Abstract
The dynamic relationship between stem cells and their niche governs self-renewal and progenitor cell deployment. The chemokine CXCL12 (C-X-C motif ligand 12) and its signaling receptor CXCR4 (C-X-C motif receptor 4) represent an important pathway that regulates homing and maintenance of stem cells in neural niches. Neural stem cells (NSCs) reside in specific niches where communication with blood vessels is regulated by CXCL12. In neurodegenerative diseases and brain tumors, reactive vasculature forms in response to diseased tissues to create new niches that secrete CXCL12, enhancing recruitment of neural progenitor cells (NPCs) to lesion sites via long-range migration. These observations suggest that the CXCL12-CXCR4 axis maintains NSCs and serves as an emergent salvage signal for initiating endogenous stem cell-based tissue repair.
Keywords: CXCL12, CXCR4, neurodegeneration, stem cells, tissue repair
Introduction
Human neurodegenerative disorders are characterized by pathological tissue injury with permanent loss of resident cells in the nervous system. Alzheimer’s disease (AD; see Glossary), Parkinson’s disease (PD) and Huntington’s disease (HD) are major causes of disability in the United States with current therapy limited to symptomatic treatments, supporting the potential utility of cell-based therapies. NSCs are functionally defined by their ability for sustained self-renewal and proliferation with multipotency towards central nervous system (CNS) lineages, being able to generate mature neurons, astrocytes or oligodendrocytes both in vitro and in vivo to populate a tissue during development or injury response [1, 2]. With greater lineage commitment, neural progenitor cells (NPCs) display partial differentiation and enhanced proliferation to specific fates, e.g. neuroblasts or oligodendrocyte progenitor cells (OPCs) [1, 2]. Due to their ability to repopulate tissues, both NSCs and NPCs have been considered as attractive sources for the development of cell-based therapies [3]. Thus, it will be essential to identify key molecular signals responsible for NSC-NPC biology to inform treatment of neurodegenerative diseases.
Chemokines represent a superfamily of small chemotactic cytokines that are commonly secreted. Chemokines control cellular motility during development, normal homeostatsis, and injury responses [4]. Chemokines are classified into the four subfamilies: CXC, CC, C and CX3C based on conserved cysteine residues near the N-terminus [5, 6]. CXCL12 (also known as pre-B-cell-growth-stimulating factor [PBSF] or stromal cell-derived factor [SDF]-1) belongs to the CXC subfamily [7]. CXCL12 is classically defined as a regulation signal for peripheral hematopoietic stem cells (HSCs) [8], but CXCL12 also serves to maintain embryonic and adult NSCs [9–11]. Considering the vital roles of NSCs during tissue repair, CXCL12 is predicted to contribute to the recruitment of NSCs to damaged regions to enhance recovery. Consistent with this hypothesis, blood vessels within damaged tissues release CXCL12 [12–15]. In this review, we will focus on recent progress highlighting conserved roles of CXCL12 in the NSC niche. We further discuss the correlation between pathological induction of vascular CXCL12 and abnormal activation of NSCs in neurodegenerative animal models. These studies suggest that CXCL12 is an important response signal for the activation of stem cell-based tissue repair after damage.
Basic functions of CXCL12
Chemokine receptors are G-protein-coupled receptors (GPCRs) characterized by seven-transmembrane domains (16–17). G-proteins are heterotrimeric protein complexes. Binding of chemokines to their receptors releases subunits from the G protein complex, which activates a series of GPCR-mediated downstream pathways. CXCR4 was the first identified receptor for CXCL12 and CXCR4 signaling pathways are mediated by pertussis toxin (PTX)-sensitive Gαi components. (4). The CXCL12-CXCR4 axis serves multiple roles in peripheral and central organs/tissues [18, 19]. The CXCL12-CXCR4 axis constitutes a basic signaling pathway for leukocyte and endothelial cell migration. In particular, CXCL12 is a potent chemoattractant for T- and B-cells, monocytes and neutrophils during host responses [20, 21]. CXCL12 also enhances vasculogenesis through the recruitment of endothelial progenitors during inflammation [22, 23] and tumor growth [24–27]. During early embryonic development, CXCL12-CXCR4 signaling is essential for organogenesis as genetic deletion of CXCL12 or CXCR4 disrupts the development of vessels, muscle, primordial germ cells (PGCs) and sensory lateral lines in both mouse and zebrafish models [28–32]. In addition, CXCL12-CXCR4 signaling regulates stem/progenitor cell homing and maintenance. Within the bone marrow niche, CXCL12 guides circulating HSCs and hematologic progenitors within the blood to the bone marrow [28, 29]. Furthermore, niche-derived CXCL12 is essential in maintaining endogenous HSCs in the bone marrow niche [33–34]. Conditional deletion of CXCR4 in HSCs results in the subsequent depletion of HSCs in the perivascular niche [33].
CXCR7, a previously orphan GPCR, also binds CXCL12 with high affinity and specificity (Box 1). In contrast to CXCR4, CXCL12 binding to CXCR7 does not activate G-protein-mediated signaling pathways [35]. Studies from zebrafish suggest that CXCR7 modulates CXCR4 function through scavenging extracellular CXCL12 [36]. Genetic studies from CXCR7 knockout (KO) mice demonstrate that CXCR7 is required for the early development of the heart [37]. In tumors, CXCR7 promotes growth [38–41] and tumor-associated vasculogenesis [35]. However, CXCL12 is not the only ligand for CXCR7. CXCL11 (interferon-inducible T cell alpha chemoattractant, I-TAC) is a second ligand for CXCR7 [35]. Like CXCL12, binding of CXCL11 to CXCR7 does not activate GPCR-mediated downstream pathways. CXCR7 and CXCR4 demonstrate overlapping but not identical expression patterns in early embryonic germline zone and early-born neurons [42]. In adult brains, expression patterns of CXCR7 are much more like that of CXCL12 and localized mainly to the vasculature, although message expression has been reported in neurons and astrocytes [42, 43].
Box 1. CXCR7 – a second receptor for CXCL12.
An orphan receptor, previously termed RDC1, has been identified as the second receptor for CXCL12 [35]; it has subsequently been termed CXCR7. CXCL11, another CXC chemokine formerly known as interferon (IFN)-inducible T cell α chemoattractant, can bind this receptor with high affinity [35]. Binding of CXCL12 to CXCR7 provides cultured, receptor-bearing cells with a growth and survival advantage and increased adhesive properties [35]. However, signaling pathways triggered by binding of CXCL12 to CXCR7 are controversial. Although CXCL12 and CXCL11 do not activate calcium flux [35, 37], CXCL12-CXCR7 axis plays some essential roles during development as well as in tumorogenesis.
In the CNS, both CXCR7 and CXCR4 demonstrate similar expression distributions in early embryonic germline zone and early-born neurons [42]. In adult brains, expression patterns of CXCR7 are much more like that of CXCL12. Expression of high level CXCR7 is detected in neurons, astrocytes and endothelial cells [42–43]. Functional analyses demonstrate that CXCR7 impacts the function of CXCR4 through generation of a local-gradient concentration of CXCL12 [34]. It is worth noting that CXCL12-secreting supportive cells have not been clearly defined in the brain. Because robust expression of CXCR7 has been detected in the brain vascular niche, it is possible for CXCR7 to regulate the behaviors of NSCs by innerving with CXCL12-CXCR4 signaling in the vascular niche. Consistent with this hypothesis, it has been reported that CXCR7 regulates the migration of primordial germ cells along the lateral line by competing with CXCR4 [106]. As the role of CXCR7 in regulating the CXCR4-CXCL12 axis is developing, future studies defining the specific role of this new receptor will be pivotal in understanding how this axis functions during degenerative and repair processes. It has been further found that microRNA 430 (miR-430) regulates endogenous CXCL12 and CXCR7 during the migration of PGCs [107]. One of miR-430’s regulatory mechanisms is to balance the expression of CXCR7 in order to avoid sequencing the CXCL12 by this receptor [107].
