All Paleolithic hominids lived by hunting and collecting wild foods, an aspect of existence that began to disappear only with the emergence of the farming and herding societies of the Neolithic ≤10,000 years ago (10 KYA). What are the roots of this remarkable economic transformation? The answer lies in equally revolutionary changes that took place within certain stone age cultures several millennia before. In 1968, Lewis R. Binford noted what appeared to be substantial diversification of human diets in middle- and high-latitude Europe at the end of the Paleolithic, roughly 12–8 KYA (1). Rapid diversification in hunting, food processing, and food storage equipment generally accompanied dietary shifts, symptoms of intensified use of habitats, and fuller exploitation of the potential foodstuffs they contained. Some of this behavior was directed to grinding, drying, and storing nuts, but it also involved small animals (2–6). Kent Flannery pushed these observations further in 1969 with his “Broad Spectrum Revolution” (BSR) hypothesis, proposing that the emergence of the Neolithic in western Asia was prefaced by increases in dietary breadth in foraging societies just before this period (7). He argued that subsistence diversification, mainly by adding new species to the diet, raised the carrying capacity of an environment increasingly constrained by climate instability at the end of the Pleistocene.
Binford's and Flannery's papers have stimulated much archaeological research over three decades. Inspired by the early works of Odum and Odum (8), Emlen (9), and MacArthur and Pianka (10), both archaeologists argued that economic change resulted from unprecedented demographic crowding in certain regions of the world. Some archaeologists have questioned the role of “population pressure” in human social evolution (6, 11), but most continue to think of demographic factors as one of several ingredients necessary to the forager-to-farmer transition or the Paleolithic to the Neolithic (5, 12–16). If density-dependent effects can play decisive roles in shaping the evolutionary histories of predator–prey systems in general (17–20), why not in cases involving human beings (21–22)? Changes in human population density are bound to influence the rates of interspecific and intraspecific contact and the availability of critical foodstuffs, as well as people's solutions for getting enough to eat. Rapid technological change and increased densities of archaeological sites during the later Paleolithic lend some credence to this position.
Evidence of increasing dietary breadth is expected to take the form of more species in the diet (7) and/or greater proportional evenness among high- and low-ranked prey items in response to declining availability of preferred types. A predator can afford to ignore lower-quality prey at little cost if the chance of finding a superior type in the near future is high, which fosters a narrower diet. As the supply of preferred prey dwindles, however, broadening the diet to include common but lower-yield prey types maximizes a predator's returns per unit expenditure by reducing search time (19).
Archaeological evidence for broadening of Paleolithic diets in Eurasia is clear from greater exploitation of energy-rich nuts and large seeds, whose nutritional benefits require considerable work and equipment to extract (5). This trend is most apparent from the proliferation of milling tools after the Last Glacial Maximum (23) and, to a lesser extent, from increasing evidence of storage facilities and preserved plant parts (24, 25). Under chronically lean conditions, people should also have become less selective about what animals to hunt, rather than going hungry. Oddly, the story from the faunal evidence was much less clear than that from plants. Measures of dietary diversity in game use based on Linnean taxonomic categories (counting species or genera) register only one economic transition—that from foragers to farmers in the early Neolithic, marked by a decline in dietary breadth (26–29). What variation could be found in the taxonomic diversity of archaeofaunas over the Middle, Upper, and Epi-Paleolithic was more easily explained by climate-driven environmental changes or geographic variation in animal and plant community composition (30–32). There seemed to be no zooarchaeological support for the BSR hypotheses of expanding diet breadth in the later Paleolithic.
The basic idea behind the BSR hypothesis remains a good one. The contradictions between data on plant and animal exploitation actually stem from how zooarchaeologists have tended to categorize prey animals (33). Because the cultures of interest are extinct, prey-ranking systems cannot be inferred from watching people make decisions. The relative values (payoffs) of prey must instead be evaluated from knowledge of modern variants of the animals whose bones occur in archaeological deposits. Species and genera present the most obvious analytical categories, and the most literal expectation of Flannery's BSR hypothesis is indeed more species in the diet and/or more even emphasis on those species. Thus diet variation normally is examined in terms of indices of taxonomic richness (N-species or N-genera) and taxonomic evenness (proportionality in abundance) (26, 28, 31, 34). Such analyses use either Kintigh's simulation-based technique (35) or a more longstanding regression approach (36) developed from the work of Fisher, Corbet, and Williams (37) and others for problems of sampling in modern community ecology.
