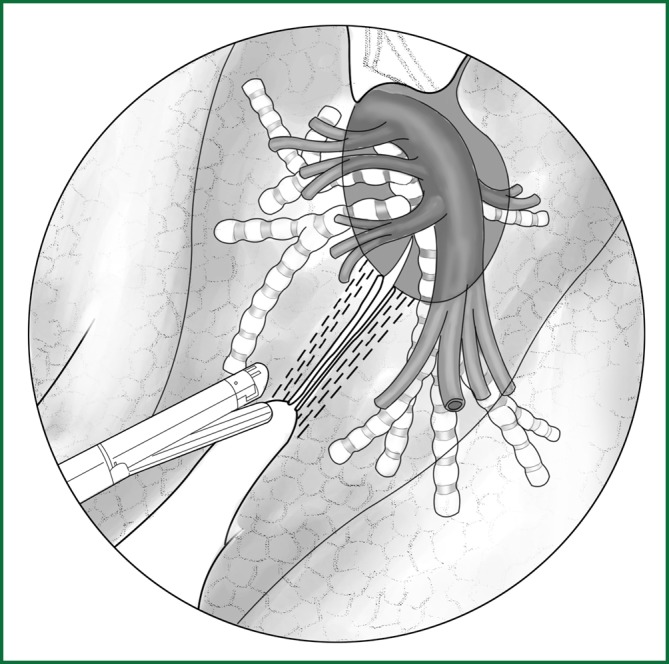
The dissection of the oblique fissure of the left lung using a cutting stapler.
Handling of the lingual segmental artery
After the lingual segmental artery of the left upper pulmonary artery is dissected in the oblique fissure, it is clamped with a vascular clip (with or without buckle) and severed (Figure 50). It can also be freed by an ordinary right-angle clamp with the suture attached at the proximal end and severed after a knot tying using a knot pusher and ligation. To save time, the lingual segmental artery can be severed using an ultrasonic scalpel after a ligation with thread at the proximal end; therefore, the ligation at the distal end is not necessary.
Figure 50.
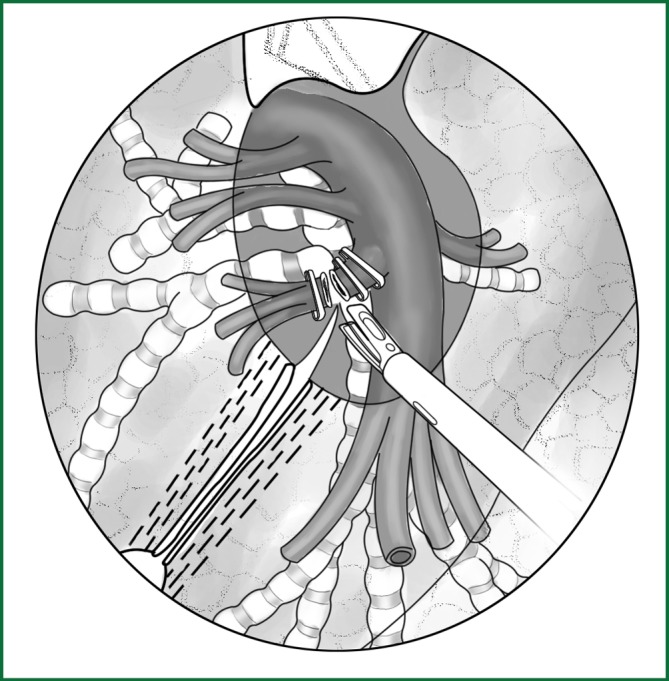
The lingual segmental artery of the left upper pulmonary artery is clamped with a vascular clip and then severed.
Handling of the lingual segmental vein
After the upper lobe is pulled backward and the hilar pleura is cut open, the upper pulmonary vein is freed, and the lowermost branch is the lingual segmental vein. It is severed after ligation or severed using an endoscopic cutting stapler (Figure 51).
Figure 51.
