Abstract
Treatment of the central nervous system (CNS) is an essential therapy component for childhood acute lymphoblastic leukaemia (ALL). Individual patient data from 47 trials addressing 16 CNS treatment comparisons were analyzed. Event-free survival (EFS) was similar for radiotherapy versus IT, and radiotherapy plus IT versus IVMTX plus IT. Triple intrathecal therapy (TIT) gave similar EFS but poorer survival than ITMTX, but additional IVMTX improved both outcomes. One trial resulted in similar EFS and survival with IVMTX plus ITMTX versus TIT alone. Radiotherapy can generally be replaced by IT therapy. TIT should be used with effective systemic therapy such as IVMTX.
Keywords: Leukaemia, Meta-analysis, childhood leukaemia, CNS, triple intrathecal therapy
INTRODUCTION
Pre-symptomatic treatment of the central-nervous-system (CNS) is an indispensable component of the successful therapy of childhood acute lymphoblastic leukaemia (ALL)1. Historically, pre-symptomatic cranial or craniospinal irradiation was a standard component of treatment for all patients. As concerns about the long-term consequences of radiation increased2, irradiation was first limited to the brain, then decreased in dose, and finally largely replaced by effective intrathecal and systemic chemotherapy, but retained for patients with high-risk disease or leukemic blasts identifiable in the cerebrospinal fluid at diagnosis. In some contemporary clinical trials, irradiation has been omitted for all patients3,4. To successfully prevent the progression or recurrence of leukaemia in the CNS, optimal systemic and intrathecal therapy is necessary1. In 2003, we performed a collaborative meta-analysis of randomized trials addressing issues of pre-symptomatic CNS therapy that initiated accrual prior to 19945. In this study, we update this work, and include additional trials that commenced before 2000 to allow adequate follow-up time.
METHODS
CNS-directed interventions include cranial or craniospinal irradiation, intrathecal drugs, intravenous methotrexate at a dose of ≥0.5 g/m2 plus leucovorin, or intravenous mercaptopurine. This paper focuses on newly diagnosed patients and excludes those who received craniospinal irradiation, a strategy no longer in use. Intravenous mercaptopurine will be reported separately.
Other drugs, which also have an effect on CNS control, in particular type of steroid6-8 will be addressed in a future study, but a summary of steroid use in the current set of trials has been recorded. Thioguanine also appears to influence CNS control, but is not currently in use in most protocols because of concerns about toxicity9.
Trials were identified following detailed search of electronic databases including MEDLINE and EMBASE. Additional hand-searching of review articles, meeting abstracts, reference lists of published trials, and the content lists of major cancer and general medical journals was undertaken. Members of the Childhood ALL Collaborative Group and other experts were consulted to ensure the completeness of the resulting list of trials. For all trials, ethical committee review and individual/ family informed consent were obtained as per national standard. Data collected on each trial included period of recruitment, eligibility criteria, randomized treatment elements and timing, and any CNS-directed treatment given in addition to the randomized question.
For all trials the following information was sought for each patient aged 21 years or younger at random assignment to treatment: date of diagnosis, age or date of birth, gender, white blood count (WBC) at diagnosis, immunophenotype, date of random assignment to treatment and treatment allocation, site of any first relapse, as well as time of first remission, relapse, death or last contact. In addition, the date and type of any secondary tumour were sought. Data were checked for internal consistency, balance between treatment groups by initial features, randomization dates, and length of follow-up, and consistency with published results.
All first event outcomes were measured from date of randomization. Primary outcomes were event-free survival (EFS) and overall survival (OS). Any induction failure, relapse or death was counted as an adverse event. As many trials did not record secondary tumours, these events were not included in analyses. Secondary outcome measures were no remission (defined as death without remission achievement), isolated CNS relapse, any CNS relapse (isolated CNS relapse plus combined relapses with CNS involvement), non-CNS relapse, and death in remission which included death due to secondary tumour. Relapse data were obtained only for first relapse. Survival from relapse was also analyzed.
Standard statistical meta-analysis methods were used. Within trials, analyses examined time from randomization to any event, with the observed minus expected (o-e) number of events and its variance (v) obtained by the log-rank method. These o-e values were then added over all trials to produce a total (T), with variance (V) equal to the sum of the separate variances. These were used to calculate an overall odds ratio (OR), or ratio of event rates, and its 95% confidence interval (CI) equal to exp(T/V±1.96/√V). Results are presented as forest plots with a square representing the point estimate of the OR and a horizontal line showing the 99% confidence interval for each trial. The size of the square is proportional to the number of events, not patients. Overall estimates are shown by a diamond with the width representing the 95% confidence interval. All p-values given are two-sided. Heterogeneity between the effects in different trials or subgroups was tested with Χ2n-1 equal to S-T2/V, where S is the sum of (o-e)2/v from each of n trials or n subgroups10.
T and V obtained by summing o-e and v from log rank analyses restricted to each one year time period were used to estimate the log OR, b, for each year. The estimated overall event rate in each time period, r, equals the number of events divided by the number of person years, and the probability of surviving event-free during that year is p=exp(-r). Descriptive survival curves were drawn from the separate probability estimates p+0.5p(p-1)b for one treatment group, and p-0.5p(p-1)b for the other treatment group10.
Trials were categorized a priori and the results combined according to the treatment comparisons and background CNS-directed treatments. We examined conventional pre-specified subgroups by gender, age group (<10, ≥10 years), WBC (<10, 10-19, 20-49, 50-99, ≥100 × 109/L) and immunophenotype (B-lineage, T-lineage).
RESULTS
Data were available for 47 trials, ten of which were not included in the previous analyses. 45 trials had a median follow-up of at least five years. Studies were grouped into 11 categories by randomized comparison. Table I gives details of the CNS-directed treatments11-56. Steroid use is given in supplementary table S1. Supplementary tables S2 and S3 show the distribution of patient and disease characteristics and median follow-up for each trial.
Table I.
Summary of CNS-directed treatments
