Abstract
Near-infrared spectroscopy (NIRS) is a non-invasive diffuse optical-imaging technique that can measure local metabolic demand in the surface of the cortex due to differential absorption of light by oxygenated and deoxygenated blood. Over the past decade, NIRS has become increasingly used as a complement to other neuroimaging techniques, such as EEG, MEG and fMRI, particularly in pediatric populations who cannot easily be tested using fMRI and MEG. In this review of empirical findings from human infants, ranging in age from birth to 12 months of age, a number of interpretive concerns are raised about what can be concluded from NIRS data. In addition, inconsistencies across studies are highlighted and strategies are proposed for enhancing the reliability of NIRS data gathered from infants. Finally, a variety of new and promising advances in NIRS techniques are highlighted.
“Shedding light” on a scientific question took on new meaning when it was discovered (Jobsis, 1977) that near-infrared light could (literally) be pumped through the skull and a small fraction of that light which returned through the skull is modulated by the underlying brain hemodynamics. Thus, just like fMRI, near-infrared spectroscopy (NIRS) provides an indirect measure of neural activity because of the correlation between blood oxygenation and local metabolic demand.
Over 20 years after this discovery, and 10 years after fMRI entered the mainstream in the field of cognitive neuroscience, developmental psychologists became excited about the potential of NIRS to complement fMRI in studies of the infant brain. 1 Seeking a complement to fMRI was a natural response to the enormous technical problems associated with gathering fMRI data from infants. Despite a small number of heroic efforts to use fMRI with infants (Anderson, Marois, Colson, Peterson, Duncan, Ehrenkranz, Konstantino, Sarofin, Schneider, Gore & Ment, 2001; Blasi, Mercune, Lloyd-Fox, Thomson, Brammer, Sauter, Deeley, Barker, Renvall, Deoni, Gasston, Williams, Johnson, Simmons & Murphy, 2011; Dehaene-Lambertz, Dehaene & Hertz-Pannier, 2002), it is now clear that the requirements of fMRI (unless the infant is asleep) are exceptionally limiting. The most daunting hurdle is the need for rigid head stabilization, although both safety concerns (RF gradients and acoustic noise) and the cost of a scanner (even at typical hourly rates) render fMRI with infants a method suitable for only a handful of research centers.
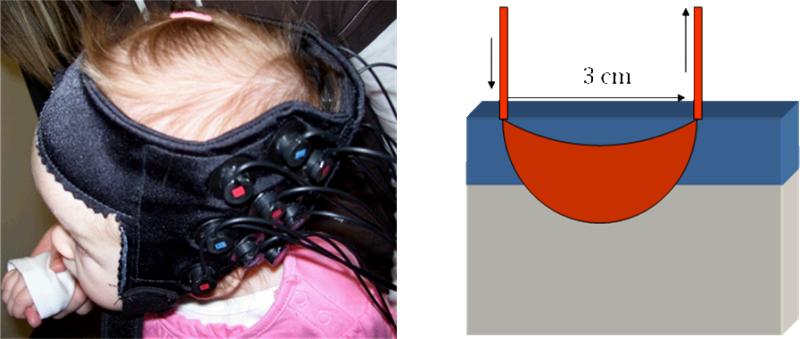
In contrast, NIRS does not demand rigid head stabilization, as the optical fibers are embedded in a “cap” attached to the infant's head (see Figure 1A), and there are no safety concerns beyond limiting the laser powers to readily acceptable levels. In addition, the cost of a NIRS system is about 1/10th of a 3T MR system. NIRS is not without its own limitations, however. Spatial resolution is approximately 1 cm2 and the primary target of sampling from the brain is the surface of the cortex (see Figure 1B). Deeper brain structures are not accessible because the detection of photons that travel further distances from the optical emitters would require laser-light intensities that damage brain tissue. Finally, in contrast to an fMRI voxel, which is typically a 3-4 mm cube, NIRS gathers data from a less precisely defined 3D volume that is captured by a 2D array of emitter-detector pairs. That is, several layers of tissue, including skin, skull, surface vasculature, and gray matter, are collapsed into the 2D samples.
Figure 1.
Left: Infant wearing a cap holding an array of 9 optical fibers to gather data from 12 NIRS channels over the left temporal cortex. Right: Schematic of a NIRS channel, showing the input and output optical fibers and the banana-shaped pathway of photons that dip into the gray matter of the brain after passing through superficial layers of skin, skull, and surface vasculature.
Given this very brief background about what NIRS is and generally how it works with infants (see extensive reviews by Gervain, Mehler, Werker, Nelson, Csibra, Lloyd-Fox, Shukla & Aslin, 2011; Hespos, Ferry, Cannistraci, Gore & Park, 2009; Lloyd-Fox, Blasi & Elwell, 2010), we now consider how NIRS has been used to address questions in the field of developmental cognitive neuroscience. Our focus here is on what kinds of conclusions about brain function and cognitive development have been reached by investigators using NIRS, as well as what kinds of conclusions could be drawn from NIRS data even under ideal circumstances. The overall goal is to push the field to go beyond rudimentary (largely confirmatory) demonstrations of neural activity to test specific hypotheses about neural organization during development.
Questions typically asked
The most basic question one could ask about a new method, especially a method applied to human infants, is whether it provides a reliable measure of neural activity. Consider the example of EEG activity and the averaging of evoked responses to a particular stimulus over repeated trials. This event-related potential (ERP) can be sampled from one electrode location on the scalp or from over 100 such electrode locations. Given uncertainty about where in the brain these ERPs originate, it would not be terribly informative to record from a single channel. Such an ERP would reveal only that some aspect of the sensory information contained in the stimulus, or some aspect of the processing of that information by one or more brain regions, was sufficient to generate an ERP that differed from a no-stimulus baseline.
The situation with NIRS is a bit less ambiguous because the origin of the hemodynamic signal must reside in brain regions proximal to the emitter-detector pair that defines a channel. However, just like a single-channel ERP response that is above a no-stimulus baseline, a reliable activation in a single NIRS channel is not very informative, particularly because the number of stimulus events typically presented to infants in a NIRS study is an order of magnitude fewer than the number presented in an ERP study. This is the natural outcome of the fact that blocked and event-related NIRS designs are sampling a sluggish hemodynamic response over many seconds, whereas ERP events can be repeated at rates up to 10 Hz. Moreover, because the NIRS signal contains both deep (brain level) and surface (non-brain level) activations, it is quite possible to obtain a NIRS activation that is no more specific than a change in heart rate or systemic blood oxygenation correlated with respiration. This is particularly likely in NIRS because of the relatively large magnitude of hemodynamic signals in the surface vasculature, an artifact not present in fMRI.2 Thus, unless some NIRS channels show a response above a no-stimulus baseline and other channels do not (or show less of a response), it is not clear that a response from a single NIRS channel provides any brain-specific information.
Here is what I mean by brain-specific. We know from behavioral methods that infants respond differently to stimuli that vary along a variety of dimensions – some well-defined such as intensity, and others less well-defined such as “face-like”. If a single NIRS channel shows reliable activation (i.e., above a no-stimulus baseline) to a face stimulus and no reliable (or less) activation to a non-face stimulus, it is seductive to conclude that this NIRS channel is sampling a region of the brain that processes faces. The problem with this conclusion is that face stimuli elicit increases in a variety of other psychological processes that are not face-specific, such as attention and arousal. Because these non-specific responses are the outcome of processing a face stimulus, and because we have no measure of attention or arousal independent of the face and non-face stimuli presented to the infant, it is unclear whether activation in a single NIRS channel is face-specific or a general index of attention or arousal.
Given the need to demonstrate at least some degree of channel-specific (i.e., regional) selectivity to conclude that a given NIRS channel is stimulus-specific and not merely reflective of attention or arousal, multiple NIRS channels provide an obvious way forward. To that end, NIRS studies have been conducted using 2 to over 100 channels, although the depth of the resultant NIRS signals is reduced if tightly packed channels have smaller emitter-detector separations on the infant's head. There are two rather obvious benefits, then, to the use of multiple NIRS channels: (1) to assess stimulus-specificity, and (2) to assess regional-selectivity. Studies focusing on this second benefit typically assess the presence and degree of lateralization, particularly in the language domain (see reviews by Minagawa-Kawai, Cristia & Dupoux, 2011; Obrig, Rossi, Telkemeyer & Wartenburger, 2010), because of substantial lateralization of function in the adult brain. However, regional-selectivity can be examined at a more fine-grained level than the two hemispheres, including any pair of NIRS channels. As we will see, conclusions about regional-selectivity, in the limit, are not immune from interpretive difficulties.
Presence vs. Absence of a Stimulus
In the early days of infant NIRS, it was exciting to see any evidence that the method worked. Meek, Firbank, Elwell, Atkinson, Braddick, and Wyatt (1998) were the first to show a NIRS response over occipital cortex in awake 1- to 3-month-olds as they viewed the presence/absence of a flickering checkerboard in 10-sec blocks. A separate group of infants tested with the same single NIRS channel over frontal cortex revealed no significant activations. Given this evidence of regional selectivity to visual stimulation, albeit in separate groups of infants, it is surprising that similar demonstrations are still being reported a decade later. Karen, Morren, Haensse, Bauschatz, Bucher, and Wolf (2008) presented a flickering LED stimulus vs. darkness in 20-sec blocks to neonates (1-week-olds) and reported significant activations in one or more of the 10 NIRS channels over occipital cortex, but no other cortical region was assessed to guard against non-selective regional activations.
One clear advantage of using visual stimuli is the presence of highly constrained anatomical pathways from the eye to the cortex, which, as we will see in a later section, leads to a very specific prediction about the pattern of regional cortical activation. The auditory system is not so constrained anatomically, with substantial pre-cortical overlap in the mapping of signals from the ears to the cortex. As a result, there is tremendous spread of activations elicited by auditory stimuli across diverse cortical regions. It is perhaps not surprising, therefore, that early infant NIRS studies reported activations that were highly variable. For example, Sakatani, Chen, Lichty, Zuo, and Wuang (1999) presented newborns with piano music versus silence while recording from a single NIRS channel over frontal cortex, and Zaramella, Freato, Amigoni, Salvadori, Marangoni, Suppjei, Schiavo, and Chiandetti (2001) presented newborns with tone frequency-sweeps while recording from a single NIRS channel over right or left temporal cortex. Although reliable differences in activation between sound and silence were obtained, the absence of other (control) NIRS channels prevented an assessment of regional selectivity.
Moreover, in contrast to studies of visual stimuli, where at a minimum the infant's eyelids must be open (i.e., quiet wakefulness), many auditory NIRS studies are conducted while the infant is asleep. There is some evidence to suggest that NIRS activations are less robust in newborns during periods of quiet sleep than during periods of quiet awake (Kotilahti, Nissila, Huotilainen, Makela, Gavrielides, Noponen, Bjorkman, Fellman & Katila, 2005). If so, then level-of-arousal could complicate interpretations of infant NIRS data in two ways. First, if arousal is low, then any regional differences may appear to be binary, with activation in one region and no activation in the other. However, as arousal increases, the gain of the “weaker” region may rise faster than the gain of the “stronger” region. As a result, what appears to be robust regional selectivity during periods of sleep may disappear during periods of wakefulness. Second, if infants at different ages are compared to ask whether activations change over post-natal development, then any age differences in arousal could contaminate what appear to be regional differences in stimulus-specificity.
Assessments of Stimulus-Specificity
It should be apparent from the previous section that simply recording from multiple NIRS channels is not a powerful way to assess stimulus-specificity at a given channel location. The presence of a stimulus likely leads to changes in attention or arousal that could elicit different activations in some NIRS channels, and these activations could be non-specific (i.e., systemic) but modulated by differences in the gain of the hemodynamic response at different channel locations. A more powerful design is to ask whether the same NIRS channel shows different levels of activation to variations along some stimulus dimension. If these stimulus variations can be assumed to avoid changes in arousal, then the differences in the gain of the hemodynamic response across channels is mitigated.
