Abstract
Prostacyclin (PGI2) is a potent vasodilator that exerts multiple vasoprotective effects in the cardiovascular system. The effects of PGI2 are mediated by activation of the cell membrane G-protein coupled PGI2-receptor (IP receptor). More recently, however, it has been suggested that PGI2 might also serve as an endogenous ligand and activator of nuclear peroxisome proliferator-activated receptor-δ (PPARδ). Consistent with this concept, studies designed to define pharmacological properties of stable PGI2 analogues revealed that beneficial effects of these compounds appear to be mediated, in part, by activation of PPARδ. This review discusses emerging evidence regarding contribution of PPARδ activation to vasoprotective and regenerative functions of PGI2 and stable analogues of PGI2.
PGI2
Pharmacology of PGI2 has been extensively studied since 1976, when PGI2 was identified as a major product of arachidonic acid metabolism within the vasculature (1). In the cardiovascular system, PGI2 is a potent vasodilator, and in some vascular beds, it functions as an endothelium-derived relaxing factor (2). In addition, PGI2 exerts an inhibitory effect on aggregation of platelets (1,2). PGI2 also inhibits white blood cells adhesion and proliferation of smooth muscle cells, thus preventing the development of atherosclerosis (3,4). Beneficial vascular effects of PGI2 produced in the endothelium are in many respects similar to the effects of endothelium-derived nitric oxide (NO). Indeed, PGI2 may compensate for the loss of NO thereby preserving endothelial function in conditions associated with impairment of NO signaling (5). It has also been recognized that biosynthesis of PGI2 is an important determinant of regenerative functions in the cardiovascular system including angiogenesis and repair of injured endothelium (6–9). In this regard, accumulating evidence continues to substantiate an important contribution of PGI2 to the regenerative capacity of endothelial progenitor cells (EPCs; 9–12).
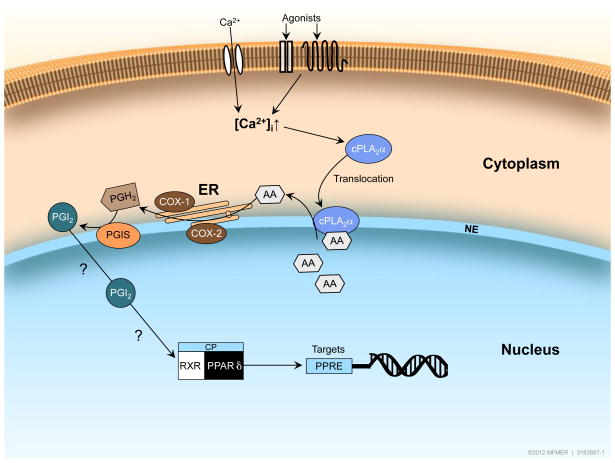
PGI2 is generated by cytosolic phospholipase A2-α (cPLA2-α)-induced mobilization of arachidonic acid which serves as a substrate for cyclooxygenase (COX) enzyme activity. Two isoforms, COX-1 and COX-2, are coupled to prostacyclin synthase (PGIS), enzyme responsible for synthesis of PGI2. Interestingly, in vascular endothelial cells, enzymes required for production of PGI2 are localized at endoplasmic reticulum and around the nuclear membrane (13–15), thereby suggesting that locally generated PGI2 might be involved in nuclear signaling. Notably, COX-1, COX-2, and PGIS have been detected on both inner and outer surfaces of the nuclear membrane (14). An increase in intracellular calcium is required for activation of cPLA2. Moreover, elevated intracellular calcium also causes translocation of cPLA2-α to the nuclear envelope, thus enabling mobilization of arachidonic acid and subsequent production of PGI2 in close proximity to nucleus (16; Figure 1). Consistent with this concept, colocalization of translocated cPLA2-α with COX-2 has been demonstrated in perinuclear region of endothelial cells (17). Recognition of ability of PGI2 (and stable analogues of PGI2) to activate nuclear PPARδ provided functional explanation for the nuclear localization of enzymes responsible for production of PGI2 (18,19). However, in the cardiovascular system, ongoing efforts are only beginning to define the stimuli (e.g. shear stress) that can activate the PGI2 nuclear signaling pathway under physiological or pathological conditions (20). The exact nature of the agonists and the physical stimuli that can activate and translocate cPLA2 to couple perinuclear generation of PGI2 with activation of nuclear receptor(s) remains to be determined. Therefore, in this review we will discuss existing evidence regarding pharmacological relevance of PPARδ activation in mediation of vascular effects of PGI2 and stable analogues of PGI2. We will also address implications of this signaling pathway for understanding of the vascular effects of clinically relevant drugs that may affect production of PGI2.
Figure 1.