| Trial | Year | Ref | CNS-directed therapy |
|---|---|---|---|
| A: TIT versus IT therapy | |||
| *CCG-1952 | 1996 | 11 | DITa × 1 + IT MTX × 1 R: TITa × 14 (f) or 18 (m) v IT MTX × 14 (f) or 18 (m) |
| B: Radiotherapy plus IT therapy versus extra IT therapy | |||
| a Radiotherapy v IT therapy | |||
| CCG-161 | 1978 | 12 | IT MTX × 6 R: 18 Gy CRT v IT MTX × 8 |
| CCG-105 | 1983 | 13 | IT MTX × 6 R: 18 Gy CRT v IT MTX × 8 (f) or 14 (m) |
| INEN-P83 | 1983 | IT MTX × 5 R: 18 Gy CRT v IT MTX × 12 | |
| CCG-1882 | 1989 | 14 | IT MTX × 14 (f) or 18 (m) + IT AraC × 1 R: 18 Gy CRT v IT MTX × 7 |
| b Radiotherapy (+ IT) v DIT or TIT therapy | |||
| †SWOG 7623/AlinC12 | 1976 | 15 | R: 24 Gy CRT + IT MTX × 5 v TITa × 22 |
| LAL 7/78 | 1978 | 16 | R: 24 Gy CRT + IT MTX × 6 v DITa × 10 |
| INS 84 | 1984 | 17 | TITa × 6 R: 18Gy CRT v TITa × 12 |
| INEN-P85 | 1985 | R: 18 Gy CRT + IT MTX × 5 v TITa × 17 | |
| c Radiotherapy + DIT v TIT therapy | |||
| *DFCI ALL 95-001 | 1996 | 18 | IT AraC × 1 + TITa × 2 R: 18Gy CRT + DITa × 6 v TITa × 12 |
| C: Addition of IV methotrexate to long-term IT therapy or radiotherapy with IT therapy | |||
| a TIT therapy +/- IV methotrexate | |||
| SJCRH Total XIIIA | 1991 | 19 | TITa × 13 or 17 + 2 g/m2IV MTX × 9 or 10 R: ± 1 g/m2 IV MTX × 1 |
| *POG 9005 | 1991 | 20 | 1 g/m2 IV mp × 17–19 + TITA × 16 R: ± 1 g/m2 IV MTX × 12 |
| FRALLE 93 LR | 1993 | 21 | TITc × 16 R: ± 1·5 g/m2 IV MTX × 6 |
| FRALLE 93 IR | 1993 | 21 | TITc × 18 R: ± 8 g/m2 IV MTX × 4 |
| *SJCRH Total XIIIB | 1994 | 22 | 1 g/m2 IV mp + TITa × 13 or 15 + 2 g/m2 IV MTX × 10 R: ± 1 g/m2 IV MTX × 1 |
| b IT therapy +/- IV methotrexate | |||
| CCG-139 | 1984 | 23 | IT MTX × 15 (f) or 20 (m) R: ± 0·5 g/m2 IV MTX × 24 (f) or 33 (m) |
| DFCI 87001 | 1987 | 24 | IT AraC × 1 + IT MTX × 10 (HR: + 18 Gy CRT) R: ± 4 g/m2 IV MTX × 1 |
| UKALLXI LWCC | 1990 | 25 | IT MTX × 16 R: ± 6–8 g/m2 IV MTX × 3 |
| c Radiotherapy with IT therapy +/- IV methotrexate | |||
| DFCI 81001 | 1981 | 26 | 18 or 28 Gy CSCRT + IT AraC × 1 + IT MTX × 8 R: ± 4 g/m2IV MTX × 1 |
| *POG 9404 | 1994 | 27 | TITa × 11 + 18 Gy CRT R: ± 5 g/m2 IV MTX × 4 |
| D: IV methotrexate + IT therapy v TIT | |||
| *POG8035/8036 | 1981 | 28 | TITa × 6 R: 1 g/m2 IV MTX × 17 + IT MTX × 4 v TITa × 17 |
| E: Addition of IT therapy to radiotherapy plus short-term IT therapy | |||
| †CALGB-7113 | 1971 | 29 | 24 Gy CRT + IT MTX × 12 R: ± IT MTX × 3 |
| CCG-162 | 1978 | 30 | 18 Gy CRT + IT MTX × 6 R: ± IT MTX × 8 |
| UKALLVII | 1979 | 31 | 18 or 24 Gy CRT + IT MTX × 5 R: ± IT MTX × 8 |
| F: Radiotherapy plus IT therapy v IV methotrexate plus IT therapy | |||
| CLB 7611 | 1976 | 32 | IT MTX × 6 R: 24 Gy CRT v 0·5 g/m2 IV MTX × 3 |
| †NCI 77-02 | 1980 | 33 | R: 18–24 Gy CRT + IT MTX × 5 v 33·6 g/m2 IV MTX × 10 |
| ALL-BFM-81 | 1981 | 34 | IT MTX × 6 R: 12–18 Gy CRT v 0·5 g/m2 IV MTX × 4 |
| Jena ALL VII 81 | 1981 | 35 | IT MTX × 8 R: 18 Gy CRT v 0·5 g/m2 IV MTX × 4 |
| JCCLSG L-874 | 1987 | 36 | DITb × 3 R: 18 Gy CRT v 2 g/m2 IV MTX × 3 + DITb × 6 |
| JCCLSG I-874 | 1987 | 36 | (2 g/m2 × 1 + 4·5 g/m2 × 20) IV MTX + DITb × 1 R: 18 Gy CRT + DITb × 2 v 4·5 g/m2 IV MTX × 3 |
| GCMTLA | 1988 | 37 | TITb × 6 R: 12–18 Gy CRT v 0·5 g/m2 IV MTX × 4 + TITb × 6 |
| †TCCSG L89-12 | 1989 | 38 | TITa × 4 + DITa × 3 R: 18 Gy CRT v 3 g/m2 IV MTX |
| UKALLXI HWCC | 1990 | 25 | IT MTX × 7 or 9 R: 24 Gy CRT v 6–8 g/m2 IV MTX × 3 + IT MTX × 8 or 9 |
| †TCCSG L92-13 HR | 1992 | 38 | DITb × 4 + TITa × 3 R: 18 Gy CRT + DITb × 1 v 3 g/m2 IV MTX × 2 |
| *MRC ALL97 | 1997 | 39 | IT MTX × 7 or 9 R: 24 Gy CRT v 6–8 g/m2 IV MTX × 3 + IT MTX × 8 or 9 |
| G: Higher dose of IV methotrexate | |||
| *POG 9405 | 1994 | 40 | TITa × 22 R: 2·5 g/m2 IV MTX × 12 v 1 g/m2 IV MTX × 12 |
| *POG 9406 | 1994 | 40 | TITa × 18 + 1 g/m2 IV mp × 6 R: 2·5 g/m2 IV MTX × 6 v 1 g/m2 IV MTX × 6 |
| H: Higher doses of radiotherapy | |||
| UKALL V | 1976 | 41 | IT MTX × 5 R: 24 Gy v 21 Gy CRT |
| UKALLVI(i) | 1978 | 42 | IT MTX × 8 or IT MTX × 6 + IT AraC × 2 + 0·5 g/m2 IV MTX × 3 R: 24 Gy v 21 Gy CRT |
| UKALLVI(ii) | 1978 | 42 | IT MTX × 8 or IT MTX × 6 + IT AraC × 2 + 0·5 g/m2 IV MTX × 3 R: 24 Gy v 18 Gy CRT |
| UKALLVII | 1979 | 31 | IT MTX × 5 R: 24 Gy v 18 Gy CRT |
| GBTLI-80 | 1980 | 43 | IT MTX × 13 R: 24 Gy v 18 Gy CRT |
| TCCSG L81-10 | 1981 | 44 | DITa × 5 R: 24 Gy v 18 Gy CRT |
| ALL-BFM-83 | 1983 | 34 | IT MTX × 8 + 0·5 g/m2 IV MTX × 4 R: 18 Gy v 12 Gy CRT |
| I: Addition of radiotherapy | |||
| a Addition to IT | |||
| †POG CNS2 | 1970 | 45 | IT MTX × 20 R: ± 24 Gy CRT |
| †CLB 7111 | 1971 | 46 | IT MTX × 6 R: ± 24 Gy CRT |
| †SWOG 690/691/Alinc9 | 1971 | 47 | TITa × 10 R: ± 18–24 Gy CRT |
| †DFCI-SFCC | 1972 | 48 | IT MTX × 9 R: ± 24 Gy CRT |
| †CLB 7411 | 1974 | 49 | IT MTX × 6 R: ± 24 Gy CRT |
| CCG-101 | 1974 | 50 | IT MTX × 6 R: ± 24 Gy CRT |
| *CCG-123 | 1983 | 51 | IT AraC × 1 + IT MTX × 6 R: ± 18Gy CRT |
| b Addition to IV with IT | |||
| EORTC 58832 | 1983 | 52 | 2·5 g/m2 IV MTX × 4 + IT MTX × 7 R: ± 16–20 Gy CRT |
| J: Higher dose of IV methotrexate v more IT or DIT therapy | |||
| FRALLE 87 | 1987 | 53 | DITc × 5 R: 8 g/m2 IV MTX × 4 v 3 g/m2 IV MTX × 4 + DITc × 5 |
| FRALLE 89 | 1989 | 21 | IT MTX × 5 R: 8 g/m2IV MTX × 4 v 3 g/m2IV MTX × 4 + IT MTX × 5 |
| K: Addition of IV methotrexate plus IT, DIT or TIT therapy to radiotherapy plus IT or TIT therapy and/or IV methotrexate | |||
| Jena ALLVII 81 | 1981 | 35 | 12 or 18 Gy CRT + IT MTX × 8 R: ± 0·5 g/m2 IV MTX × 4 + IT MTX × 4 |
| TCCSG L84-11 SR | 1984 | 38 | 18 Gy CRT + TITa × 5 + 0·5 g/m2 IV MTX × 4 + IT MTX × 4 R: ± 0·5 g/m2 IV MTX × 3 + DITb × 6 |
| TCCSG L84-11 HR | 1984 | 38 | 24 Gy CRT + TITa × 5 + 0·5g/m2 IV MTX × 12 + DITb × 12 R: ± 0·5 g/m2 IV MTX × 3 + TITa × 6 |
| Other comparisons without data | |||
| †ALGB 6801 | 1968 | 54 | R: IT MTX × 15 |
| †GATLA-70 | 1970 | 55 | R: ± 24 Gy CRT + IT MTX × 5 |
| †NCI 72-1 | 1971 | 56 | 24 Gy CRT R: IT AraC × 38 v IT MTX × 35 |
| †NCI-84-C-153A | 1984 | R: 33·6 g/m2 IV MTX × 10 v IT MTX × 8 | |
Data not available;
Not in previous paper; AraC, cytosine arabinoside; CRT, cranial irradiation; CSCRT, craniospinal irradiation; DITa, IT MTX + IT AraC; DITb, IT MTX + IT Hc; DITc, IT MTX + IT P; Dx, dexamethasone; f, female; Hc, hydrocortisone; IT, intrathecal; m, male; mp, mercaptopurine; MTX, methotrexate; P, prednisone; R, randomization; TITa, IT MTX + IT AraC + IT Hc; TITb, IT MTX + IT AraC + IT Dx; TITc, IT MTX + IT AraC + IT P.
Grouping of trials
Triple intrathecal therapy (TIT) with methotrexate, hydrocortisone and cytarabine versus intrathecal (IT) methotrexate (IT MTX) therapy. One new trial, involving 2029 NCI standard-risk (age<10 years and WBC<50 × 109/L) patients, addressed this question.
-
Radiotherapy plus IT therapy versus extra IT therapy. Nine trials, including one new trial, addressed this question. Data were available for eight of these trials, involving 2995 patients, which compared treatment regimens with either 18 or 24 Gy cranial irradiation (CRT) plus some IT treatment with the same regimen without CRT but with additional doses of IT MTX or TIT, the use of double IT (DIT) in place of IT MTX, or TIT in place of double IT.