The main weakness of diversity approaches that rely on Linnean taxonomic units is their insensitivity to physical and behavioral differences among prey animals. The only qualification normally added to these analyses is prey body size, because all game animals are composed of similar tissues, and large animals yield much more food than small ones, even if they are more difficult to catch. This practice potentially overlooks great differences in prey-handling costs and the long-term price of heavy exploitation among animals that are broadly equivalent in food content and package size. In fact, some distantly related taxa are nearly equivalent from the viewpoint of handling costs because of their locomotor habits or ways of avoiding predators: both tortoises and rock-dwelling marine shellfish are sluggish or immobile; hares and partridges, although similar in body weight to tortoises or an armful of shellfish, are quick and maneuverable.
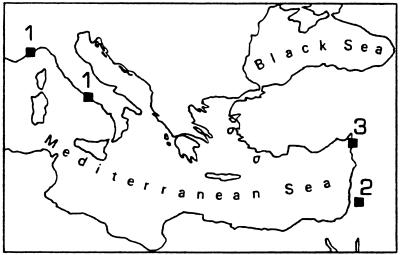
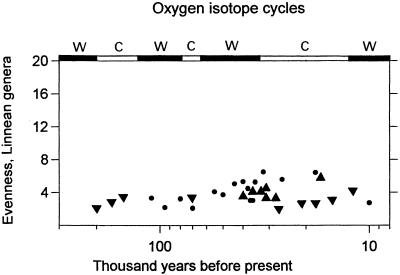
Differing prey-type classification systems greatly affect archaeologists' perceptions of change in prehistoric diet breadth and have obscured critical information. This can be demonstrated with a simple measure of diversity to three faunal series from the Mediterranean Basin, the Reciprocal of Simpson's Index, or 1/Σ(ρi)2, where ρ represents the proportion of each prey type for array i in an assemblage (38, 39). The Mediterranean faunal series include a total of 32 assemblages from shelter sites in northern Israel (200–11 KYA) (40, 41), western Italy (110–9 KYA) (32), and south-central Turkey (41–17 KYA) (42) (Fig. 1). Application of the index to assemblages that potentially contain about 20 genera (Fig. 2) yields consistently low levels of evenness in dietary breadth over a 200 KYA time span. There is only a very weak correlation with time (r = 0.386, P = 0.05, n = 32), and there is no correspondence to the 6–7 climatic oscillations indicated by oxygen isotope data from deep sea cores (e.g., ref. 43). Although sample size varies among the assemblages, it does not explain the pattern in Fig. 2. As usual, no support for the BSR hypothesis is found here.
Figure 1.
Geographic origins of Paleolithic shelter sites in the three Mediterranean faunal series: (1) western coast of Italy, with 16 assemblages; (2) Wadi Meged, inland Galilee of Israel, 9 assemblages; (3) Hatay coast of south-central Turkey, 7 assemblages.
Figure 2.
Evenness in the representation of Linnean genera for the faunal series from Italy (●), Israel (▾), and Turkey (▴), by using the Reciprocal of Simpson's Index (20 = most even). Time is expressed on a logged scale, as are oxygen isotope climate cycles (41); c, cold stage; w, warm stage.
What about small animal exploitation? This is where Binford and Flannery expected to see the greatest changes in game use. We know that small animals were important to human diets in the Mediterranean Basin from at least the early Middle Paleolithic, some 200 KYA onward (32, 44, 45). The proportional contribution of small game to total meat intake varied from staple to supplement, with no trend, however, except a sharp increase at the close of certain Paleolithic sequences (16, 41). The spectrum of animal taxa eaten by Paleolithic peoples in the Mediterranean Basin did not vary much either (32, 46) and included tortoises, marine shellfish, large legless lizards, ostrich eggs, game birds such as partridges, hares, and rabbits, in addition to ungulates such as deer, gazelles, and wild cattle. However, the relative emphasis that humans placed on three general types of small animals changed dramatically with time. Middle Paleolithic foragers seldom bothered with small prey unless they could be obtained easily—not so from the early Upper Paleolithic onward.