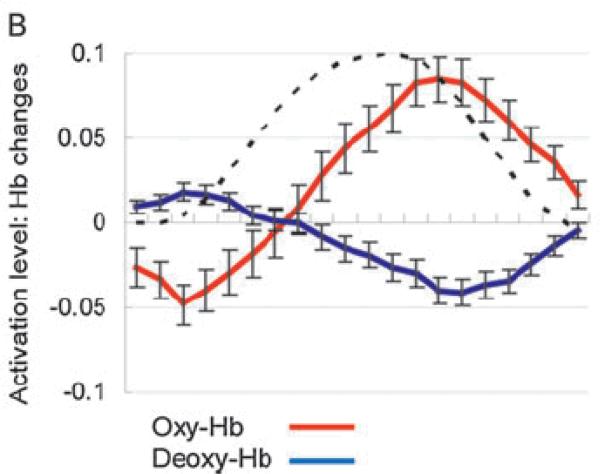
A canonical NIRS design consists of two blocks of stimuli (A and B) as well as a no-stimulus baseline. In a later section we will discuss the limitations of two-stimulus designs, but for the moment we focus on the power of such designs and how results should be (cautiously) interpreted. There are two general strategies employed in infant NIRS studies that utilize 2-stimulus designs: (1) a hypothesis-driven prediction based on adult data, or (2) an exploratory search for stimulus specificity and/or regional selectivity. Both of these strategies have their limitations. For example, the hypothesis-driven strategy makes it seductive to conclude, when there is a correspondence between infants and adults in how certain classes of stimuli activate the two hemispheres, that there is similarity of processing by these lateralized brain regions across development. That is a risky inference because, as noted earlier, evidence of differential activation across NIRS channels can only be interpreted as support for specialization if the gain of every NIRS channel's hemodynamic response function (HRF) is the same. This assumption is made in fMRI studies to deal with a whole-brain consisting of over 100,000 voxels, each of which is presumed to be minimally contaminated by systemic vascular responses and surface (non-brain) vasculature. In NIRS studies with only two-dozen or so channels, all of which are contaminated by surface vasculature, this uniform-gain assumption is extremely unlikely. Moreover, in fMRI the HRF can be measured empirically by presenting adults with a very brief stimulus and recording the resultant response in a low-level sensory area of the cortex (see Boynton, Engel, Glover & Heeger, 1996). Although in principle this same technique could be used to measure the HRF in single NIRS channels, this has only been estimated in one infant study using the Finite Impulse Response (FIR) method of Friston, Holmes, Worsley, Poline, Frith, and Frackowiak (1995). Minagawa-Kawai, van der Lely, Ramus, Sato, Mazuka, and Dupoux (2011) concluded that the HRF of 4-month-olds, at least in temporal cortex, has the same shape as the adult HRF, but is delayed 2.8 sec (see Figure 2).3 Because HRFs are likely to be different across brain regions, the mere presence of a lateralized response to the same stimulus is rather weak evidence of functional brain lateralization, unless it is accompanied by variations in stimulus-specificity. Despite these limitations, the hypothesis-driven strategy is preferable because of the multiple-comparison problem. As we will see later, when many NIRS activations are compared to chance, it is essential to rule out false-positives, and statistical corrections for multiple comparisons have not always been conducted appropriately.
Figure 2.
Estimates of the Hemodynamic Response Functions from the newborn temporal cortex (dashed line = adult HRF from fMRI). [Reprinted with permisson from Minagawa-Kawai et al., 2011].
The exploratory-search strategy is much more common in the infant NIRS literature. This is the natural outcome of knowing less about what to predict, especially since the infant brain may not be organized in the same way, or with the same specificity, as the adult brain. As a result, most of the infant NIRS literature consists of studies that conform to the following logic. First, activations are gathered simultaneously from many NIRS channels (e.g., 24) located over two or more regions of the cortex. Second, each infant is presented with two stimulus conditions (and a no-stimulus baseline). Third, activations in each channel are compared across stimulus conditions, and often clusters of channels are averaged to create regions of interest (ROIs). Fourth, significant differences present in individual channels or ROIs are interpreted as evidence of stimulus specificity and/or regional selectivity, often with reference to (a) prior behavioral or ERP studies of infants, (b) behavioral, ERP, and fMRI studies of adults, or (c) studies of animals (outside the language domain). This fourth and final step is the most problematic because the “explanation” of the obtained differences in NIRS activations is made post hoc.
Exploratory-search NIRS Studies: Audition
The vast majority of infant NIRS studies have investigated activations elicited by auditory stimuli, largely because these studies have been conducted with sleeping newborns, thereby reducing movement artifacts. However, there have been several studies of activations to visual stimuli, including simple checkerboards like those used by Meek et al. (1998), complex objects varying along various dimensions, and “social” events such as facial expressions. There is even a recent study of newborns’ differential response to the olfactory stimulus of their mother's breast milk compared to artificial (formula) milk (Aoyama, Toshima, Saito, Konishi, Motoshige, Ishikawa, Nakamura & Kobayashi, 2010). They reported greater activation over orbito-frontal cortex (implicated in odor perception by adults) to mother's milk than to formula milk. This study is illustrative of several limitations discussed earlier. For example, there were only two NIRS channels (right and left orbito-frontal locations), with no comparison channels to assess regional selectivity. Thus, the differential activation to the two odor stimuli could be an arousal effect and not a brain-specific effect (as previously defined).
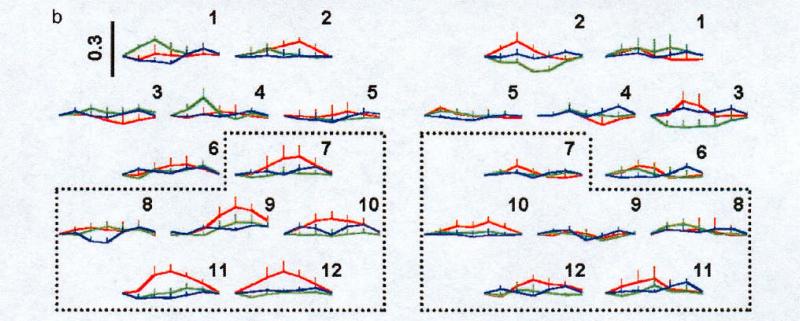
The first study to deal effectively with the stimulus-specificity and regional-selectivity issues for auditory stimuli was reported by Pena, Maki, Kovacic, Dehaene-Lambertz, Koizumi, Bouquet, and Mehler (2003). They presented newborns with two types of speech stimuli (forward and backward sentences) as well as a no-stimulus control (baseline). An array of 12 NIRS channels was positioned over the left temporal cortex and 12 channels over the right temporal cortex. Activations were greater to forward than to backward speech, but only over the left hemisphere. A more detailed analysis (see Figure 3), which defined two 6-channel ROIs above and below the Silvian fissure in each hemisphere, revealed that the left-hemisphere effect for forward versus backward speech was carried by the posterior ROI (i.e., below the Silvian fissure). Moreover, channel-by-channel comparisons between the two hemispheres showed that one channel in the left ventral-anterior temporal cortex was differentially activated to forward versus backward speech.
Figure 3.
Data from Pena et al. (2003), showing 12 NIRS channels from the left and right temporal cortices. Each channel provided a measure of oxyhemogloben (red), deoxy (blue, and total (green) to forward vs. backward speech. Dashed area in each hemisphere indicates targeted ROI (posterior temporal). [Reprinted with permission].
These are important findings for two reasons. First, forward and backward speech have the same average spectrum and are matched in intensity. Thus, the activation difference between stimulus conditions cannot be a low-level effect of arousal. Second, the activation difference between stimulus conditions was regionally selective (i.e., posterior channels in the left hemisphere), arguing against a more complicated “outcome of arousal” effect. There are, however, some interpretative limitations. Although speech is preferentially processed in the left hemisphere of the adult brain (in both frontal and temporal areas), similar activations in newborns does not confirm that same underlying processing. It could be that pathways from primary auditory cortex (A1 is too small to measure in isolation using NIRS) radiate to higher-level processing areas in the left temporal cortex for acoustic features that are “language relevant” and to the right temporal cortex for more general properties that are shared with language stimuli. And in fact two recent studies of newborns (Telkemeyer, Rossi, Koch, Nierhaus, Steinbrink, Poeppel, Obrig & Wartenburger, 2009) and 3- and 6-month-olds (Telkemeyer, Rossi, Nierhaus, Steinbrink, Obrig & Wartenburger, 2011) provide evidence of greater NIRS activations over right-hemisphere temporal cortex for slowly modulated acoustic events than for rapid events, which show bilateral activations (see also Minagawa-Kawai, Cristia, Vendelin, Cabrol & Dupoux, 2011). Thus, cortical areas in infants that will develop language specializations in adults could be viewed as proto-areas rather than as fully homologous to the adult brain.
Another concern with Pena et al. (2003) is that alternative ROIs were not tested. It is certainly acceptable to collapse NIRS channels based on an anatomical landmark (like the Silvian fissure), but there are a large number of alternative landmarks and other ROIs that were not examined. The proper way to test the significance of any ROI is to conduct a Monte Carlo procedure in which all possible ROIs of size N are examined, including channels that are not spatially adjacent. Nevertheless, the Pena et al. (2003) study is a landmark in providing convincing evidence of both stimulus specificity and regional selectivity, unconfounded by low-level arousal effects.
A follow-up to the Pena et al. (2003) study asked whether newborns would respond differently to native versus non-native language stimuli when they were presented in forward versus backward conditions. In contrast to the infants in Italy who were presented with forward and backward Italian sentences, May, Byers-Heinlein, Gervain, and Werker (2011) presented infants in Canada with forward and backward sentences in English (their native language) and Tagalog (a foreign language). The same ROIs used by Pena et al. (above/below the Silvian fissure) revealed no differences between activations for forward and backward speech in English, and no hemisphere differences, thereby failing to replicate the findings from Pena et al. for the infants’ native language. However, May et al. low-pass filtered their sentences, whereas Pena et al. did not. Thus, the segmental information was masked but the prosodic information was not, perhaps rendering the acoustic differences between forward and backward speech less salient. The results from Tagalog showed greater activation to backward than forward speech, a pattern opposite to what Pena et al. reported for the infants’ native language. But again, with low-pass filtered speech, the very different prosody of Tagalog compared to English may have resulted in primary attention to the different prosody and not the different directions of the speech signals. Sato, Hirabayashi, Tsubokura, Kanai, Ashida, Konishi, Uchida-Ota, Konishi, and Maki (2011) did not use filtered speech and replicated Pena et al. (2003) in Japanese infants presented with Japanese forward and backward speech, but not when Japanese infants were presented with English forward and backward speech. Thus, familiarity with native-language speech may be a requirement for the differential findings using forward and backward speech in newborns.
Several studies extended the work of Pena et al. (2003) by contrasting two types of forward speech: normally intonated and monotone. Unfortunately, these studies have failed to clarify our understanding of how the infant brain processes speech stimuli because the pattern of results does not conform to a simple role for the two hemispheres. Saito, Kondo, Aoyama, Fukumoto, Konishi, Kakamura, Kobayashi, and Toshima (2007) reported that newborns have a greater activation over frontal cortex to intonated versus monotone speech. However, the use of only a single NIRS channel renders these results subject to an arousal interpretation rather than a specific role for the frontal cortex in the processing of prosody.
Homae, Watanabe, Nakano, Asakawa, and Taga (2006) presented intonated and monotone speech to 3-month-olds and recorded 48 NIRS channels, 24 over the left-hemisphere temporal-parietal areas and 24 over the right-hemisphere areas. Consistent with data from adults, which suggest that segmental (typically rapid) speech cues preferentially activate the left hemisphere and prosodic (slow) speech cues preferentially activate the right hemisphere, they reported greater NIRS activation over the right hemisphere for intonated than for monotone speech.4 An identical follow-up experiment with 10-month-olds (Homae, Watanabe, Nakano & Taga, 2007) revealed exactly the opposite effect compared to the 3-month-olds: right-hemisphere channels showed greater activation to monotone speech than to intonated speech. The problem here is not that these studies failed to demonstrate stimulus specificity or regional selectivity, but rather that the pattern of results across development does not conform to any simple prediction about what these NIRS activations mean.