Hypothetical model of the cellular localization of enzymes responsible for PGI2 synthesis and subsequent activation of PPARδ. Increases in intracellular Ca2+ levels cause activation and translocation of cPLA2α from the cytoplasm to the nuclear envelope. (Complexity of cPLA2α activation has been simplified to include only activation by increased Ca2+ levels). Mobilization of arachidonic acid from phospholipids by cPLA2α provides substrate for enzyme activity of COX-1 or COX-2 and PGIS resulting in production of PGI2 and subsequent activation of nuclear receptor PPARδ. PPARδ-RXR heterodimers undergo a conformational shift and this causes dismissal of the co-repressor complex in exchange for co-activator proteins thereby resulting in enhanced PPARδ target gene expression. Exact nature of agonists and physical stimuli responsible for PGI2-induced activation of PPARδ remains to be determined (denoted by ? in the figure). cPLA2=cytosolic phospholipase A2; ER = endoplasmic reticulum; NE = nuclear envelope; AA = arachidonic acid; PGI2 = prostacyclin; PGH2 = prostaglandin H2; PGIS = prostacyclin synthase; RXR = retinoid X receptor; CP = co-activator proteins; PPRE = PPARδ responsive element.
Vascular protective effects of PGI2
In mice, genetic inactivation of PGIS causes significant alterations in vascular wall architecture including thickening of arterial media attended by increased arterial blood pressure (21). Moreover, phenotypic changes in the cardiovascular system substantially progress as PGIS null mice become older. These findings suggest that, in the cardiovascular system, loss of PGI2 exacerbates phenotypic characteristics of aging.
The vasoprotective function of PGI2 was also corroborated by studies of IP-deficient mice. Under basal conditions, IP-deficient mice had normal blood pressure and did not suffer from spontaneous thrombosis (22,23). However, deletion of IP receptors predisposes mice to an exaggerated response to thrombotic stimuli, accelerated atherosclerosis, and augmented intimal hyperplasia after vascular injury (22,23). Moreover, hypertensive response to a high-salt diet is exacerbated in IP-deficient mice (24). These findings indicate that production of PGI2 and activation of IP receptors are essential mechanisms responsible for preservation of normal vascular function. Additional support for vasoprotective function of PGI2 is provided by the results of gene delivery studies demonstrating that elevation of PGI2 biosynthesis by expression of recombinant COX-1 and PGIS attenuates aberrant remodeling of arterial wall after injury, exerts beneficial effect on pulmonary hypertension, and prevents arterial thrombosis in ischemic cerebrovascular disease (25–28). Traditionally, protective vascular effects of PGI2 have been ascribed to the activation of IP receptors located on cell membrane. However, more recently, it has been recognized that activation of PPARδ represents a previously unappreciated nuclear signaling mechanism that might contribute to vasoprotective and regenerative effects of PGI2.
Vascular protective effects of PPARδ
PPARδ is a nuclear receptor that functions as a ligand-activated transcription factor. Ligand-induced activation of PPARδ causes formation of heterodimers with a retinoid X receptor and subsequent binding to specific PPAR responsive elements in promoter regions of target genes (29,30; Figure 1). PPARδ is ubiquitously expressed in various tissues, including vascular endothelial and smooth muscle cells (7). Vascular effects of PPARδ activation involve: a) direct effects induced by activation of PPARδ in blood vessel wall, and b) indirect effects mediated by systemic alterations in glucose, lipid, and lipoprotein metabolism (31). PPARδ agonists exert anti-atherosclerotic effects in murine models of hypercholesterolemia (32,33). In the vascular endothelium, major direct effects of PPARδ activation are anti-inflammatory effects, prevention of apoptosis, and stimulation of angiogenesis (32–38). In smooth muscle cells, PPARδ is responsible for inhibition of migration, and neointimal formation however controversial results have been reported regarding the effect on smooth muscle cells proliferation (39,40). Moreover, activation of PPARδ in smooth muscle cells exerts anti-inflammatory effects (41) and attenuates apoptotic cell death induced by oxidized low-density lipoprotein (42). PGI2 released by shear stress from endothelium may activate PPARδ in smooth muscle cells thereby promoting conversion of synthetic to contractile phenotype (20). Generally, the effects on vascular wall mediated by activation of PPARδ are considered beneficial, with the exception of angiogenesis, which under certain conditions might contribute to tumor growth (31–45).
Preclinical studies in insulin-resistant primates have demonstrated that a PPARδ agonist, GW501516, decreases plasma triglyceride, LDL cholesterol, and increases levels of HDL cholesterol (46). Based on these observations, clinical development of PPARδ agonists has been mostly directed towards treatment of dyslipidemia (47–50). Prevention of dyslipidemia and the beneficial effects of PPARδ activation on insulin resistance and glucose homeostasis protect vascular endothelium from well-established risk factors responsible for initiation of endothelial dysfunction and progression of vascular disease, including atherosclerosis. In this regard, it is interesting to note that recent findings demonstrate that stable analogue of PGI2, beraprost, exerts beneficial effects on metabolic syndrome and dyslipidemia (51,52). Currently, the exact mechanisms underlying these effects are poorly understood, but could be in part mediated by activation of nuclear receptor(s) including PPARδ. Thus, both direct effects of PPARδ activation on blood vessel wall and indirect effects mediated by normalization of metabolic functions are essential for protection of vascular wall (31). Interventions designed to increase production of endogenous PPARδ ligands or treatment with selective PPARδ agonists are currently being studied as novel therapeutic strategies. However, efforts in drug development designed to harness therapeutic effects of PPARδ agonists have yet to produce a drug approved for specific therapeutic application in general population.