In the light of the results of the comparison of TIT versus IT MTX therapy, this group of trials was split post-hoc into three subgroups: (a) CRT + IT versus extra IT, (b) CRT + IT MTX versus DIT or TIT, and (c) CRT + DIT versus TIT. We found heterogeneity among subgroups for CNS relapse (p=0.01).
-
Addition of IV methotrexate (doses ≥ 500 mg/m2) to long-term IT therapy or radiotherapy with IT therapy. Ten trials with 4140 patients addressed this question, including two new trials. One trial (CCG-163d) previously included was discovered to be confounded, with other chemotherapy differences between the arms with and without IV MTX, and so it has now been excluded in the analysis. One trial (POG9005), which was previously in the ‘other comparisons without data’ category, is now included because most protocols specified some additional low dose oral MTX in the control arm and this is considered not an important confounding factor.
Again, in the light of the IT MTX versus TIT results, this comparison of the addition of IV methotrexate was split into three groups according to the background treatment: (a) TIT therapy, sometimes plus IV mercaptopurine (MP) and/or some IV methotrexate, (b) IT MTX, and (c) CRT plus IT MTX or TIT therapy. Heterogeneity of effect between subgroups was seen for EFS (p=0.01). Excluding group c, heterogeneity was seen between groups a and b for both CNS (p=0.06) and non-CNS relapse (p=0.008).
IV methotrexate plus IT MTX therapy versus triple IT therapy. Data were newly available for one trial, previously included in the ‘other comparison’ group, involving 1159 patients.
Addition of IT therapy to treatment including radiotherapy and short term IT therapy. Data were available for 1174 patients in two trials out of the four addressing this, previously included in the ‘other comparisons group’.
Radiotherapy plus IT therapy versus IV methotrexate plus IT therapy. Trials comparing CRT with IV MTX were grouped together on the assumption that small differences in IT therapy do not have a major impact. Data for eight trials (including one new trial), involving 1635 patients, were available.
Higher dose of IV methotrexate. Two new trials, both including TIT therapy for all patients and totally involving 1071 patients, addressed this treatment.
Higher doses of radiotherapy. Data were available for all eight trials, including one previously without data (GBTLI-80), involving 905 patients. Comparisons were of 24 Gy versus 21 or 18 Gy, except for one trial comparing 18 Gy versus 12 Gy.
Addition of radiotherapy. Data, involving 664 patients, were available for three of the eight trials addressing this question, including one new trial (CCG-123). These were previously included in the ‘other comparison’ group. Significant heterogeneity was found for the effect on CNS relapse between (a) CCG trials, which used only IT therapy, and (b) the EORTC 58832 trial, which included IV MTX in both arms (p=0.0003). Therefore this comparison was split.
Higher dose of IV methotrexate versus more IT therapy. Two trials, involving 700 patients, previously included in ‘other comparisons’ are grouped here. One used double IT and the other IT MTX.
Addition of IV methotrexate plus IT therapy to radiotherapy plus IT therapy and/or IV methotrexate. Three trials involving 511 patients addressed this.
Outcomes
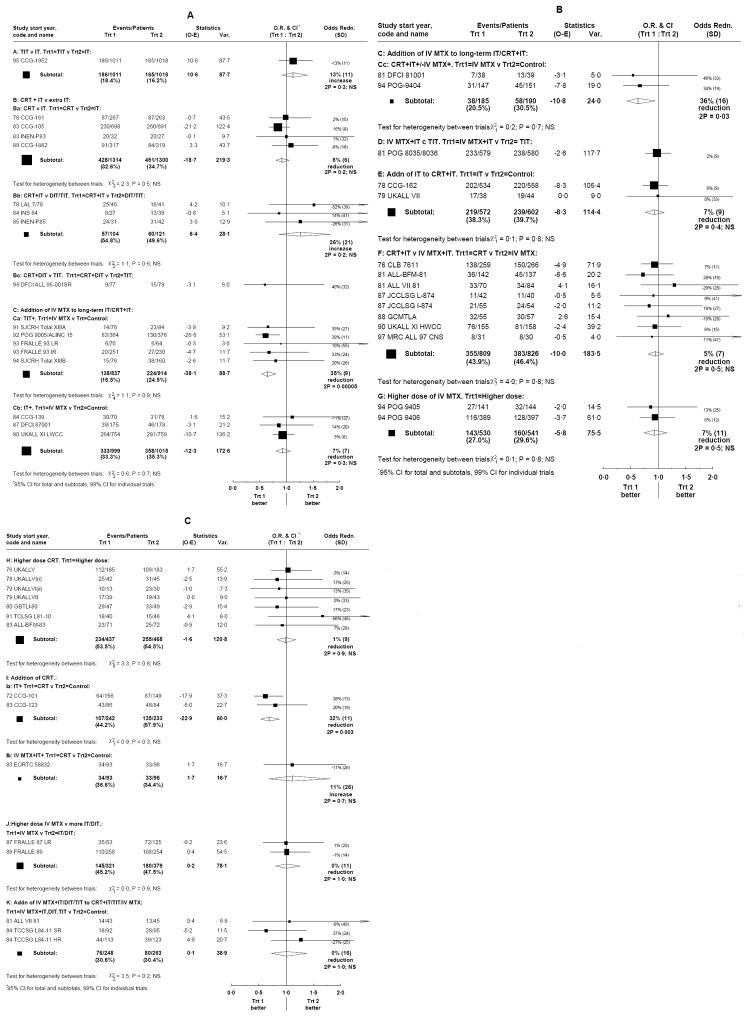
Except as stated, treatment effects were similar in different gender, age, WBC, and immunophenotype subgroups. Figures 1A (groups A, Ba, Bb, Ca, Cb), 1B (groups Cc, D, E, F, G), and 1C (groups H, Ia, Ib, J, K) show the treatment effects on EFS for each trial, and for each comparison group. Table S4 shows outcome for all event types according to treatment and results are summarized in Table II. Only 12 trials reported secondary tumour data, which, if included, would have added 21 events.
Figure 1.
Effects of treatments on event free survival. Ratios of annual event rates with each trial result represented by a square, with larger squares indicating more information, and the overall result for each comparison represented by a diamond.
Table II.
Summary results
| Trial | Background CNS treatment | Arm 1 | Arm 2* | Conclusions |
|---|---|---|---|---|
| A: TIT versus IT therapy (n=2029) | ||||
| CCG-195211 | DIT × 1 + IT × 1 | TIT × 14 (f) or 18 (m) | IT × 14 (f) or 18 (m) | TIT reduced CNS relapse compared with IT but an increase in non-CNS relapse resulted in similar EFS and worse OS (OR=1·50; p=0·01). |
|
| ||||
| Ba: Radiotherapy plus short-term IT therapy versus extra IT therapy (n=2614) | ||||
| CCG-16112 | IT × 6 | CRT | IT × 8 | Adding CRT to short-term IT reduced CNS relapses (OR=0·68; p=0·01) compared with adding more IT doses, but did not substantially affect EFS (OR=0·92; 95% CI = 0·80 1·05; p=0·2). |
| CCG-10513 | IT × 6 | CRT | IT × 8 (f) or 14 (m) | |
| INEN-P83 | IT × 5 | CRT | IT × 12 | |
| CCG-188214 | IT × 15 (f) or 19 (m) | CRT | IT × 7 | |
|
| ||||
| Bb: Radiotherapy plus short-term IT therapy versus DIT or TIT (n=234) | ||||
| LAL 7/7816 | CRT + IT × 6 | DIT × 10 | Small numbers make results uncertain but OS was worse with CRT (OR=1·63; p=0·02) | |
| INS 8417 | TIT × 6 | CRT | TIT × 12 | |
| INEN-P85 | CRT + IT × 5 | TIT × 17 | ||
|
| ||||
| Bc: Radiotherapy plus DIT versus extra TIT (n=155) | ||||
| DFCI ALL 95-00118 | IT × 1 + TIT × 2 | CRT + DIT × 6 | TIT × 12 | Insufficient data to draw any conclusions. |
|
| ||||
| Ca: Addition of IV methotrexate to TIT or TIT with IV methotrexate (n=1751) | ||||
| SJCRH Total XIIIA19 | TIT × 13 or 17 + IV × 9 or 10 | IV × 1 | Control | Adding IV MTX to TIT reduced both CNS (OR=0·48; p=0·0009) and non-CNS relapse (OR=0·65; p=0·002), giving better EFS (OR=0·65; p=0·00005) and OS (OR=0·71; p=0·02) |
| POG 900520 | IV mp × 17–19 + TIT × 16 | IV × 12 | Control | |
| FRALLE 93 LR21 | TIT × 16 | IV × 6 | Control | |
| FRALLE 93 IR21 | TIT × 18 | IV × 4 | Control | |
| SJCRH Total XIIIB22 | IV mp + TIT × 13 or 15 + IV × 10 | IV × 1 | Control | |
|
| ||||
| Cb: Addition of IV methotrexate to long-term IT therapy (n=2014) | ||||
| CCG-13923 | IT × 15 (f) or 20 (m) | IV × 24 (f) or 33 (m) | Control | Adding IV MTX to long-term IT probably reduced CNS relapse (OR=0·78; p=0·06), but did not improve EFS or OS. |
| DFCI 8700124 | IT × 11 (HR: + CRT) | IV × 1 | Control | |
| UKALLXI LWCC25 | IT × 16 | IV × 3 | Control | |
|
| ||||
| Cc: Addition of IV methotrexate to radiotherapy with TIT or IT therapy (n=375) | ||||