Homae et al. (2007) went to great pains to offer a variety of plausible explanations for the flip in activations between 3 and 10 months of age. Perhaps the 10-month-olds have a greater expectation (via listening experience) to hear infant-directed speech, thereby sending a violation of expectation signal (from frontal cortex) to the right temporal cortex. Perhaps 10-month-olds find it more difficult to process monotone speech and activate right-hemisphere temporal areas that are involved in processing intonation. Perhaps the right hemisphere is involved in processing the emotional content of speech signals and the monotone stimulus triggers a negative emotional response. All of these hypotheses are plausible, but they are also unverifiable in these two experiments. And they highlight a problem that is not unique to these two particular NIRS studies. The problem is the linking hypothesis between the level of NIRS activation and the underlying (inferred) psychological process that the activation is presumed to measure. If a study can report statistically significant effects without a prior constraints on what interpretation will be given to those results, then researchers are simply making a post hoc story about what they think the results could mean. In later sections, I will argue that we must move beyond these “just so” stories about our data and into a new regime where specific predictions, and their required linking hypotheses, are stated clearly before the data are collected. It is not clear what we learn from studies that compare two stimuli (speech and music) that differ on so many dimensions, making it nearly impossible to know what specific difference led to greater left-hemisphere activation for speech (e.g., Kotilahti, Nissila, Nasi, Lipiainen, Naponen, Merilainen, Huotilainen & Fellman, 2010). To be clear, this criticism is not unique to NIRS studies, to neuroimaging methods, or to studies of infants. Every dependent measure, whether behavioral, neural, or hemodynamic, provides the researcher with data that must be interpreted relative to some underlying assumptions about unobserved psychological processes (e.g., attention, learning, discrimination). The clearer the linking hypothesis between the data and the unobserved process, the more confidence we have in the interpretation. Behavioral and ERP studies of infants are not immune from these interpretative issues, nor are ERP or fMRI studies of adults.
Exploratory-search NIRS Studies: Vision
The first follow-up to the Meek et al. (1998) study, using a large number of NIRS channels and the simultaneous recording of activations over targeted and control regions of cortex, was published by Taga, Asakawa, Maki, Konishi, and Koizumi (2003). Using 12 NIRS channels over occipital cortex and 12 (control) channels over frontal cortex, Taga et al. presented 2- to 4-month-olds with a flickering checkerboard. The design also moved from the lengthy stimulus blocks (10-20 sec) used with auditory stimuli to 3-sec stimuli and jittered interstimulus intervals as in event-related fMRI designs. The predicted pattern of results was that occipital but not frontal channels would show significant activations. Those expectations were confirmed, thereby replicating Meek et al. within individual infants. The Taga et al. study also highlighted an important methodological issue that must be dealt with when using visual stimuli rather than auditory stimuli. Because the infants were awake rather than asleep, there was a greater incidence of movement artifacts. These movements were exacerbated during the no-stimulus (control) condition if no visual stimulus was available to maintain the infant's attention. Thus, a non-target (i.e., minimally interesting) visual stimulus was presented during the control periods. This means that NIRS studies using visual stimuli are minimally comparing two conditions (target stimulus vs. control stimulus), and referencing both activations to a short pre-stimulus baseline.
The Taga et al. (2003) study provided compelling evidence for regional selectivity, but minimal evidence for stimulus specificity. A subsequent study by Watanabe, Homae, Nakano, and Taga (2008) recorded 48 NIRS channels over expanded occipital-parietal and fronto-prefrontal regions as 3-month-olds viewed a flickering checkerboard, a complex motion (mobile) stimulus, and a non-target (dim fireworks) control. Consistent with expectations, a central region over occipital cortex showed reliable activations to both of the visual stimuli (checkerboard and mobile). However, the mobile stimulus showed a much more extensive pattern of activation across many channels over lateral occipital, prefrontal, and frontal areas compared to the checkerboard stimulus. And the checkerboard stimulus also activated a few channels in the pre-frontal areas. There were no significant lateralization effects. Watanabe et al. controlled for multiple comparisons by using the False Discovery Rate (FDR) method that is also used in fMRI studies (Benjamini & Hochberg, 1995; see excellent review in Ashby, 2011).
Although Watanabe et al. (2008) provided evidence of stimulus specificity (checkerboard vs. mobile), in addition to replicating and extending the regional selectivity of Taga et al. (2003), the pattern of regional selectivity was complex and subject to a variety of post hoc interpretations. Perhaps the lateral-occipital cortex (LOC) activations to the mobile but not the checkerboard are reflective of similar “object” responses in adult fMRI studies. Perhaps the greater temporal activation in the mobile condition results from the pairing of sounds with all of the visual events, but with infants having a greater expectation that mobiles and sounds will be paired than checkerboards and sounds (due to experience with mobiles in their homes). But why would both the checkerboard and the mobile elicit activations in prefrontal cortex? Is this a region that is involved with stimulus comparison, temporal expectation, or some other psychological process? Again, there are many plausible but untested post hoc explanations of these effects that can only be resolved by future hypothesis-driven experiments.
There are two additional lines of NIRS research with infants that employ visual stimuli, and both of them ask questions about the neural correlates of high-level cognitive and social-cognitive events. The first line of research presents 6-month-olds with a variety of object-occlusion events. Consider a red ball that moves behind an occluding surface and re-emerges on the opposite side of the box as an object with a different color (green ball) a different shape (red cube) or a change in both color and shape (green cube). Behavioral studies have shown that infants at this age are more attentive (surprised) by a change in the object during occlusion than by the absence of a change. However, this preference to attend longer to the change is more pronounced when the occluding surface is narrow than when it is wide. The explanation for this occluder-size effect is that a wide occluder could hide a second object that was hidden while the first object moved behind the occluder, whereas the narrow occluder could not accommodate both objects. Thus, the wide occluder event is “possible” whereas the narrow occluder event is “impossible”.
Wilcox and colleagues have conducted a series of four experiments to assess NIRS activations during these occlusion events. In all studies, two NIRS channels are located over the occipital cortex and reveal activations that do not differ by stimulus condition. These results suggest that the events are equally complex at the level of early visual cortex. Two additional NIRS channels over left temporal cortex reveal no difference in activations to the possible and impossible events (Wilcox, Bortfeld, Woods, Wruck & Boas, 2005), but significant differences if the object undergoes a shape or shape+color change during occlusion (the color change was marginally significant; Wilcox, Bortfeld, Woods, Wruck, & Boas, 2008). In addition, if the wide occluder (possible event) depicted the sudden jump of the object from one side to the other (rendering the event impossible), then this spatiotemporally impossible event elicited a temporal activation different from the control (possible) condition. Interestingly, possible events involving a change in shape+color elicited activations in more anterior temporal regions than impossible events involving spatiotemporal discontinuities (Wilcox, Bortfeld, Woods, Wruck, Armstrong & Boas, 2009). Finally, additional NIRS channels over parietal cortex revealed a complex pattern of temporal and parietal activations to changes in shape, color, spatiotemporal discontinuity, and invisible spatial displacement events (occipital activations remained invariant across all stimulus conditions; Wilcox, Haslup & Boas, 2010).
These four studies by Wilcox and colleagues involve many different levels of stimulus processing that could reflect a variety of underlying cognitive mechanisms. Low-level visual stimulation associated with each visual display, such as changes in local luminance and contrast, is reflected in the occipital activations. However, the temporal and parietal activations could reflect object tracking, motion perception, change detection, violation of expectation, visual working memory, or other components of cognition. As with Homae et al. (2006, 2007) in the speech domain, and Watanabe et al. (2008) in the visual object domain, teasing apart these alternative hypotheses awaits future experiments that control (or manipulate) all but one alternative explanation.
The second line of research that examines NIRS responses to high-level visual events involves the presentation of stimuli that contain a social or social-cognitive component. Grossmann, Johnson, Lloyd-Fox, Blasi, Deligianni, Elwell, and Csibra (2008) presented 4-month-olds with dynamic facial events depicting either mutual gaze or averted gaze. Sixteen NIRS channels were recorded over prefrontal and temporal locations on each hemisphere. Two channels (one frontal and one posterior temporal), both in the right hemisphere, showed differential activations to the two gaze conditions.
Minagawa-Kawai, Matsuoka, Dan, Naoi, Nakamura, and Kojima (2009) asked whether the infants own mother is responded to differently than another infant's mother. Four NIRS channels were recorded over the frontal cortex of 12-month-olds and results revealed greater activation to the infant's own mother's face, but only when the mother was smiling. The region showing the greatest mother-stranger effect was over the orbital-frontal cortex, which has been implicated in studies of positive emotional responses to social stimuli in adult fMRI studies.
Lloyd-Fox, Blasi, Volein, Everdell, Elwell, and Johnson (2009) presented 5-month-olds with two dynamic visual events – a face with occluding hands playing the peek-a-boo game, and a rotating three-dimensional multi-part object – as well as a low-salience visual baseline condition. Ten NIRS channels were collected from prefrontal-temporal-parietal locations on each hemisphere (20 channels total). Results showed greater activation to the dynamic face than to the dynamic non-face object in a subset of temporal and prefrontal channels. Because one of these temporal channels corresponds to the superior temporal sulcus (STS), which is known to be activated by biological motion and dynamic social stimuli in adult fMRI studies, it is seductive to conclude that the infant brain is organized in a similar stimulus-specific manner. However, there are many differences between the dynamic face and non-face stimuli, including their patterns of motion. Thus, it is not possible to know for certain that the social nature of the face event is driving this effect in 5-month-olds. A follow-up experiment by Lloyd-Fox, Blasi, Everdell, Elwell, and Johnson (2011) contrasted three different dynamic face events (moving gaze, moving mouth, moving hand) with the same dynamic non-face comparison event. Again, 5-month-olds were tested with 10 NIRS channels over prefrontal-temporal-parietal locations in each hemisphere. Results indicated a gradient of activations bilaterally, with prefrontal regions dominated by the moving hand stimulus, prefrontal-temporal regions by the moving gaze stimulus, and middle temporal regions by the moving mouth stimulus. Thus, the face-nonface comparison in Lloyd-Fox et al. (2009) cannot be the simple result of the presence/absence of facial features, as all three conditions in Lloyd-Fox et al. (2011) contained facial features but elicited regionally specific responses.
Exploratory-search NIRS Studies: Visual-auditory interactions
Another approach to the question of regional selectivity is to ask how NIRS activations are distributed over cortical areas known to be involved in particular sensory modalities. For example, the traditional view of primary sensory pathways is that they carry modality-specific information from the sensory receptors (e.g., retina for vision and cochlea for hearing) to the respective primary cortical areas (e.g., V1 for vision and A1 for hearing). If this separation of sensory information were present in the infant brain, then one would predict little or no cross-talk between modality-specific activations.
Bortfeld, Wruck, and Boas (2007) located two NIRS channels over occipital cortex and two channels over left temporal cortex while 5-month-olds were presented with visual stimuli on half the blocks and a combination of visual and auditory stimuli on the other blocks. The first prediction was that occipital channels would be activated in both conditions because a visual stimulus was present in each, and this prediction was confirmed. The second prediction was that temporal channels would only be activated in the visual+auditory condition and not in the visual-only condition. This prediction was partially supported – temporal channels were activated in the visual+auditory condition, but these same channels were negatively activated in the visual-only condition. This unexpected finding could be the result of “stealing” oxygenated blood from temporal areas when the visual-only stimulus was presented, or it could be the result of sluggish attentional switching from the multi-modal to the uni-modal conditions. In the absence of an auditory-only condition, these two possibilities remain unresolved.
Bortfeld, Fava, and Boas (2009) conducted a follow-up experiment with 6- to 9-month-olds who were presented with visual-only stimuli and visual+auditory stimuli, just as in Bortfeld et al. (2007). However, now NIRS channels were positioned over each hemisphere's temporal cortex, rather than over occipital and left-temporal cortices. Activations were reliable only over the left temporal channels in the visual+auditory condition. In contrast to Bortfeld et al. (2007), there was no significant negative activation over left temporal cortex in the visual-only condition. These results suggest that the left temporal cortex is specialized for speech processing and that there is minimal cross-talk between modalities.