PGI2:aputative endogenous ligand for PPARδ
Vascular endothelium has the highest production of PGI2 under basal conditions as well as during activation of arachidonic acid metabolism by vascular injury (22,53). Although smooth muscle cells and adventitia may generate PGI2, the levels in these layers are lower as compared to endothelium. Consistent with role of PGI2 in nuclear signaling, enzymes required for synthesis of PGI2 in endothelium localize around the nucleus (Figure 1; 15). Prior studies have provided evidence that under in vitro conditions PPARδ can be activated by several different products of arachidonic acid metabolism and stable analogues of PGI2 (54,55). However, inability of exogenous PGI2 itself to activate PPARδ under some in vitro conditions (55) is most likely result of PGI2 instability in neutral and acidic buffer. PGI2 is quickly hydrolyzed (30–120 s) to a pharmacologically inactive product, 6-ketoPGF1α. Nevertheless, relevant to the concept of PGI2 as an endogenous PPARδ ligand in endothelium, several studies have demonstrated that stable analogues of PGI2 [including carbaprostacyclin (cPGI2) and iloprost] behave as PPARδ ligands (18,55). The first evidence supporting the role of PGI2 as an endogenous ligand for PPARδ in vivo was reported in studies of mechanisms responsible for embryo implantation (18). These findings provided impetus for re-assessment of signaling mechanisms that may help to explain beneficial effects of PGI2 in different tissues including cells of blood vessel wall. As mentioned above, studies with stable analogues of PGI2 consistently demonstrated that these compounds activate PPARδ, thereby providing support for the importance of endogenous PGI2 in activation of PPARδ. In agreement with this concept, a more recent study presented the crystal structure of the PPARδ ligand-binding domain bound to iloprost thus establishing evidence for direct interaction between iloprost with PPARδ (56).
It appears that significantly higher concentrations of PGI2 analogues are required for activation of nuclear PPARδ receptor as compared to concentrations that are able to stimulate cell membrane IP receptors (57). Interestingly, cicaprost (stable PGI2 analogue) is a highly selective IP agonist that does not activate PPARδ even in very high (μM) concentrations (9,18,55). This pharmacological property of cicaprost has been successfully employed in studies designed to dissect contribution of IP receptors and PPARs to the vascular effects of stable PGI2 analogues (9,58). Although iloprost and cPGI2 stimulated angiogenesis in vivo, cicaprost had no effect, suggesting that activation of PPARs might be the mechanism underlying the angiogenic effects of PGI2 analogues (58).
We would also like to point out that although available evidence is consistent with the concept that PGI2 signaling might depend in part on activation of PPARδ, direct in vivo evidence supporting this hypothesis is currently missing. In this regard, future studies in IP-receptor-deficient mice may help to define the effects of endogenous PGI2 dependent on activation of PPARδ. Of note, reported lack of PPARδ ligands effects on embryonic development in IP-receptor deficient mice (60), suggests that, in some cells, activation of PPARδ might depend on intact function of IP receptors. Several prior studies demonstrated that drugs causing an increase in cyclic AMP or activation of protein kinase A (PKA) enhance basal and stimulated activity of PPARδ (61–64). Because activation of IP receptors is coupled to increased production of cyclic AMP, it is possible that PGI2-induced elevation of cyclic AMP might participate in regulation of activity of PPARδ. Because complex mechanisms underlying PPARδ signaling involve dissociation of co-repressor proteins and recruitment of co-activator proteins, the exact molecular targets affected by elevation of cyclic AMP or activation of PKA are currently unknown. Thus, additional studies are required to determine the mechanisms underlying interaction between IP and PPARδ signaling in blood vessel walls.
Although the majority of studies suggest that PGI2 is a ligand for PPARδ, there are also reports demonstrating an ability of PGI2 to activate PPARα. Previous in vitro studies established that stable analogues of PGI2, including iloprost and cPGI2 (but not cicaprost), may also behave as ligands for PPARα (55) Consistent with this concept, it has been demonstrated that PGI2 released from endothelium by shear stress causes synthetic-to contractile phenotypic modulation in smooth muscle cells by activation of both PPARα and PPARδ (20). In addition, it has been suggested that the angiogenic effects of PGI2 in some experimental conditions are dependent on activation of PPARα (59). Relevant to this concept, crystal structures of PPARα ligand domains bound to iloprost have been reported, thus providing structural basis for the recognition of PPARα by iloprost (56). Thus, it appears that PGI2 might influence vascular function by activation of PPARα. The exact conditions and mechanisms responsible for activation of this signaling mechanism remain to be determined.