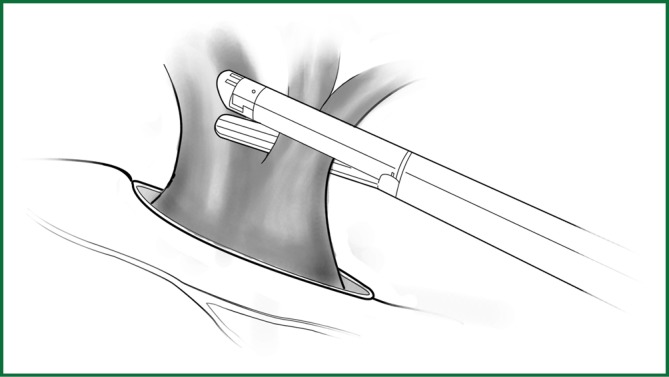
The lingual segmental vein of the upper pulmonary vein is freed and then severed using a cutting stapler.
Handling of the lingual segmental bronchus
The lingual segmental bronchus of the left upper lobar bronchus is freed and then ligated or severed using an endoscopic cutting stapler (Figure 52).
Figure 52.
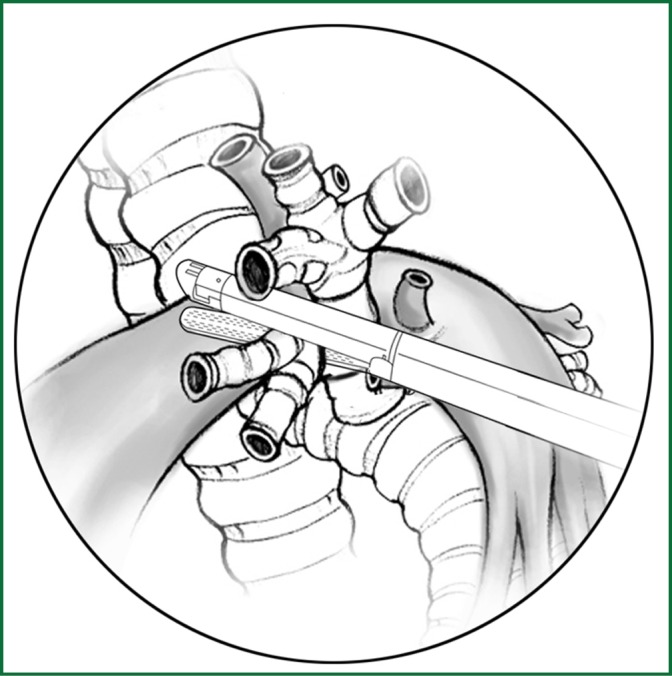
Severing of the lingual segmental bronchus using an endoscopic cutting stapler.
Resection of the lingual segment of lung tissue
The anesthetist helps to inflate the lungs for observation. There is a clear boundary between the intrinsic upper lobe of the left upper lung and the lingual segment of lung (Figure 53). The lingual segment is continuously severed along this boundary using an endoscopic cutting stapler.
Figure 53.
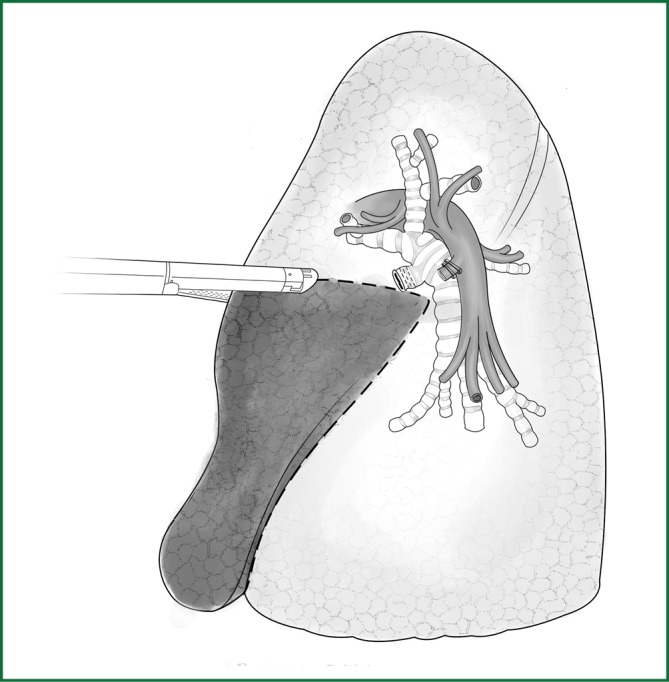
The lingual segment without inflation expansion is continuously severed using an endoscopic cutting stapler.
