SUMMARY
Prostate cancer (PC) is the most common non-cutaneous malignancy affecting men in North America. Despite significant efforts, conventional imaging of PC does not contribute to patient management as much as imaging performed for other common cancers. Given the lack of specificity in conventional imaging techniques, one possible solution is to screen for PC specific antigenic targets and generate agents able to specifically bind. Prostate specific membrane antigen (PSMA) is over-expressed in PC tissue, with low levels of expression in the small intestine, renal tubular cells and salivary gland. The first clinical agent for targeting PSMA was 111In-capromab, involving an antibody recognizing the internal domain of PSMA. The second- and third-generation humanized PSMA binding antibodies have the potential to overcome some of the limitations inherent to capromab pendetide i.e. inability to bind to live PC cells. One example is the humanized monoclonal antibody J591 (Hu mAb J591) that was developed primarily for therapeutic purposes but also has interesting imaging characteristics including the identification of bone metastases in PC.
The major disadvantage of use of mAb for imaging is slow target recognition and background clearance in an appropriate timeframe for diagnostic imaging. Urea-based compounds such as small molecule inhibitors may also present promising agents for PC imaging with SPECT and PET. Two such small-molecule inhibitors targeting PSMA, MIP-1072 and MIP-1095, have exhibited high affinity for PSMA. The uptake of 123I-MIP-1072 and 123I-MIP-1095 in PC xenografts have imaged successfully with favorable properties amenable to human trials. While advances in conventional imaging will continue, Ab and small molecule imaging exemplified by PSMA targeting have the greatest potential to improve diagnostic sensitivity and specificity.
Keywords: Prostate specific membrane antigen, prostate cancer, molecular imaging, monoclonal antibody, single-photon emission computed tomography, positron emission tomography
INTRODUCTION
Prostate cancer (PC) is the most common non-cutaneous malignancy affecting men in North America. In 2011, approximately 240,890 patients were diagnosed with PC and 33,720 died from the disease in the United States [1]. The large majority of PC cases have clinically localized low-risk disease and high cure rates. The remaining patients present with advanced disease that is not completely characterized by standard-of-care clinical algorithms or conventional imaging. There is a considerable interest in developing an accurate non-invasive imaging biomarker that will ideally quantify aggressiveness, extent and burden of disease.
The role of imaging in PC is divided into detection of recurrent and/or metastatic disease and lesion localization [2]. Despite significant efforts, conventional imaging of PC does not contribute to patient management as much as imaging performed for other common cancers. In addition, these imaging tests yield little information to differentiate aggressive from indolent disease. The first post-diagnostic imaging test is often an extent-of-disease evaluation with magnetic resonance imaging (eMRI-endorectal coil) or computed tomography (CT) to evaluate the prostate and/or prostate bed, locoregional lymphadenopathy, solid organ, or bony involvement in high-risk patients. Bone scintigraphy with 99mTc-MDP or more recently, 18F-NaF is widely used as an adjunct for the detection of bone metastases. Positron emission tomography (PET) with fluorodeoxyglucose (FDG) has no role in early diagnosis of PC because of low and heterogeneous utilization of glucose by PC and it has a limited role in late stage cancers [3]. Other non-specific PET agents such as acetate and choline (11C and 18F-labelled) or MR-based nanoparticles, diffusion weighted imaging and spectroscopy may have a future role, however the performance of these agents remains to be determined in randomized controlled clinical trials.
ANTIGEN-BASED IMAGING
Given the lack of specificity in conventional imaging techniques, one possible solution is to screen for PC specific antigenic targets and develop agents capable of specific binding. In the case of PC, initial attempts began in the 1980s with monoclonal antibodies (mAbs) to prostate specific antigen (PSA) and prostatic acid phosphatase (PAP) [4]. While the relevance and specificity of these antigens is appropriate, PSA and PAP are secreted antigens precluding cell-associated antibody binding. Furthermore, the presence of PSA and PAP in plasma effectively blocks specific antibody binding at the tumor site.
One future direction in PC imaging involves the development of imaging biomarkers and the exploitation of existing biomarkers to improve the accuracy of detecting prostate disease at every stage. Recently, various markers of PC have been identified which includes cell surface proteins, glycoprotein, receptors, enzymes and peptides [5]. Prostate-specific membrane antigen (PSMA) is the most well established, highly specific prostate epithelial cell membrane antigen known [6–10]. Pathology studies have indicated that virtually all PC express PSMA [11–14]. The expression of PSMA increases progressively in higher-grade cancers, metastatic disease, and castration-resistant prostate cancer (CRPC) [8, 15–17].
PROSTATE SPECIFIC MEMBRANE ANTIGEN IMAGING
Despite its name, PSMA is expressed in other tissues including normal (benign) prostate epithelium, the small intestine, renal tubular cells and salivary gland [18, 19]. This “non-target” expression is fortunately 100–1000 fold less than baseline expression in PC [10]. Furthermore, antibodies generally do not cross intact basement membrane and tight junctions required to access these sites of non-PC PSMA expression. Unlike other prostate-specific antigens like PSA, PSMA is not secreted and is membrane bound [9]. The unique functional characteristics, prostate cancer specificity and antigenic access, makes PSMA an ideal extracellular target for various imaging and therapeutic agents.
MONOCLONAL ANTIBODY TARGETING OF PSMA EXPRESSION
PSMA has several optimal characteristics for targeting by antibodies. First, it is a highly expressed prostate-restricted non-secreted protein anchored to the plasma membrane. Second, its expression increases as tumor grade increases with concurrent increases in metastatic sites and CRPC [20]. In addition, the 19 amino acid cytoplasmic domain contains a novel MXXXL internalization motif resulting in its internalization and endosomal recycling which increases the deposition of conjugated radiometals into the cell. This last quality potentially improves both imaging and therapeutic efficacy [21].
PSMA - INTRACELLULAR EPITOPE IMAGING
The first clinical agent for targeting PSMA in PC was 111In-capromab [22]. It consists of a murine antibody 711E-C5.3 (mAb7E11) labeled with 111In. This mAb had affinity directed against the short intracellular epitope of the protein (amino acids 1–18) and was developed for pre-surgical staging and the evaluation of PSA relapse after local therapy. In pre-surgical patients with high-risk disease, but negative conventional imaging, capromab penditide was able to identify a subset of patients with occult local nodal disease. It was assumed that this upstaging of disease and sparing of unnecessary surgery would lead to diverging outcomes, but no studies have been performed to determine whether high risk patients with negative capromab scans fare better. In fact, capromab penditide scans fail to image bone metastases which are frequently the initial site of metastasis in 72% of patients on can assume a significant false negative rate in the setting of PSA relapse [23]. These findings highlight the main controversy with capromab detection. It has shown varied amount of efficacy with an average sensitivity and specificity of 60% and 70% respectively [24]. The poor efficacy associated with radionuclide imaging has been associated to binding of mAb7E11 to a receptor located inside the PC cell. Thus, only nonviable cells who have damaged cell membranes bind mAb7E11, which limits its use as a good imaging agent [25]. Capromab use in a SPECT study suggested that higher sensitivities can be obtained, but with persistent limitations in detection of bone metastases [22]. A promising next generation antibody (J591) that targets the extracellular domain of PSMA may provide significant benefits to the imaging of PC.