Angiogenesis
The importance of arachidonic acid metabolism via the COX pathway in angiogenesis was recognized more than 20 years ago (65,66). Initially, it was observed that pro-angiogenic proteins, including angiogenin and acidic and basic fibroblast growth factors (aFGF and bFGF), stimulate production of PGI2 and that this effect is an important component of their ability to promote angiogenesis. These studies were followed by reports demonstrating that vascular endothelial growth factor (VEGF) is also a potent stimulator of PGI2 production (6,67). By contrast, it was demonstrated that a stable PGI2 analogue, SM-10902, promotes wound healing by stimulation of angiogenesis (68). Further analysis supported the role of PGI2 as possible ligand for nuclear receptors, leading to speculation that activation of PPARδ in endothelial cells could be important for PGI2 signaling (69). Moreover, in an in vivo study designed to examine contribution of nuclear receptors to the stimulatory effect of stable PGI2 analogues on angiogenesis, the authors found that iloprost and cPGI2 upregulated expression of VEGF mRNA and protein (58). Inactivation of VEGF signaling inhibited the pro-angiogenic effect of iloprost and cPGI2. Based on prior studies demonstrating that iloprost and cPGI2 are ligands for PPARδ (and in some tissues for PPARα), the authors proposed that activation of PPAR(s) is the most likely mechanism of the observed angiogenic effects (58). This concept was further extended and refined by studies in human EPCs (9). Since specific molecular markers for identification of EPCs have not been established, studies were performed on so-called “late outgrowth” EPCs. Obtained findings suggest that these cells possess intrinsically high production of PGI2 (9). One possibility is that the angiogenic function of EPCs is dependent on PGI2-induced activation of PPARδ. Indeed, in vitro and in vivo analysis of angiogenic function of EPCs in response to endogenous PGI2 or treatment with iloprost pointed to PPARδ as a major molecular target (9). Further research revealed that the angiogenic effect of PPARδ activation in human EPCs is dependent on increased production of tetrahydrobiopterin, an enzymatic cofactor (70). The regenerative effect of tetrahydrobiopterin was independent of endothelial nitric oxide synthase (eNOS) activation thus suggesting that tetrahydrobiopterin has effects on EPCs that could not be explained by its role as a cofactor for eNOS (70).
Existing evidence supports the concept that PPARδ (unlike PPARα and PPARγ) is pro-angiogenic (38). Treatment of cultured human endothelial cells with GW501516, a selective PPARδ agonist, stimulates proliferation and increases expression and production of VEGF (36). Consistent with in vitro observations, activation of PPARδ in vivo also causes angiogenesis (36). Other target genes that might be responsible for PPARδ-induced stimulation of angiogenesis have been identified including angiopoietin-like protein 4, calcineurin, cyclin-dependent kinase inhibitor 1c (Cdkn 1c), and Cl− intracellular channel protein 4 (CLIC4; 38). However, PPARδ activation has an inhibitory effect on some anti-angiogenic proteins including thrombospondin-1 and cellular retinol-binding protein-1 (CRBP1; 38). More recent studies indicate that PPARδ activation enhances regenerative and angiogenic capacity of EPCs (9,70–72).
Efforts to harness potentially beneficial vascular (and metabolic) effects of PPARδ agonists have been hampered by the tumorigenic effects of this signal transduction pathway. Although the exact role of PGI2/ PPARδ signaling in pathogenesis in cancer remains to be determined (73,74), existing literature suggests that tumor angiogenesis could be stimulated by activation of PPARδ (31, 43–45). This issue has to be approached with high degree of caution, and requires very careful monitoring of patients entering clinical trials designed to test therapeutic safety and efficacy of compounds that may activate PPARδ.
Pulmonary hypertension
Stable analogues of PGI2 are essential drugs used in therapy of pulmonary hypertension. It is generally accepted that the beneficial effects of these compounds are, for the most part, mediated by activation of IP receptors on vascular smooth muscle cells and the resulting decrease in pulmonary vascular resistance (75). In addition, these salutary effects are dependent on the ability of PGI2 analogues to arrest and reverse pathological remodeling of pulmonary vascular wall. Emerging experimental evidence suggests that some of the long-term benefits could be mediated by activation of PPARδ receptors. For instance, activation of PPARδ in the lungs inhibits proliferation of fibroblasts and the subsequent aberrant vascular remodeling responsible for narrowing of pulmonary blood vessel diameter (57). In addition, more recent findings indicate that, in children with pulmonary hypertension, the PGI2 analogue treprostinil increases the number and angiogenic potential of EPCs (12), thereby stimulating endothelial repair and formation of new blood vessels. These observations may help to explain clinically observed long-term therapeutic effects of PGI2 analogues that could not be attributed to pulmonary vasodilatation in patients resistant to the acute vasodilator effect of PGI2 (75,76).
Adverse cardiovascular effects of COX inhibitors
Non-steroidal anti-inflammatory drugs (NSAIDs) exert their therapeutic effects by inhibiting COX isoforms. However, long-term use of NSAIDs as anti-inflammatory agents and analgesics is associated with adverse cardiovascular effects (77). Existing evidence suggests that older patients with cardiovascular risk factors are particularly prone to develop adverse effects including high blood pressure, edema, and congestive heart failure. Moreover, the detrimental effects of these drugs on cardiovascular function predispose treated patients to development of stroke and myocardial infarction (77). Although the mechanisms underlying adverse effects are quite complex and incompletely understood, inactivation of PGI2 synthesis is considered a major mechanism contributing to development of adverse cardiovascular effects of COX inhibitors (78). In this regard, recognition of nuclear PPARδ receptors as potentially important target for PGI2 signaling may provide new insights. For example, emerging evidence suggests that loss of PGI2/PPARδ signaling causes apoptosis of endothelial cells (79) and impairment of regenerative capacity of EPCs (9,72). In addition, aging is associated reduced expression of PPARδ (80). This may increase the vulnerability of an aging population to drugs that interfere with activation of PPARδ. However, the exact contribution of diminishing PGI2/PPARδ signaling to adverse cardiovascular effects of COX inhibitors in humans is unknown and remains to be determined.