The mRNA expression levels of CXCR7 mRNA are increased in malignant gliomas compared with the normal brain tissues [108]. CXCR7 promotes tumor growth and metastases, making it a potential target for cancer intervention. CXCR7 was found to be abundantly expressed on tumor-associated blood vessels and malignant cells but not normal vasculature [35]. Abnormal CXCR7 mRNA expression potentially relies on the hypoxic conditions within tumors [109]. The CXCR7-specific small molecule compound CCX771 decreases the tumor growth in xenograft animals through inhibiting tumor-associated angiogenesis [35]. In breast cancers, evidence from bioluminescence-based in vivo imaging has suggested that CXCR7-expressing tumor cells regulate the release of CXCL12 in the tumor microenvironment and enhance the growth of CXCR4-expresisng tumor cells [110]. CXCR7-specific antagonist limits the growth of CXCR4-expressing tumor cells, suggesting CXCR7-dependent ligand scavenging potentially impact the roles of CXCR4 in the tumor cells.
Niche-dependent signaling of CXCL12
Tissue-specific stem cells reside in anatomically defined microenvironments, or niches, that are structurally composed of supportive cells, vascular network and extracellular matrix [44]. The niche not only maintains a restricted population of stem cells but also instructs the delicate balance of self-renewal and differentiation [44]. In Drosophila melanogaster, the ovary microenvironment has been well characterized for germline stem cells [45], which rely on adjacent somatic cells (such as terminal filament cells, cap cells and escort cells) to maintain their stemness [45]. In mammals, the perivascular niche supports stem cell maintenance across different tissues [46, 47]. Chemokines and in particular, CXCL12, is readily identified in all vertebrates but not invertebrate species (such as D. melanogaster). CXCL12 is a conserved signaling component of the stem cell niche that functionally regulates stem cells. The important roles of CXCL12 in stem cell niches are summarized in Figures 1–3.
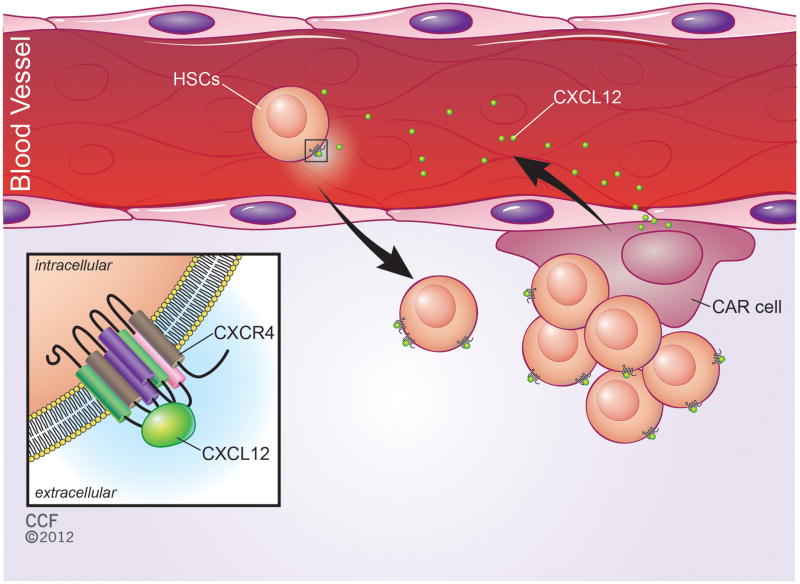
Figure 1. CXCL12 signaling in the bone marrow stem cell niche.
Hematopoietic stem cells (HSCs) are maintained in a highly-specialized microenvironment (niche) in the bone marrow. CXCL12-abundant-reticular cells (CARs) are completely-differentiated and supportive cells in the bone marrow niche. CARs secrete the small molecule chemokine CXCL12 [16, 24]. CXCL12 functions as a chemoattractant signal to recruit peripheral HSCs from blood to the niche (a procedure referred as “homing”) [38–39, 43] and maintain their quiescent state in the perivascular niches [9–12, 43, 58]. HSCs express CXCL12’s cognate receptor CXCR4 on their surface (see inset).
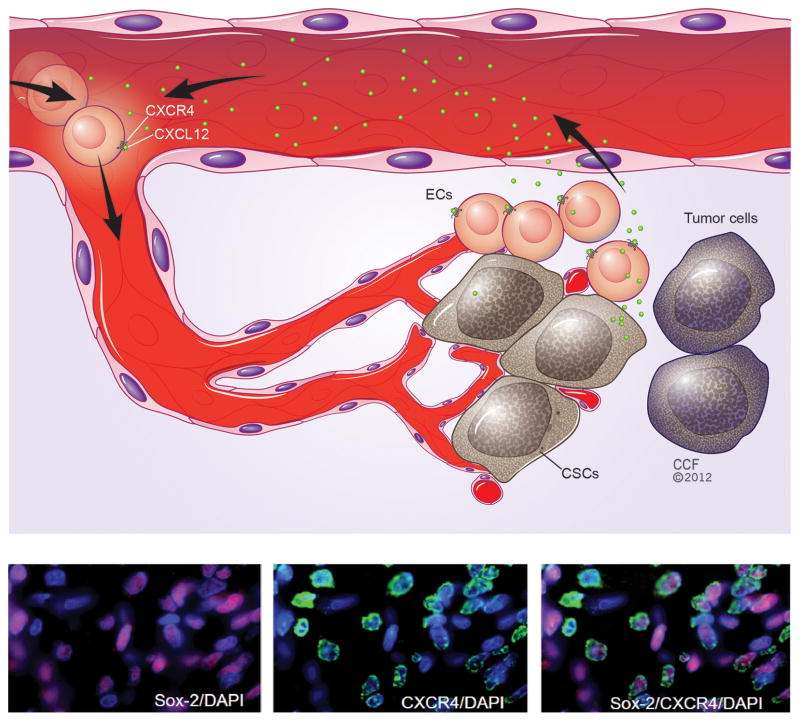
Figure 3. CXCL12 signaling in the pathological niche (eg. brain tumors).
(a) In human glioma, cancer stem cells (CSCs) reside within a specialized microenvironment – the perivasular niche - that is close to the vessels. CSCs rely on extracellular signals to maintain their stemness and tumorigenic potential. CSCs may directly secrete CXCL12 in order to regulate the growth of CSCs. Meanwhile, CSC-associated CXCL12 recruits peripheral endothelial progenitor cells (ECs) and further enhances tumor angiogenesis. (b) CXCR4 (green) is co-expressed with Sox2 (purple), a marker for glioblastoma CSCs, as demonstrated by staining of human glioblastoma tissue sections and the counter-staining of cell nuclei by DAPI [75]. Adapted, with permission, from 75 (b).