Taga and Asakawa (2007) recorded 12 NIRS channels over each hemisphere's temporal cortex in 2- to 4-month-olds. Stimuli consisted of auditory (speech) and visual (flashing checkerboards), each of which was presented for 3 sec, but out of phase with each other (12 sec ISI for auditory; 15 sec ISI for visual). The auditory stimulus activated several temporal channels bilaterally, but the visual stimulus activated none of the temporal channels (the FDR correction method was used to control for multiple comparisons). These results are somewhat different from Bortfeld et al. (2007) in that visual-only stimuli did not lead to a decrease in temporal activations. However, Taga and Asakawa used shorter stimulus durations (3-sec vs. 20-sec), tested younger infants (2-4 months vs. 5 months), and did not present the auditory and visual stimuli synchronously.
One other study bears on the question of multimodal interaction, although it was not designed specifically for that purpose. As summarized earlier, Watanabe et al. (2008) presented 3-month-olds with two different visual stimuli (flickering checkerboard and dynamic mobile) and recorded NIRS activations over 48 channels in occipital-parietal and prefrontal-frontal areas. They presented a uniform auditory stimulus in synchrony with the onset of each visual stimulus (5-sec duration; 10-sec ISI). In a follow-up experiment, they increased the number of NIRS channels from 48 to 60 and focused their coverage on temporal and occipital cortex. Results indicated that both the occipital and temporal cortices, bilaterally, were activated by the combined visual+auditory stimuli, and that the two visual stimuli (checkerboard+sound and mobile+sound) elicited equivalent activations in both left and right hemispheres.
Hypothesis-driven NIRS Studies
The majority of infant NIRS studies employ an exploratory strategy of gathering activations from multiple channels elicited by the presentation or two or more stimuli. The goal is to determine whether there are differential activations to specific stimuli and to selective regions of the brain. The interpretation of these findings is largely post hoc, with a variety of plausible accounts consistent with the pattern of results. We turn now to the second major strategy: hypothesis-driven predictions about NIRS activations in infants. One limitation of exploratory studies is that they rely on properties of the infant brain that have been established prior to entering the experiment. Thus, unless the experimenter has clear evidence of early experiences attained by some infants and not by others, the only way to gain experimental control over the predicted NIRS activations is to have a strong hypothesis on which to base that prediction. These strong hypotheses have so far been limited to studies of discrimination, learning, and focal activation.
Studies of Discrimination
There is a long tradition of behavioral research with infants that relies on measures of preference, typically using looking time as the dependent variable (see review by Aslin, 2007). When infants show longer looking times to stimulus X over stimulus Y, that constitutes clear evidence of discrimination as well as preference. However, when stimulus X and stimulus Y are equally preferred, it is not clear whether that negative effect is the result of a failure to discriminate X from Y. It is quite possible for infants to discriminate two stimuli but not to prefer one over the other. The same interpretive limitation is present for NIRS activations. The absence of differential activation to stimulus X over stimulus Y does not of necessity imply that a given NIRS channel, and the brain region it samples, is incapable of processing the difference between X and Y. Looking-time studies progressed beyond simple preference paradigms to “entice” infants to exhibit a latent preference by repeating stimulus X and then asking if they prefer stimulus Y over stimulus X. This is the habituation paradigm, and in many cases it reveals the ability to discriminate X and Y when a preference test fails to do so.
It is surprising, in light of the extensive behavioral literature on infants’ discrimination abilities using the habituation paradigm, that it has been used in only a few NIRS studies (see Turk-Browne, Scholl & Chun, 2008, for a discussion of the parallels between behavioral habituation and fMRI adaptation). Minagawa-Kawai, Mori, Naoi, and Kojima (2007) asked whether infants could discriminate a speech contrast (vowel duration) that is used phonemically in their native Japanese language, and compared their performance to a non-phonemic contrast. They tested a broad age range (3- to 28-month-olds) using 4 NIRS channels over each hemisphere's temporal cortex. Each infant was presented with alternating 20-sec blocks of two types: a single repeating stimulus versus a random mixture of two stimuli. Each infant was tested in two sessions: with the phonemic contrast (discriminable by Japanese adults), and with the non-phonemic contrast (within-category and therefore not discriminable by Japanese adults). The prediction of this modified habituation paradigm is that infants who can discriminate the speech contrast should show greater activation to the mixed block than to the uniform block. That prediction was supported by a main-effect of block type (mixed > uniform), and it did not interact with whether the speech contrast was phonemic or not. In addition, the language-relevance effect (phonemic vs. non-phonemic) was present only in activations from the left hemisphere, and only in 3 of the 5 age groups (6-7 months, 11-14 months, and 25-28 month, but not 3-4 months or 10-11 months). The absence of the language-relevance effect in the youngest age group could be attributed to the subtle nature of the phonemic contrast, and the absence of the effect in the 10- to 11-month-old group was due to exceptionally high between-subject variance. These results, therefore, provide convincing evidence for the utility of the mixed versus uniform stimulus-block paradigm as a measure of discrimination, as well as for the development of sensitivity to language-relevant phonemic information.
A subsequent study by Sato, Sogabe, and Mazuka (2009) examined a different speech contrast in Japanese infants. Both 4- and 10-month-olds were tested with a pitch-accent contrast (high-low versus low-high) in a set of 14 words as NIRS activations were gathered from 12 channels over each temporal cortex. As in Minagawa-Kawai et al. (2007), each infant was presented with two types of blocks: uniform in which all 14 words had the same pitch-accent, and mixed in which half of the 14 words were high-low and the other half low-high, in random order. The uniform blocks were 20-25 sec long and the mixed blocks were 10 sec long. The prediction was that infants should show greater activation to the mixed than to the uniform blocks. The results from the 10-month-olds were consistent with this prediction: there was significantly greater activation to the mixed than to the uniform blocks. In addition, this discrimination effect was greater in the left hemisphere than in the right hemisphere. For the 4-month-olds there was also significantly greater activation to the mixed than to the uniform blocks, but there was no hemisphere difference. Sato et al. also presented the same high-low pitch contrast in a non-word context (pure-tones). Interestingly, the 10-month-olds did not show the same pattern of left-hemisphere enhancement of the mixed vs. uniform block-effect as they did for the word stimuli. The 4-month-olds showed no hemisphere effect, consistent with their performance with the word stimuli.
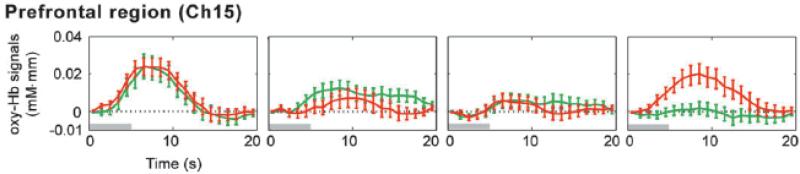
Nakano, Watanabe, Homae, and Taga (2008) extended these results from the domain of vowel duration and pitch contrasts to that of consonants by asking whether 3- to 4-month-old Japanese infants could discriminate a /ba/-/pa/ contrast. The experimental design was virtually identical to what has traditionally been used with behavioral measures. Each infant was presented with 15 trials during a habituation phase, with each 5-sec trial consisting of 10 repetitions of either /ba/ or /pa/ followed by 15 sec of silence. Infants were then presented with 5 post-habituation test trials, each consisting of 5-sec trials with a different repeating syllable (e.g., a shift from /ba/ to /pa/) or the very same syllable presented during the habituation phase (i.e., a no-shift control). Separate infants were assigned either to the experimental (shift) or to the control (no-shift) groups. During the habituation phase, infants in both groups showed widespread activations across most of the 48 NIRS channels, which were positioned over temporal and prefrontal cortices. Both groups also showed highly significant decreases over the 15 habituation trials (see Figure 4), consistent with behavioral measures such as looking time and sucking. The key question was whether infants in the experimental (shift) group showed increases in activation from the final set of habituation trials to the test trials, and whether these increases were absent (or less reliable) in the control (no-shift) group. Again, there was clear evidence of recovery of activation in a small set of NIRS channels (bilateral prefrontal) that was greater in the experimental group than in the control group.
Figure 4.
A single NIRS channel over prefrontal cortex from Nakano et al. (2008) showing decreasing activations in 3 blocks of 5 trials to a speech category, following by recovery to a novel speech category (orange) in the 4th block but not to a no-change control group (green). [Reprinted with permission].
The Nakano et al. (2008) study is an excellent example of hypothesis-driven research. The pattern of NIRS activations was not determined by pre-existing differences in the responsiveness of the infant brain, but rather to the repeated presentation of a particular speech stimulus (counterbalanced across infants) during the habituation phase of the experiment. Thus, the differences in NIRS activations cannot be due to acoustic differences per se, but must be due to the change in sensitivity to these acoustic differences induced by the habituation phase that preceded the test phase. It is interesting to note that all of the infants in this study were asleep, thereby providing clear evidence that a state of wakefulness is not required for infants up to 4 months of age to exhibit speech discrimination.
A final NIRS study of speech discrimination was reported by Arimitsu, Uchida-Ota, Yagihashi, Kojima, Watanabe, Hokuto, Ikeda, Takahashi and Minagawa-Kawai (2011). They presented sleeping newborns with two Japanese speech contrasts, one that was phonemic (vowel duration) and one that was prosodic (pitch rise/fall). Again, the uniform versus mixed design was used with 15-sec blocks. Twelve NIRS channels over each temporal cortex revealed significant activations to both the phonemic and prosodic contrasts. Although there was evidence of a right-hemisphere bias for the prosodic contrast (see Telkemeyer et al., 2009), there was no significant evidence of a left-hemisphere bias for the phonemic contrast, perhaps because this contrast did not involve rapid acoustic cues. The Arimitsu et al. study reported on individual NIRS channels as well as several ROIs. However, the rationale for choosing a particular ROI was based more on a visual inspection of the data than on an objective criterion. As noted earlier, ROIs should never be selected post hoc, but should be defined either by using anatomical or statistical (i.e., Monte Carlo) criteria.
Studies of Learning
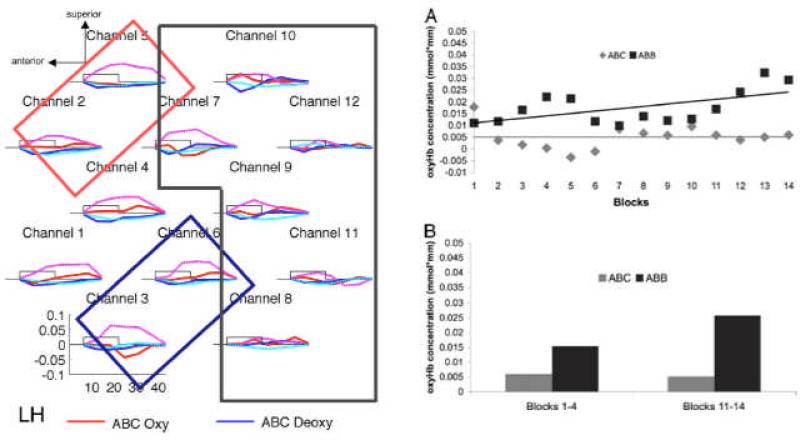
Another potential outcome of exposure to stimuli in the laboratory, that contrasts with habituation and recovery to a novel stimulus, is learning from the stimuli and showing an increase in activation across blocks. Gervain, Macagno, Cogoi, Pena, and Mehler (2008) asked whether newborns could discriminate between 3-syllable sequences that conformed to an ABB or an ABC pattern. Twelve NIRS channels were positioned over each temporal cortex, and alternating 18-sec blocks of ABB and ABC patterns, each involving a new set of syllables, were presented with 25-35 sec of silence between blocks. Results showed greater activation to ABB blocks than to ABC blocks from most channels, with a greater activation difference for these two patterns in the left hemisphere (see Figure 5). Most relevant to the question of learning, Gervain et al. also found that this activation difference between ABB and ABC was not significant in block 1, but became significant and increased over the 14 alternating blocks. Interestingly, this learning effect for the ABB pattern was present in the left frontal ROI, but not in the right frontal or either of the two temporal ROIs.