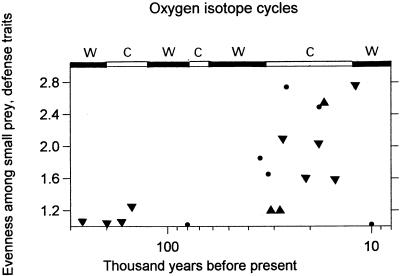
Variation within the small game fraction of each series (Fig. 3) reveals a clear trend toward more even dependence on high- and low-ranked small prey types, confirmation of expanding dietary breadth during the later part of the Mediterranean Paleolithic. This regrouping of the data simply distinguishes between slow-moving easily collected types (mostly tortoises and shellfish), fast-running mammals (mostly lagomorphs), and quick-flying game birds. Only 18 assemblages contain small game components large enough to be compared, with four from the early Middle Paleolithic of Italy collapsed into one to increase sample size. Removing large game from the comparison allows clear expression of expanding diet breadth in small game exploitation (r = 0.606, P = 0.01, n = 18) and also shows that most of the expansion took place during a cold period (Stage 2). This is the opposite of what is expected to result from climate-driven changes in animal community composition, wherein the number of small animal species and population sizes tends to increase in warmer environments (19). The evidence indicates a categorical change in how humans interacted with small animal populations after about 40–50 KYA but surprisingly little change in how humans interacted with large animal populations.
Figure 3.
Evenness among three prey categories within the small game fraction only, based on prey defense mechanisms (slow game, quick-running terrestrial mammals, and quick-flying birds), by using the Reciprocal of Simpson's Index (3 = most even). Symbols as in Fig. 2.
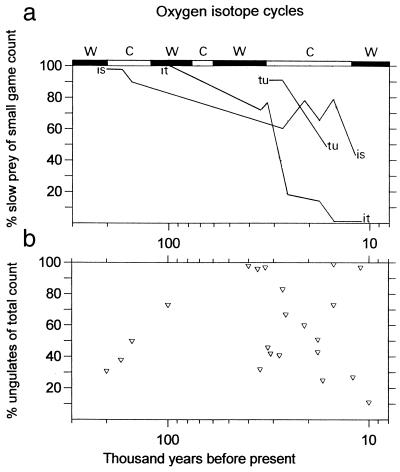
If different ways of categorizing prey in studies of Paleolithic diet breadth produce contradictory results, which approach is more appropriate? The answer depends on how we think foragers should have ranked prey according to expected energy returns. Linnean taxonomy is a powerful tool in biology and zooarchaeology, not least because there is considerable agreement about what animals should be called and how they are related to one another. However, foragers' perceptions of prey do not necessarily follow the rules of biological systematics, and variation in the relative abundances of species or genera does not seem to be sensitive to behavioral changes in prehistoric human predators. Prey body size should be a valuable nontaxonomic criterion for ranking the potential returns of prey, but it too has its limits because of the additional complications of capture costs and, in some cases, also processing costs. The large-to-small body-size contrast in the three Mediterranean series, expressed as the percentage of ungulates in the total count for each assemblage in Fig. 4, appears trendless (r = 0.276, P = 1, n = 18). The proportion of slow animals within the small game fraction of each assemblage clearly declines with time (r = 0.572, 0.02 > P > 0.01, n = 18), the converse of which is increased reliance on small quick animals. Prey body size must have had some economic significance, but it seems that the absolute differences in prey size often were recalibrated from the foragers' point of view by differing capture costs among small prey animals.
Figure 4.
(a) Trends by region (lines) in the percentage of slow small prey within the small game fraction of each assemblage; is, Israel; it, Italy; and tu, Turkey. (b) The percentage of ungulate remains in the total count of each assemblage.