CXCL12 in the bone marrow niche
Two locations, the osteoblastic and perivascular microenvironments, harbor HSCs in the bone marrow [46]. In both niches, a subtype of supportive reticular cells, CXCL12-abundant-reticular (CAR) cells (Figure 1), expresses high levels of CXCL12 [33, 48]. CARs physically contact HSCs in the bone marrow niches [48]. In both pharmacological studies and knockout (KO) mouse model studies, CXCL12-CXCR4 signaling maintains quiescent HSCs, suggesting bone marrow niche-derived CXCL12 is necessary to establish the early stem cell colony via regulation of HSCs homing from blood to bone marrow [33, 34, 49, 50].
CXCL12 in the neural niche
Two major types of niches that have been described in the adult rodent brain include the (i) subventricular zone (SVZ) adjacent to the lateral ventricles, and (ii) the subgranular zone (SGZ) located in the dentate gyrus (DG) of the hippocampus [47, 51]. In SVZ, the stem cell niche for NSCs is composed of a highly-organized cellular microenvironment, including astrocytes, ependymal cells and NSCs [47] (Figure 2A). NSCs extend a single small process to directly contact the ventricle and also extend a long ciliated basal process to blood vessels in the SVZ [52]. Besides the cellular microenvironment, a specialized vascular niche may exist in the SVZ [53–54]. Cellular expression of CXCL12 and CXCR4 are mutually exclusive during the early development of the ventricular zone supporting a paracrine signaling paradigm [11, 55, 56]. CXCR4 is expressed by NSCs while CXCL12 is predominantly expressed in the meninges suggesting that spatially restricted CXCL12 regulates the positioning of early NSCs (Figure 2C). In addition, CXCL12 increases NSC proliferation in response to growth factors during early CNS development [11]. In the adult SVZ, proliferating progenitor cells depend on vessel-derived CXCL12 to migrate from the ependymal cell layer to the juxtavascular localization [9]. CXCL12 enhances the expression of both epidermal growth factor receptor (EGFR) and integrin α6 – the dominant receptor for the laminins – in NSCs, resulting in enhanced binding of NSCs to extracellular matrix proteins on vessels (specifically members of the laminin family) [9].
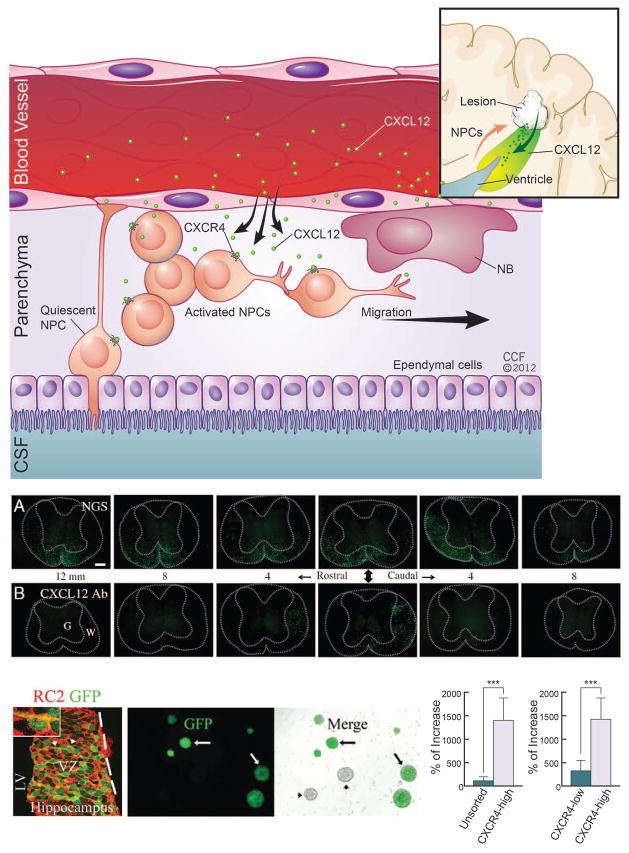
Figure 2. CXCL12 signaling in the SVZ.
(a) A stem cell niche in the SVZ is required to maintain the self-renewal and multipotency of neural stem cells (NSCs). The perivascular space provides a microenvironment to influence the normal roles of NSCs for adult neurogenesis. As suggested in previous studies [9], vessels secrete CXCL12 and regulate the activation of NSCs, which constitutively express CXCR4 receptors. In this hypothesis, NSCs are transformed from an original quiescent state to an activated state. Activated NSCs partially differentiate into neural progenitor cells (NPCs). In addition, vascular niches can be newly generated within damage tissue in many neurodegenerative diseases (inset). These pathologically-associated vascular niches may potentially provide inducible CXCL12 (green arrow) and form a gradient ligand concentration (colored by green) to recruit NPCs or neuronal blast cells (NBs) to enhance stem cell-based tissue repair (red arrow). (b) CXCL12 is critical for NSC engraftment in the spinal cord in a JHM strain of mouse hepatitis virus (JHMV) mouse model. NSC engraftment, as visualized by genetically-labeled green fluorescent protein (GFP) reporter, was observed to be reduced in the presence of a CXCL12 blocking antibody (CXCL12 Ab; bottom panels) as compared to a protein control (normal goat serum, NGS; top panels) [96]. Starting from implanted sites (middle panels pointed by arrow heads), GFP-NSCs migrate toward both rostral and caudal spinal cords. Each panel has 4μM distance. (c) CXCR4 is expressed in early NSCs in vitro and in vivo as demonstrated using a CXCR4-GFP transgenic mouse model. Panel i: GFP-NSCs co-express an early radial cell marker RC2 (clone RC2). Panels ii-iii: GFP-NSCs can be cultured and visualized under fluorescent microscopy. Panels iv-v: GFP-NSCs can be enriched via flow cytometry-based cell sorting (FACS) for sphere formation [11]. CSF (cerebrospinal fluid). Adapted, with permission, from [96] (b) and [11] (c).
The DG of the hippocampus also displays a complementary expression of CXCL12 and CXCR4 [57]. CXCL12 is expressed in the meninges, Cajal-Retzius and dentate granule neurons, while expression of CXCR4 is found in broad spectrum of cells, including radial-glial-like cells in the DG. Transgenic studies with reporters driven by nestin (a marker for early NSCs and NPCs) or CXCR4 have demonstrated that nestin-expressing cells co-localize with CXCR4 and glial fibrillary acidic protein (GFAP), a marker for both adult NSCs and astrocytes [58, 59]. CXCR4 is also critical for embryonic hippocampal development as evidenced by CXCR4 KO mice displaying abnormal DG development with an aberrant migration phenotype [57, 60]. CXCR4-expressing NSCs may rely on niche-provided CXCL12 signals to maintain their “stemness” or regulate the migration of their neuronal progeny. These observations indicate functions of CXCL12-CXCR4 signaling on NSCs in the DG (an alternative location for adult neurogenesis). Proliferating NPCs in the DG exhibit a cluster behavior associated with the recruitment of endothelial cells and vascular remodeling [51]. DG blood vessels may directly provide CXCL12 for NSCs or NPCs as vessels are the main CXCL12 sources in the adult brain [43]. Neurons also provide CXCL12 and potentially regulate the behaviors of NSCs or NPCs [57, 61]. DG neurons directly synapse with proliferating nestin-expressing NPCs and CXCL12 is present in synaptic vesicles [62]. NPCs receive both tonic and phasic GABAergic inputs. Both transmission and growth-regulatory signals from GABAergic neurons can be enhanced by CXCL12 [62].