Concluding remarks
Existing evidence is consistent with the concept that stable analogues of PGI2 might exert vascular effects in part by activation of PPARδ. More recent findings suggest that this signaling pathway may also contribute to vascular regenerative functions of these compounds. However, direct in vivo evidence demonstrating ability of endogenous PGI2 to behave as PPARδ ligand in vascular tissue remains to be established. Further improvements in understanding of PGI2/PPARδ signaling in the human cardiovascular system will certainly help to optimize a number of therapeutic interventions dependent on modulation of arachidonic acid metabolism and subsequent activation of nuclear receptor(s). Future efforts in this area might also provide important new insights into molecular mechanisms underlying cardiovascular effects of widely used COX inhibitors.
Acknowledgments
This work was supported by the National Institutes of Health grants HL-91867 and HL-111062 to Z.S.K., by the American Heart Association Scientist Development Grants 08-35436N to A.S., and 09-DG2190046 to T.H., and by the Mayo Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moncada S, et al. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976;263:663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- 2.Shepherd JT, Katusic ZS. Endothelium-derived vasoactive factors: I. Endothelium-dependent relaxation. Hypertension. 1991;18:III76–III85. doi: 10.1161/01.hyp.18.5_suppl.iii76. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi T, et al. Roles of thromboxane A2 and prostacyclin in the development of atherosclerosis in apoE-deficient mice. J Clin Invest. 2004;114:784–794. doi: 10.1172/JCI21446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egan KM, et al. COX-2-derived prostacyclin confers atheroprotection on female mice. Science. 2004;306:1954–1957. doi: 10.1126/science.1103333. [DOI] [PubMed] [Google Scholar]
- 5.Sun D, et al. Enhanced release of prostaglandins contributes to flow-induced arteriolar dilation in eNOS knockout mice. Circ Res. 1999;85:288–293. doi: 10.1161/01.res.85.3.288. [DOI] [PubMed] [Google Scholar]
- 6.Murohara T, et al. Vascular endothelial growth factor/vascular permeability factor enhances vascular permeability via nitric oxide and prostacyclin. Circulation. 1998;97:99–107. doi: 10.1161/01.cir.97.1.99. [DOI] [PubMed] [Google Scholar]
- 7.Kawabe J, et al. Prostaglandin I2 promotes recruitment of endothelial progenitor cells and limits vascular remodeling. Arterioscler Thromb Vasc Biol. 2010a;30:464–470. doi: 10.1161/ATVBAHA.109.193730. [DOI] [PubMed] [Google Scholar]
- 8.Kawabe J, et al. Prostacyclin in vascular diseases. Recent insights and future perspectives. Circ J. 2010b;74:836–843. doi: 10.1253/circj.cj-10-0195. [DOI] [PubMed] [Google Scholar]
- 9.He T, et al. Angiogenic function of prostacyclin biosynthesis in human endothelial progenitor cells. Circ Res. 2008;103:80–88. doi: 10.1161/CIRCRESAHA.108.176057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santhanam AV, et al. Endothelial progenitor cells stimulate cerebrovascular production of prostacyclin by paracrine activation of cyclooxygenase-2. Circ Res. 2007;100:1379–1388. doi: 10.1161/01.RES.0000265848.55035.5d. [DOI] [PubMed] [Google Scholar]
- 11.Di Stefano R, et al. The prostacyclin analogue iloprost increases circulating endothelial progenitor cells in patients with critical limb ischemia. Thromb Haemost. 2008;100:871–877. [PubMed] [Google Scholar]
- 12.Smadja DM, et al. Treprostinil increases the number and angiogenic potential of endothelial progenitor cells in children with pulmonary hypertension. Angiogenesis. 2011;14:17–27. doi: 10.1007/s10456-010-9192-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spencer AG, et al. Subcellular localization of prostaglandin endoperoxide H synthases-1 and -2 by immunoelectron microscopy. J Biol Chem. 1998;273:9886–9893. doi: 10.1074/jbc.273.16.9886. [DOI] [PubMed] [Google Scholar]
- 14.Liou JY, et al. Colocalization of prostacyclin synthase with prostaglandin H synthase-1 (PGHS-1) but not phorbol ester-induced PGHS-2 in cultured endothelial cells. J Biol Chem. 2000;275:15314–15320. doi: 10.1074/jbc.275.20.15314. [DOI] [PubMed] [Google Scholar]
- 15.Hirabayashi T, Shimizu T. Localization and regulation of cytosolic phospholipase A2. Biochim Biophys Acta. 2000;1488:124–138. doi: 10.1016/s1388-1981(00)00115-3. [DOI] [PubMed] [Google Scholar]
- 16.Schievella AR, et al. Calcium-mediated translocation of cytosolic phospholipase A2 to the nuclear envelope and endoplasmic reticulum. J Biol Chem. 1995;270:30749–30754. doi: 10.1074/jbc.270.51.30749. [DOI] [PubMed] [Google Scholar]