PSMA - EXTRACELLULAR EPITOPE IMAGING
The second- and third-generation humanized PSMA binding antibodies have the potential to overcome some of the limitations inherent to capromab pendetide. One example is the humanized monoclonal antibody J591 (hereafter referenced as J591) that was developed primarily for therapeutic purposes but also has interesting imaging characteristics including the identification of bone metastases in PC [26]. J591 has been studied extensively in preclinical models where is has demonstrated excellent binding characteristics and tumor-to-background signal in PC xenografts. It has been demonstrated that PSMA-specific internalizing antibodies such as J591 and J415 may be the ideal mAbs for the development of novel therapeutic methods to target the delivery of beta-emitting radionuclides, which include 131In, 90Y, and 177Lu for the treatment of PSMA-positive tumors [27]. In addition, J591 is specific to external domain of PSMA, thus targeting viable tumor. These immunoconjugates are better candidates for both imaging and targeted therapy than are antibodies targeting PSMA internally.
In addition to J591, three additional mAbs (3/A12, 3/E7 and 3/F11) have been characterized [28]. These three IgG mAbs bind to different epitopes of the extracellular domain and have slightly different pharmacokinetics, but all have some potential for future development [29]. These antibodies (3/A12 in particular) are labeled with 64Cu and have demonstrated good in vivo tumor-to-background rations required in a PET ligand [30]. Another new mAb, 3C6, targeting the extracellular epitope of PSMA has been labeled with 111In- for the imaging of PC xenografts and eventually patients in a clinical setting [31]. Furthermore, antibody fragments and minibodies are in development for immuno-PET imaging.
PSMA – SMALL MOLECULE INHIBITORS
The major disadvantage of use of mAb for imaging is slow target recognition and background clearance in an appropriate timeframe for diagnostic imaging. In general, radiopharmaceuticals that have thrived in the clinic have superior safety profiles, low radiation dose, and allow for administration and imaging in the same day. Based in part on homology to the PSMA receptors enzymatic moiety to NAALDase, Maresca et al described the design and synthesis of a series of small molecule inhibitors of PSMA with the potential to image PC with improved pharmacokinetics [32]. To this end, radiolabeled PSMA inhibitor N-[N-[(S)-1,3-dicarboxypropyl]carbamoyl]-S-[11C]methyl-l-cysteine (DCFBC) has been successfully used for PET imaging of human PSMA expressing xenografts [33]. This work has been extended by preparing and testing a PSMA inhibitor of the same class labeled with 18F [34]. Biodistribution and imaging studies showed high uptake of 18F-DCFBC in PSMA positive with little to no uptake in PSMA negative tumors. Urea-based compounds may also present promising agents for PC imaging with SPECT and PET [35]. Two such urea-based small-molecule inhibitors targeting PSMA, MIP-1072 and MIP-1095, have exhibited high affinity for PSMA [36]. The uptake of 123I-MIP-1072 and 123I-MIP-1095 in PC xenografts have imaged successfully with favorable properties amenable to human trials.
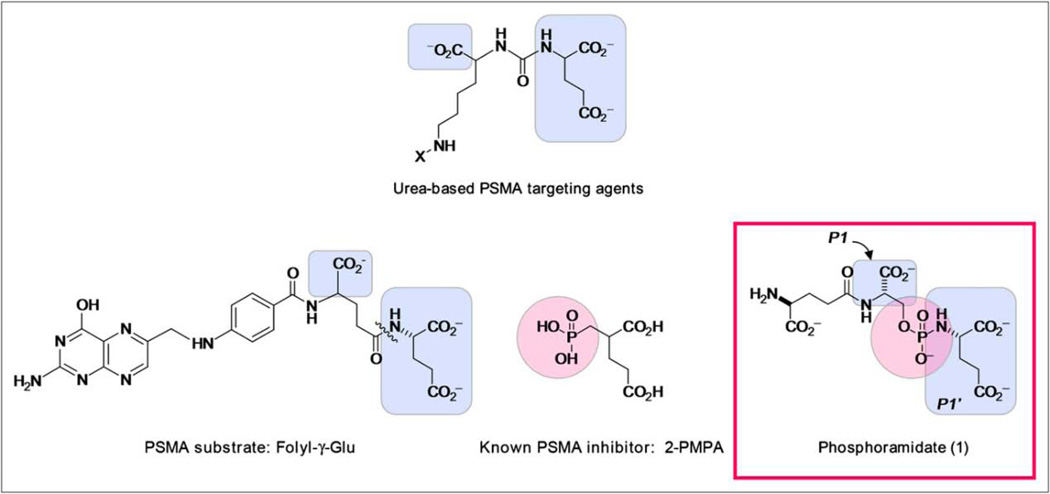
Functionally, PSMA is a proteolytic enzyme with high affinity to γ-glutamyl folic acid derivatives and N-acetylaspartylglutamate, as well as dipeptides similar to these compounds. Another class of PSMA inhibitors was created by utilizing and editing the above reference dipeptide motif and systematically pruning the molecule to pseudo-irreversibly bind to PSMA (Figure 1).
FIGURE 1.
Structural elements of known PSMA substrate and inhibitors, compared with phosphoramidate (1). Highlighted portions indicate structural features similar to phosphoramidate design.
Permission granted. THE JOURNAL OF NUCLEAR MEDICINE Vol. 50 No. 12 December 2009
These phosphoramidates localize, bind and internalize in PSMA-positive cells in vitro and have been fluorinated to function as a PET tracer in a murine xenograft model, and biodistribution data in murine xenografts has been reported [37].
CLINICAL ROLE OF PSMA TARGETED IMAGING
111IN-CAPROMAB IMAGING OF METASTATIC DISEASE
The initial excitement following capromab imaging was that it would detect sites of soft tissue primary disease and help in pre-surgical staging following biochemical relapse. The following clinical studies were designed in the context of standard-of-care management to assess performance in defined cases where the sensitivity, specificity and positive/negative predictive value could be ethically determined.
In a clinical trial radioimmunoscintigraphy localized residual or metastatic PC in 15 patients after prostatectomy and lymphadenectomy for PC with rising serum PSA. All patients had negative pre-study radiographic abdominal and pelvic cross-sectional images, and there were no adverse effects related to 111In-capromab pendetide infusion and little human antimouse antibody response [38]. An additional study with 7E11 radiolabeled with 111In and therapeutic nuclide 90Y demonstrated a similar relationship with conventional imaging in patients with known metastatic PC [39].