Thoracoscopic-assisted pneumonectomy
Introduction
The use of a thoracoscopic pulmonary wedge resection and lobectomy has been widespread, but the number of thoracoscopic pneumonectomy cases is still small. In 2003, Conland et al. first reported on cases of thoracoscopic pneumonectomy. The thoracoscopic pneumonectomy technique is simpler than that of a thoracoscopic lobectomy because the variations of vascular locations that need to be handled are smaller for the pneumonectomy and the fissure also does not need to be handled. However, surgeons often avoid the pneumonectomy in clinical practice. Additionally, the patients who need a pneumonectomy often have hilum-invading lesions and thus cannot undergo a cuff-like resection, or the lung damage is accompanied by a significant intrathoracic adhesion. All of these situations increase the risk of a thoracoscopic pneumonectomy. Therefore, most surgeons often use a thoracoscopic-assisted small incision when performing the thoracoscopic pneumonectomy, and the incision length is different, ranging from 6-16 cm. Moreover, the degree of rib expansion is also different with the goal of obtaining partial direct vision, which is conducive to the operation. We believe that a thoracoscopic-assisted pneumonectomy with a small incision, under the premise of ensuring the treatment effect, provides a better balance of being minimally invasive, efficient, and safe.
The indications for surgery
(I) Damaged lung;
(II) For patients with a central lung cancer who cannot undergo bronchial molding or angioplasty surgery, pneumonectomy can be used to completely resect the lesions.
The preoperative preparation
The preoperative preparation is essentially the same as that of the thoracoscopic lobectomy. Testing for pulmonary function and the blood gas analysis are performed to critically evaluate whether the cardiopulmonary function is sufficient to tolerate a pneumonectomy.
Anesthesia
A double-lumen tube intubation combined with intravenous anesthesia is performed.
Position
The patients usually lie on the side of the healthy lung (Figure 54). The waist bridge is elevated so that the intercostal space is expanded as much as possible to facilitate the operation. Moreover, the surgical operating table can be rotated to facilitate the operation during the surgery.
Figure 54.
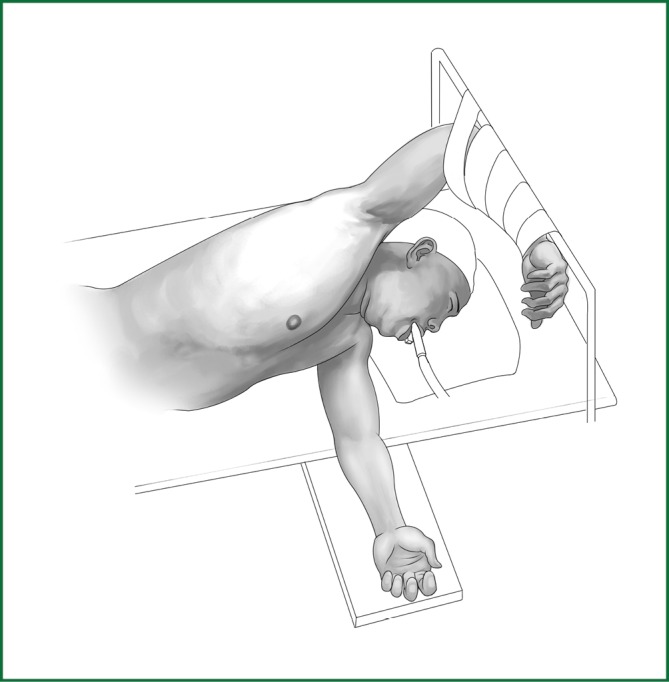
Patient’s position.
The surgical procedures
Thoracoscopic-assisted pneumonectomy includes several steps, as described below.
Incision
The observation incision is 1.2 cm in length and is located at the midaxillary line or posterior-axillary line of the 6th or 7th intercostal space.
The operation incision is approximately 5-10 cm in length and is located between the posterior-axillary line and the mid-scapular line at the 4th or 5th intercostal space. The length of the auxiliary incision depends on the size of the resected lobe, the degree of pleural adhesion, the degree of fissure development, the operational skills of the surgeon, and the financial situation of the patient. The incision is cut and then expanded.
Right pneumonectomy
The mediastinal pleura is cut around the pulmonary root, which is followed by a blunt dissection toward the lung to expose the hilar blood vessels (Figure 55).
Figure 55.