| DFCI 8100126 | CSCRT + IT × 9 | IV × 1 | Control | Adding IV MTX to CRT with short-term IT or TIT reduced CNS relapses (OR=0·39; p=0·008) and improved EFS (OR=0·64; p=0·03), but small numbers make these results less reliable. |
| POG 940427 | TIT × 11 + CRT | IV × 4 | Control | |
|
| ||||
| D: IV methotrexate + IT therapy + TIT versus extra TIT (n=1159) | ||||
| POG8035/803628 | TIT × 6 | IV × 17 + IT × 4 | TIT × 17 | IV MTX was less effective than extra TIT in preventing CNS relapse (OR=1·64; p=0·01) but the reduction in non-CNS relapse (OR=0·82; p=0·08) resulted in equivalent EFS and OS. |
|
| ||||
| E: Addition of IT therapy to radiotherapy plus short-term IT therapy (n=1174) | ||||
| CCG-16230 | CRT + IT × 6 | IT × 8 | Control | Adding extra IT therapy to CRT with short-term IT did not give benefit. |
| UKALLVII31 | CRT + IT × 5 | IT × 8 | Control | |
|
| ||||
| F: Radiotherapy versus IV methotrexate: short-term IT, DIT in both arms (IV MTX in one trial) and some additional IT or TIT in the IV MTX arm (n=1635) | ||||
| CLB 761132 | IT × 6 | CRT | IV × 3 | CRT given with short-term IT, DIT or TIT, reduced CNS relapse (OR=0·43; p<0·00001) compared with giving IV MTX, with or without extra IT, but non-CNS relapses were increased (OR=1·54; p=0·00001), resulting in no difference in EFS or OS. |
| ALL-BFM-8134 | IT × 6 | CRT | IV × 4 | |
| Jena ALL VII 8135 | IT × 8 | CRT | IV × 4 | |
| JCCLSG L-87436 | DIT × 3 | CRT | IV × 3 + TIT × 6 | |
| JCCLSG I-87436 | IV × 21+ DIT × 1 | CRT + DIT × 2 | IV × 3 | |
| GCMTLA37 | TIT ×6 | CRT | IV × 4 + TIT × 6 | |
| UKALLXI HWCC25 | IT × 7 or 9 | CRT | IV × 3 + IT × 8 or 9 | |
| MRC ALL9739 | IT × 7 or 9 | CRT | IV × 3 + IT × 8 or 9 | |
|
| ||||
| G: Higher dose of IV methotrexate (n=1071) | ||||
| POG 940540 | TIT × 22 | 2·5 g/m2 IV × 12 | 1 g/m2 IV × 12 | No benefit from higher dose of IV MTX. |
| POG 940640 | TIT × 18 + 1 g/m2 IV mp × 6 | 2·5 g/m2 IV × 6 | 1 g/m2 IV × 6 | |
|
| ||||
| H: Higher doses of radiotherapy (n=905) | ||||
| UKALL V41 | IT × 5 | 24 Gy CRT | 21 Gy CRT | No benefit from higher doses of CRT. |
| UKALLVI(i)42 | IT × 10 or IT × 8 + IV × 3 | 24 Gy CRT | 21 Gy CRT | |
| UKALLVI(ii)42 | IT × 10 or IT × 8 + IV × 3 | 24 Gy CRT | 18 Gy CRT | |
| UKALLVII31 | IT × 5 | 24 Gy CRT | 18 Gy CRT | |
| GBTLI-8043 | IT × 13 | 24 Gy CRT | 18 Gy CRT | |
| TCCSG L81-1044 | DIT × 5 | 24 Gy CRT | 18 Gy CRT | |
| ALL-BFM-8334 | IT × 8 + IV × 4 | 18 Gy CRT | 12 Gy CRT | |
|
| ||||
| Ia: Addition of radiotherapy to short-term IT therapy (n=475) | ||||
| CCG-10150 | IT × 6 | CRT | Control | Adding CRT to 6 or 7 doses of IT reduced CNS relapses (OR=0·28; p <0·00001) and improved EFS (OR=0·68; p=0·003) |
| CCG-12351 | IT × 7 | CRT | Control | |
|
| ||||
| Ib: Addition of radiotherapy to IV methotrexate plus IT therapy (n=189) | ||||
| EORTC 5883252 | IV × 4 + IT × 7 | CRT | Control | Insufficient data to draw any conclusions. |
|
| ||||
| J: Higher dose of IV methotrexate versus more IT or DIT therapy (n=700) | ||||
| FRALLE 8753 | DIT × 5 | 8 g/m2 IV × 4 | 3 g/m2 IV × 4 + DIT × 5 | There was no evidence of a difference between high dose and a lower dose with extra IT or DIT. |
| FRALLE 8921 | IT × 5 | 8 g/m2IV × 4 | 3 g/m2IV × 4 + IT × 5 | |
|
| ||||
| K: Addition of IV methotrexate plus IT, DIT or TIT therapy to radiotherapy plus IT or TIT therapy and/or IV methotrexate (n=511) | ||||
| Jena ALLVII 8135 | CRT + IT × 8 | IV × 4 + IT × 4 | Control | There was no evidence of a benefit from adding IV MTX with extra IT, DIT or TIT to a treatment which included CRT. |
| TCCSG L84-11 SR38 | CRT + TIT × 5 + IV × 4 + IT × 4 | IV × 3 + DIT × 6 | Control | |
| TCCSG L84-11 HR38 | CRT + TIT × 5 + IV × 12 + DIT × 12 | IV × 3 + TIT × 6 | Control | |
Arm 2 = Control indicates that no CNS treatment was given additional to the background.
CRT, cranial irradiation; CSCRT, craniospinal irradiation; DIT, double intrathecal; f, female; IT, intrathecal; IV, intravenous methotrexate; IV mp, intravenous mercaptopurine; m, male; TIT, triple intrathecal.
Radiotherapy
Early trials, mostly of 24 Gy versus 18 or 21 Gy, indicated no evidence that higher dose CRT offers any advantage (comparison H). No significant differences were found for any endpoints (Tables II, S4, Figure 1C).
Where only short-term IT was used (i.e. 6 or 7 doses) (Comparison Ia), CRT significantly reduced the CNS relapse rate (OR=0.28; 95% CI=0.18-0.43; p<0.00001) (Tables II, S4). The non-CNS relapse rates were similar and hence the overall event rate was reduced (p=0.003), resulting in a long term EFS benefit (Figure 1C). There was evidence of possible heterogeneity (p=0.02) between those aged <10 and ≥ 10 years, with the larger effect in younger patients where a larger proportion of the relapses involved the CNS. Survival from relapse was poorer for those relapsing after CRT so that OS was not significantly improved. Replacing CRT with additional IT doses (Comparison Ba) showed that CRT reduced CNS relapse (OR=0.68; 95% CI=0.51-0.92; p=0.01) with no difference in non-CNS relapse (Tables II, S4). As CNS relapse was a relatively rare event in these trials, no significant EFS difference emerges (Figure 1A). Survival from CNS relapse was non-significantly worse following CRT (p=0.08), while there was no difference in survival from non-CNS relapse, resulting in no difference in OS (Table S4).
Alternatively, in trials replacing CRT with IV MTX (Comparison F), CRT reduced CNS relapse (OR=0.43; 95% CI 0.34-0.56; p<0.0001) but was less effective than IV MTX for non-CNS relapse (OR=1.54; 95% CI 1.27-1.86; p=0.00001) (Tables II, S4). This resulted in similar overall EFS rates (OR=0.95; 95% CI=0.81-1.09, Figure 1B). Survival from relapse was non-significantly worse for CNS relapse after CRT compared with after IV methotrexate, but better for non-CNS relapse, and there was no difference in OS.
Comparisons of CRT plus IT with DIT or TIT (Comparison Bb) or CRT plus DIT with TIT (Comparison Bc) included only small numbers (n=225, 156, respectively). In the first group, CRT appeared to result in poorer OS (OR=1.53; 95% CI=1.01-2.30; p=0.04) (Tables II, S4) but no significant differences were found for any other endpoint. In the second group, CRT plus DIT yielded better CNS control (OR=0.13; 95% CI=0.02-0.93) but there was no difference in EFS or OS (Tables II, S4) and this is based on only one trial including 156 patients and four CNS relapses. When IV MTX was given, no benefit was seen with CRT (Comparison Ib), but fewer than 200 patients were randomized (Tables II, S4).