CXCL12 in the neoplastic neural niche
Malignant gliomas are the most prevalent primary brain tumor and rank among the most lethal cancers with current therapies limited to palliation [63, 64]. Gliomas display a cellular hierarchy with self-renewing, tumorigenic cancer stem cells (CSCs) at the apex [75–81]. CSCs are resistant to conventional radiotherapy and chemotherapy [65–69], strongly supporting the clinical significance for these cells. CSCs are enriched in the perivascular niche (Figure 3) with specialized signaling promoting CSC maintenance through growth factors, including vascular endothelial growth factor (VEGF) [70] and adhesion molecules, such as integrin α6 [71]. Tumor vessels supply essential nutrients and oxygen to support tumor growth with a dynamic process characterized by recruitment of peripheral endothelial progenitors. Tumor-associated angiogenesis is considered to be a restriction point for tumor growth beyond one mm in size.
In glioblastoma multiforme (GBM), expression of CXCL12 or CXCR4 positively correlates with the increased tumor grade [73]. CXCL12-CXCR4 signaling facilitates tumorigenesis through increased proliferation of tumor cells [73, 74]. In tissue culture experiments, CXCL12 administration stimulates a significant proliferative response in tumor-related progenitors but not in differentiated tumor cells [75]. Tumor-secreted CXCL12 recruits peripheral endothelial progenitors to mitotic neovasculature, where the microenvironment influences the differentiation of endothelial progenitors [76]. Consistent with these findings, CXCL12 enhances the release of VEGF from CSCs, leading to tumor-growth-induced angiogenesis [77]. These studies suggest that tumor-associated CXCL12 is an important niche signal for the growth of GBM CSCs (Figure 3).
Activation of NSCs in neurodegenerative diseases
Permanent tissue injury represents the main pathological change that occurs in neurodegenerative diseases. Both NSCs and the microenvironment adapt to these pathological changes in the CNS of patients with neurodegenerative disorders and in representative animal models (Table 1). During the course of neurodegenerative diseases, damaged brain influences not only the maintenance of the normal NSC pool but also their differentiation states. For example, several lines of evidence suggest that NSCs generate partially-differentiated NPCs that are recruited to sites of focal tissue damage, enhancing the endogenous tissue repair [78–89]. This stem cell-based tissue repair is summarized in Figure 2A (inset).
Table 1.
Manipulations of the CXCL12 signaling pathway and associated effects on neurogenesis and/or neurodegenerative disorders in animal modelsa
| Mouse model | Treatment/Manipulation | Effects on CXCL12-CXCR4 axis | Pathology/phenotype | Regions | Diseases | Refs |
|---|---|---|---|---|---|---|
| WT | CXCR4 antagonist AMD3100 | ↓ | Blockage of CXCR4 directly impairs learning and memory | Hippocampus | AD | [100] |
| CXCL12 | ↑ | Intracranial injection of CXCL12 enhances neurogenesis and memory | Hippocampus | AD | [105] | |
| Demyelination/MS models | ||||||
| JHMV | ↑ | Pathologically-induced CXCL12 enhances migration, proliferation, and differentiation of the cells into OPCs | Spinal cord | MS | [96] | |
| CXCL12 antibody | ↓ | Antibody internalization of CXCL12 impairs migration and proliferation of engrafted stem cells | Spinal cord | MS | [96] | |
| CXCR4 antagonist AMD3100 | ↓ | Inhibiting CXCR4 impairs migration and proliferation of engrafted stem cells | Spinal cord | MS | [96] | |
| Cuprizone | ↑ CXCL12 | Induced CXCL12 enhances migration and proliferation of OPCs | CC, brain | MS | [95] | |
| CXCR4 KD | KD of CXCR4 decreases OPCs maturation and impairs the remyelination in the lesion sites | CC, brain | MS | [95] | ||
| CXCR4 antagonist AMD3100 | Inhibiting CXCR4 decreases OPCs maturation and impairs the remyelination in the lesion sites | CC, brain | MS | [95] | ||
| AD models | ||||||
| Tg2576 | ↓ | Down-regulated CXCL12 is related to the impaired learning and memory | DG, Hippocampus | AD | [100] | |
| Exercise | ↑ | Exercising up-regulated CXCL12, which is associated with improved learning and memory | DG, Hippocampus | AD | [103] | |
Abbreviations: AD, Alzheimer’s disease; APP, amyloid Precursor Protein; CC, Corpus callosum; DG, dentate gyrus ; JHMV, JHM strain of mouse hepatitis virus; KD, knockdown; MS, multiple sclerosis; Tg2576. APP “Swedish mutation” transgenic mice; WT, wild-type mice.
Multiple sclerosis (MS)
MS, the most common inflammatory disease of the CNS, results in demyelination, gliosis, axonal and neuritic injury, and ultimately loss of oligodendrocytes. Previous studies have reported that both density and proliferation of NSCs in the SVZ are significantly increased in MS patients [78]. Similar observations were obtained from an experimental autoimmune encephalomyelitis (EAE) rodent model, which mimics the pathologic demyelination seen in MS patients (Table 1). Abnormal NSC proliferation is found in the germinal zones of EAE mouse brains and spinal cords [79, 80]. It has been suggested that partially-differentiated NPCs migrate from the SVZ to damaged sites to generate new oligodendrocytes to repair the damaged myelin sheath [81, 82].
Stroke
A stroke is a transient neurological injury, that can be subdivided into two major subtypes: (1) brain ischemias, which are caused by thrombosis, embolism or systemic hypoperfusion, and (2) brain hemorrhages caused by intracerebral hemorrhage or subarachnoid hemorrhaging. Pathological changes have been widely described in the SVZ of both stroke patients and relevant animal models (Table 1). Abnormal activation of astrocytes/microglia affects neurogenesis [83] and increases cell apoptosis [83, 84] as observed in the SVZ of stroke patients and models (Table 1). Tissue damage from stroke induces the migration, proliferation and differentiation of NPCs [85–87].
Alzheimer’s disease (AD)
AD is the most common neurodegenerative disorder, primarily affecting older adults. The pathogenesis of AD is associated with extracellular deposits of fibrillar β-amyloid (Aβ) in plaques. In experimental AD animals, Aβ deposits impair cell proliferation, causing altered neurogenesis in the SVZ/SGZ [88]. Mechanistic studies suggested that Aβ deposits deplete the endogenous NSC pool through regulating the p75 neurotrophin receptor, leading to a rapid decline in neurogenesis [89]. Familial AD (FAD) can be associated with mutant amyloid precursor protein (APP) or mutated presenilin-1 (PS1) or PS2. The effects of mutated PS1 on NSCs might be related to defective Notch signaling in these cells [90]or microglial activation [91].
Preclinical studies relevant to stem cell-based therapy for AD patients are currently underway, focusing on both animal models (Table 1) and in vitro cell cultures. In an AD mouse model, transplanted mesenchymal stem cells (MSCs) rescue neuronal death caused by Aβ deposition [92–94]. Transplanted MSCs attenuate Aβ-induced memory impairment and apoptosis and mediate the processing of Aβ rather than the direct differentiation of MSCs into neuronal cells [92, 93].