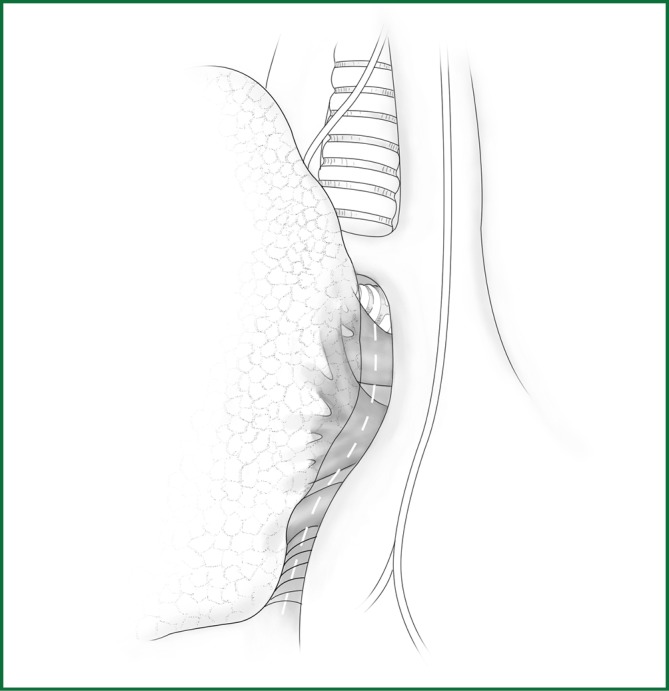
Cutting the mediastinal pleura.
The lower lobe is pulled forward and upward, and the lower lung ligament is cut open and pulled upward. Then, the mediastinal pleura is cut to free the lower pulmonary vein, which is severed using an endoscopic cutting stapler (Figure 56).
Figure 56.
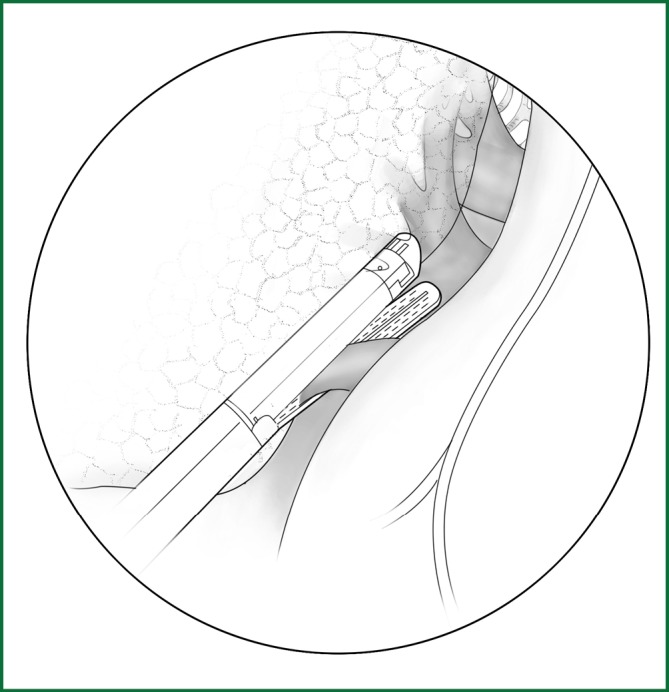
Severing of the right lower pulmonary vein.
The upper and lower lobes are pulled backward to expose the anterior edge of the hilum. The upper pulmonary vein on the shallow surface is freed first and severed using an endoscopic cutting stapler (Figure 57). The upper and middle pulmonary veins can also be severed.
Figure 57.

Severing of the right upper pulmonary vein.
After the right pulmonary artery trunk is freed, it is clamped with a stapler and severed after the distal end is ligated. If it is difficult to directly free the right pulmonary artery trunk, the first branch of the pulmonary artery trunk (i.e., apicoanterior segmental artery) can be ligated and severed first to fully expose the right pulmonary artery trunk (Figure 58).
Figure 58.
Handling of the right pulmonary artery trunk. (A) A stapler is used to clamp the right pulmonary artery trunk and (B) the stapler is used to clamp the first branch of the right pulmonary artery trunk.
The upper lobe is pulled downward, and the right main bronchus is freed beneath the arch of the azygos vein and severed at a distance of 0.5-0.8 cm from the carina of the trachea after being clamped with a stapler (Figure 59).
Figure 59.