This is not to say that all faunal series examined in the future will adhere to the patterns found in the three Mediterranean series examined here. Animal communities vary in their composition, as do the choices of prey species available to human hunters. On the other hand, the Mediterranean Basin represents a major part of the total geographic range of Paleolithic humans. By experimenting with prey type categories, albeit with attention to independently documented characteristics of the subject species, the evidence for increased diet breadth during the later Paleolithic of the Mediterranean region springs into focus. Independent standards for prey classification were isolated from wildlife data (40) and linked to demographic increases by examination of stature reduction (diminution effects) in certain slow-growing species and predator–prey simulation modeling (2, 33, 47). That small prey animals differ tremendously in their development rates also permits an unusually clear view of how increases in Paleolithic diet breadth shifted with local demographic growth. This is not a matter of how much small game animals contributed to total game intake so much as how certain very sensitive species serve as symptoms of threshold effects in predator–prey systems—like fume-sensitive canaries carried into coal shafts by 19th century miners.
Canary in a Coal Mine
In the Mediterranean Basin, a simple distinction in the “catchability” of small animals happens to correspond to great differences in prey population resilience, the latter governed mainly by individual maturation rate (40). Slow-moving Mediterranean tortoises (maturing at 8–12 years) and some shellfish (1–5 years) are especially susceptible to overharvesting because of their slow maturation rates (48–52). It is unlikely that Paleolithic foragers viewed prey in terms of their potentials for population recovery, but they certainly would have been aware of declining availability of prey and thus declining returns for the same level of foraging effort.
Because tortoises and shellfish grow throughout much of their life span, overharvesting also causes diminution or a reduction in the mean size of individuals subsequently available to foragers. Humans' strong preference for the largest individuals, which may disproportionately represent older, more productive females, exerts exceptionally harsh impacts on the size and sex structures of tortoise and certain shellfish populations (48, 49). Interestingly, slow-growing, slow-moving tortoises and marine mollusks dominate the small game fractions of the early Middle Paleolithic record, constituting up to half of all identified specimens in several of the assemblages (40). What is more, the individuals taken by Middle Paleolithic foragers were large on average. Body-size diminution occurred for tortoises by the late Middle Paleolithic or earliest Upper Paleolithic in Israel (≥44 KYA) and was sustained over multiple climate cycles thereafter. Limpet diminution began by about 23 KYA in Italy (33) but considerably later to the west in Spain (2). The timing and duration of prey diminution are largely independent of global climate trends—the other potential cause of diminution—and thus point to a human cause (33).
With evidence of harvesting pressure on “low-turnover” prey populations during the later Paleolithic, there was a corresponding increase in the exploitation of agile warm-blooded small animals, mainly birds such as partridges and lagomorphs (hares and rabbits). These quick small animals mature in ≤1 year, and their populations rebound easily from heavy hunting by humans. Predator–prey simulations indicate that hare and partridge populations can support 7 to 10 times the annual offtake that tortoise populations can support (40). Limpets and large predatory mollusks (e.g., Thais) are only somewhat more resilient than tortoises. Thus greater dependence on slow-growing animals during the Middle Paleolithic, and on larger individuals on average, implies that these early human populations were very small and dispersed (33). Paleolithic foragers' emphasis on slow (highly ranked) and quick (lower ranked) small prey grew more “even” with time, the predicted outcome of hunting pressure and demographic increase in the absence of a correlation with climate warming.
Implications
Thirty years later, and contrary to the results of interim studies, the data on small game use in southern Europe and western Asia support Flannery's “Broad Spectrum Revolution” hypothesis of expanding dietary breadth in response to demographic packing during the late Pleistocene. However, indications of more even dependence on high- and low-ranked prey were obtained only when small animals were classified according to development rates and predator escape strategies, rather than by counting species or genera or organizing prey taxa along a body-size gradient. Diet breadth models are useful in research on human evolution only if anomalies in archaeological data are recognized and prehistoric ranking systems are treated as points of investigation rather than as given knowledge.
The findings for the three Mediterranean faunal series diverge from Flannery's original predictions as to when the BSR began in the eastern end of this vast region. Early diet expansion and demographic pulses may associate with the spread of Upper Paleolithic cultures from Asia into Europe, the same general path as the spread of Neolithic adaptations after 10 KYA (53, 55). The dietary shifts identified by Binford and Flannery between 12 and 8 KYA were merely the last in a longer series of economic changes. That these changes began earlier in the eastern Mediterranean Basin than at its northern and western ends reinforces the likelihood that prehistoric human populations were largest in the semiarid subtropical to tropical latitudes of Asia and Africa (5, 21).