Combining additional CNS therapy with CRT
If CRT was included in treatment, along with some IT therapy, EFS rates were not improved with addition of further IT doses (OR=0.93; 95% CI=0.77-1.12) (Comparison E, Tables II, S4, Figure 1B). No significant differences were found for any endpoints (Table S4).
No significant differences were found for any endpoints in the comparison of CRT plus IT therapy and/or IV MTX with IV MTX plus IT therapy, although numbers are limited (Comparison K, Tables I, S4).
Two trials looked at the addition of IV MTX to therapy including CRT, one trial with craniospinal irradiation plus IT MTX therapy and the other with cranial irradiation plus TIT (Comparison Cc). Although only 375 patients were randomized there was a reduction in CNS relapse (OR=0.39; 95% CI=0.20-0.79), EFS benefit (OR=0.64; 95% CI=0.43-0.95; p=0.03), and possibly improved OS (p=0.09) (Tables II, S4, Figure 1B). The majority of patients in this comparison were T lineage ALL.
Treatment without CRT
The one large trial of TIT versus IT MTX (Comparison A) showed that CNS relapses were reduced by one third with TIT (OR=0.66; 95% CI 0.47-0.93; p=0.02), but non-CNS relapses were increased by three quarters (OR=1.73; 95% CI 1.30-2.29; p=0.0002) (Tables II, S4), and there was no difference in EFS (p=0.3) (Figure 1A). TIT resulted in worse OS (OR=1.50; 95% CI 1.09-2.06; p=0.01; 2.8% absolute difference) (Figure 2), due partly to worse post-relapse survival for non-CNS as opposed to CNS relapse with either treatment, but also to poorer survival for each relapse type after relapse on TIT (OR=1.84; 95% CI 1.01-3.35; p=0.05 after CNS, OR=1.30; 95% CI 0.83-2.05; p=0.3 after non-CNS relapse).
Figure 2.
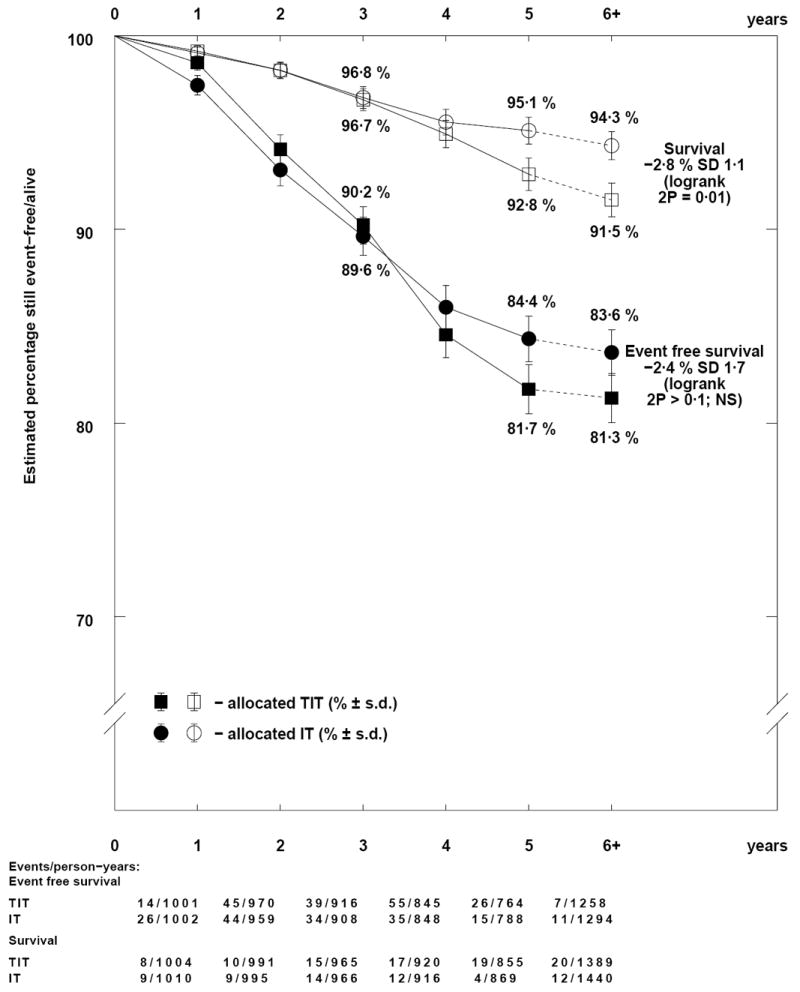
Descriptive event free and overall survival curves for TIT versus IT therapy.
Adding IV MTX to TIT (Comparison Ca) reduced the overall event rate by a third (OR=0.65; 95% CI=0.53-0.80; p=0.00005) (Figure 1A) due to a reduction in both CNS and non-CNS relapses (Tables II, S4), and resulting in absolute difference of 8.2% in EFS and 3.6% in OS (Figure 3). Where all patients received IT MTX therapy (Comparison Cb), there was no apparent benefit for IV MTX (EFS: OR=0.93; 95% CI=0.80-1.08; p=0.3; Figures 1A, 4), although the rate of CNS relapses may be somewhat lower (OR=0.78; 95% CI=0.60-1.01; p=0.06) (Tables II, S4). No differences were found for survival from relapse or OS in either of these comparisons.
Figure 3.
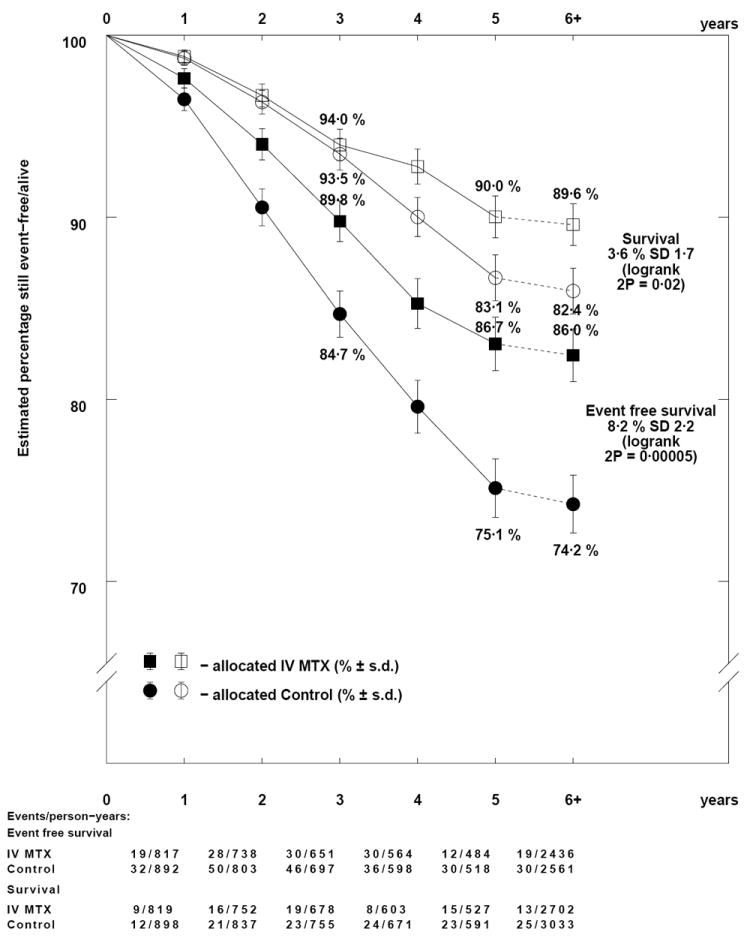
Descriptive event free and overall survival curves for the addition of IV methotrexate to TIT.
Figure 4.
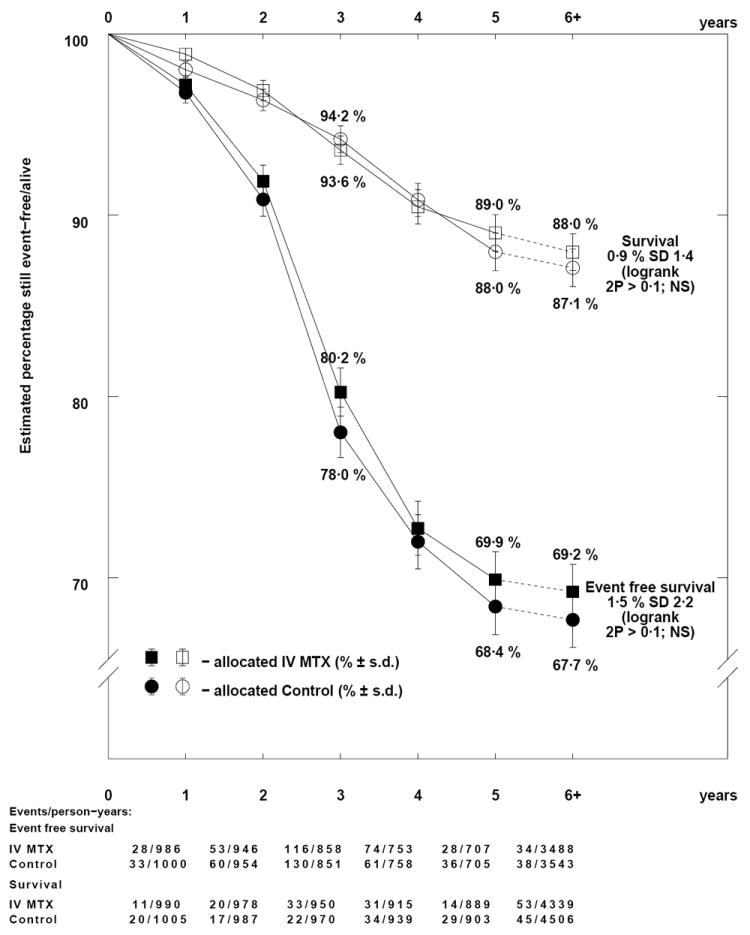
Descriptive event free and overall survival curves for the addition of IV methotrexate to IT therapy.