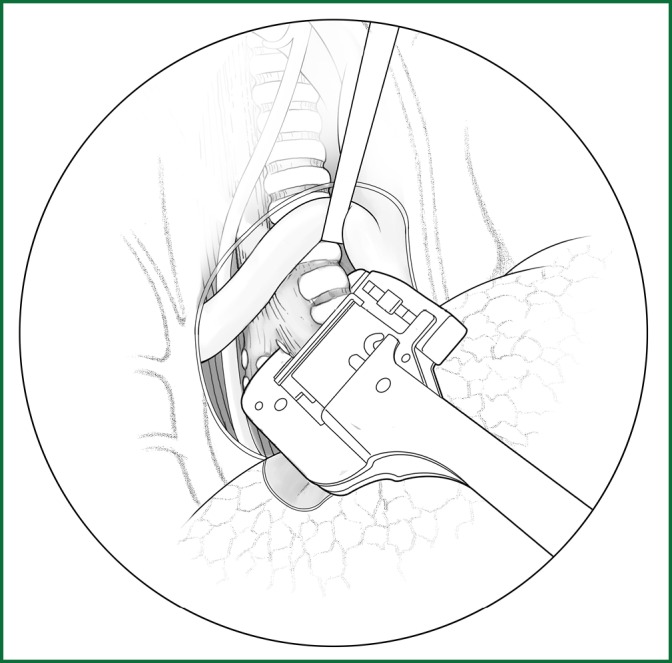
The clamping of the right main pulmonary bronchus with a stapler.
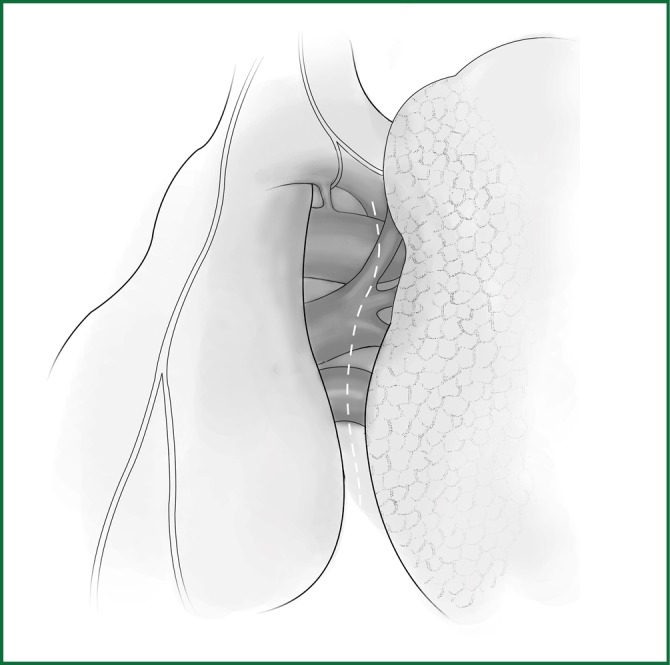
After verifying that there is no leak in the bronchial stump by examination with an inflation of the lungs, the thoracic cavity is flushed, the drainage tube is placed, and the incision is sutured.
Left pneumonectomy
The left pneumonectomy and the right pneumonectomy are similar, as briefly illustrated with the following figures.
The mediastinal pleura is cut around the pulmonary root, which is followed by a blunt dissection to expose the hilar blood vessels (Figure 60).
Figure 60.
Cutting the hilar mediastinal pleura of the left lung.
The left lower lobe is pulled forward and upward, and the left lower lung ligament is cut open. The mediastinal pleura is cut open with an electric surgical knife or an ultrasound scalpel, and the left lower pulmonary vein is freed with a right-angle clamp. After the distal end is ligated, the vein is ligated with a stapler and then severed, or it is directly severed using an endoscopic cutting stapler (Figure 61).
Figure 61.