111IN-CAPROMAB IN PATIENTS WITH BIOCHEMICAL RELAPSE AND NEGATIVE CONVENTIONAL IMAGING
Although 111In-capromab failed to detect many of the bone scan positive lesions and CT positive soft tissue lesions, there are somewhat counter-intuitive successes of capromab in the setting of otherwise negative imaging. These studies include patients who have a lower burden and prevalence of disease. The main two clinical settings are presurgical staging and postsurgical PSA relapse. In the presurgical studies, capromab and surgical pathology of resected lymph nodes were compared with no attempt to identify possible bony lesions. In studies on high-risk patients (high pre-surgical PSA, high Gleason score/clinical stage) capromab’s performance was significantly better than CT scans. In this study, 152 patients (64/152 with positive nodes on pathology) capromab scans showed a sensitivity of 62%, sensitivity of 72%, PPV of 62%, NPV of 2% and an overall accuracy of 68% [40]. In comparison, CT had sensitivity of 4% and specificity of 100%. Interestingly, the 62% sensitivity in these soft tissue lesions that are too-small-to-characterize lesions on CT/MR is similar to the sensitivity is the large lesions in the MPC studies. This would suggest that the main indication for 111In-capromab is to detect diminutive soft tissue lesions. Once the lesions are large or within the bones, the advantage disappears as anatomic imaging becomes more relevant. Improved visualization of these scintigraphic findings by improved radiotracer detection or a mAb affinity would increase the relevance of PSMA imaging dramatically.
111IN-CAPROMAB IN EXTENT-OF-DISEASE ANALYSIS
The second relevant clinical setting for capromab imaging is distinguishing local versus systemic extent-of-disease in patients with a PSA relapse after radical prostatectomy. Approximately 30% of patients develop PSA relapse following prostatectomy face the clinical dilemma of whether to initiate salvage external beam radiotherapy (EBRT) to the prostate bed or opt for systemic therapy. This quandary exists because to date there is no reliable way to determine extent-of-disease on relapse in PC.
In part because of the availability of PSA to reliably monitor for early recurrence, unlike many other cancers, MRI and CT +/− PET are usually not the initial diagnostic test detecting recurrence and are unreliable modality, determine the extent of disease. In a study of 32 men with residual biochemical evidence of disease after radical prostatectomy, Kahn et al used capromab scans to attempt to identify men most likely to have EBRT-induced PSA response [41]. Capromab scans demonstrated metastasis in 9/32 (28%) with disseminated disease and 23/32 with local disease. Of the patients with local disease, 61% had a durable EBRT response while only 22% with disseminated disease had a similar response. This result was highly suggestive of a role for capromab for extent-of-disease selection. However, the size of the cohorts and questions about how similar the groups of responders and non-responder were, continue to plague this study. Another, study by Levesque et al. produced similar results suggesting that capromab is useful in selecting patients for salvage EBRT [42]. Unfortunately, other studies have been contradictory. In Wilkenson’s study, 42 patients had rising PSA levels after prostatectomy and 15/42 had limited disease. Unlike the prior studies, only 7/14 (42%) had a durable PSA response at followup [43]. Similarly, Thomas et al conducted a study with 192 patients. Thirty of them received salvage radiotherapy (RT) but there was no statistically significant difference between the findings of the capromab scan and the likelihood of responding to salvage RT [44].