In one large trial comparing IV MTX plus IT therapy with TIT alone (Comparison D), CNS relapse was higher with IV MTX plus IT MTX (OR=1.64; 95% CI 1.13-2.39; p=0.01) (Tables II, S4) but the lower non-CNS relapse rate, albeit not significant, resulted in similar EFS (OR=0.98; 95% CI=0.82-1.17, Figure 1B) and OS.
In the comparison of higher dose IV MTX (i.e. 2.5 g/m2 versus 1 g/m2) (Comparison G), no significant differences were found for any endpoint, including EFS (OR=0.93; 95% CI=0.74-1.16) (Tables II, S4, Figure 1B).
Similarly, comparisons of high dose IV MTX (8g/m2) with lower dose (3g/m2) plus IT MTX or DIT (Comparison J) showed no significant differences for any endpoint (Tables II, S4).
DISCUSSION
This study demonstrated that effective systemic and IT chemotherapy can yield EFS at least comparable to that of CRT with or without additional IT therapy; the findings extend those of the previous meta-analysis showing that CRT could generally be replaced by long term IT therapy, with some additional benefit from the addition of IV MTX5. Historically, the introduction of CRT was a major breakthrough, but there is no evidence that higher dose CRT offers any advantage. Although the addition of CRT to short term IT (≤8 doses) treatment showed benefit in early trials, alternative therapy, such as giving additional IT doses or IV MTX can be as effective in terms of EFS and OS. In CCG-105, the omission of CRT diminished EFS for those receiving standard (minimal) therapy but not for those receiving more intensive BFM-based treatment13. Therefore the ability to omit CRT depends on systemic as well as IT therapy. Even if CRT is used, there was some evidence, mainly in T-lineage disease, that the addition of IV MTX to CRT might improve EFS further for some patients.
There was no direct evidence on the comparative effectiveness of IT MTX, DIT and TIT in the previous meta-analysis. The CCG-1952 trial has now provided some evidence on TIT versus IT MTX which has suggested that other comparison groups should be split, and this has been confirmed by heterogeneity tests between the revised subgroups. On the other hand, different IT therapies are grouped together in the comparisons between trials with CRT and those with IV MTX on the assumption that small differences in IT MTX, DIT, TIT therapies will have minor effects compared with those of CRT and IV MTX. Direct evidence comparing doses of IV MTX has also become available.
When neither CRT nor IV MTX are used, CCG 1952 study showed that, compared with IT MTX, TIT reduced the frequency of isolated CNS relapse. The impact of TIT on isolated CNS relapse was especially pronounced in patients with CNS2 status such that it reduced 6-year cumulative risk to 7.7%±5.3% as compared to 23.0%±9.5% among those treated with IT MTX (P=0.004); the 6-year cumulative risk of isolated CNS relapse among patients with CNS1 status was 3.1%±1.0% versus 5.1%±1.2% between those treated with TIT or IT MTX (P=0.03)10. However, TIT treatment was unexpectedly associated with an increased frequency of bone marrow and testicular relapse, resulting in similar EFS and poorer OS. One explanation for this seemingly paradoxical finding is that cytarabine or hydrocortisone in TIT interferes with egress of MTX from cerebrospinal fluid into blood such that patients receiving TIT had less systemic exposure to MTX10. Another explanation is that CNS relapse and other relapses are competing events so that improved CNS control by TIT might have led to leukemic relapse in other sites. In this regard, adding IV MTX to TIT improved outcome by reducing both CNS and non-CNS relapses, whereas adding IV to IT had little effect. Only one trial compared IV MTX plus IT with TIT, with no EFS or OS difference. It may be that more effective systemic therapy is needed before the full benefit of TIT can be realized. COG is re-examining the question of TIT in the B-precursor higher risk trial, AALL1131. All patients will receive IV MTX at 5 g/m2 with leucovorin and the more favourable patients by presenting features and response will be randomized to receive IT MTX versus TIT.
That higher dose of IV MTX failed to significantly improve EFS in some studies might be due to excessive leucovorin rescue24,57,58. In this regard, it is of special interest that even lower dose IV MTX, without leucovorin, on CCG-1991 (100-300 mg/m2) can reduce CNS relapse59. This trial was in NCI standard risk patients and compared two courses of interim maintenance with five doses of vincristine plus IV MTX at 100 mg/m2 escalated by 50 mg/m2 every ten days for four doses as tolerated, versus oral mercaptopurine, oral methotrexate plus ten doses of dexamethasone at six mg/m2 and reported an EFS advantage with escalating IV MTX. However, the recent COG AALL0232 trial in NCI high risk patients up to the age of 30 years shows a substantial EFS advantage for IV MTX at 5 g/m2 with leucovorin rescue compared with asparaginase plus escalating IV MTX without rescue60,61. This trial also randomized between dexamethasone and prednisone. The final report is not yet available, but there appears to be an interaction suggesting EFS benefit only for the high dose IV MTX plus dexamethasone combination. In this regard, when high dose IV MTX is used, attention needs to be paid to the leucovorin rescue, keeping it to the minimum necessary.
Despite impressive gains from the treatment of childhood ALL, there is still a need to improve management of CNS disease so that cranial irradiation can be avoided in all children. In this regard, both intrathecal and systemic therapy must be optimized in order to achieve the goal. Dexamethasone, high dose cytarabine and asparaginase may all be relevant. With improved systemic therapy, future studies should determine the optimal number of intrathecal therapy because this treatment modality can also adversely affect neurocognitive function. However, a certain number of intrathecal therapy doses are necessary because high dose methotrexate alone, even at very high dose (33.6 g/m2), was inadequate for CNS control62. To answer outstanding questions it would be helpful for future trials to be designed so that randomized comparisons address single drugs as far as possible.
No clear subgroup differences exist, but information is limited, particularly for T-lineage ALL. An effective CNS-directed treatment strategy without concomitant adequate systemic therapy may lead to increased bone marrow relapse and poorer OS. Thus, the combination of CNS and systemic effects, as well as outcome post relapse, need to always be borne in mind.
Supplementary Material
Acknowledgments
We thank the following for providing trial data: French ALL Cooperative group: A Baruchel, M Auclerc; Instituto Nacional de Enfermedades Neoplasicas (INEN), Peru: C Perez, A Solidaro; Jena University, Germany: F Zintl, I Schiller; Tokyo Children’s Cancer Study Group, Japan: S Nakazawa, M Tsuchida.
Footnotes
CONFLICT OF INTEREST STATEMENT
All members of the writing committee declare there are no conflicts of interest.