- 17.Grewal S, et al. Cytosolic phospholipase A2-α and cyclooxygenase-2 localize to intracllular membranes of EA. hy.926 endothelial cells that are distinct from the endoplasmic reticulum and the Golgi apparatus. FEBS Lett. 2005;272:1278–1290. doi: 10.1111/j.1742-4658.2005.04565.x. [DOI] [PubMed] [Google Scholar]
- 18.Lim H, et al. Cyclo-oxygenase-2-derived prostacyclin mediates embryo implantation in the mouse via PPARδ. Genes Develop. 1999;13:1561–1574. doi: 10.1101/gad.13.12.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim H, Dey SK. PPARδ functions as a prostacyclin receptor in blastocyst implantation. Trends Endocrinol Metab. 2000;11:137–142. doi: 10.1016/s1043-2760(00)00243-5. [DOI] [PubMed] [Google Scholar]
- 20.Tsai MC, et al. Shear stress induces synthetic-to-contractile phenotypic modulation in smooth muscle cells via peroxisome proliferator-activated receptor alpha/delta activations by prostacyclin released by sheared endothelial cells. Circ Res. 2009;105:471–480. doi: 10.1161/CIRCRESAHA.109.193656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yokoyama C, et al. Prostacyclin-deficient mice develop ischemic renal disorders, including nephrosclerosis and renal infarction. Circulation. 2002;106:2397–2403. doi: 10.1161/01.cir.0000034733.93020.bc. [DOI] [PubMed] [Google Scholar]
- 22.Murata T, et al. Altered pain perception and inflammatory response in mice lacking prostacyclin receptor. Nature. 1997;388:678–682. doi: 10.1038/41780. [DOI] [PubMed] [Google Scholar]
- 23.Cheng Y, et al. Role of prostacyclin in the cardiovascular response to thromboxane A2. Science. 2002;296:539–541. doi: 10.1126/science.1068711. [DOI] [PubMed] [Google Scholar]
- 24.Francois H, et al. Prostacyclin protects against elevated blood pressure and cardiac fibrosis. Cell Metab. 2005;2:201–207. doi: 10.1016/j.cmet.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Zoldhelyi P, et al. Prevention of arterial thrombosis by adenovirus-mediated transfer of cyclooxygenase gene. Circulation. 1996;93:10–17. doi: 10.1161/01.cir.93.1.10. [DOI] [PubMed] [Google Scholar]
- 26.Nagaya N, et al. Gene transfer of human prostacyclin synthase ameliorates monocrotaline-induced pulmonary hypertension in rats. Circulation. 2000;102:2005–2010. doi: 10.1161/01.cir.102.16.2005. [DOI] [PubMed] [Google Scholar]
- 27.Yamada M, et al. Prostacyclin synthase gene transfer modulates cyclooxygenase-2 derived prostanoid synthesis and inhibits neointimal formation in rat balloon-injured arteries. Arterioscler Thromb Vasc Biol. 2002;22:256–262. doi: 10.1161/hq0202.104123. [DOI] [PubMed] [Google Scholar]
- 28.Lin H, et al. Cyclooxygenase-1 and bicistronic cyclooxygenase-1/prostacyclin synthase gene transfer protect against ischemic cerebral infarction. Circulation. 2002;105:1962–1969. doi: 10.1161/01.cir.0000015365.49180.05. [DOI] [PubMed] [Google Scholar]
- 29.Barish GD, et al. PPARδ: a dagger in the heart of the metabolic syndrome. J Clin Invest. 2006;116:590–597. doi: 10.1172/JCI27955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brunelli L, et al. Peroxisome proliferator-activated receptor-δ upregulates 14–3–3ε in human endothelial cells via CCAAT/enhancer binding protein-β. Circ Res. 2007;100:e59–e71. doi: 10.1161/01.RES.0000260805.99076.22. [DOI] [PubMed] [Google Scholar]
- 31.Wang D, et al. Crosstalk between peroxisome proliferator-activated receptor δ and VEGF stimulates cancer progression. Proc Natl Acad Sci USA. 2006;103:19069–19074. doi: 10.1073/pnas.0607948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barish GD, et al. PPARdelta regulates multiple proinflammatory pathways to suppress atherosclerosis. Proc Natl Acad Sci USA. 2008;105:4271–4276. doi: 10.1073/pnas.0711875105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takata Y, et al. PPARdelta-mediated antiinflammatory mechanisms inhibit angiotensin II-accelerated atherosclerosis. Proc Natl Acad Sci USA. 2008;105:4277–4282. doi: 10.1073/pnas.0708647105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee CH, et al. Transcriptional repression of atherogenic inflammation: modulation by PPARdelta. Science. 2003;302:453–457. doi: 10.1126/science.1087344. [DOI] [PubMed] [Google Scholar]
- 35.Liou JY, et al. Protection of endothelial survival by peroxisome proliferator-activated receptor-delta mediated 14–3–3 upregulation. Arterioscler Thromb Vasc Biol. 2006;26:1481–1487. doi: 10.1161/01.ATV.0000223875.14120.93. [DOI] [PubMed] [Google Scholar]
- 36.Piqueras L, et al. Activation of PPARbeta/delta induces endothelial cell proliferation and angiogenesis. Arterioscler Thromb Vasc Biol. 2007;27:63–69. doi: 10.1161/01.ATV.0000250972.83623.61. [DOI] [PubMed] [Google Scholar]