Demographic pulses emanated from southwestern Asia into Europe several times. The surge in bird exploitation is an early symptom of Upper Paleolithic demographic expansion, as is tortoise diminution in the east. Lagomorph exploitation instead seems more diagnostic of the later stages of this process (56). Middle Paleolithic people rarely hunted lagomorphs in ecosystems where other predators, such as denning wolves, frequently did (32). Upper Paleolithic people exploited lagomorphs in modest quantities, but Epipaleolithic and Mesolithic people hunted them most of all.
An interesting quality of small prey populations that rebound quickly is their greater reliability as a food source if capture costs can be reduced artificially. Any forager population that can grow faster on low-value but more resilient foods will have a demographic advantage over competing populations. In the late Pleistocene, this involved a lowering of humans' position in the food chain in some arid low-latitude regions, largely because greater plant use was also part of the BSR. Large-seeded plants permit more direct access to primary production in ecosystems, despite higher collection and processing costs, and thus may support humans at higher population densities (5, 21). Small quick animals also present this possibility to a lesser extent but to the same end and often complementary to the intensified use of plant seeds in the absence of domesticated ungulates.
The results of small game exploitation in the Paleolithic raise two larger issues that, although beyond the immediate scope of this review, are unlikely to go away any time soon. The first of these is the proposed role of humans in the extinction of large mammals during the late Pleistocene. Most of the shifts in predator–prey dynamics noted here concern small animals, not big ones. A second issue is the relation between demographic packing and the peculiar acceleration in social and technological complexity among Paleolithic cultures. How, for example, do the patterns in subsistence evolution demonstrated above relate to the spectacular radiations in food-getting equipment of the later Paleolithic? Early indications of expanding diets in the eastern Mediterranean precede rather than follow the evolution of the kinds of tools (specialized projectile tips, nets, and other traps) needed to capture quick small animals efficiently (40). Industrial Age culture encourages us to view technology as the doorway to new worlds but, in deep time, technological evolution may more often represent uniquely human responses to preexisting challenges.
One contribution of the zooarchaeological data on small game use can be realized immediately. Most information about human population history is obtained via comparisons of modern human genetic diversity, which on the whole suggest several demographic pulses originating from western Asia and/or Africa that ultimately affected peripheral populations of Europe and elsewhere (53–55, 57). Time is the most difficult variable to control in these studies; biological clocks inferred from mutation rates are not very accurate, and thus there is much variety in their interpretation. Demographic pulses are also evidenced by the zooarchaeological record from 40–50 KYA onward. Although this evidence involves different scales of observation than those generally obtained from DNA research, faunal indications of demographic pulses can be dated by radiometric techniques across geographic gradients, permitting independent tests of prehistoric human population dynamics and history.
Acknowledgments
This research was supported by grants from the National Science Foundation Archaeology Program. Thanks to H. Harpending and S. L. Kuhn for comments on earlier drafts of this manuscript.