References
- 1.Pui CH, Howard SC. Current management and challenges of malignant disease in the CNS in paediatric leukaemia. Lancet Oncol. 2008;9:57–68. doi: 10.1016/S1470-2045(08)70070-6. [DOI] [PubMed] [Google Scholar]
- 2.Mody R, Li S, Dover DC, et al. Twenty-five-year follow-up among survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. Blood. 2008;111:5515–23. doi: 10.1182/blood-2007-10-117150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veerman AJ, Kamps WA, van den Berg H, et al. Dexamethasone-based therapy for childhood acute lymphoblastic leukaemia: results of the prospective Dutch Childhood Oncology Group (DCOG) protocol ALL-9 (1997-2004) Lancet Oncol. 2009;10:957–66. doi: 10.1016/S1470-2045(09)70228-1. [DOI] [PubMed] [Google Scholar]
- 4.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–41. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke M, Gaynon P, Hann I, et al. CNS-directed therapy for childhood acute lymphoblastic leukemia: Childhood ALL Collaborative Group overview of 43 randomized trials. J Clin Oncol. 2003;21(9):1798–809. doi: 10.1200/JCO.2003.08.047. [DOI] [PubMed] [Google Scholar]
- 6.Bostrom BC, Sensel MR, Sather HN, et al. Dexamethasone versus prednisone and daily oral versus weekly intravenous mercaptopurine for patients with standard-risk acute lymphoblastic leukemia: a report from the Children’s Cancer Group. Blood. 2003;101:3809–17. doi: 10.1182/blood-2002-08-2454. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell CD, Richards SM, Kinsey SE, et al. Benefit of dexamethasone compared with prednisolone for childhood acute lymphoblastic leukaemia: results of the UK Medical Research Council ALL97 randomized trial. British Journal of Haematology. 2005;129:734–45. doi: 10.1111/j.1365-2141.2005.05509.x. [DOI] [PubMed] [Google Scholar]
- 8.Inaba H, Pui CH. Glucocorticoid use in acute lymphoblastic leukaemia. Lancet Oncol. 2010;11:1096–106. doi: 10.1016/S1470-2045(10)70114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Escherich G, Richards S, Stork LC, Vora AJ. Meta-analysis of randomised trials comparing thiopurines in childhood acute lymphoblastic leukaemia. Leukemia. 2011;25(6):953–9. doi: 10.1038/leu.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Early Breast Cancer Trialists’ Collaborative Group. Treatment of Early Breast Cancer : Worldwide Evidence 1985-1990. Vol. 1. Oxford, UK: Oxford University Press; 1990. p. 207. [Google Scholar]
- 11.Matloub Y, Lindemulder S, Gaynon PS, et al. Intrathecal triple therapy decreases central nervous system relapse but fails to improve event-free survival when compared with intrathecal methotrexate: results of the Children’s Cancer Group (CCG) 1952 study for standard-risk acute lymphoblastic leukemia, reported by the Children’s Oncology Group. Blood. 2006;108:1165–73. doi: 10.1182/blood-2005-12-011809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Littman P, Coccia P, Bleyer WA, et al. Central nervous system (CNS) prophylaxis in children with low risk acute lymphoblastic leukemia (ALL) Int J Radiat Oncol Biol Phys. 1987;13:1443–9. doi: 10.1016/0360-3016(87)90308-7. [DOI] [PubMed] [Google Scholar]
- 13.Tubergen DG, Gilchrist GS, O’Brien RT, et al. Prevention of CNS disease in intermediate-risk acute lymphoblastic leukemia: comparison of cranial radiation and intrathecal methotrexate and the importance of systemic therapy: a Childrens Cancer Group report. J Clin Oncol. 1993;11:520–6. doi: 10.1200/JCO.1993.11.3.520. [DOI] [PubMed] [Google Scholar]
- 14.Nachman J, Sather HN, Cherlow JM, et al. Response of children with high-risk acute lymphoblastic leukemia treated with and without cranial irradiation: a report from the Children’s Cancer Group. J Clin Oncol. 1998;16:920–30. doi: 10.1200/JCO.1998.16.3.920. [DOI] [PubMed] [Google Scholar]
- 15.van Eys J, Berry D, Crist W, et al. A comparison of two regimens for high-risk acute lymphocytic leukemia in childhood. A Pediatric Oncology Group Study. Cancer. 1989;63:23–9. doi: 10.1002/1097-0142(19890101)63:1<23::aid-cncr2820630104>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 16.Ortega JJ, Javier G, Olive T. Treatment of standard- and high-risk childhood acute lymphoblastic leukaemia with two CNS prophylaxis regimens. Haematol Blood Transfus. 1987;30:483–92. doi: 10.1007/978-3-642-71213-5_85. [DOI] [PubMed] [Google Scholar]
- 17.Stark B, Nirel R, Avrahami G, et al. Long-term results of the Israeli National Studies in childhood acute lymphoblastic leukemia: INS 84, 89 and 98. Leukemia. 2010;24:419–24. doi: 10.1038/leu.2009.254. [DOI] [PubMed] [Google Scholar]
- 18.Moghrabi A, Levy DE, Asselin B, et al. Results of the Dana-Farber Cancer Institute ALL Consortium Protocol 95-01 for children with acute lymphoblastic leukemia. Blood. 2007;109:896–904. doi: 10.1182/blood-2006-06-027714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masson E, Relling MV, Synold TW, et al. Accumulation of methotrexate polyglutamates in lymphoblasts is a determinant of antileukemic effects in vivo. A rationale for high-dose methotrexate. J Clin Invest. 1996;97:73–80. doi: 10.1172/JCI118409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahoney DHJ, Shuster JJ, Nitschke R, et al. Acute neurotoxicity in children with B-precursor acute lymphoid leukemia: an association with intermediate-dose intravenous methotrexate and intrathecal triple therapy--a Pediatric Oncology Group study. J Clin Oncol. 1998;16:1712–22. doi: 10.1200/JCO.1998.16.5.1712. [DOI] [PubMed] [Google Scholar]
- 21.Schaison GS, Baruchel A, Leblanc TAM, Leverger G. Therapy of childhood acute lymphoblastic leukemia. Int J Pediatr Hematol Oncol. 1998;5:145–54. [Google Scholar]
- 22.Pui CH, Sandlund JT, Pei D, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Total Therapy Study XIIIB at St Jude Children’s Research Hospital. Blood. 2004;104:2690–6. doi: 10.1182/blood-2004-04-1616. [DOI] [PubMed] [Google Scholar]
- 23.Lange BJ, Blatt J, Sather HN, Meadows AT. Randomized comparison of moderate-dose methotrexate infusions to oral methotrexate in children with intermediate risk acute lymphoblastic leukemia: a Childrens Cancer Group study. Med Pediatr Oncol. 1996;27:15–20. doi: 10.1002/(SICI)1096-911X(199607)27:1<15::AID-MPO4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 24.LeClerc JM, Billett AL, Gelber RD, et al. Treatment of childhood acute lymphoblastic leukemia: results of Dana-Farber ALL Consortium Protocol 87-01. J Clin Oncol. 2002;20:237–46. doi: 10.1200/JCO.2002.20.1.237. [DOI] [PubMed] [Google Scholar]
- 25.Hill FG, Richards S, Gibson B, et al. Successful treatment without cranial radiotherapy of children receiving intensified chemotherapy for acute lymphoblastic leukaemia: results of the risk-stratified randomized central nervous system treatment trial MRC UKALL XI (ISRC TN 16757172) Br J Haematol. 2004;124:33–46. doi: 10.1046/j.1365-2141.2003.04738.x. [DOI] [PubMed] [Google Scholar]
- 26.Niemeyer CM, Reiter A, Riehm H, et al. Comparative results of two intensive treatment programs for childhood acute lymphoblastic leukemia: The Berlin-Frankfurt-Munster and Dana-Farber Cancer Institute protocols. Ann Oncol. 1991;2:745–9. doi: 10.1093/oxfordjournals.annonc.a057856. [DOI] [PubMed] [Google Scholar]
- 27.Asselin BL, Devidas M, Wang C, et al. Effectiveness of high-dose methotrexate in T-cell lymphoblastic leukemia and advanced-stage lymphoblastic lymphoma: a randomized study by the Children’s Oncology Group (POG 9404) Blood. 2011;118:874–83. doi: 10.1182/blood-2010-06-292615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pullen J, Boyett J, Shuster J, et al. Extended triple intrathecal chemotherapy trial for prevention of CNS relapse in good-risk and poor-risk patients with B-progenitor acute lymphoblastic leukemia: a Pediatric Oncology Group study. J of Clin Oncol. 1993;11:839–49. doi: 10.1200/JCO.1993.11.5.839. [DOI] [PubMed] [Google Scholar]
- 29.Henderson ES, Scharlau C, Cooper MR, et al. Combination chemotherapy and radiotherapy for acute lymphocytic leukemia in adults: results of CALGB protocol 7113. Leuk Res. 1979;3(6):395–407. doi: 10.1016/0145-2126(79)90036-5. [DOI] [PubMed] [Google Scholar]
- 30.Bleyer WA. Intrathecal methotrexate versus central nervous system leukemia. Cancer Drug Deliv. 1984;1:157–67. doi: 10.1089/cdd.1984.1.157. [DOI] [PubMed] [Google Scholar]
- 31.Medical Research Council leukaemia trial, UKALL VII. A report to the Council by the Working Party on Leukaemia in Childhood. Arch Dis Child. 1985;60:1050–4. doi: 10.1136/adc.60.11.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freeman AI, Boyett JM, Glicksman AS, et al. Intermediate-dose methotrexate versus cranial irradiation in childhood acute lymphoblastic leukemia: a ten-year follow-up. Med Pediatr Oncol. 1997;28:98–107. doi: 10.1002/(sici)1096-911x(199702)28:2<98::aid-mpo3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 33.Poplack DG, Reaman GH, Bleyer WA, et al. CNS preventive therapy with high-dose methotrexate in acute lymphoblastic leukemia: a preliminary report. Proc Am Soc Clin Oncol. 1984;3(204) abstr 797. [Google Scholar]