111IN-CAPROMAB SPECT/CT IMAGING
Recent studies have focused on the use of 111In-capromab SPECT/CT fusion imaging and/or fusion of SPECT images with contemporaneous MRI to enhance lesion detection in PC [45]. Schettino et al performed 58 capromab scans and compared the findings of the capromab only to the capromab-MR/CT fusion to determine whether greater accuracy is conferred [46]. The study revealed a significant difference in the findings of 47% of the patients (27/58). Interestingly, 46% were re-classified as negative, uncovering a high false positive rate rather than decreasing the known false negative rate. Sodee et al suggested that with experience in over 600 cases and a detailed case report of 5 patients this technique is likely to improve the high false negative rate, but there is scant pathology proven evidence to the contrary [47]. Using the fusion techniques Ellis et al have reported a sensitivity of 79% and specificity of 80% when the capromab-CT [48].
CLINICAL TRIALS AND FUTURE PROSPECTS
J591 IMAGING
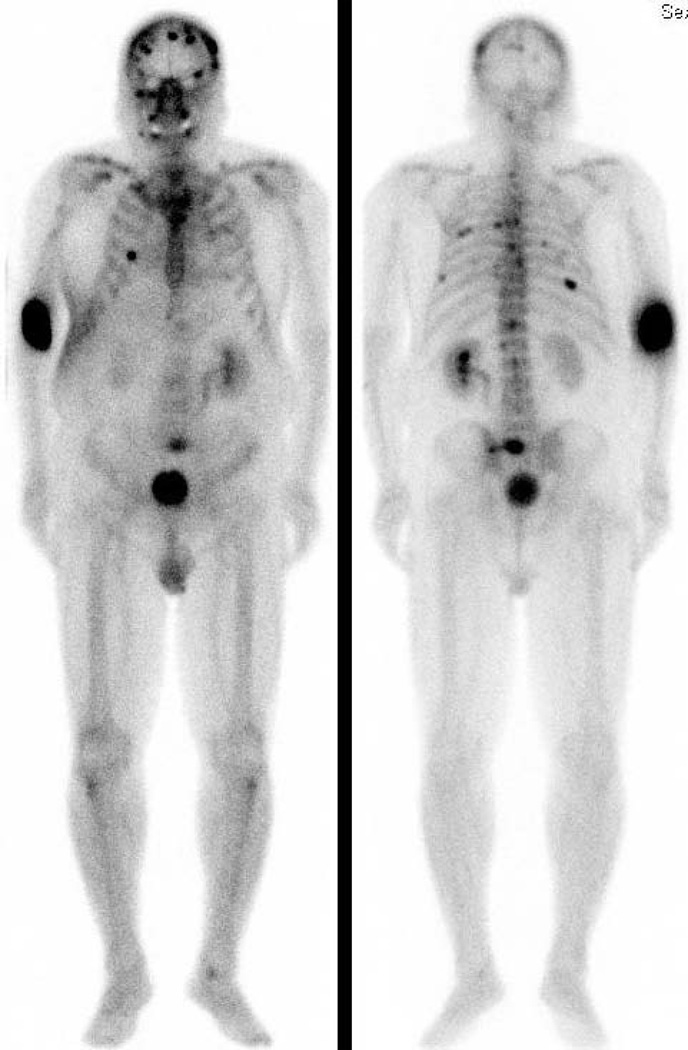
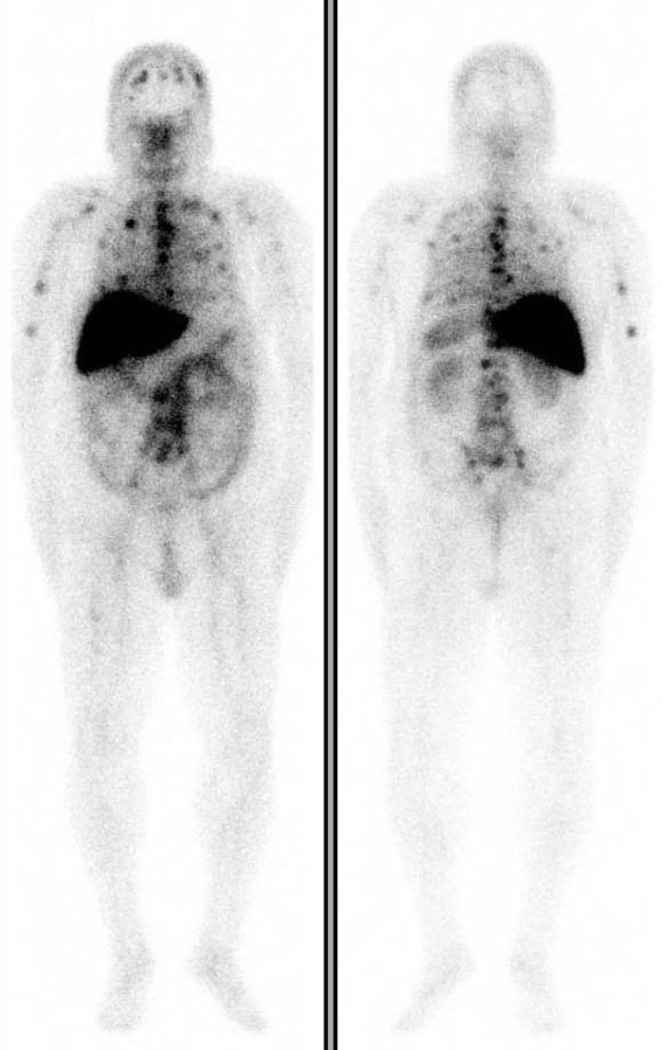
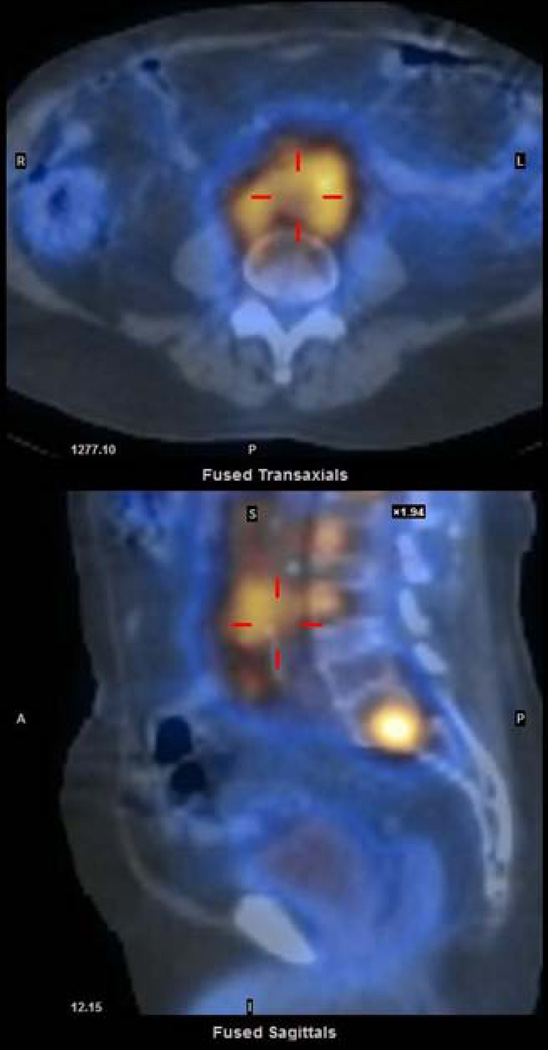
While no formal prostate imaging studies of humanized J591 have been conducted, two independent phase I therapeutic trials have been completed where imaging was performed. The primary goal of these trials was to define the maximum tolerated dose of the therapeutic nuclides 90Y and 177Lu conjugated to J591. In these trials, imaging was performed to assess antibody targeting of known sites of metastases seen on conventional imaging. J591 imaging has demonstrated superior targeting compared to historical capromab penditide controls. In the initial phase I study, 177Lu-J591 was able to target (image) all known sites of disease in all treated subjects [49] A subsequent phase II study demonstrated 94% tumor targeting [26]. 111In-J591 imaging prior to 90Y-J591 treatment revealed 89% of known bony lesions and the majority (69%) of soft-tissue lesions [50]. In a few selected cases, J591 demonstrated lesions that were not apparent on the bone scan but were identified on MR or conventional imaging as the lesion progressed [51]. (Figure 2) SPECT images have confirmed both osseous as well as soft tissue uptake (Figure 3).
FIGURE 2.
(A) Anterior and Posterior planar 99mTc-MDP Bone Scan demonstrating multifocal osseous metastasis.
(B) Anterior and Posterior planar 177Lu-huJ591demonstrating excellent tumor targeting to sites clearly seen on the bone scan and a few that are not clearly identified on bone scan. Abdominopelvic uptake was suspicious for soft tissue metastasis.
FIGURE 3.
Axial (top) and sagittal (bottom) reconstruction after treatment dose of 177Lu-J591 SPECT/CT demonstrating localization in retroperitoneal lymph nodes as well as lumbar vertebrae.
In a recent retrospective review of the initial decade of experienced with radiolabeled J591 has revealed that it targets 86.4% of known lesions on planar imaging [52]. As all of the described work has utilized SPECT and therapeutic nuclides, the next generation of J591 imaging will require the conjugation of a PET nuclide such as 89Zr as exhibited in a murine model by Holland et al. [53]. Other PSMA antibodies have been conjugated with a PET nuclide as was done in Regino et al, but huJ591 currently is the lead agent as the antibody has extensive safety data in human subjects and has been deimmunized [31].
89Zr-DFO-labeled mAbs show exceptional promise as radiotracers for immunoPET of human cancers. 89Zr-DFO-J591displays high tumor-to-background tissue contrast in immunoPET and can be used to delineate and quantify PSMA-positive PC in vivo [53].