References
- 1.Binford L R. In: New Perspectives in Archaeology. Binford S R, Binford L R, editors. Chicago, IL: Aldine; 1968. pp. 313–341. [Google Scholar]
- 2.Clark G A, Straus L G. In: Hunter–Gatherer Economy in Prehistory. Bailey G, editor. Cambridge, U.K.: Cambridge Univ. Press; 1983. pp. 131–148. [Google Scholar]
- 3.Coles B, editor. The Wetland Revolution in Prehistory. Exeter, U.K.: The Prehistoric Society; 1992. [Google Scholar]
- 4.Jochim M. A Hunter–Gatherer Landscape: Southwest Germany in the Late Paleolithic and Mesolithic. New York: Plenum; 1998. [Google Scholar]
- 5.Keeley L H. J Anthropol Arch. 1988;7:373–411. [Google Scholar]
- 6.Price T D, Gebauer A B. In: Last Hunters—First Farmers. Price T D, Gebauer A G, editors. Santa Fe, NM: School of American Research Press; 1995. pp. 3–19. [Google Scholar]
- 7.Flannery K V. In: The Domestication and Exploitation of Plants and Animals. Ucko P J, Dimbleby G W, editors. Chicago, IL: Aldine; 1969. pp. 73–100. [Google Scholar]
- 8.Odum E P, Odum H T. Fundamentals of Ecology. Philadelphia, PA: Saunders; 1959. [Google Scholar]
- 9.Emlen J. Am Nat. 1966;100:611–617. [Google Scholar]
- 10.MacArthur R H, Pianka E. Am Nat. 1966;100:603–609. [Google Scholar]
- 11.Hayden B. In: Last Hunters—First Farmers. Price T D, Gebauer A B, editors. Santa Fe, NM: School of American Research; 1995. pp. 273–299. [Google Scholar]
- 12.Bar-Yosef O, Meadow R H. In: Last Hunters—First Farmers. Price T D, Gebauer A B, editors. Santa Fe, NM: School of American Research; 1995. pp. 39–94. [Google Scholar]
- 13.Binford L R. Proc Br Acad. 1999;101:1–35. [Google Scholar]
- 14.Redding R. J Anthropol Archaeol. 1988;7:56–97. [Google Scholar]
- 15.Watson P J. In: Last Hunters—First Farmers. Price T D, Gebauer A B, editors. Santa Fe, NM: School of American Research; 1995. pp. 21–37. [Google Scholar]
- 16.Davis S J M, Lernau O, Pichon J. In: Le site de Hatoula en Judée occidentale, Israel, Mémoires et Travaux du Centre de Recherche Français de Jérusalem. Lechevallier M, Ronen A, editors. Paris: Association Palérient; 1994. pp. 83–100. [Google Scholar]
- 17.Boutin S. J Wildl Manage. 1992;56:116–127. [Google Scholar]
- 18.Gavin A. J Wildl Manage. 1991;55:760–766. [Google Scholar]
- 19.Pianka E R. Evolutionary Ecology. New York: Harper & Row; 1978. [Google Scholar]
- 20.Sinclair A R E. J Wildl Manage. 1991;55:767–773. [Google Scholar]
- 21.Harpending H, Bertram J. Am Antiq. 1975;40:82–91. [Google Scholar]
- 22.Winterhalder B, Goland C. Curr Anthropol. 1993;34:710–715. [Google Scholar]
- 23.Wright K I. Am Antiq. 1994;59:238–263. [Google Scholar]
- 24.Hillman G, Colledge C S, Harris D R. In: Foraging and Farming: The Evolution of Plant Exploitation. Hillman G C, Harris D R, editors. London: Unwin Hyman; 1989. pp. 240–266. [Google Scholar]
- 25.Miller N F. In: The Origins of Agriculture: An International Perspective. Cowan C W, Watson P J, editors. Washington, DC: Smithsonian Institution Press; 1992. pp. 39–58. [Google Scholar]
- 26.Edwards P C. Antiquity. 1989;63:225–246. [Google Scholar]
- 27.Horwitz L K. Archaeozoologia. 1996;8:53–70. [Google Scholar]
- 28.Neeley M P, Clark G A. In: Hunting and Animal Exploitation in the Later Paleolithic and Mesolithic of Eurasia, Archeological Papers of the American Anthropological Association. Peterkin G L, Bricker H, Mellars P, editors. Vol. 4. Washington, DC: American Anthropological Association; 1993. pp. 221–240. [Google Scholar]
- 29.Davis S. Paléorient. 1982;8:5–14. [Google Scholar]
- 30.Bar-Oz G, Dayan T, Kaufman D. J Archaeol Sci. 1999;26:67–82. [Google Scholar]