CXCL12-CXCR4 signaling in NSC-based tissue repair
In neurodegenerative diseases, a local vascular microenvironment is established in response to damaged tissue, and CXCL12 from this vascular microenvironment influences local pathogenesis in multiple ways. Since CXCL12 is important to NSCs and NPCs, CXCL12 may regulate NSC-based tissue repair (Figure 2A, inset). Consistent with this hypothesis, some studies have found that CXCL12-CXCR4 signaling mediates NSC-based remyelination through the regulation of NSC activation and recruitment of NPCs [95, 96].
CXCL12-CXCR4 signaling in demyelination
In cuprizone-induced demyelination animal models, which mimic the pathology of MS, CXCR4 inhibition or silencing CXCR4 mRNA expression impairs the differentiation of OPCs, resulting in failed remyelination [95]. Loss of CXCR4 function not only impairs local replacement of oligodendrocytes but also impacts NPC migration. This observation is associated with an abnormal accumulation of NPCs in the SVZ of cuprizone-treated mice [95], suggesting that delayed migration of NPCs could contribute to failed remyelination (Table 1). Treatment with anti-CXCL12 serum decreases migration and proliferation of implanted NSCs or NPCs and impaired remyelination in a hepatitis virus demyelination mouse model [96]. Furthermore, blocking CXCR4 with antagonist AMD3100 decreased the ability of implanted NSCs or NPCs for tissue repair [96]. These findings suggest that CXCL12 is an emergency response signal for initiating endogenous NSC-based repair (Figure 2B).
CXCL12-CXCR4 signaling in stroke
Similarly, CXCL12-CXCR4 signaling may be involved in NSC pathological activation during a stroke. In rodent models with temporary middle cerebral artery suture occlusion (MCAo), migration of transplanted NSCs or NPCs to lesion sites is directly dependent on CXCL12-CXCR4 signaling [97]. Endothelial cells and perivascular astrocytes within damaged tissue have been proposed as the sources of CXCL12, and injury-induced CXCL12 recruits endogenous NSCs/NPCs and peripheral stem cells as well [98]. In addition, other factors may mediate CXCL12-CXCR4 signaling during the tissue repair. For example, DETA-NONOate, a nitric oxide donor, directly up-regulates CXCR4 expression in peripheral stromal cells and further increases their engraftment in MCAo injured brains [99].
CXCL12-CXCR4 signaling in AD
CXCR4 is up-regulated while CXCL12 is down-regulated in AD patients [100, 101]. Transgenic mice Tg2576 that express the human “Swedish” mutation in APP (APP695) develop memory deficits and Aβ deposits [102], providing a useful model to study the function of CXCL12-CXCR4 signaling in AD related diseases (Table 1). Both CXCL12 and CXCR4 are down-regulated in Tg2576 mice perhaps related to the impaired learning and memory caused by the dysfunctional DG [100]. In short-term voluntary wheel running experiments, aged Tg2576 transgenic mice demonstrated improved spatial learning as compared to sedentary controls and this corresponded with an increase in chemokines CXCL12 and CXCL1 [103]. Chronic treatment of wild type mice with the CXCR4 antagonist AMD3100 selectively impairs learning and memory [100]. Therefore, inhibited CXCL12-CXCR4 signaling in AD patients might impact the normal neuronal survival advantage conferred by CXCL12. Pretreatment with CXCL12 in culture can significantly protect neurons from Aβ-induced dendritic regression and apoptosis through activation of AKT and ERK1/2 pathways [104]. Intra-cerebroventricular injection of Aβ reduces dendritic length and spine density of pyramidal neurons in the hippocampus CA1 area, and this deleterious effect can be significantly inhibited by direct application of CXCL12 [104]. These studies suggest that increasing the ambient CNS concentration of CXCL12 may be considered as a strategy to treat AD patients, since CXCL12 is down-regulated in both patients and experimental animals. Indeed, intracerebral administration of CXCL12 into the brains of AD genetically engineered models enhances recruitment of mesenchymal stem cells (MSCs), which significantly rescues the memory deficit although Aβ levels do not change [105]. A NSC-driven tissue repair can be promoted by exogenous Aβ which enhances the migration of NPCs [101]. However, whether CXCL12-CXCR4 is involved in the Aβ-mediated migration of NPCs remains to be determined.
Perspective
Cellular-based therapies (either exogenous or endogenous) represent a promising intervention for complex human CNS diseases. The successful development of stem cell-based therapies relies both on the extent of our knowledge of stem cell replacement mechanisms for injured cells and extrinsic signals that are required for stem cell-based tissue repair in order to instruct their functional integration. Activation of endogenous adult NSCs in neurodegenerative diseases provides a unique model to explore these challenging questions(Box 2).
Box 2. Outstanding questions.
What is the detailed distribution of CXCL12 in the normal vascular and the niche?
How does niche-dependent CXCL12 regulate the survival and maintenance of endogenous NSCs or NPCs?
Does niche-dependent CXCL12 regulate stem cell self-renewal and/or differentiation?
Does vascular CXCR7 balance the concentration of CXCL12? If so, how does niche-dependent CXCR7 regulate the behaviors of endogenous NSCs or NPCs?
How is pathologically-enhanced CXCL12 associated with the activation of endogenous NSCs or NPCs during neurodegeneration, and what is the underlying molecular mechanism?
What is the mechanism of CXCL12-driven recruitment of endogenous NPCs to injury sites?
Is the CXCL12 signal directly involved in the proliferation, differentiation and maturation of endogenous NSCs or NPCs after tissue damage?
Expression of CXCL12 is significantly upregulated in damaged tissues of patients with neuroinflammatory, neoplastic, ischemic and neurodegenerative diseases. Pathologically-induced CXCL12 facilitates the recruitment of peripheral endothelial progenitor cells to lesion sites, consequently enhancing vasculogenesis which may promote repair of damaged tissue or augment tumorigenesis, depending on the disease context. In this regard, a growing body of evidence has shown that endogenous NSCs or NPCs express CXCR4 and depend on niche-generated CXCL12. More importantly, these CXCR4-expressing NPCs can be recruited from the stem cell niche to damaged tissue through long-distance migration. It seems that the first two steps in NSC-based tissue repair (activation and migration) are under partial control of CXCL12-CXCR4 signaling. Thus, CXCL12 may be a critical response and homing signal for guiding stem cell-based tissue repair. Future studies directly modulating this signaling axis in stem cell repair models of neurodegenerative diseases will be critical for understanding homing, integration and repair processes.
Interrogating the roles of CXCL12 signaling in NSCs will extend our current knowledge about the molecular mechanisms underlying the normal maintenance of NSCs in the niche. Furthermore, studying CXCL12 and its cognate receptors will be helpful in isolating and developing therapeutic stem cells for patients with clinical neurodegeneration. Moving forward, the CXCL12 signaling pathway may provide targets to screen drugs that control key steps in cell-based therapies.
Acknowledgments
We regret that many important studies related to chemokines and stem cell niches were not discussed due to the limited space in this review. We thank Joe Pangrace for his assistance in preparing illustrations and Dhurata Brega for editorial assistance. J.D.L. is supported by the Voices Against Brain Cancer and a National Institutes of Health (NIH) K99/R00 Pathway to Independence Award CA157948. J.N.R. is supported by the James D. Foundation and the NIH (grants CA112958, CA116659 and CA154130). R.R. acknowledges funding support for research that is relevant for this review by the NIH (R01NS32151, K24NS51400, R21NS74820), the National Multiple Sclerosis (MS) Society and the Williams Family Fund for MS research.