In patients with PC, a positive surgical margin is associated with an increased risk of cancer recurrence and poorer outcome, yet the margin status cannot be reliably determined during the surgery. Recently, an activatable mAb–fluorophore conjugate consisting of a hu-J591 linked to an indocyanine green (ICG) derivative was used as a tracer. After binding to PSMA an 18-fold activation was observed permitting the specific detection of PSMA+ tumors up to 10 days after injection of a low dose (0.25 mg/kg) of the reagent [54]. This agent demonstrates a promising in-vivo method to image the extent of PC and can assist with real-time resection of extracapsular extension of tumor and positive lymph nodes.
As PSMA expression is downstream of the androgen receptor (AR), J591 has also shown potential as an imaging agent to predict changes in AR signaling after MDV3100, abiraterone, or other AR-targeted therapeutics. Relative changes in PSMA expression levels can be quantitatively measured using a human-ready imaging reagent and could serve as a biomarker of AR signaling to noninvasively evaluate AR activity in patients with CRPC. The changes are measured in vivo in human PC xenograft models through PET imaging using 64Cu-J591 [55].
SMALL MOLECULE INHIBITORS
In vitro biochemical studies of MIP-1072 and MIP-1095 demonstrated that they inhibit NAALADase activity in lysates from PSMA expressing tumors. Binding studies with intact PSMA-expressing cells demonstrated that both 123I-MIP-1072 and 123I-MIP-1095 exhibit saturable and competitive binding. In contrast, no binding was observed in cells that do not express PSMA. Furthermore, a time- and temperature-dependent increase in cell association of MIP-1072 and MIP-1095 indicated internalization via endocytosis.
A series of high affinity radiolabeled PSMA inhibitors have been developed that localize specifically to PSMA-avid PC in preclinical models, two of which were shown to detect both bone and soft tissue metastases in PC patients. These radiopharmaceuticals, which are currently in clinical trials, may be valuable for patient management including the diagnosis, staging and potential treatment of PC [56]. In initial Phase 1 clinical trials in patients with histologically confirmed metastatic PC, 123I-MIP-1072 and 123I-MIP-1095 detected both bone and soft tissue PC metastases at 1–4 hours post-injection.
We have recently evaluated novel 99mTc-labeled small molecule inhibitors of the enzymatic domain of PSMA. Preclinical studies with PSMA positive LNCaP cells and xenografts demonstrate that these compounds (99mTc-MIP-1404 and 99mTc-MIP-1405) bind to PSMA with high affinity. In early Phase I human studies, these molecules localize in tumors rapidly and identified a greater number of lesions than bone scans and rapidly detected soft tissue PC lesions including sub-cm lymph nodes [57]. Given the apparent high sensitivity of these agents, future work is planned in patients with high risk localized PC to more accurately assess the sensitivity/specificity of this agent for occult disease.
CONCLUSION
Imaging is an emerging component of diagnostic and therapeutic management of PC. While advances in conventional imaging will continue, Ab and small molecule imaging exemplified by PSMA targeting have the greatest potential to improve diagnostic sensitivity and specificity. To date, the most successful targeted PC imaging is demonstrated with PSMA.
111In-Capromab remains the only FDA-approved imaging agent for PC imaging, but indirect evidence demonstrates clear inferiority to the multiple investigational PSMA-targeted agents. Its inability to image bone lesions, which is a common and early site of metastatic spread, is hindrance to clinical metrics and the agent’s future development.
Early experience with a mAb to the extracellular domain of PSMA confirms that an Ab to an extracellular epitope will have superior in vivo detection of tumor although there is no data available to compare these entities. Ultimately, a direct comparison of 111In-Capromab and 111In-huJ591 on the same patients contemporaneously will be required to establish the superiority of the agent. Ideally, the next step will be a direct comparison of 111In-huJ591 and 89Zr-J591 to determine whether immunoPET confers greater lesion detection and ultimately gives quantitative information about tumor targeting which has been indirect to-date. When whole Ab imaging is optimized in human subjects the questions in the future will likely include a comparison between whole Abs and small molecule agents, which is more practical for clinical use, has better imaging characteristics and is better suited to guide therapeutic options. In a similar timeframe, non-specific investigational agents may have been FDA-approved or at least deemed worthy of regular use in PC patients and some of the MRI based or optical imaging tracers such as quantum dots.
| Prostate Cancer Imaging Agents | |||||
|---|---|---|---|---|---|
| Imaging agent |
Targeting domain |
Radiotracer | Current Status | Advantages | Disadvantages |
| 7E11 | Intracellular epitope of PSMA |
111 In | pre-surgical staging and the evaluation of PSA relapse after local therapy |
In pre-surgical patients with high- risk disease, but negative conventional imaging, capromab penditide was able to identify patients with occult local nodal disease. |
Significant false negative rate in the setting of PSA relapse [23]. Varied amount of efficacy with an average sensitivity and specificity of 60% and 70% respectively [24]. Imaging has been associated to binding of mAb7E11 to a receptor located inside the PC cell. Thus, only non-viable cells bind mAb7E11, which limits its use as a good imaging agent [25]. |
| Hu mAb J591 |
Extracellular domain of PSMA |
111In, 90Y, 177Lu |
PSMA-specific internalizing antibodies such as J591 and J415 are ideal mAbs for the development of novel therapeutic methods to target the delivery of beta-emitting radionuclides, which include 131In, 90Y, and 177Lu for the treatment of PSMA-positive tumors [27]. |
J591 is specific to external domain of PSMA, thus targeting viable tumor. These immunoconjugates are better candidates for both imaging and targeted therapy than are antibodies targeting PSMA internally. |
Slow target recognition and background clearance in an appropriate timeframe for diagnostic imaging |
| mAb 3/A12 [28, 30] |
Extracellular domain of PSMA |
64Cu | microPET images of mice with PSMA-positive tumors revealed a high uptake of the mAbs 24 and 48 hrs. post infusion |