- 31.Simek J F, Snyder L M. In: Upper Pleistocene Prehistory of Western Eurasia, University Museum Monographs no. 54. Dibble H L, Montet-White A, editors. Philadelphia, PA: Univ. of Pennsylvania; 1988. pp. 321–332. [Google Scholar]
- 32.Stiner M C. Honor Among Thieves: A Zooarchaeological Study of Neandertal Ecology. Princeton, NJ: Princeton Univ. Press; 1994. [Google Scholar]
- 33.Stiner M C, Munro N D, Surovell T A, Tchernov E, Bar-Yosef O. Science. 1999;283:190–194. doi: 10.1126/science.283.5399.190. [DOI] [PubMed] [Google Scholar]
- 34.Grayson D K, Delpech F. J Archaeol Sci. 1998;25:1119–1129. [Google Scholar]
- 35.Kintigh K W. Am Antiq. 1984;49:4454. [Google Scholar]
- 36.Grayson D K. Quantitative Zooarchaeology. Orlando, FL: Academic; 1984. [Google Scholar]
- 37.Fisher R A, Corbet A S, Williams C B. J Anim Ecol. 1943;12:42–58. [Google Scholar]
- 38.Simpson E H. Nature (London) 1949;163:688. [Google Scholar]
- 39.Levins R. Evolution in Changing Environments: Some Theoretical Explorations. Princeton, NJ: Princeton Univ. Press; 1968. [Google Scholar]
- 40.Stiner M C, Munro N D, Surovell T A. Curr Anthropol. 2000;41:39–73. [PubMed] [Google Scholar]
- 41.Munro N D. In: Zooarchaeology of the Pleistocene/Holocene Boundary, BAR International Series 800. Driver J, editor. Oxford, U.K.: British Archaeological Reports; 1999. pp. 37–45. [Google Scholar]
- 42.Kuhn S L, Stiner M C, Güleç E. Antiquity. 1999;73:505–517. [Google Scholar]
- 43.Shackleton N J, Opdyke N D. Quart Res. 1973;3:39–55. [Google Scholar]
- 44.Stiner M C, Tchernov E. In: Neanderthals and Modern Humans in West Asia. Akazawa T, Aoki K, Bar-Yosef O, editors. New York: Plenum; 1998. pp. 241–262. [Google Scholar]
- 45.Klein R G, Scott K. J Archaeol Sci. 1986;13:515–542. [Google Scholar]
- 46.Tchernov E. In: Late Quaternary Chronology and Paleoclimates of the Eastern Mediterranean, Radiocarbon 1994. Bar-Yosef O, Kra R S, editors. Tucson, AZ: Univ. of Arizona Radiocarbon Lab.; 1994. pp. 333–350. [Google Scholar]
- 47.Klein R G, Cruz-Uribe K. S Afr Archaeol Bull. 1983;38:26–30. [Google Scholar]
- 48.Blasco M, Crespillo E, Sanchez J M. Isr J Zool. 1986–1987;34:139–147. [Google Scholar]
- 49.Dye A H, Branch G M, Castilla J C, Bennett B A. In: Rocky Shores: Exploitation in Chile and South Africa. Siegfried W R, editor. Berlin: Springer; 1994. pp. 131–154. [Google Scholar]
- 50.Hailey A, Wright J, Steer E. Herpetol J. 1988;1:294–301. [Google Scholar]
- 51.Hockey P A R. In: Rocky Shores: Exploitation in Chile and South Africa. Siegfried W R, editor. Berlin: Springer; 1994. pp. 17–31. [Google Scholar]
- 52.Siegfried W R, Hockey P A R, Branch G M. In: Rocky Shores: Exploitation in Chile and South Africa. Siegfried W R, editor. Berlin: Springer; 1994. pp. 1–15. [Google Scholar]
- 53.Ammerman A J, Cavalli-Sforza L S. The Neolithic Transition and the Genetics of Populations in Europe. Princeton, NJ: Princeton Univ. Press; 1984. [Google Scholar]
- 54.Barbujani G, Bertorelle G. Proc Natl Acad Sci USA. 2001;98:22–25. doi: 10.1073/pnas.98.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reich D E, Goldstein D B. Proc Natl Acad Sci USA. 1998;95:8119–8123. doi: 10.1073/pnas.95.14.8119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuhn S L, Stiner M C. In: Hunter–Gatherers: Interdisciplinary Perspectives. Panter-Brick C, Layton R H, Rowley-Conwy P A, editors. Cambridge, U.K.: Cambridge Univ. Press; 2001. pp. 99–142. [Google Scholar]
- 57.Relethford J H. Annu Rev Anthropol. 1998;27:1–23. [Google Scholar]