Glossary
- Alzheimer’s disease (AD)
is the most common neurodegenerative disorder, which primarily affects older adults. The main clinical manifestations of AD are learning and selective memory impairment due to neuronal death. The pathogenesis of AD is related to the extracellular deposits of Aβ, also known as neuritic plaques
- Chemokines
are chemotactic cytokines that functionally guide the migration of leukocytes during normal and inflammatory conditions. Most (if not) all chemokines are small (8–10 kilodaltons) secreted proteins, that are classified into four subfamilies (CXC, CC, C or CX3C). Binding of chemokines to their receptors (GPCRs) induces G-protein mediated signaling pathways
- Demyelinating diseases
are caused by the loss of mature oligodendrocytes that normally provide the myelin sheath to protect neuron axons in the CNS. Occurrence of pathological demyelination is due to many factors, including genetic mutations, infection, inflammation and chemical toxicity
- Experimental autoimmune encephalomyelitis (EAE)
is an animal model for human CNS inflammation. Neuroinflammation-mediated demyelination is the major pathological change in both brains and spinal cords
- Glioblastoma multiforme (GBM)
is the most popular and aggressive malignant primary brain tumor in humans. GBMs are resistant to clinical chemo- and radio-therapies
- G protein-coupled receptors (GPCRs)
are seven-transmembrane (7TM) domain receptors. Binding of ligand to a GPCR can induce G-protein-mediated down-stream signaling pathways. A G-protein is composed of α, β and γ three subunits. G-protein-mediated pathways directly depend on the α subunit involved (e.g., Gαs, Gαi/o, Gαq/11, Gα12/13)
- Multiple sclerosis (MS)
is the most common autoimmune inflammatory disease that results in the demyelination of the CNS. The pathologic features of MS include multifocal demyelination due to the loss of mature oligodendrocytes, peripheral infiltrated cells as well as neuronal axon damage. The pathogenesis of MS remains unknown
Footnotes
Disclosure statement
R.R.is on the scientific advisory board of ChemoCentryx.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–8. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 2.Temple S. The development of neural stem cells. Nature. 2001;414:112–7. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- 3.Baetge EE. Neural stem cells for CNS transplantation. Ann N Y Acad Sci. 1993;695:285–91. doi: 10.1111/j.1749-6632.1993.tb23068.x. [DOI] [PubMed] [Google Scholar]
- 4.Sallusto F, Baggiolini M. Chemokines and leukocyte traffic. Nat Immunol. 2008;9:949–52. doi: 10.1038/ni.f.214. [DOI] [PubMed] [Google Scholar]
- 5.Murphy PM, et al. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52:145–76. [PubMed] [Google Scholar]
- 6.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–7. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 7.Strieter RM, et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem. 1995;270:27348–57. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- 8.Lapidot T, et al. How do stem cells find their way home? Blood. 2006;106:1901–10. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 9.Kokovay E, et al. Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signaling. Cell Stem Cell. 2010;7:163–73. doi: 10.1016/j.stem.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo Y, et al. SDF1alpha/CXCR4 signaling, via ERKs and the transcription factor Egr1, induces expression of a 67-kDa form of glutamic acid decarboxylase in embryonic hippocampal neurons. J Biol Chem. 2008;283:24789–800. doi: 10.1074/jbc.M800649200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li M, et al. Chemokine receptor CXCR4 signaling modulates the growth factor-induced cell cycle of self-renewing and multipotent neural progenitor cells. Glia. 2011;59:108–18. doi: 10.1002/glia.21080. [DOI] [PubMed] [Google Scholar]
- 12.Rostasy K, et al. SDF-1alpha is expressed in astrocytes and neurons in the AIDS dementia complex: an in vivo and in vitro study. Neuropathol Exp Neurol. 2003;62:617–26. doi: 10.1093/jnen/62.6.617. [DOI] [PubMed] [Google Scholar]
- 13.Ambrosini E, et al. Astrocytes produce dendritic cell-attracting chemokines in vitro and in multiple sclerosis lesions. J Neuropathol Exp Neurol. 2005;64:706–15. doi: 10.1097/01.jnen.0000173893.01929.fc. [DOI] [PubMed] [Google Scholar]
- 14.Calderon TM, et al. A role for CXCL12 (SDF-1alpha) in the pathogenesis of multiple sclerosis: regulation of CXCL12 expression in astrocytes by soluble myelin basic protein. J Neuroimmunol. 2006;177:27–39. doi: 10.1016/j.jneuroim.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Miller JT, et al. The neuroblast and angioblast chemotaxic factor SDF-1 (CXCL12) expression is briefly up regulated by reactive astrocytes in brain following neonatal hypoxic-ischemic injury. BMC Neurosci. 2005;6:63. doi: 10.1186/1471-2202-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fredriksson R, et al. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmac. 2003;63:1256–72. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- 17.Kawasawa Y, et al. G protein-coupled receptor genes in the FANTOM2 database. Genome Res. 2003;13:1466–77. doi: 10.1101/gr.1087603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- 19.Klein RS, Rubin JB. Immune and nervous system CXCL12 and CXCR4: parallel roles in patterning and plasticity. Trends Immunol. 2004;25:306–14. doi: 10.1016/j.it.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Bleul CC, et al. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996;184:1101–9. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petty JM, et al. Pulmonary stromal-derived factor-1 expression and effect on neutrophil recruitment during acute lung injury. J Immunol. 2007;178:8148–57. doi: 10.4049/jimmunol.178.12.8148. [DOI] [PubMed] [Google Scholar]
- 22.Möhle R, et al. The chemokine receptor CXCR-4 is expressed on CD34+ hematopoietic progenitors and leukemic cells and mediates transendothelial migration induced by stromal cell-derived factor-1. Blood. 1998;91:4523–30. [PubMed] [Google Scholar]
- 23.Jo DY, et al. Chemotaxis of primitive hematopoietic cells in response to stromal cell-derived factor-1. J Clin Invest. 2000;105:101–11. doi: 10.1172/JCI7954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orimo A, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–48. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 25.Du R, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–20. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kubota Y, et al. Isolation and function of mouse tissue resident vascular precursors marked by myelin protein zero. J Exp Med. 2011;208:949–60. doi: 10.1084/jem.20102187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ping YF, et al. The chemokine CXCL12 and its receptor CXCR4 promote glioma stem cell-mediated VEGF production and tumour angiogenesis via PI3K/AKT signalling. J Pathol. 2011;224:344–54. doi: 10.1002/path.2908. [DOI] [PubMed] [Google Scholar]