No human data available | |
| mAb 3/E7 [28] |
Extracellular domain of PSMA |
64Cu | microPET images of mice with PSMA-positive tumors revealed a high uptake of the mAbs 24 and 48 hrs. post infusion |
No human data available | |
| mAb 3/F11 [28] |
Extracellular domain of PSMA |
64Cu | microPET images of mice with PSMA-positive tumors revealed a high uptake of the mAbs 24 and 48 hrs. post infusion |
Planar gamma- scintigraphic images obtained for xenografted model demonstrated targeting for PSMA positive tumors suggesting possible applications in imaging and for targeted radiation therapy |
No human data available |
| N-[N-[(S)- 1,3- Dicarboxypr opyl]carbam oyl]-4- [18F]fluorob enzyl-L- cysteine (DCFBC) [34] |
Small molecule inhibitor of enzymatic active site of extracellular domain of PSMA |
18F | Toxicity studies are under way en route to clinical implementation |
Suitable physical half-life It can be synthesized with relatively ease in reasonable radiochemical yield and specific radioactivity. Low deflourination. |
High renal uptake |
| MIP-1072 & MIP-1095 [56] |
Urea based small molecule inhibitor of enzymatic domain of PSMA |
123I | Due to the high specificity of 123I-MIP-1072 for PC trials are underway to monitor tumor progression in patients before, during, and after chemotherapy. |
Superior safety profile. Low radiation dose. Faster target detection with saturable and competitive binding |
Renal toxicity |
| MIP-1404 & MIP-1405 [57] |
Urea based small molecule inhibitor of enzymatic domain of PSMA |
99Tc |
99mTc-MIP-1404 has minimal activity in the bladder, further work is planned to correlate imaging findings with histopathology in patients with high risk clinically- localized PC. |
Both agents cleared the blood rapidly with MIP-1404 demonstrating significantly lower urinary activity (7%) compared to MIP- 1405 (26%). More bone lesion detected as compared to bone scan |
Renal toxicity |
| 111In- Capromab Studies: | |||||
|---|---|---|---|---|---|
| Study | Agent used |
Number of patients |
Type of patient population | Result | Comments |
| Sodee DB et al [38] |
111In- 7E11 |
15 | Localized residual or metastatic PC post prostatectomy and lymphadenectomy with rising serum PSA. All patients had negative pre- study radiographic abdominal and pelvic cross-sectional images. |
One patient with low PSA had normal imaging results and 14 patients had scintigraphic evidence of residual prostatic bed or metastatic prostatic carcinoma. Two patients with borderline abnormal bone scans had abnormal activity in the same regions on capromab |
|
| Deb N et al [39] |
111In &90Y- CYT356 |
12 | CRPC | Myelosuppression was the dose-limiting toxicity. No patient attained a complete or partial response based on PSA and/or radiological criteria. |
Patients treated on higher dose of 90Y-CYT356 had greater progression free survival |
| Manyak MJ et al [40] |
111In- 7E11 |
152 | High pre-surgical PSA, high Gleason score/clinical stage |
Capromab scans showed a sensitivity of 62%, sensitivity of 72%, PPV of 62%, NPV of 2% and an overall accuracy of 68%. In comparison, CT had sensitivity of 4% and specificity of 100%. |
Improved visualization of these scintigraphic findings by improved radiotracer detection or a mAb affinity increase the relevance of PSMA imaging. |
| Kahn et al [41] |
111In- 7E11 |
32 | Residual biochemical evidence of disease after radical prostatectomy |
Capromab scans demonstrated metastasis in 9/32 (28%) with disseminated disease and 23/32 with local disease |
The size of the cohorts and questions about how similar the groups of responders and non-responder were, continue to plague this study. |
| Levesque et al [42] |
111In- CYT356 |
48 | Recurrent PC detected by PSA following radical retropubic prostatectomy |
The scans showed monoclonal uptake in pelvic, abdominal, and extrapelvic retroperitoneal sites beyond the region of limited obturator node dissections. |
The study suggested a good predicting tool for selecting patients for salvage EBRT |
| Wilkensen et al [43] |
111In- Capromab |
42 | Patients showing biochemical progression after radical prostatectomy. |
42 patients underwent imaging for biochemical progression after radical prostatectomy. Abnormal accumulation of mAb was seen in 36 patients. Of these patients 16 (38.1%) subsequently completed a course of salvage RT. |
A regular PSA screening in combination with 111In- Capromab imaging may result in significant improvement in rates of biochemical failure in PC patients |
| Thomas et al [44] |
30 | Patients who had 111In- Capromab scan and underwent salvage radiotherapy for recurrent disease after prostatectomy. |
In patients who had prostatectomy and biochemical relapse and received salvage RT, presalvage RT In- Capromab scan findings outside the prostate fossa were not predictive of biochemical control after RT. |
||
Acknowledgments
Supported by: Prostate Cancer Foundation, NIH ULI RR024996, Robert H. McCooey Memorial Cancer Research Fund
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: S. Vallabhajosula has served as a consultant to Molecular Insight Pharmaceuticals
REFERENCES
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Turkbey B, Pinto PA, Choyke PL. Imaging techniques for prostate cancer: implications for focal therapy. Nat Rev Urol. 2009;6:191–203. doi: 10.1038/nrurol.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Segall G, Delbke D, Stabin MG, et al. SNM practice guideline for sodium 18F-fluoride PET/CT bone scans 1.0. J Nuc Med. 2010;51:1813–1820. doi: 10.2967/jnumed.110.082263. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh A, Heston WD. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J Cell Biochem. 2004;91:528–539. doi: 10.1002/jcb.10661. [DOI] [PubMed] [Google Scholar]
- 5.Ross JS, Gray KE, Webb IJ, et al. Antibody-based therapeutics: focus on prostate cancer. Cancer Metastasis Rev. 2005;24:521–537. doi: 10.1007/s10555-005-6194-0. [DOI] [PubMed] [Google Scholar]
- 6.Horoszewicz JS, Kawinski E, Murphy GP. Monoclonal antibodies to a new antigenic marker in epithelial prostatic cells and serum of prostatic cancer patients. Anticancer Res. 1987;7:927–935. [PubMed] [Google Scholar]
- 7.Israeli RS, Powell CT, Fair WR, Heston WD. Molecular cloning of a complementary DNA encoding a prostate-specific membrane antigen. Cancer Res. 1993;53:227–230. [PubMed] [Google Scholar]
- 8.Israeli RS, Powell CT, Corr JG, Fair WR, Heston WD. Expression of the prostate-specific membrane antigen. Cancer Res. 1994;54:1807–1811. [PubMed] [Google Scholar]