Figure 5.
Data from Gervain et al. (2008) showing left anterior responses and a learning effect. [Reprinted with permission].
Gervain et al. (2008) conducted a follow-up experiment that compared ABA patterns to ABC patterns to determine whether the immediate repetition of the B element in ABB patterns was easier for newborns to extract than the non-adjacent repetition of the A element in the ABA patterns. They found no evidence of differential activation to ABA and ABC patterns, and no learning effect across alternating blocks. Gervain, Berent, and Werker (2011) then asked whether the immediate repetition could be equally effective for learning in the initial syllables (AAB) as in the final syllables (ABB). Their results replicated the ABB versus ABC comparison in Gervain et al. (2008), with greater activation to AAB than to ABC and a bias for this differential effect to be stronger in the left hemisphere. Gervain et al. (2011) then asked whether newborns could discriminate early AAB from late ABB repetitions. Instead of alternating blocks of AAB and ABB, they used the mixture versus uniform design of Minagawa-Kawai et al. (2007). That is, half of the blocks consisted of either AAB or ABB patterns, and the other half of the blocks consisted of both AAB and ABB patterns. Newborns showed reliable evidence of discrimination, but the direction of the effect was unexpected, with greater activation elicited by the uniform blocks (i.e., AAB or ABB) than the mixture blocks (i.e., AAB and ABB). In a final experiment, they reverted to the alternating uniform-block design and contrasted AAB with ABB to determine whether early versus late repetition provided any advantage. However, there was no significant differential activation to the initial and final repetition patterns.
Unfortunately, in none of the follow-up experiments to Gervain et al. (2008) was there a learning effect over blocks. Thus, it is not clear if the initial report of this effect was spurious or if such learning effects are less robust when the patterns being contrasted are more difficult to discriminate. The comparison of ABB and ABC patterns was examined in 7- and 9-month-olds by Wagner, Fox, Tager-Flusberg, and Nelson (2011) to determine whether older infants would more robustly show the learning effects. They used the same stimuli and 12-channel arrays over left and right temporal cortex, but found no significant effects of pattern, hemisphere, or cortical ROI using the same oxy-hemogloben measure as Gervain et al. (2008, 2011). However, there were effects of pattern and hemisphere in the deoxy-hemogloben measure (which is typically small in magnitude and often unreported in infant studies; see Telkemeyer et al., 2009, 2011 for exceptions). The direction of deoxy-hemogloben responses is opposite to that of oxyhemogloben responses, and therefore the significant effect reported by Wagner et al. in the 7-month-olds was consistent with the newborns in the original Gervain et al. (2008) study – activations to ABB patterns were greater (negative deoxy) than to ABC patterns. However, this pattern was evident only in the right hemisphere, not the left hemisphere as in newborns (although it was present in frontal and not temporal ROIs, as in newborns). Moreover, this pattern reversed in the 9-month-olds – activations to ABC patterns were greater (negative deoxy) than to ABB patterns. Finally, there was a hint of a learning effect, with a significant reduction in the activations to the ABC pattern over blocks in both ages. These diverse results across age cannot be attributed to state of arousal (sleeping newborns vs. awake 7- and 9-month-olds) because the 7- to 9-month shift was present despite similar states of wakefulness.
Studies of Focal Activation
The foregoing studies of pattern learning have the distinct flavor of “the more we study X, the less we know about X”. Results that appear to be consistent within a given experiment do not replicate, or do not make a coherent story as more data are collected under similar conditions. Certainly one expects differences across age, but within an age one would hope that proposed explanations for effects are supported when the next experiment is conducted to examine that explanation. At present, it is very difficult to make specific predictions as one embarks on a new line of research, perhaps because we are trying to study complex cognitive phenomena before we fully understand what NIRS activations mean. That is, the field suffers from placing little emphasis on the specific linking hypothesis for how changes in oxy- or deoxy-hemogloben indicate an underlying perceptual, cognitive, or language process. In general, the history of infant NIRS studies is peculiar in that it began by tackling high-level cognition rather than focusing first on low-level (i.e., less interesting to some researchers) sensory and motor phenomena. The reason such a bottom-up strategy might have been more fruitful is that sensory and motor systems are well studied in adults and animals and they have considerable uniformity across age and species. Thus, the predictions one can make about these systems are quite constrained, thereby limiting the predicted outcomes if NIRS activations are valid measures of these low-level processes.
There are three domains within which specific predictions based on clear anatomical constraints have been made for infant NIRS studies: somatosensory cortex, visual cortex, and the intraparietal sulcus. Somatosensory cortex not only has a well-defined spatial organization, but pathways from the sensory periphery send information contralaterally to termination sites in somatosensory cortex. For example, tactile stimulation of the palm of the right hand activates the primary somatosensory cortex in the left hemisphere. The same contralateral organization holds for the motor cortex, leading many NIRS labs to use a finger tapping or tactile stimulus on the palm as a “sanity check” on the recording equipment. Surprisingly, there is only one such study of infants (Kusaka, Isobe, Miki, Ueno, Koyano, Nakamura, Nakamura, Konishi, Kuboi, Kato, Okubo, Yasuda, Nishida & Itoh, 2011). They stimulated sleeping newborns by passively moving the elbow or the knee while 12 NIRS channels were recorded over the right and left somatosensory cortex, and reported stronger contralateral than ipsilateral activations (although the statistics were poorly specified). These results are consistent with an earlier fMRI study of sleeping newborns using similar stimulation (Erberich, Panigrahy, Friedlich, Seri, Nelson & Gilles, 2006).
The second domain in which NIRS signals are constrained by sensory anatomy is the visual system. There is a longstanding sub-field of fMRI research that uses flickering checkerboards that are limited to portions of the visual field. Because any visual stimulus on the right half of the visual field (i.e., the two left hemi-retinas) projects only to the left primary visual cortex, and any visual stimulus on the left half of the visual field projects only to the right visual cortex, activations must conform to the timing of these visual stimuli. Many investigators have used this method to elicit fMRI activations in visual cortex while an adult fixates the center of a display. In the simplest case, a checkerboard fills either the right- or the left-half of the visual field. The expected pattern of activation is for the right-half stimulus to activate the left visual cortex, and the left-half stimulus to activate the right visual cortex. A more complex case is when the checkerboard fills only one quadrant of the visual field and rotates slowly (i.e., upper-right, lower-right, lower-left, upper-left). In this case the expected pattern of activation is a 180 deg phase-shift (i.e., inferior-left cortex, superior-left cortex, superior-right cortex, inferior-right cortex). These are precisely the patterns of activation that have been obtained from many fMRI studies (see Wandell, Dumoulin & Brewer, 2007).
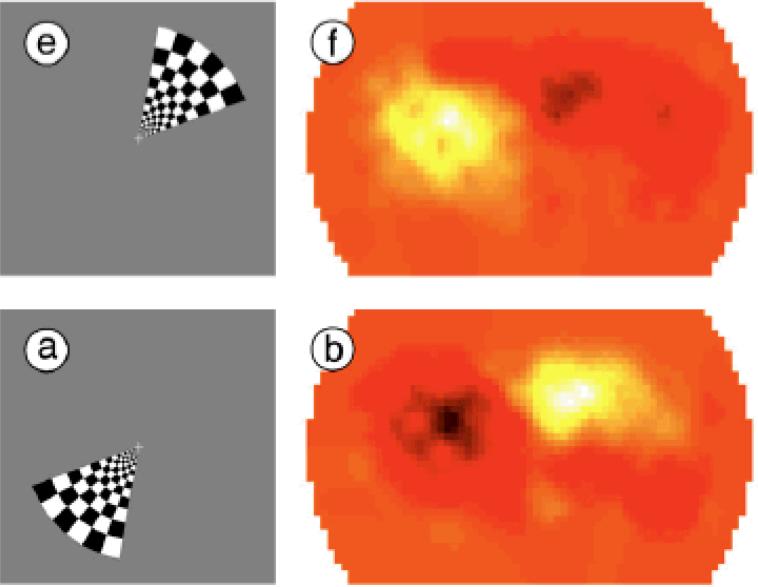
Two reports using NIRS in adults (Plichta, Heinzel, Ehlis, Pauli & Fallgatter, 2007; Zeff, White, Dehghani, Schlaggar & Culver, 2007) demonstrated that flickering checkerboards in subsets of the visual field activate NIRS channels over the occipital cortex in the same way as they activate fMRI voxels in the visual cortex. Moreover, adult NIRS activations show the characteristic 180-deg phase shift to a rotating checkerboard wedge (see Figure 6) or expanding checkerboard ring that have been obtained with fMRI (see review by White & Culver, 2010).
Figure 6.
Data from White and Culver (2010) gathered over occipital NIRS channels as a flickered checkerboard wedge rotates around the visual field. NIRS activations (right panels) are 180 deg out of phase with the location of the wedge stimulus. [Reprinted with permission].
A key requirement of these visual-field stimulation studies is for the subject to maintain central fixation while the flickering checkerboard is presented in the retinal periphery. Infants are likely to direct their gaze to this flickering checkerboard rather than attending consistently to a small central fixation target. However, pilot data from my lab suggest that this problem can be overcome. Custom software enables an eye-tracker (Tobii T60XL) to monitor the infant's gaze on-line and to move the central fixation target to compensate for gaze shifts into the periphery where the checkerboard is located. This is not a perfect image stabilization technique because there is a 100-200 msec lag in detecting gaze deviations from the central fixation target and moving the display accordingly to re-center it at the infant's current (eccentric) gaze position. If the infant's gaze moves off screen, then the display automatically re-centers itself on the screen before the checkerboard is re-introduced. We have gathered eye-tracking data from several 6-month-olds whose gaze was directed to the central fixation target for more than 50% of the 2-minute display. Thus, we are encouraged that retinotopic mapping of the visual cortex will be possible, at least for some infants, using this on-line gaze-centering paradigm and an array of NIRS channels over occipital cortex.
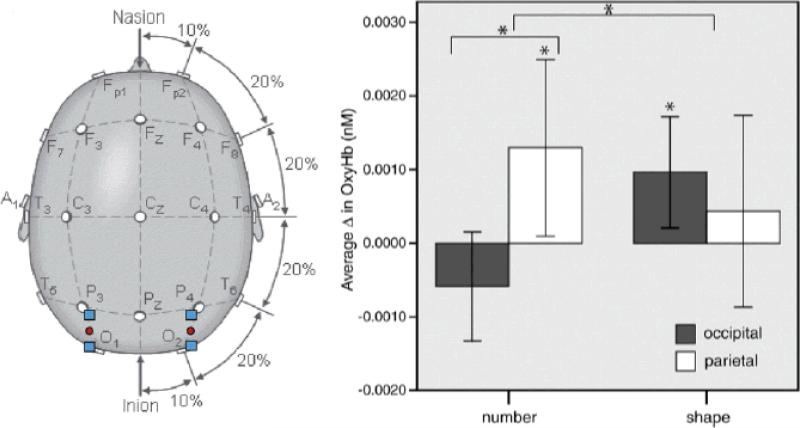
A third domain in which NIRS activations are constrained by prior knowledge of cortical anatomy is a region of parietal cortex specialized for numerosity judgments – the intraparietal sulcus (IPS). Based on extensive fMRI studies in children and adults, as well as single unit recording in animals, the IPS is uniquely activated when processing differences in number, even when there are correlated variations in element size, area, perimeter, and density (Cantlon, Brannon, Carter & Pelphrey, 2006). Hyde, Boas, Blair, and Carey (2010) used a focal array of 4 NIRS channels, two centered on bilateral IPS and two centered on occipital cortex (control). Six-month-old infants were presented with visual arrays consisting of 16 elements that were flashed for 500 msec, with an ISI of 500-1000 msec. Every 5-11 flashes, a novel number of elements (either 8 or 32) was presented for a single flash, thereby serving as an odd-ball event with respect to the more predictable base-rate of 16-element events. A separate control group was presented with the same set of displays, except that the odd-ball events consisted of the same number of elements but of a different shape (squares rather than circles). Greater activations to the odd-ball events, either number or shape, were only significant in the right hemisphere. Although both lateral-occipital areas are known to be responsive to shape changes, only the right IPS is responsive to number changes. This pattern of results was present in the infant NIRS data: right parietal activations were greater than right occipital activations for a change in number (see Figure 7), but there was no significant difference in left parietal and left occipital activations for a change in number. Moreover, right occipital activations differed from baseline for a change in shape (it is not clear why left occipital activations were not above baseline for a change in shape). These results from 6-month-olds closely mirror those obtained with fMRI from children and adults, suggesting that the right IPS is specialized for numerosity processing from a very early age. However, further control studies are needed, especially with a larger number of NIRS channels, to verify that right parietal channels located near the IPS are the only ones that show this effect.