- 28.Tachibana K, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–4. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 29.Zou YR, et al. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–9. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 30.David NB, et al. Molecular basis of cell migration in the fish lateral line: role of the chemokine receptor CXCR4 and of its ligand, SDF1. Proc Natl Acad Sci U S A. 2002;99:16297–302. doi: 10.1073/pnas.252339399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Odemis V, et al. Mice deficient in the chemokine receptor CXCR4 exhibit impaired limb innervation and myogenesis. Mol Cell Neurosci. 2005;30:494–505. doi: 10.1016/j.mcn.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 32.Sapède D, et al. Role of SDF1 chemokine in the development of lateral line efferent and facial motor neurons. Proc Natl Acad Sci U S A. 2005;102:1714–8. doi: 10.1073/pnas.0406382102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sugiyama T, et al. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–88. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 34.Nie Y, et al. CXCR4 is required for the quiescence of primitive hematopoietic cells. J Exp Med. 2006;205:777–83. doi: 10.1084/jem.20072513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burns JM, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201–13. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naumann U, et al. CXCR7 functions as a scavenger for CXCL12 and CXCL11. PLoS One. 2010;5:e9175. doi: 10.1371/journal.pone.0009175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sierro F, et al. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc Natl Acad Sci U S A. 2007;104:14759–64. doi: 10.1073/pnas.0702229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hawkins OE, Richmond A. The dynamic yin-yang interaction of CXCR4 and CXCR7 in breast cancer metastasis. Breast Cancer Res. 2012;14:103. doi: 10.1186/bcr3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luker KE, et al. Scavenging of CXCL12 by CXCR7 promotes tumor growth and metastasis of CXCR4-positive breast cancer cells. Oncogene. 2012 doi: 10.1038/onc.2011.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu H, et al. Alteration of CXCR7 expression mediated by TLR4 promotes tumor cell proliferation and migration in human colorectal carcinoma. PLoS One. 2011;6:e27399. doi: 10.1371/journal.pone.0027399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miao Z, et al. CXCR7 (RDC1) promotes breast and lung tumor growth in vivo and is expressed on tumor-associated vasculature. Proc Natl Acad Sci U S A. 2007;104:15735–40. doi: 10.1073/pnas.0610444104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schönemeier B, et al. Regional and cellular localization of the CXCl12/SDF-1 chemokine receptor CXCR7 in the developing and adult rat brain. J Comp Neurol. 2008;510:207–20. doi: 10.1002/cne.21780. [DOI] [PubMed] [Google Scholar]
- 43.Schönemeier B, et al. Enhanced expression of the CXCl12/SDF-1 chemokine receptor CXCR7 after cerebral ischemia in the rat brain. J Neuroimmunol. 2008;198:39–45. doi: 10.1016/j.jneuroim.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 44.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–9. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 45.Tran J, et al. Somatic control over the germline stem cell lineage during Drosophila spermatogenesis. Nature. 2000;407:754–7. doi: 10.1038/35037613. [DOI] [PubMed] [Google Scholar]
- 46.Kiel MJ, Morrison SJ. Maintaining hematopoietic stem cells in the vascular niche. Immunity. 2006;25:862–4. doi: 10.1016/j.immuni.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 47.Doetsch F, et al. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–61. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tokoyoda K, et al. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20:707–18. doi: 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 49.Broxmeyer HE, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–18. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Larochelle A, et al. AMD3100 mobilizes hematopoietic stem cells with long-term repopulating capacity in nonhuman primates. Blood. 2006;107:3772–8. doi: 10.1182/blood-2005-09-3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palmer TD, et al. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–94. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 52.Mirzadeh Z, et al. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–78. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tavazoie M, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–88. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shen Q, et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tissir F, et al. Expression of the chemokine receptor Cxcr4 mRNA during mouse brain development. Brain Res Dev Brain Res. 2004;149:63–71. doi: 10.1016/j.devbrainres.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 56.Tran PB, et al. Chemokine receptors are expressed widely by embryonic and adult neural progenitor cells. J Neurosci Res. 2004;76:20–34. doi: 10.1002/jnr.20001. [DOI] [PubMed] [Google Scholar]
- 57.Berger O, et al. Expression of SDF-1 and CXCR4 during reorganization of the postnatal dentate gyrus. Dev Neurosci. 2007;29:48–58. doi: 10.1159/000096210. [DOI] [PubMed] [Google Scholar]
- 58.Tran PB, et al. Chemokine receptor expression by neural progenitor cells in neurogenic regions of mouse brain. J Comp Neurol. 2007;500:1007–33. doi: 10.1002/cne.21229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mignone JL, et al. Neural stem and progenitor cells in nestin-GFP transgenic mice. J Comp Neurol. 2004;469:311–24. doi: 10.1002/cne.10964. [DOI] [PubMed] [Google Scholar]
- 60.Lu M, et al. Abnormal development of the hippocampal dentate gyrus in mice lacking the CXCR4 chemokine receptor. Proc Natl Acad Sci U S A. 2002;99:7090–5. doi: 10.1073/pnas.092013799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tham TN, et al. Developmental pattern of expression of the alpha chemokine stromal cell-derived factor 1 in the rat central nervous system. Eur J Neurosci. 2001;13:845–56. doi: 10.1046/j.0953-816x.2000.01451.x. [DOI] [PubMed] [Google Scholar]
- 62.Bhattacharyya BJ, et al. The chemokine stromal cell-derived factor-1 regulates GABAergic inputs to neural progenitors in the postnatal dentate gyrus. J Neurosci. 2008;28:6720–30. doi: 10.1523/JNEUROSCI.1677-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Denysenko T, et al. Glioblastoma cancer stem cells: heterogeneity, microenvironment and related therapeutic strategies. Cell Biochem Funct. 2010;28:343–51. doi: 10.1002/cbf.1666. [DOI] [PubMed] [Google Scholar]
- 64.Iacob G, Dinca EB. Current data and strategy in glioblastoma multiforme. J Med Life. 2009;2:386–93. [PMC free article] [PubMed] [Google Scholar]
- 65.Ignatova TN, et al. Human cortical glialtumors contain neural stem -like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39:193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- 66.Liu G, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kang MK, Kang SK. Tumorigenesis of chemotherapyeutic drug-resistant cancer stem-like cells in brain glioma. Stem Cells Dev. 2007;16:837–47. doi: 10.1089/scd.2007.0006. [DOI] [PubMed] [Google Scholar]
- 68.Galli R, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–21. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 69.Bao S, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–60. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 70.Bao S, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–8. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 71.Lathia JD, et al. Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell. 2010;6:421–32. doi: 10.1016/j.stem.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mieno S, et al. Role of stromal-derived factor-1alpha in the induction of circulating CD34+CXCR4+ progenitor cells after cardiac surgery. Circulation. 2006;114:186–92. doi: 10.1161/circulationaha.105.001610. [DOI] [PubMed] [Google Scholar]
- 73.Oh JW, et al. CXC chemokine receptor 4 expression and function in human astroglioma cells. J Immunol. 2001;166:2695–704. doi: 10.4049/jimmunol.166.4.2695. [DOI] [PubMed] [Google Scholar]
- 74.Rubin JB, et al. A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc Natl Acad Sci U S A. 2003;100:13513–8. doi: 10.1073/pnas.2235846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ehtesham M, et al. CXCR4 mediates the proliferation of glioblastoma progenitor cells. Cancer Lett. 2009;274:305–12. doi: 10.1016/j.canlet.2008.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aghi M, et al. Tumor stromal-derived factor-1 recruits vascular progenitors to mitotic neovasculature, where microenvironment influences their differentiated phenotypes. Cancer Res. 2006;66:9054–64. doi: 10.1158/0008-5472.CAN-05-3759. [DOI] [PubMed] [Google Scholar]