- 37.Fan Y, et al. Suppression of pro-inflammatory adhesion molecules by PPAR-delta in human vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:315–321. doi: 10.1161/ATVBAHA.107.149815. [DOI] [PubMed] [Google Scholar]
- 38.Bishop-Bailey D. PPARs and angiogenesis. Biochem Soc Trans. 2011;39:1601–1605. doi: 10.1042/BST20110643. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J, et al. Peroxisome proliferator-activated receptor delta is upregulated during vascular lesion formation and promotes post-confluent cell proliferation in vascular smooth muscle cells. J Biol Chem. 2002;277:11505–11512. doi: 10.1074/jbc.M110580200. [DOI] [PubMed] [Google Scholar]
- 40.Lim HJ, et al. PPAR delta agonist L-165041 inhibits rat vascular smooth muscle cell proliferation and migration via inhibition of cell cycle. Atherosclerosis. 2009;202:446–454. doi: 10.1016/j.atherosclerosis.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 41.Kim HJ, et al. Transforming growth factor-β1 is a molecular target for the peroxisome proliferator-activated receptor δ. Circ Res. 2008;102:193–200. doi: 10.1161/CIRCRESAHA.107.158477. [DOI] [PubMed] [Google Scholar]
- 42.Kim HJ, et al. Peroxisome proliferator-activated receptor {delta} regulates extracellular matrix and apoptosis of vascular smooth muscle cells through the activation of transforming growth factor-{beta}1/Smad3. Circ Res. 2009;105:16–24. doi: 10.1161/CIRCRESAHA.108.189159. [DOI] [PubMed] [Google Scholar]
- 43.Abdollahi A, et al. Transcriptional network governing the angiogenic switch in human pancreatic cancer. Proc Natl Acad Sci USA. 2007;104:12890–12895. doi: 10.1073/pnas.0705505104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bishop-Bailey D, Swales KE. The role of PPARs in the endothelium: implications for cancer therapy. PPAR Res. 2008:article ID 904251, 12. doi: 10.1155/2008/904251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wagner KD, Wagner N. Peroxisome proliferator-activated receptor beta/delta (PPARβ/δ) acts as regulator of metabolism linked to multiple cellular functions. Pharmacol Therap. 2010;125:423–435. doi: 10.1016/j.pharmthera.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 46.Oliver WR, Jr, et al. A selective peroxisome proliferator-activated receptor δ agonist promotes reverse cholesterol transport. Proc Natl Acad Sci USA. 2001;98:5306–5311. doi: 10.1073/pnas.091021198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sprecher DL, et al. Triglyceride:high-density lipoprotein cholesterol effects in healthy subjects administered a peroxisome proliferator activated receptor δ agonist. Arterioscler Thromb Vasc Biol. 2007;27:359–365. doi: 10.1161/01.ATV.0000252790.70572.0c. [DOI] [PubMed] [Google Scholar]
- 48.Risérus U, et al. Activation of peroxisome proliferator-activated receptor (PPAR)δ promotes reversal of multiple metabolic abnormalities, reduces oxidative stress, and increases fatty acid oxidation in moderately obese men. Diabetes. 2008;57:332–339. doi: 10.2337/db07-1318. [DOI] [PubMed] [Google Scholar]
- 49.Brown JD, et al. VLDL hydrolysis by hepatic lipase regulates PPARδ transcriptional responses. PLoS ONE. 2011;6:1–13. doi: 10.1371/journal.pone.0021209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ooi EMM, et al. Mechanism of action of a peroxisome proliferator-activated receptor (PPAR)-δ agonist on lipoprotein metabolism in dyslipidemic subjects with central obesity. J Clin Endocrinol Metab. 2011;96:E1568–E1576. doi: 10.1210/jc.2011-1131. [DOI] [PubMed] [Google Scholar]
- 51.Sato N, et al. The prostacyclin analog beraprost sodium ameliorates characteristics of metabolic syndrome in obese Zucker (fatty) rats. Diabetes. 2010;59:1092–1100. doi: 10.2337/db09-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watanabe M, et al. Improvement of dyslipidemia in OLETF rats by the prostaglandin I2 analog beraprost sodium. Prostaglandins Other Lipid Mediat. 2010;93:14–19. doi: 10.1016/j.prostaglandins.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 53.Moncada S. Adventures in vascular biology: a tale of two mediators. Philos Trans R Soc Lond B Biol Sci. 2008;361:735–759. doi: 10.1098/rstb.2005.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hertz R, et al. Activation of gene transcription by prostacyclin analogues is mediated by the peroxisome-proliferators-activated receptor (PPAR) Eur J Biochem. 1996;15:242–247. doi: 10.1111/j.1432-1033.1996.00242.x. [DOI] [PubMed] [Google Scholar]