- 34.Moricke A, Zimmermann M, Reiter A, et al. Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia. 2010;24:265–84. doi: 10.1038/leu.2009.257. [DOI] [PubMed] [Google Scholar]
- 35.Zintl F, Plenert W, Malke H. Results of acute lymphoblastic leukemia therapy in childhood with a modified BFM protocol in a multicenter study in the German Democratic Republic. Haematol Blood Transfus. 1987;30:471–9. doi: 10.1007/978-3-642-71213-5_83. [DOI] [PubMed] [Google Scholar]
- 36.Tsurusawa M, Katano N, Yamamoto Y, et al. Improvement in CNS protective treatment in non-high-risk childhood acute lymphoblastic leukemia: report from the Japanese Children’s Cancer and Leukemia Study Group. Med Pediatr Oncol. 1999;32:259–6. doi: 10.1002/(sici)1096-911x(199904)32:4<259::aid-mpo4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 37.Viana MB, Murao M, Ramos G, et al. Malnutrition as a prognostic factor in lymphoblastic leukaemia: a multivariate analysis. Arch Dis Child. 1994;71:304–10. doi: 10.1136/adc.71.4.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsuchida M, Ohara A, Manabe A, et al. Long-term results of Tokyo Children’s Cancer Study Group trials for childhood acute lymphoblastic leukemia, 1984-1999. Leukemia. 2010;24:383–96. doi: 10.1038/leu.2009.260. [DOI] [PubMed] [Google Scholar]
- 39.Hann I, Vora A, Richards S, et al. Benefit of intensified treatment for all children with acute lymphoblastic leukaemia: results from MRC UKALL XI and MRC ALL97 randomised trials. UK Medical Research Council’s Working Party on Childhood Leukaemia. Leukemia. 2000;14:356–63. doi: 10.1038/sj.leu.2401704. [DOI] [PubMed] [Google Scholar]
- 40.Salzer WL, Devidas M, Carroll WL, et al. Long-term results of the pediatric oncology group studies for childhood acute lymphoblastic leukemia 1984-2001: a report from the children’s oncology group. Leukemia. 2010;24:355–70. doi: 10.1038/leu.2009.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chessells JM, Durrant J, Hardy RM, Richards S. Medical Research Council leukaemia trial--UKALL V: an attempt to reduce the immunosuppressive effects of therapy in childhood acute lymphoblastic leukemia. Report to the Council by the Working Party on Leukaemia in Childhood. J Clin Oncol. 1986;4:1758–64. doi: 10.1200/JCO.1986.4.12.1758. [DOI] [PubMed] [Google Scholar]
- 42.Eden OB, Lilleyman JS, Richards S. Testicular irradiation in childhood lymphoblastic leukaemia. Medical Research Council Working Party on Leukemia in Childhoods. Br J Haematol. 1990;75:496–8. doi: 10.1111/j.1365-2141.1990.tb07788.x. [DOI] [PubMed] [Google Scholar]
- 43.Brandalise S, Odone V, Pereira W, et al. Treatment results of three consecutive Brazilian cooperative childhood ALL protocols: GBTLI-80, GBTLI-82 and -85. ALL Brazilian Group. Leukemia. 1993;7(Suppl 2):S142–5. [PubMed] [Google Scholar]
- 44.Tsuchida M, Akatsuka J, Bessho F, et al. Treatment of acute lymphoblastic leukemia in the Tokyo Children’s Cancer Study Group--preliminary results of L84-11 protocol. Acta Paediatr Jpn. 1991;33:522–32. [PubMed] [Google Scholar]
- 45.Duttera MJ, Bleyer WA, Pomeroy TC, et al. Irradiation, methotrexate toxicity, and the treatment of meningeal leukaemia. Lancet. 1973;2(7831):703–7. doi: 10.1016/s0140-6736(73)92539-7. [DOI] [PubMed] [Google Scholar]
- 46.Jones B, Freeman AI, Shuster JJ, et al. Lower incidence of meningeal leukemia when prednisone is replaced by dexamethasone in the treatment of acute lymphocytic leukemia. Med Pediatr Oncol. 1991;19:269–75. doi: 10.1002/mpo.2950190411. [DOI] [PubMed] [Google Scholar]
- 47.Komp DM, Fernandez CH, Falletta JM, et al. CNS prophylaxis in acute lymphoblastic leukemia: comparison of two methods a Southwest Oncology Group study. Cancer. 1982;50:1031–6. doi: 10.1002/1097-0142(19820915)50:6<1031::aid-cncr2820500602>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 48.Dritschilo A, Cassady JR, Camitta B, et al. The role of irradiation in central nervous system treatment and prophylaxis for acute lymphoblastic leukemia. Cancer. 1976;37:2729–35. doi: 10.1002/1097-0142(197606)37:6<2729::aid-cncr2820370624>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 49.Brecher ML, Berger P, Freeman AI, et al. Computerized tomography scan findings in children with acute lymphocytic leukemia treated with three different methods of central nervous system prophylaxis. Cancer. 1985;56:2430–3. doi: 10.1002/1097-0142(19851115)56:10<2430::aid-cncr2820561017>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 50.Ortega JA, Nesbit ME, Sather HN, et al. Long-term evaluation of a CNS prophylaxis trial--treatment comparisons and outcome after CNS relapse in childhood ALL: a report from the Childrens Cancer Study Group. J Clin Oncol. 1987;5:1646–54. doi: 10.1200/JCO.1987.5.10.1646. [DOI] [PubMed] [Google Scholar]
- 51.Steinherz PG, Gaynon PS, Breneman JC, et al. Treatment of patients with acute lymphoblastic leukemia with bulky extramedullary disease and T-cell phenotype or other poor prognostic features: randomized controlled trial from the Children’s Cancer Group. Cancer. 1998;82:600–12. doi: 10.1002/(sici)1097-0142(19980201)82:3<600::aid-cncr24>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 52.Vilmer E, Suciu S, Ferster A, et al. Long-term results of three randomized trials (58831, 58832, 58881) in childhood acute lymphoblastic leukemia: a CLCG-EORTC report. Children Leukemia Cooperative Group. Leukemia. 2000;14:2257–66. doi: 10.1038/sj.leu.2401960. [DOI] [PubMed] [Google Scholar]
- 53.Donadieu J, Auclerc MF, Baruchel A, et al. Critical study of prognostic factors in childhood acute lymphoblastic leukaemia: differences in outcome are poorly explained by the most significant prognostic variables. Fralle group. French Acute Lymphoblastic Leukaemia study group. Br J Haematol. 1998;102:729–39. doi: 10.1046/j.1365-2141.1998.00818.x. [DOI] [PubMed] [Google Scholar]
- 54.Holland JF, Glidewell O. Chemotherapy of acute lymphocytic leukemia of childhood. Cancer. 1972;30:1480–7. doi: 10.1002/1097-0142(197212)30:6<1480::aid-cncr2820300611>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 55.Sackmann Muriel F, Pavlovsky S, Penalver JA, et al. Evaluation of induction of remission, intensification, and central nervous system prophylactic treatment in acute lymphoblastic leukemia. Cancer. 1974;34:418–26. doi: 10.1002/1097-0142(197408)34:2<418::aid-cncr2820340227>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 56.Moss HA, Nannis ED, Poplack DG. The effects of prophylactic treatment of the central nervous system on the intellectual functioning of children with acute lymphocytic leukemia. Am J Med. 1981;71:47–52. doi: 10.1016/0002-9343(81)90257-6. [DOI] [PubMed] [Google Scholar]
- 57.Borsi JD, Wesenberg F, Stokland T, Moe PJ. How much is too much? Folinic acid rescue dose in children with acute lymphoblastic leukaemia. European Journal of Cancer. 1991;27:1006–9. doi: 10.1016/0277-5379(91)90269-j. [DOI] [PubMed] [Google Scholar]
- 58.Skarby TV, Anderson H, Heldrup J, et al. High leucovorin doses during high-dose methotrexate treatment may reduce the cure rate in childhood acute lymphoblastic leukemia. Leukemia. 2006;20:1955–62. doi: 10.1038/sj.leu.2404404. [DOI] [PubMed] [Google Scholar]
- 59.Matloub Y, Bostrom BC, Hunger SP, et al. Escalating intravenous methotrexate improves event-free survival in children with standard-risk acute lymphoblastic leukemia: a report from the Children’s Oncology Group. Blood. 2011;118:243–51. doi: 10.1182/blood-2010-12-322909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Winick NJ, Salzer WL, Devidas M, Nachman JB. Dexamethasone (DEX) versus prednisone (PRED) during induction for children with high-risk acute lymphoblastic leukemia (HR-ALL): A report from the Children’s Onocology Group Study AALL0232. J Clin Oncol. 2011;29(suppl) abstr 9504. [Google Scholar]
- 61.Larsen EC, Salzer WL, Devidas M, et al. Comparison of high-dose methotrexate (HD-MTX) with Capizzi methotrexate plus asparaginse (C-MTX/ASNase) in children and young adults with high-risk acute lymphoblastic leukemia (HR-ALL): A report from the Children’s Oncology Group Study AALL0232. J Clin Oncol. 2011;29(suppl) doi: 10.1200/JCO.2015.62.4544. abstr 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nathan PC, Whitcomb T, Wolters PL, et al. Very high-dose methotrexate (33.6 g/m(2)) as central nervous system preventive therapy for childhood acute lymphoblastic leukemia: results of National Cancer Institute/Children’s Cancer Group trials CCG-191P, CCG-134P and CCG-144P. Leuk Lymphoma. 2006;47:2488–504. doi: 10.1080/10428190600942769. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.