- 9.Troyer JK, Beckett ML, Wright GL., Jr Detection and characterization of the prostate-specific membrane antigen (PSMA) in tissue extracts and body fluids. Int J Cancer. 1995;62:552–558. doi: 10.1002/ijc.2910620511. [DOI] [PubMed] [Google Scholar]
- 10.Sokoloff RL, Norton KC, Gasior CL, Marker KM, Grauer LS. A dual-monoclonal sandwich assay for prostate-specific membrane antigen: levels in tissues, seminal fluid and urine. Prostate. 2000;43:150–157. doi: 10.1002/(sici)1097-0045(20000501)43:2<150::aid-pros10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 11.Bostwick DG, Pacelli A, Blute M, Roche P, Murphy GP. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer. 1998;82:2256–2261. doi: 10.1002/(sici)1097-0142(19980601)82:11<2256::aid-cncr22>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 12.Kusumi T, Koie T, Tanaka M, et al. Immunohistochemical detection of carcinoma in radical prostatectomy specimens following hormone therapy. Pathol Int. 2008;58:687–694. doi: 10.1111/j.1440-1827.2008.02294.x. [DOI] [PubMed] [Google Scholar]
- 13.Mannweiler S, Amersdorfer P, Trajanoski S, Terrett JA, King D, Mehes G. Heterogeneity of prostate-specific membrane antigen (PSMA) expression in prostate carcinoma with distant metastasis. Pathol Oncol Res. 2009;15:167–172. doi: 10.1007/s12253-008-9104-2. [DOI] [PubMed] [Google Scholar]
- 14.Ananias HJ, van den Heuvel MC, Helfrich W, de Jong IJ. Expression of the gastrin-releasing peptide receptor, the prostate stem cell antigen and the prostate-specific membrane antigen in lymph node and bone metastases of prostate cancer. Prostate. 2009;69:1101–1108. doi: 10.1002/pros.20957. [DOI] [PubMed] [Google Scholar]
- 15.Wright GL, Jr, Haley C, Beckett ML, Schellhammer PF. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urol Oncol. 1995;1:18–28. doi: 10.1016/1078-1439(95)00002-y. [DOI] [PubMed] [Google Scholar]
- 16.Wright GL, Jr, Grob BM, Haley C, et al. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology. 1996;48:326–334. doi: 10.1016/s0090-4295(96)00184-7. [DOI] [PubMed] [Google Scholar]
- 17.Sweat SD, Pacelli A, Murphy GP, Bostwick DG. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology. 1998;52:637–640. doi: 10.1016/s0090-4295(98)00278-7. [DOI] [PubMed] [Google Scholar]
- 18.Troyer JK, Beckett ML, Wright GL. Detection and characterization of the prostate-specific membrane antigen (PSMA) in tissue extracts and body fluids. Int J Cancer. 1995;62:552–558. doi: 10.1002/ijc.2910620511. [DOI] [PubMed] [Google Scholar]
- 19.Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardon C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–85. [PubMed] [Google Scholar]
- 20.Laidler P, Dulińska J, Lekka M, Lekki J. Expression of prostate specific membrane antigen in androgen-independent prostate cancer cell line PC-3. Arch Biochem Biophys. 2005;435:1–14. doi: 10.1016/j.abb.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Rajasekaran SA, Anilkumar G, Oshima E, et al. A novel cytoplasmic tail MXXXL motif mediates the internalization of prostate-specific membrane antigen. Mol Biol Cell. 2003;14:4835–4845. doi: 10.1091/mbc.E02-11-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elsasser-Beile U, Wolf P, Gierschner D, Buhler P, Schultze-Seemann W, Wetterauer U. A new generation of monoclonal and recombinant antibodies against cell-adherent prostate specific membrane antigen for diagnostic and therapeutic targeting of prostate cancer. Prostate. 2006;66:1359–1370. doi: 10.1002/pros.20367. [DOI] [PubMed] [Google Scholar]
- 23.Wynant GE, Murphy GP, Horoszewicz JS, et al. Immunoscintigraphy of prostatic cancer: preliminary results with 111In-labeled monoclonal antibody 7E11-C5.3 (CYT-356) Prostate. 1991;18:229–241. doi: 10.1002/pros.2990180305. [DOI] [PubMed] [Google Scholar]
- 24.Apolo AB, Pandit-Taskar N, Morris MJ. Novel tracers and their development for the imaging of metastatic prostate cancer. J Nucl Med. 2008;49:2031–2041. doi: 10.2967/jnumed.108.050658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Troyer JK, Beckett ML, Wright GL., Jr Location of prostate-specific membrane antigen in the LNCaP prostate carcinoma cell line. Prostate. 1997;30:232–242. doi: 10.1002/(sici)1097-0045(19970301)30:4<232::aid-pros2>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 26.Tagawa ST, Beltran H, Vallabhajosula S, et al. Anti-prostate-specific membrane antigen-based radioimmunotherapy for prostate cancer. Cancer. 2010;116:1075–1083. doi: 10.1002/cncr.24795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith-Jones PM, Vallabhajosula S, Navarro V, Bastidas D, Goldsmith SJ, Bander NH. Radiolabeled monoclonal antibodies specific to the extracellular domain of prostate-specific membrane antigen: preclinical studies in nude mice bearing LNCaP human prostate tumor. J Nucl Med. 2003;44:610–617. [PubMed] [Google Scholar]
- 28.Alt K, Wiehr S, Ehrlichmann W, et al. High-resolution animal PET imaging of prostate cancer xenografts with three different 64Cu-labeled antibodies against native cell-adherent PSMA. Prostate. 2010;70:1413–1421. doi: 10.1002/pros.21176. [DOI] [PubMed] [Google Scholar]
- 29.Wolf P, Freudenberg N, Buhler P, et al. Three conformational antibodies specific for different PSMA epitopes are promising diagnostic and therapeutic tools for prostate cancer. Prostate. 2010;70:562–569. doi: 10.1002/pros.21090. [DOI] [PubMed] [Google Scholar]
- 30.Elsasser-Beile U, Reischl G, Wiehr S, et al. PET imaging of prostate cancer xenografts with a highly specific antibody against the prostate-specific membrane antigen. J Nucl Med. 2009;50:606–611. doi: 10.2967/jnumed.108.058487. [DOI] [PubMed] [Google Scholar]
- 31.Regino CA, Wong KJ, Milenic DE, et al. Preclinical evaluation of a monoclonal antibody (3C6) specific for prostate-specific membrane antigen. Curr Radiopharm. 2009;2:9–17. doi: 10.2174/1874471010902010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maresca KP, Hillier SM, Femia FJ, et al. A series of halogenated heterodimeric inhibitors of prostate specific membrane antigen (PSMA) as radiolabeled probes for targeting prostate cancer. J Med Chem. 2009;52:347–357. doi: 10.1021/jm800994j. [DOI] [PubMed] [Google Scholar]
- 33.Foss CA, Mease RC, Fan H, et al. Radiolabeled small-molecule ligands for prostate-specific membrane antigen: in vivo imaging in experimental models of prostate cancer. Clin Cancer Res. 2005;11:4022–4028. doi: 10.1158/1078-0432.CCR-04-2690. [DOI] [PubMed] [Google Scholar]