Figure 7.
Placement of the NIRS channels in Hyde et al. (2010) and the resultant activations to changes in number or shape over right hemisphere occipital and parietal locations in 6-month-olds. [Reprinted with permission].
Questions that should be asked in the future
So far we have reviewed nearly 50 infant NIRS studies, many of which fall short of answering the questions that motivated their authors. Findings across studies are remarkably consistent and inconsistent at the same time. They are consistent in finding some channels that are activated by suprathreshold stimuli, but this is hardly surprising – surely there is some region of cortex that responds to just about any stimulus. The most important question one can ask about NIRS findings from infants is: what have we learned that we didn't know already from other measures? That is, what is the “value-added” of NIRS for studies of infant perception, cognition, and language? The answer, unfortunately, is rather modest. Almost all infant NIRS studies are largely confirmatory and fail to clarify the underlying neural mechanism, beyond specifying its regional selectivity.
Consider evidence that NIRS activations show hemisphere lateralization. If brain area X is activated by stimulus Y, and this regional selectivity mirrors what is present in adults, does this imply a similar functional role for area X? The problem with such an interpretation – that there is developmental continuity of regional brain function – is that hemodynamic responses are slow. Thus, they could reflect feed-forward activation (processing the stimulus), feedback activation (sending information from somewhere else), or some combination of the two (comparing inputs and stored representations). One clear way of overcoming this interpretive limitation is to present a stimulus that can only activate certain NIRS channels because of known anatomical constraints, but this research strategy, as noted earlier, is extremely rare in the literature (see Minagawa-Kawai, Cristia & Dupoux, 2011, for a developmental theory of lateralization).
A similar concern can be raised about stimulus specificity in a given brain region. Typically conclusions about stimulus specificity are weak – brain area X is activated by stimulus Y but not (or less so) by stimulus Z. But how specific is this pattern of activation – what stimuli other than Y activate area X, and what stimuli other than Z fail to activate area X? Clearly, there is a need to move away from 2-stimulus designs and focus on multiple levels along a particular stimulus dimension to get a better handle on the linking hypothesis between NIRS activation and underlying psychological processes. As noted by Aslin and Fiser (2005), when only two levels along a stimulus dimension are used, and one observes an increase (or decrease) in a dependent measure (e.g., NIRS activation), it is often concluded that the relevant NIRS activation indicates a brain region that represents that stimulus dimension. But if so, one would expect a third level along that stimulus dimension to show a further increase (or decrease) in activation. If that does not happen, then it calls into question the validity of the conclusion about what the NIRS activation means. In other words, it may be less important to know whether a given brain area is activated by a stimulus than to know that a change in a stimulus is correlated with a change in NIRS activation. This is because the assumption that brain areas are independent is very unlikely, leading to substantial “cross talk” across areas. The challenge here is to understand how these interactions among brain areas are organized, and to differentiate such interactions from measurement errors stemming from systemic hemodynamic responses.
Finally, developmental questions are rarely asked in infant NIRS studies or in any neuroimaging modality (see Karmiloff-Smith, 2010). Rather, a single age is often the target of a given study, which makes comparisons across studies difficult because of variations in stimuli, testing designs, probe placements, criteria for data rejection, signal processing, and statistical analysis. The best studies begin with robust behavioral evidence that ability X is absent at age 1 and present at age 2. Then one can ask if NIRS activation emerges between age 1 and age 2. If so, then it is seductive to conclude that the emerging NIRS activation mediates that behavioral change. But of course this correlation does not confirm causality. Moreover, if there is no change in neural activation between ages 1 and 2, then it suggests either an insensitive neural measure or a non-localist mechanism responsible for the developmental change.
There is a caveat to the foregoing picture of NIRS as a technique that has so far under-achieved on its promise of revealing brain mechanisms of infant development. One can view neural signals – ERP, MEG, fMRI, NIRS – as merely dependent measures and not as brain-specific per se. If one's goal is to find some measure of detection, discrimination, or learning, and NIRS provides such a reliable signal, then it has potential utility in answering questions about when during development an underlying process is present. Of course, the presence of a neural response does not necessarily imply that it is functional in supporting perception or influencing behavior. A reliable neural signal may be necessary but not sufficient to enable a neural network to rise above some critical threshold required to support behavior. If NIRS signals were more reliably present at a given age than any other dependent measure, and/or NIRS signals were easier to record from infants than these other measures, then it would be a clear win to use NIRS even with weak linking hypotheses between neural signals and behavior.
Prospects for technical advances
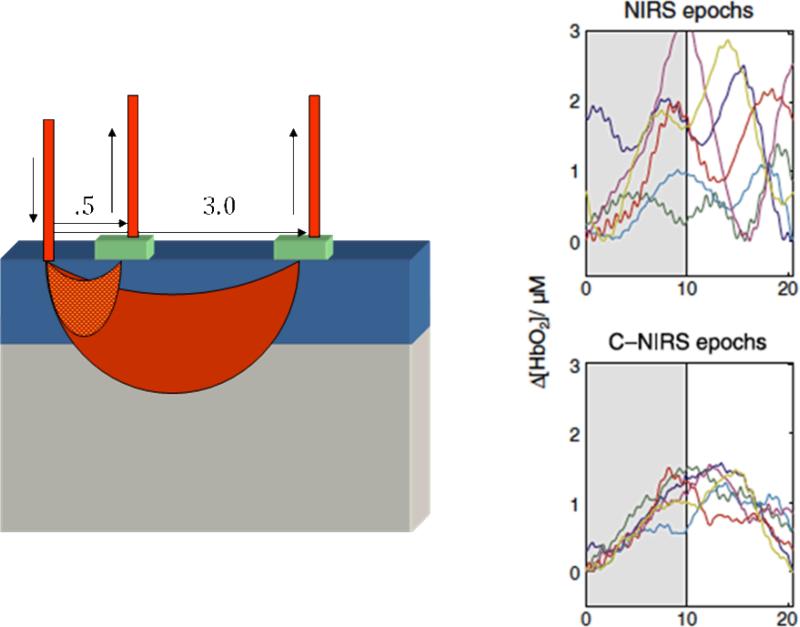
At least some of the foregoing problems can be reduced or eliminated by technical improvements in the reliability of NIRS signals. One such promising technique has been described by Saager and Berger (2005). They reasoned that because optical signals must pass through the surface vasculature to provide estimates of brain-based hemodynamic responses, it would improve the signal-to-noise ratio of the brain-based responses if the surface “noise” could be estimated. They placed a detector at a near-distance from the emitter to measure this surface vascular noise (see Figure 8) and used regression techniques to examine the residual signals after this surface noise was “subtracted off”. In simulations, subsequently verified by NIRS measurements from adults (Saager & Berger, 2008, 2011), they showed that this near-channel regression technique improved signal/noise by a factor of 3. Liao, Gregg, White, Zeff, Bjerkaas, Inder and Culver (2010) capitalized on this technique in a study of sleeping newborns who received visual stimulation (flashes) through their closed eyelids. Their results confirmed a 3-fold improvement in signal/noise, which also means that the same statistical power could be achieved in fewer than half the stimulus events.
Figure 8.
Configuration of near and far channel separations from Saager, Telleri, and Berger (2011) and the resultant reduction in variance for individual subjects in far-only vs. near-corrected C-NIRS activations. [Reprinted with permission].
A natural outcome of improved signal/noise in NIRS activations is greater use of event-related than blocked designs. This same trend occurred in the fMRI literature as pulse-sequences and gradient strengths were improved and 3T magnets replaced 1.5T magnets. Surprisingly few infant NIRS studies have employed event-related designs (see Hyde et al., 2010; Taga et al., 2003; and Watanabe et al., 2008 for exceptions).
Analysis techniques in the NIRS literature are surprisingly primitive compared to the fMRI literature. Only a few NIRS studies have used the standard GLM tools from fMRI (Minagawa-Kawai, van der Lely, Ramus, Sato, Mazuka & Dupoux, 2010; Obrig et al., 2010; Telkemeyer et al., 2011), despite modifications to widely used fMRI analysis packages such as SPM (Koh, Glaser, Flandin, Kiebel, Butterworth, Maki, Delpy, & Elwell, 2007).
Another potentially fruitful approach to studying brain development is to gather estimates of what in the fMRI literature is called functional connectivity. The basic idea is that voxels whose BOLD activations are highly correlated are somehow functionally related, even if the anatomical pathways that connect these voxels are quite indirect (i.e., multiple synapses away). There is evidence in adults that, in the absence of any task, clusters of voxels are correlated with other clusters of voxels, forming a so-called resting-state network of functionally connected modules (Raichle & Snyder, 2007). Two recent infant studies have demonstrated the feasibility of estimating functional connectivity using 94 NIRS channels, thereby providing coverage of most of the cortex. Homae, Watanabe, Otobe, Nakano, Go, Konishi, and Taga (2010) measured pairwise correlations among all 94 NIRS channels while newborns, 3-month-olds, and 6-month-olds were asleep. As in adults, correlations were clustered within hemispheres (e.g., occipital, temporal, prefrontal, etc.). More interesting, correlations were also clustered between hemispheres (e.g., left and right temporal), and there was a progressive increase in the consistency of these between-hemisphere correlations across age (see Figure 9).
Figure 9.
Functional connectivity among 94 NIRS channels during sleep gathered from newborns, 3-, and 6-month-olds by Homae et al. (2010). [Reprinted with permission].
The second infant study examined how functional connectivity changed between states of sleep and wakefulness. Studies of adults using fMRI have shown that resting-state functional connectivity is modulated when the state of wakefulness varies in adults (Larson-Prior, Zempel, Nolan, Prior, Snyder & Raichle, 2009), perhaps indicating that the network properties undergo reorganization to differentially recruit specialized neural computations (e.g., to process information that is task-relevant and to prepare and execute a motor response). Homae, Watanabe, Nakano, and Taga (2011) recorded 94 NIRS channels from 3-month-olds across three periods consisting of: no-stimulus, speech stimulation, and no-stimulus. Not only did they find similar resting-state connectivity in the initial no-stimulus period as in Homae et al. (2010), but during the speech stimulation period they found a substantial reorganization of functional connectivity, with greater ipsilateral connectivity between temporal and frontal areas (as well as some connectivity with occipital areas). Finally, in the third period when no stimulus was presented, there continued to be significant ipsilateral connectivity, suggesting that further processing of the stimulus-elicited activations during the second period continued to occur after the stimulus had ended. Taga, Watanabe, and Homae (2011) replicated all of these patterns of functional connectivity in 3- to 4-month-olds with pure-tone stimuli, suggesting that these effects are not specific to language. Although functional connectivity during sleep has been assessed using fMRI in 21-month-olds (Redcay, Haist & Courchesne, 2008), it has proven virtually impossible to gather fMRI data from awake children at this young age. The importance of the Homae et al. (2011) study is that it shows that NIRS can be used to measure both resting-state and stimulus-elicited functional connectivity in awake infants.
A final set of improvements in the reliability of NIRS will come from conducting test-retest studies in which the optodes are removed and repositioned on the infant's head. This has never been done in a systematic way and provides a crucial measure of the replicability of NIRS signals from particular channels in the same infant.5 Because the precision of repositioning the NIRS probes will be subject to some error, it will be essential to measure this error with an objective alignment system that is referenced to external landmarks on the infant's skull. In addition, there are systems that can provide a detailed measure of where the NIRS probes are located with respect to the infant's brain rather than relying on skull landmarks. A structural MRI of a sleeping infant provides a 3D image of the skull and the brain that can be mapped, in a subsequent testing session, with a stylus that documents the skull landmarks and the location of the NIRS probes on the skull (see https://www.rogue-research.com/TMS/Brainsight2.html).