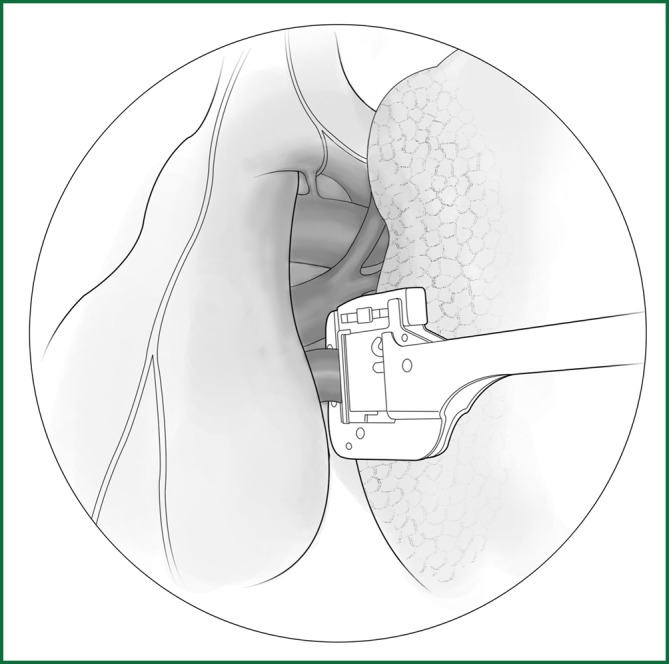
Severing of the left lower pulmonary vein.
The upper lobe is pulled backward to free the upper pulmonary vein, which is severed after the ligation of the distal end and the clamping with the stapler. Alternatively, the vein is directly severed using an endoscopic cutting stapler (Figure 62).
Figure 62.

Severing of the left upper pulmonary vein.
The left pulmonary artery trunk is freed beneath the aortic arch. If the left pulmonary artery trunk is short, the apicoposterior segmental artery is handled before the ligation and severing of the left pulmonary artery trunk (Figure 63). Before the ligation and severing of the left pulmonary artery trunk, a blocking test should also be carried out.
Figure 63.

Severing of the left pulmonary artery trunk.
After the left main bronchus is freed, it is severed and sutured at a distance of 0.5-0.8 cm from the carina of the trachea. It can also be clamped with a stapler and then severed (Figure 64).
Figure 64.
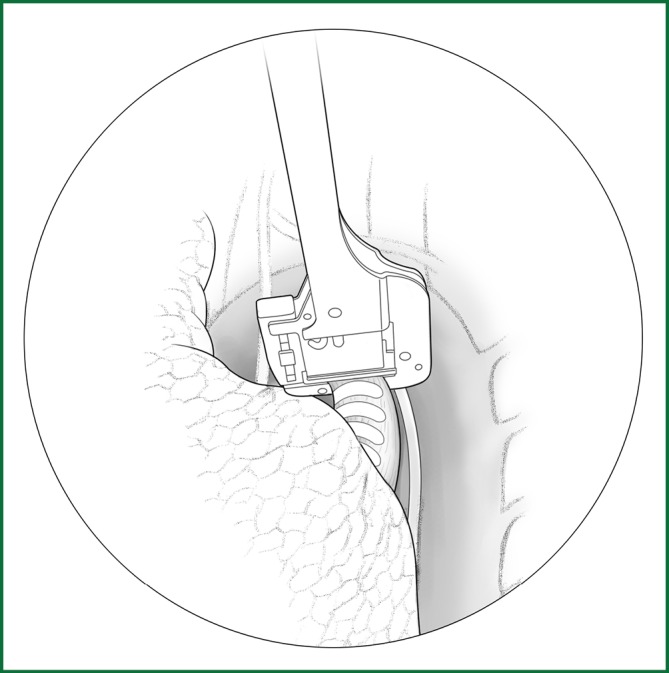
Severing of the left main pulmonary bronchus.
After verifying that there is no leak in the bronchial stump, which is examined following inflation of the lungs, the thoracic cavity is flushed, the drainage tube is placed, and the incision is sutured.
Precautions
The operational skills for a pneumonectomy are certainly important, but the assessment of the residual pulmonary function and the postoperative treatment of the patient are even more important. As nearly half of the pulmonary vascular bed is lost, the postoperative change in the hemodynamics in the patient is significant. Moreover, the respiratory function is undertaken entirely by the remaining single lobe. Therefore, attention is needed regarding two issues after a pneumonectomy. The first is to avoid heart failure, and the second is to prevent lung infections. The postoperative strategies include the following: ensuring a sufficient supply of oxygen; maintaining airway patency; closely monitoring the changes in body temperature, pulse rate, respiration rate, and blood pressure; observing the cardiac signs; and treating arrhythmia. The speed of intravenous infusion and the fluid intake and output should be controlled. The daily infusion volume usually does not exceed 1,500 mL, and an infusion drip rate of 2 mL per min is appropriate. The urine output is monitored to maintain the fluid and electrolyte balance.
Acknowledgements
Disclosure: The authors declare no conflict of interest.