- 77.Põlajeva J, et al. Mast cell accumulation in glioblastoma with a potential role for stem cell factor and chemokine CXCL12. PLoS One. 2011;6:e25222. doi: 10.1371/journal.pone.0025222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nait-Oumesmar B, et al. Activation of the subventricular zone in multiple sclerosis: evidence for early glial progenitors. Proc Natl Acad Sci U S A. 2007;104:4694–9. doi: 10.1073/pnas.0606835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Calzà L, et al. Proliferation and phenotype regulation in the subventricular zone during experimental allergic encephalomyelitis: in vivo evidence of a role for nerve growth factor. Proc Natl Acad Sci U S A. 1998;95:3209–14. doi: 10.1073/pnas.95.6.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nait-Oumesmar B, et al. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur J Neurosci. 1999;11:4357–66. doi: 10.1046/j.1460-9568.1999.00873.x. [DOI] [PubMed] [Google Scholar]
- 81.Picard-Riera N, et al. Experimental autoimmune encephalomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in adult mice. Proc Natl Acad Sci U S A. 2002;99:13211–6. doi: 10.1073/pnas.192314199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Magalon K, et al. Enriched environment promotes adult neural progenitor cell mobilization in mouse demyelination models. Eur J Neurosci. 2007;25:761–71. doi: 10.1111/j.1460-9568.2007.05335.x. [DOI] [PubMed] [Google Scholar]
- 83.Parent JM, et al. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–13. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- 84.Jin K, et al. Ischemia-induced neurogenesis is preserved but reduced in the aged rodent brain. Aging Cell. 2004;3:373–7. doi: 10.1111/j.1474-9728.2004.00131.x. [DOI] [PubMed] [Google Scholar]
- 85.Thored P, et al. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24:739–47. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- 86.Dempsey RJ, et al. Stroke-induced progenitor cell proliferation in adult spontaneously hypertensive rat brain: effect of exogenous IGF-1 and GDNF. J Neurochem. 2003;87:586–97. doi: 10.1046/j.1471-4159.2003.02022.x. [DOI] [PubMed] [Google Scholar]
- 87.Zhang R, et al. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab. 2004;24:441–8. doi: 10.1097/00004647-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 88.Rodríguez JJ, et al. Impaired adult neurogenesis in the dentate gyrus of a triple transgenic mouse model of Alzheimer’s disease. PLoS One. 2008;3:e2935. doi: 10.1371/journal.pone.0002935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sotthibundhu A, et al. Abeta(1–42) stimulates adult SVZ neurogenesis through the p75 neurotrophin receptor. Neurobiol Aging. 2009;30:1975–85. doi: 10.1016/j.neurobiolaging.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 90.Veeraraghavalu K, et al. Presenilin 1 mutants impair the self-renewal and differentiation of adult murine subventricular zone-neuronal progenitors via cell-autonomous mechanisms involving notch signaling. J Neurosci. 2010;30:6903–15. doi: 10.1523/JNEUROSCI.0527-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Choi SH, et al. Non-cell-autonomous effects of presenilin 1 variants on enrichment-mediated hippocampal progenitor cell proliferation and differentiation. Neuron. 2008;59:568–80. doi: 10.1016/j.neuron.2008.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee JK, et al. Intracerebral transplantation of bone marrow-derived mesenchymal stem cells reduces amyloid-beta deposition and rescues memory deficits in Alzheimer’s disease mice by modulation of immune responses. Stem Cells. 2010;28:329–43. doi: 10.1002/stem.277. [DOI] [PubMed] [Google Scholar]
- 93.Lee JK, et al. Bone marrow-derived mesenchymal stem cells attenuate amyloid β-induced memory impairment and apoptosis by inhibiting neuronal cell death. Curr Alzheimer Res. 2010;7:540–8. doi: 10.2174/156720510792231739. [DOI] [PubMed] [Google Scholar]
- 94.Habisch HJ, et al. Efficient processing of Alzheimer’s disease amyloid-Beta peptides by neuroectodermally converted mesenchymal stem cells. Stem Cells Dev. 2010;19:629–33. doi: 10.1089/scd.2009.0045. [DOI] [PubMed] [Google Scholar]
- 95.Patel JR, et al. CXCR4 promotes differentiation of oligodendrocyte progenitors and remyelination. Proc Natl Acad Sci U S A. 2010;107:11062–7. doi: 10.1073/pnas.1006301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Carbajal KS, et al. Migration of engrafted neural stem cells is mediated by CXCL12 signaling through CXCR4 in a viral model of multiple sclerosis. Proc Natl Acad Sci U S A. 2010;107:11068–73. doi: 10.1073/pnas.1006375107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Imitola J, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004;101:18117–22. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang Y, et al. SDF-1alpha/CXCR4-mediated migration of systemically transplanted bone marrow stromal cells towards ischemic brain lesion in a rat model. Brain Res. 2008;1195:104–12. doi: 10.1016/j.brainres.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 99.Cui X, et al. Nitric oxide donor upregulation of stromal cell-derived factor-1/chemokine (CXC motif) receptor 4 enhances bone marrow stromal cell migration into ischemic brain after stroke. Stem Cells. 2007;25:2777–85. doi: 10.1634/stemcells.2007-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Parachikova A, Cotman CW. Reduced CXCL12/CXCR4 results in impaired learning and is downregulated in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2007;28:143–53. doi: 10.1016/j.nbd.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang C, et al. β-amyloid 42 induces desensitization of CXC chemokine receptor-4 via formyl peptide receptor in neural stem/progenitor cells. Biol Pharm Bull. 2012;35:131–8. doi: 10.1248/bpb.35.131. [DOI] [PubMed] [Google Scholar]
- 102.Hsiao K, et al. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 103.Parachikova A, et al. Short-term exercise in aged Tg2576 mice alters neuroinflammation and improves cognition. Neurobiol Dis. 2008;30:121–9. doi: 10.1016/j.nbd.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Raman D, et al. Chemokines, macrophage inflammatory protein-2 and stromal cell-derived factor-1α, suppress amyloid β-induced neurotoxicity. Toxicol Appl Pharmacol. 2011;256:300–13. doi: 10.1016/j.taap.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shin JW, et al. Combined effects of hematopoietic progenitor cell mobilization from bone marrow by granulocyte colony stimulating factor and AMD3100 and chemotaxis into the brain using stromal cell-derived factor-1α in an Alzheimer’s disease mouse model. Stem Cells. 2011;29:1075–89. doi: 10.1002/stem.659. [DOI] [PubMed] [Google Scholar]
- 106.Boldajipour B, et al. Control of chemokine-guided cell migration by ligand sequestration. Cell. 2008;132:463–73. doi: 10.1016/j.cell.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 107.Staton AA, et al. miRNA regulation of Sdf1 chemokine signaling provides genetic robustness to germ cell migration. Nat Genet. 2011;43:204–11. doi: 10.1038/ng.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Calatozzolo C, et al. Expression of the new CXCL12 receptor, CXCR7, in gliomas. Cancer Biol Ther. 2011;11:242–53. doi: 10.4161/cbt.11.2.13951. [DOI] [PubMed] [Google Scholar]
- 109.Schutyser E, et al. Hypoxia enhances CXCR4 expression in human microvascular endothelial cells and human melanoma cells. Eur Cytokine Netw. 2007;18:59–70. doi: 10.1684/ecn.2007.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Luker KE, et al. Scavenging of CXCL12 by CXCR7 promotes tumor growth and metastasis of CXCR4-positive breast cancer cells. Oncogene. 2012 doi: 10.1038/onc.2011.633. [DOI] [PMC free article] [PubMed] [Google Scholar]