Concluding Remarks
The present article has provided a summary of findings obtained from infants using NIRS. It is both exciting and sobering to review these findings. The excitement comes from the potential of a new non-invasive neuroimaging technique that does not require rigid head stabilization, has no acoustic noise, and can be purchased for a fraction of the cost of an MRI system. The sobering nature of this review is that the application of NIRS to address fundamental questions in perceptual, cognitive, and language development is still in the very early stages. Not only have very few studies assessed the reliability of NIRS activations to highly constrained (predictable) stimulus events, but the “value added” of NIRS data for understanding the organization of the infant brain or the mechanisms that lead to changes in behavior has so far been minimal. There are, however, improvements on the horizon that raise the prospect of solving many of the current limitations of NIRS research with infants, including the advances summarized in the previous section of this review.
It is with these encouraging prospects in mind that we conclude by describing a particularly compelling application of NIRS that addressed a fundamental question of functional brain organization. The parents of deaf children, over 90% of whom are hearing and therefore do not use a sign language, seek to restore auditory function in their child. It is now quite common for deaf children to receive a surgically implanted device that converts sound into electrical signals and transduces those signals onto the auditory nerve. These children who receive a cochlear implant often gain the ability to sense sound, but less reliably gain the full appreciation of speech, with highly variable language outcomes even after years of post-implant training (Peterson, Pisoni & Miyamoto, 2010). A key question is whether the implant, even if it stimulates the auditory nerve, transmits those peripheral neural signals to the auditory cortex. The implant precludes the use of fMRI to answer this question because the implant poses a safety hazard in the high magnetic field. Thus, NIRS provides an alternative neuroimaging method to address this question. Sevy, Bortfeld, Huppert, Beauchamp, Tonini, and Oghalai (2010) provided compelling evidence that young cochlear implant patients, even in the first few hours after the implant has been activated, receive inputs to auditory cortex. Moreover, by using bilateral NIRS probes, they discovered that the dominant cortical activation was in the ipsilateral temporal cortex. This finding runs counter to the expectation that the primary auditory pathway is contralateral, and it may change the accepted strategy of implanting the right ear because it is contralateral to the left hemisphere which is language-dominant. This study illustrates the power of using NIRS to address a fundamental question, and we can hope that such clear hypothesis-driven studies serve as a model for future NIRS studies of infants and children.
Acknowledgments
Preparation of this article was supported in part by grants from NIH (HD-37082) and the McDonnell Foundation (220020096) to the author, and from NSF (CBET-0931687) to Andrew Berger. Helpful comments on an earlier draft were provided by Andrew Berger, Mohinish Shukla, and two anonymous reviewers.
Footnotes
Direct measures of neural activity from surface electrodes on the scalp have been used with infants since the 1960s, but that voluminous literature is beyond the scope of the present review.
Systemic vascular responses can be measured directly using a pulse oximeter, but recent evidence from adults suggests that regressing out these global responses only marginally improves the signal/noise of fMRI (Cooper, Gagnon, Goldenholz, Boas & Greve, 2011).
Note that this delayed HRF in infants could be the result, at least in part, of the contaminating effects of surface vascular signals. Minagawa-Kawai et al. (2011) did not measure the adult HRF with NIRS to make a direct comparison with infants.
The statistical tests of hemisphere differences for the two stimulus conditions do not appear to have been corrected for multiple comparisons. However, even a conservative Bonferroni correction would render the most significant p-value of <.0005 equal to <.025.
Tsuzuki, Jurcak, Singh, Okamoto, Watanabe & Dan (2007) provided evidence, in adults, that external skull landmarks provide a reasonable alternative to structural MRI data for transforming NIRS probe locations to MNI space.
References
- Anderson AW, Marois R, Colson ER, Peterson BS, Duncan CC, Ehrenkranz RA, Konstantino M, Sarofin H, Schneider KC, Gore JC, Ment LR. Neonatal Auditory Activation Detected by Functional Magnetic Resonance Imaging. Magnetic Resonance Imaging. 2001;19:1–5. doi: 10.1016/s0730-725x(00)00231-9. [DOI] [PubMed] [Google Scholar]
- Aoyama S, Toshima T, Saito Y, Konishi N, Motoshige K, Ishikawa N, Nakamura K, Kobayashi M. Maternal breast milk odour induces frontal lobe activation in neonates: A NIRS study. Early Human Development. 2010;86:541–545. doi: 10.1016/j.earlhumdev.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Arimitsu T, Uchida-Ota M, Yagihashi T, Kojima S, Watanabe S, Hokuto I, Ikeda K, Takahashi T, Minagawa-Kawai Y. Functional hemisphere specialization in processing phonemic and prosodic auditory changes in neonates. Frontiers in Psychology. 2011;2(202) doi: 10.3389/fpsyg.2011.00202. [doi: 10.3389/fpsyg.2011.00202] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby FG. Statistical Analysis of fMRI Data. MIT Press; Cambridge. MA: 2011. [Google Scholar]
- Aslin RN. What's in a look? Developmental Science. 2007;10:48–53. doi: 10.1111/J.1467-7687.2007.00563.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslin RN, Fiser J. Methodological challenges for understanding cognitive development in infants. Trends in Cognitive Sciences. 2005;9:92–98. doi: 10.1016/j.tics.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B. 1995;57:289–300. [Google Scholar]
- Blasi A, Mercune E, Lloyd-Fox S, Thomson A, Brammer M, Sauter D, Deeley Q, Barker GJ, Renvall V, Deoni S, Gasston D, Williams SCR, Johnson MH, Simmons A, Murphy DGM. Early specialization for voice and emotion processing in the infant brain. Current Biology. 2011;21:1220–1224. doi: 10.1016/j.cub.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Bortfeld H, Fava E, Boas DA. Identifying cortical lateralization of speech processing in infants using near-infrared spectroscopy. Developmental Neuropsychology. 2009;34:52–65. doi: 10.1080/87565640802564481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortfeld H, Wruck E, Boas DA. Assessing infants’ cortical response to speech using near-infrared spectroscopy. NeuroImage. 2007;34:407–415. doi: 10.1016/j.neuroimage.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. Journal of Neuroscience. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantlon JF, Brannon EM, Carter EJ, Pelphrey KA. Functional imaging of numerical processing in adults and four-year-old children. PLoS Biology. 2006;4(5):e125. doi: 10.1371/journal.pbio.0040125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper RJ, Gagnon L, Goldenholz D, Boas DA, Greve DN. The utility of near-infrared spectroscopy in the regression of low-frequency physiological noise from functional magnetic resonance imaging data. NeuroImage. 2011 doi: 10.1016/j.neuroimage.2011.11.028. on-line Eprint version [doi:10.1016/j.neuroimage.2011.11.028] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science. 2002;298:2013–2015. doi: 10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- Erberich SG, Panigrahy A, Friedlich P, Seri I, Nelson MD, Gilles F. Somatosensory lateralization in the newborn brain. NeuroImage. 2006;29:155–161. doi: 10.1016/j.neuroimage.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Gervain J, Berent I, Werker JF. Binding at birth: The newborn brain detects identity relations and sequential position in speech. Journal of Cognitive Neuroscience. 2011 doi: 10.1162/jocn_a_00157. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervain J, Macagno F, Cogoi S, Pena M, Mehler J. The neonate brain detects speech structure. Proceedings of the National Academy of Sciences of the USA. 2008;105:14222–14227. doi: 10.1073/pnas.0806530105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervain J, Mehler J, Werker JF, Nelson CA, Csibra G, Lloyd-Fox S, Shukla M, Aslin RN. Near-infrared spectroscopy: A report from the McDonnell Infant Methodology Consortium. Developmental Cognitive Neuroscience. 2011;1:22–46. doi: 10.1016/j.dcn.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann T, Johnson MH, Lloyd-Fox S, Blasi A, Deligianni F, Elwell C, Csibra G. Proceedings of the Royal Society B, Biological Sciences. 2008;275:2803–2811. doi: 10.1098/rspb.2008.0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hespos SJ, Ferry AL, Cannistraci C, Gore J, Park S. Optical imaging on human infants. In: Roe AW, editor. Imaging the brain with optical methods. Springer; New York: 2009. pp. 159–176. [Google Scholar]
- Homae F, Watanabe H, Nakano T, Asakawa K, Taga G. The right hemisphere of sleeping infant perceives sentential prosody. Neuroscience Research. 2006;54:276–280. doi: 10.1016/j.neures.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Homae F, Watanabe H, Nakano T, Taga G. Prosodic processing in the developing brain. Neuroscience Research. 2007;59:29–39. doi: 10.1016/j.neures.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Homae F, Watanabe H, Nakano T, Taga G. Large-scale brain networks underlying language acquisition in early infancy. Frontiers in Psychology. 2011;2(93) doi: 10.3389/fpsyg.2011.00093. [doi:10.3389/fpsyg.2011.00093] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homae F, Watanabe H, Otobe T, Nakano T, Go T, Konishi Y, Taga G. Development of global cortical networks in early infancy. Journal of Neuroscience. 2010;30:4877–4882. doi: 10.1523/JNEUROSCI.5618-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde DC, Boas DA, Blair C, Carey S. Near-infrared spectroscopy shows right parietal specialization for number in pre-verbal infants. NeuroImage. 2010;53:647–652. doi: 10.1016/j.neuroimage.2010.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobsis FF. Noninvasive infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science. 1977;198:1264–1267. doi: 10.1126/science.929199. [DOI] [PubMed] [Google Scholar]
- Karen T, Morren G, Haensse D, Bauschatz AS, Bucher HU, Wolf M. Hemodynamic response to visual stimulation in newborn infants using functional near-infrared spectroscopy. Human Brain Mapping. 2008;29:453–460. doi: 10.1002/hbm.20411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmiloff-Smith A. Neuroimaging of the developing brain: Taking “developing” seriously. Human Brain Mapping. 2010;31:934–941. doi: 10.1002/hbm.21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh PH, Glaser DE, Flandin G, Kiebel S, Butterworth B, Maki A, Delpy DT, Elwell CE. Functional optical signal analysis: a software tool for near-infrared spectroscopy data processing incorporating statistical parametric mapping. Journal of Biomedical Optics. 2007;12:064010. doi: 10.1117/1.2804092. [DOI] [PubMed] [Google Scholar]
- Kotilahti K, Nissila I, Nasi T, Lipiainen L, Naponen T, Merilainen P, Huotilainen M, Fellman V. Hemodynamic responses to speech and music in newborn infants. Human Brain Mapping. 2010;31:595–603. doi: 10.1002/hbm.20890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotilahti K, Nissila I, Huotilainen M, Makela R, Gavrielides N, Noponen T, Bjorkman P, Fellman V, Katila T. Bilateral hemodynamic responses to auditory stimulation in newborn infants. NeuroReport. 2005;16:1373–1377. doi: 10.1097/01.wnr.0000175247.35837.15. [DOI] [PubMed] [Google Scholar]
- Kusaka T, Isobe K, Miki T, Ueno M, Koyano K, Nakamura S, Nakamura M, Konishi Y, Kuboi T, Kato I, Okubo K, Yasuda S, Nishida T, Itoh S. Functional lateralization of sensorimotor cortex in infants measured using multichannel near-infrared spectroscopy. Pediatric Research. 2011;69:430–435. doi: 10.1203/PDR.0b013e3182125cbd. [DOI] [PubMed] [Google Scholar]