- 34.Mease RC, Dusich CL, Foss CA, et al. N-[N-[(S)-1,3-Dicarboxypropyl]carbamoyl]-4-[18F]fluorobenzyl-L-cysteine, [18F]DCFBC: a new imaging probe for prostate cancer. Clin Cancer Res. 2008;14:3036–3043. doi: 10.1158/1078-0432.CCR-07-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y, Dhara S, Banerjee SR, et al. A low molecular weight PSMA-based fluorescent imaging agent for cancer. Biochem Biophys Res Commun. 2009;390:624–629. doi: 10.1016/j.bbrc.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hillier SM, Maresca KP, Femia FJ, et al. Preclinical evaluation of novel glutamate-urea-lysine analogues that target prostate-specific membrane antigen as molecular imaging pharmaceuticals for prostate cancer. Cancer Res. 2009;69:6932–6940. doi: 10.1158/0008-5472.CAN-09-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lapi SE, Wahnishe H, Pham D, et al. Assessment of an 18F-labeled phosphoramidate peptidomimetic as a new prostate-specific membrane antigen-targeted imaging agent for prostate cancer. J Nucl Med. 2009;50:2042–2048. doi: 10.2967/jnumed.109.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sodee DB, Conant R, Chalfant M, et al. Preliminary imaging results using In-111 labeled CYT-356 (Prostascint) in the detection of recurrent prostate cancer. Clin Nucl Med. 1996;21:759–767. doi: 10.1097/00003072-199610000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Deb N, Goris M, Trisler K, et al. Treatment of hormone-refractory prostate cancer with 90Y-CYT-356 monoclonal antibody. Clin Cancer Res. 1996;2:1289–1297. [PubMed] [Google Scholar]
- 40.Manyak MJ, Hinkle GH, Olsen JO, et al. Immunoscintigraphy with indium-111-capromab pendetide: evaluation before definitive therapy in patients with prostate cancer. Urology. 1999;54:1058–1063. doi: 10.1016/s0090-4295(99)00314-3. [DOI] [PubMed] [Google Scholar]
- 41.Kahn D, Williams RD, Manyak MJ, et al. 111Indium-capromab pendetide in the evaluation of patients with residual or recurrent prostate cancer after radical prostatectomy. The ProstaScint Study Group. J Urol. 1998;159:2041–2046. doi: 10.1016/S0022-5347(01)63239-7. [DOI] [PubMed] [Google Scholar]
- 42.Levesque PE, Nieh PT, Zinman LN, Seldin DW, Libertino JA. Radiolabeled monoclonal antibody indium 111-labeled CYT-356 localizes extraprostatic recurrent carcinoma after prostatectomy. Urology. 1998;51:978–984. doi: 10.1016/s0090-4295(98)00025-9. [DOI] [PubMed] [Google Scholar]
- 43.Wilkinson S, Chodak G. The role of 111indium-capromab pendetide imaging for assessing biochemical failure after radical prostatectomy. J Urol. 2004;172:133–136. doi: 10.1097/01.ju.0000132138.02846.08. [DOI] [PubMed] [Google Scholar]
- 44.Thomas CT, Bradshaw PT, Pollock BH, et al. Indium-111-capromab pendetide radioimmunoscintigraphy and prognosis for durable biochemical response to salvage radiation therapy in men after failed prostatectomy. J Clin Oncol. 2003;21:1715–1721. doi: 10.1200/JCO.2003.05.138. [DOI] [PubMed] [Google Scholar]
- 45.DeWyngaert JK, Noz ME, Ellerin B, Kramer EL, Maguire GQ, Jr, Zeleznik MP. Procedure for unmasking localization information from ProstaScint scans for prostate radiation therapy treatment planning. Int J Radiat Oncol Biol Phys. 2004;60:654–662. doi: 10.1016/j.ijrobp.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 46.Schettino CJ, Kramer EL, Noz ME, Taneja S, Padmanabhan P, Lepor H. Impact of fusion of indium-111 capromab pendetide volume data sets with those from MRI or CT in patients with recurrent prostate cancer. AJR Am J Roentgenol. 2004;183:519–524. doi: 10.2214/ajr.183.2.1830519. [DOI] [PubMed] [Google Scholar]
- 47.Sodee DB, Sodee AE, Bakale G. Synergistic value of single-photon emission computed tomography/computed tomography fusion to radioimmunoscintigraphic imaging of prostate cancer. Semin Nucl Med. 2007;37:17–28. doi: 10.1053/j.semnuclmed.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 48.Ellis RJ, Kim EY, Conant R, et al. Radioimmunoguided imaging of prostate cancer foci with histopathological correlation. Int J Radiat Oncol Biol Phys. 2001;49:1281–1286. doi: 10.1016/s0360-3016(00)01582-0. [DOI] [PubMed] [Google Scholar]
- 49.Bander NH, Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ. Phase I trial of 177lutetium-labeled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. J Clin Oncol. 2005;23:4591–4601. doi: 10.1200/JCO.2005.05.160. [DOI] [PubMed] [Google Scholar]
- 50.Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ, Bander NH. Phase I trial of yttrium-90-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for androgen-independent prostate cancer. J Clin Oncol. 2004;22:2522–2531. doi: 10.1200/JCO.2004.09.154. [DOI] [PubMed] [Google Scholar]
- 51.Bander NH. Technology insight: monoclonal antibody imaging of prostate cancer. Nat Clin Pract Urol. 2006;3:216–225. doi: 10.1038/ncpuro0452. [DOI] [PubMed] [Google Scholar]
- 52.Akhtar NH, Nanus DM, Osborne JR, et al. Antiprostate-specific membrane antigen (PSMA)-based radioimmunotherapy: A combined analysis of radiolabeled-J591 studies. J Clin Oncol. 2011;29(suppl 7) abstr 136. [Google Scholar]
- 53.Holland JP, Divilov V, Bander NH, Smith-Jones PM, Larson SM, Lewis JS. 89Zr-DFO-J591 for immunoPET of prostate-specific membrane antigen expression in vivo. J Nucl Med. 2010;51:1293–1300. doi: 10.2967/jnumed.110.076174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakajima T, Mitsunaga M, Bander NH, Heston WD, Choyke PL, Kobayashi H. Targeted, activatable, in vivo fluorescence imaging of prostate-specific membrane antigen (PSMA) positive tumors using the quenched humanized J591 antibody-indocyanine green (ICG) conjugate. Bioconjug Chem. 2011;22:1700–1705. doi: 10.1021/bc2002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Evans MJ, Smith-Jones PM, Wongvipat J, et al. Noninvasive measurement of androgen receptor signaling with a positron-emitting radiopharmaceutical that targets prostate-specific membrane antigen. Proc Natl Acad Sci U S A. 2011;108:9578–9582. doi: 10.1073/pnas.1106383108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barrett J, LaFrance N, Coleman RE, et al. Targeting metastatic prostate cancer [PCa] in patients with 123I-MIP1072 & 123I-MIP1095. J Nucl Med. 2009;50:522. [Google Scholar]
- 57.Osborne JR, Akhtar NH, Vallabhajosula S, et al. Tc-99m labeled small-molecule inhibitors of prostate-specific membrane antigen (PSMA): New molecular imaging probes to detect metastatic prostate adenocarcinoma (PC) J Clin Oncol. 2012;30(Suppl 5) Abst 173. [Google Scholar]