- Larson-Prior LJ, Zempel JM, Nolan TS, Prior FW, Snyder AZ, Raichle ME. Cortical network functional connectivity in the descent to sleep. Proceedings of the National Academy of Sciences, USA. 2009;106:4489–4494. doi: 10.1073/pnas.0900924106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao SM, Gregg NM, White BR, Zeff BW, Bjerkaas KA, Inder TE, Culver JP. Neonatal hemodynamic response to visual cortex activity: high-density near-infrared spectroscopy study. Journal of Biomedical Optics. 2010;15:026101. doi: 10.1117/1.3369809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Fox S, Blasi A, Elwell CE. Illuminating the developing brain: The past, present and future of functional near infrared spectroscopy. Neuroscience and Biobehavioral Reviews. 2010;34:269–284. doi: 10.1016/j.neubiorev.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Lloyd-Fox S, Blasi A, Everdell N, Elwell CE, Johnson MH. Selective cortical mapping of biological motion processing in young infants. Journal of Cognitive Neuroscience. 2011;23:2521–2532. doi: 10.1162/jocn.2010.21598. [DOI] [PubMed] [Google Scholar]
- Lloyd-Fox S, Blasi A, Volein A, Everdell N, Elwell CE, Johnson MH. Social perception in infancy: A near-infrared spectroscopy study. Child Development. 2009;80:986–999. doi: 10.1111/j.1467-8624.2009.01312.x. [DOI] [PubMed] [Google Scholar]
- May L, Byers-Heinlein K, Gervain J, Werker JF. Language and the newborn brain: does prenatal language experience shape the neonate neural response to speech? Frontiers in Psychology. 2011;2(222) doi: 10.3389/fpsyg.2011.00222. [doi: 10.3389/fpsyg.2011.00222] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek JH, Firbank M, Elwell CE, Atkinson J, Braddick O, Wyatt JS. Regional hemodynamic responses to visual stimulation in awake infants. Pediatric Research. 1998;43:840–843. doi: 10.1203/00006450-199806000-00019. [DOI] [PubMed] [Google Scholar]
- Minagawa-Kawai Y, Cristia A, Dupoux E. Cerebral lateralization and early speech acquisition: A developmental scenario. Developmental Cognitive Neuroscience. 2011;1:217–232. doi: 10.1016/j.dcn.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa-Kawai Y, Cristia A, Vendelin I, Cabrol D, Dupoux E. Assessing signal-driven mechanisms in neonates: Brain responses to temporally and spectrally different sounds. Frontiers in Psychology. 2011;2(135) doi: 10.3389/fpsyg.2011.00135. [doi: 10.3389/fpsyg.2011.00135] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa-Kawai Y, Matsuoka S, Dan I, Naoi N, Nakamura K, Kojima S. Prefrontal activation associated with social attachment: Facial-emotion recognition in mothers and infants. Cerebral Cortex. 2009;19:284–292. doi: 10.1093/cercor/bhn081. [DOI] [PubMed] [Google Scholar]
- Minagawa-Kawai Y, Mori K, Naoi N, Kojima S. Neural attunement processes in infants during the acquisition of a language-specific phonemic contrast. Journal of Neuroscience. 2007;27:315–321. doi: 10.1523/JNEUROSCI.1984-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa-Kawai Y, van der Lely H, Ramus F, Sato Y, Mazuka R, Dupoux E. Optical brain imaging reveals general auditory and language-specific processing in early infant development. Cerebral Cortex. 2011;21:254–261. doi: 10.1093/cercor/bhq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T, Watanabe H, Homae F, Taga G. Prefrontal cortical involvement in young infants’ analysis of novelty. Cerebral Cortex. 2008;19:455–463. doi: 10.1093/cercor/bhn096. [DOI] [PubMed] [Google Scholar]
- Obrig H, Rossi S, Telkemeyer S, Wartenburger I. From acoustic segmentation to language processing: Evidence from optical imaging. Frontiers in Neuroenergetics. 2010;2(13) doi: 10.3389/fnene.2010.00013. [doi: 10.3389/fnene.2010.00013] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena M, Maki A, Kovacic D, Dehaene-Lambertz G, Koizumi H, Bouquet F, Mehler J. Sounds and silence: An optical topography study of language recognition at birth. Proceedings of the National Academy of Sciences of the USA. 2003;100:11702–11705. doi: 10.1073/pnas.1934290100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson NR, Pisoni DB, Miyamoto RT. Cochlear implants and spoken language processing abilities: Review and assessment of the literature. Restorative Neurology and Neuroscience. 2010;28:237–250. doi: 10.3233/RNN-2010-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plichta MM, Heinzel S, Ehlis A-C, Pauli P, Fallgatter AJ. Model-based analysis of rapid event-related functional near-infrared spectroscopy (NIRS) data: A parametric validation study. NeuroImage. 2007;35:625–634. doi: 10.1016/j.neuroimage.2006.11.028. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. NeuroImage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Redcay E, Haist F, Courchesne E. Functional neuroimaging of speech perception during a pivotal period in language acquisition. Developmental Science. 2008;11:237–252. doi: 10.1111/j.1467-7687.2008.00674.x. [DOI] [PubMed] [Google Scholar]
- Saager RB, Berger AJ. Direct characterization and removal of interfering absorption trends in two-layer turbid media. Journal of the Optical Society of America, A. 2005;22:1874–1882. doi: 10.1364/josaa.22.001874. [DOI] [PubMed] [Google Scholar]
- Saager RB, Berger AJ. Measurement of layer-like hemodynamic trends in scalp and cortex: implications for physiological baseline suppression in functional near-infrared spectroscopy. Journal of Biomedical Optics. 2008;13:034017. doi: 10.1117/1.2940587. [DOI] [PubMed] [Google Scholar]
- Saager RB, Telleri NL, Berger AJ. Two-detector corrected near infrared spectroscopy (C-NIRS) detects hemodynamic activation responses more robustly than single-detector NIRS. NeuroImage. 2011;55:1679–1685. doi: 10.1016/j.neuroimage.2011.01.043. [DOI] [PubMed] [Google Scholar]
- Saito Y, Kondo T, Aoyama S, Fukumoto R, Konishi N, Kakamura K, Kobayashi M, Toshima T. The function of the frontal lobe in neonates for response to a prosodic voice. Early Human Development. 2007;83:225–230. doi: 10.1016/j.earlhumdev.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Sakatani K, Chen S, Lichty W, Zuo H, Wuang Y. Cerebral blood oxygenation changes induced by auditory stimulation in newborn infants measured by near-infrared spectroscopy. Early Human Development. 1999;55:229–236. doi: 10.1016/s0378-3782(99)00019-5. [DOI] [PubMed] [Google Scholar]
- Sato H, Hirabayashi Y, Tsubokura H, Kanai M, Ashida T, Konishi I, Uchida-Ota M, Konishi Y, Maki A. Cerebral hemodynamics in newborn infants exposed to speech sounds: A whole-head optical topography study. Human Brain Mapping. 2011 doi: 10.1002/hbm.21350. Eprint on-line [DOI: 10.1002/hbm.21350] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Sogabe Y, Mazuka R. Development of hemispheric specialization for lexical pitch-accent in Japanese infants. Journal of Cognitive Neuroscience. 2009;22:2503–2513. doi: 10.1162/jocn.2009.21377. [DOI] [PubMed] [Google Scholar]
- Sevy A, Bortfeld H, Huppert T, Beauchamp M, Tonini R, Oghalai J. Neuroimaging with near-infrared spectroscopy demonstrates speech-evoked activity in the auditory cortex of deaf children following cochlear implantation. Hearing Research. 2010;270:39–47. doi: 10.1016/j.heares.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taga G, Asakawa K. Selectivity and localization of cortical response to auditory and visual stimulation in awake infants aged 2 to 4 months. NeuroImage. 2007;36:1246–1252. doi: 10.1016/j.neuroimage.2007.04.037. [DOI] [PubMed] [Google Scholar]
- Taga G, Asakawa K, Maki A, Konishi Y, Koizumi H. Brain imaging in awake infants by near-infrared optical topography. Proceedings of the National Academy of Sciences of the USA. 2003;100:10722–10727. doi: 10.1073/pnas.1932552100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taga G, Watanabe H, Homae F. Spatiotemporal properties of cortical haemodynamic response to auditory stimuli in sleeping infants revealed by multi-channel near-infrared spectroscopy. Philosophical Transactions of the Royal Society A, Mathematical, Physical & Engineering Sciences. 2011;369:4495–4511. doi: 10.1098/rsta.2011.0238. [DOI] [PubMed] [Google Scholar]
- Telkemeyer S, Rossi S, Koch SP, Nierhaus T, Steinbrink J, Poeppel D, Obrig H, Wartenburger I. Sensitivity of newborn auditory cortex to the temporal structure of sounds. Journal of Neuroscience. 2009;29:14726–14733. doi: 10.1523/JNEUROSCI.1246-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telkemeyer S, Rossi S, Nierhaus T, Steinbrink J, Obrig H, Wartenburger I. Acoustic processing of temporally modulated sounds in infants: Evidence from a combined near-infrared spectroscopy and EEG study. Frontiers in Psychology. 2011;2(62) doi: 10.3389/fpsyg.2011.00062. [doi: 10.3389/fpsyg.2011.00062] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzuki D, Jurcak V, Singh AK, Okamoto M, Watanabe E, Dan I. Virtual spatial registration of stand-alone functional NIRS data to MNI space. NeuroImage. 2007;34:1506–1518. doi: 10.1016/j.neuroimage.2006.10.043. [DOI] [PubMed] [Google Scholar]
- Turk-Browne NB, Scholl BJ, Chun MM. Babies and brains: Habituation in infant cognition and functional neuroimaging. Frontiers in Human Neuroscience. 2008;2:16. doi: 10.3389/neuro.09.016.2008. [doi: 10.3389/neuro.09.016.2008] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JB, Fox SE, Tager-Flusberg H, Nelson CA. Neural processing of repetition and non-repetition grammars in 7- and 9-month-old infants. Frontiers in Psychology. 2011;2(168) doi: 10.3389/fpsyg.2011.00168. [doi: 10.3389/fpsyg.2011.00168] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandell BA, Dumoulin SO, Brewer AA. Visual field maps in visual cortex. Neuron. 2007;56:366–383. doi: 10.1016/j.neuron.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Homae F, Nakano T, Taga G. Functional activation in diverse regions of the developing brain of human infants. NeuroImage. 2008;43:346–357. doi: 10.1016/j.neuroimage.2008.07.014. [DOI] [PubMed] [Google Scholar]
- White BR, Culver JP. Phase-encoded retinotopy as an evaluation of diffuse optical neuroimaging. NeuroImage. 2010;49:568–577. doi: 10.1016/j.neuroimage.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox T, Bortfeld H, Woods R, Wruck E, Boas DA. Hemodynamic response to featural changes in the occipital and inferior temporal cortex in infants: a preliminary methodological exploration. Developmental Science. 2008;11:361–370. doi: 10.1111/j.1467-7687.2008.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox T, Bortfeld H, Woods R, Wruck E, Armstrong J, Boas DA. Hemodynamic changes in the infant cortex during the processing of featural and spatiotemporal information. Neuropsychologia. 2009;47:657–662. doi: 10.1016/j.neuropsychologia.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox T, Bortfeld H, Woods R, Wruck E, Boas DA. Using near-infrared spectroscopy to assess neural activation during object processing in infants. Journal of Biomedical Optics. 2005;10:011010. doi: 10.1117/1.1852551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox T, Haslup JA, Boas DA. Dissociation of processing of featural and spatiotemporal information in the infant cortex. NeuroImage. 2010;53:1256–1263. doi: 10.1016/j.neuroimage.2010.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaramella P, Freato F, Amigoni A, Salvadori S, Marangoni P, Suppjei A, Schiavo B, Chiandetti L. Brain auditory activation measured by near-infrared spectroscopy (NIRS) in neonates. Pediatric Research. 2001;49:213–219. doi: 10.1203/00006450-200102000-00014. [DOI] [PubMed] [Google Scholar]
- Zeff BW, White BR, Dehghani H, Schlaggar BL, Culver JP. Retinotopic mapping of adult human visual cortex with high-density diffuse optical tomography. Proceedings of the National Academy of Sciences of the USA. 2007;104:12169–12174. doi: 10.1073/pnas.0611266104. [DOI] [PMC free article] [PubMed] [Google Scholar]