- 55.Forman BM, et al. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci USA. 1997;94:4312–4317. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jin L, et al. Structural basis for Iloprost as a dual peroxisome proliferator-activated receptor α/δ agonist. J Biol Chem. 2011;286:31473–31479. doi: 10.1074/jbc.M111.266023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ali FY, et al. Role of prostacyclin versus peroxisome proliferator-activated receptor β receptors in prostacyclin sensing by lung fibroblasts. Am J Respir Cell Mol Biol. 2006;34:242–246. doi: 10.1165/rcmb.2005-0289OC. [DOI] [PubMed] [Google Scholar]
- 58.Pola R, et al. Comparative analysis of the in vivo angiogenic properties of stable prostacyclin analogs: a possible role for peroxisome proliferator-activated receptors. J Molec Cell Cardiol. 2004;36:363–370. doi: 10.1016/j.yjmcc.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 59.Biscetti F, et al. Peroxisome proliferator-activated receptor alpha is crucial for iloprost-induced in vivo angiogenesis and vacular endothelial growth factor upregulation. J Vasc Res. 2008;46:103–108. doi: 10.1159/000143793. [DOI] [PubMed] [Google Scholar]
- 60.Huang JC, et al. Prostacyclin receptor signaling and early embryo development in the mouse. Human Reproduc. 2007;22:2851–2856. doi: 10.1093/humrep/dem304. [DOI] [PubMed] [Google Scholar]
- 61.Lazennec G, et al. Activation of peroxisome proliferator-activated receptors (PPARs) by their ligands and protein kinase A activators. Molec Endocrinol. 2000;14:1962–1975. doi: 10.1210/mend.14.12.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hansen JB, et al. Peroxisome proliferator-activated receptor δ (PPARδ)-mediated regulation of preadipocyte proliferation and gene expression is dependent on cAMP signaling. J Biol Chem. 2001;276:3175–3182. doi: 10.1074/jbc.M005567200. [DOI] [PubMed] [Google Scholar]
- 63.Krogsdam AM, et al. Nuclear receptor corepressor-dependent repression of peroxisome-proliferator-activated receptor δ-mediated transactivation. Biochem J. 2002;363:157–165. doi: 10.1042/0264-6021:3630157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blanquart C, et al. Peroxisome proliferator-activated receptors: regulation of transcriptional activities and roles in inflammation. J Steroid Biochem Molec Biol. 2003;85:267–273. doi: 10.1016/s0960-0760(03)00214-0. [DOI] [PubMed] [Google Scholar]
- 65.Kuwashima L, et al. Stimulation of endothelial cell prostacyclin release by retina-derived factors. Invest Ophthalmol Vis Sci. 1988;29:1213–1220. [PubMed] [Google Scholar]
- 66.Bicknell R, Vallee BL. Angiotensin stimulates endothelial cell prostacyclin secretion by activation of phospholipase A2. Proc Natl Acad Sci USA. 1989;86:1573–1577. doi: 10.1073/pnas.86.5.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.He H, et al. Vascular endothelial growth factor signals endothelial cell production of nitric oxide and prostacyclin through flk-1/KDR activation of c-Src. J Biol Chem. 1999;274:25130–25135. doi: 10.1074/jbc.274.35.25130. [DOI] [PubMed] [Google Scholar]
- 68.Yamamoto T, et al. Effect of topical application of a stable prostacyclin analogue, SM-10902 on wound healing in diabetic mice. Eur J Pharmacol. 1996;302:53–60. doi: 10.1016/0014-2999(96)00019-2. [DOI] [PubMed] [Google Scholar]
- 69.Spisni E, et al. Colocalization of prostacyclin (PGI2) synthase-caveolin-1 in endothelial cells and new roles for PGI2 in angiogenesis. Exp Cell Res. 2001;266:31–43. doi: 10.1006/excr.2001.5198. [DOI] [PubMed] [Google Scholar]
- 70.He T, et al. Activation of peroxisome proliferator-activated receptor-δ enhances regenerative capacity of human endothelial progenitor cells by stimulating biosynthesis of tetrahydrobiopterin. Hypertension. 2011;58:287–294. doi: 10.1161/HYPERTENSIONAHA.111.172189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Han JK, et al. Peroxisome proliferator-activated receptor-δ agonist enhances vasculogenesis by regulating endothelial progenitor cells through genomic and nongenomic activations of the phosphatidylinositol 3-kinase/Akt pathway. Circulation. 2008;118:1021–1033. doi: 10.1161/CIRCULATIONAHA.108.777169. [DOI] [PubMed] [Google Scholar]
- 72.Han JK, et al. Peroxisome proliferator-activated receptor-δ activates endothelial progenitor cells to induce angiomyogenesis through matrix metalol-proteinase-9-mediated insulin-like growth factor-1 paracrine networks. Eur Heart J. 2011 doi: 10.1093/eurheartj/ehr365. in press. [DOI] [PubMed] [Google Scholar]
- 73.Foreman JE, et al. Regulation of peroxisome proliferator-activated receptor-β/δ by the APC/β-CATENIN pathway and nonsteroidal antiinflammatory drugs. Mol Carcinog. 2009;48:942–952. doi: 10.1002/mc.20546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu KK, Liou JY. Cyclooxygenase inhibitors induce colon cancer cell apoptosis via PPARdelta →14–3–3epsilon pathway. Methods Mol Biol. 2009;512:295–307. doi: 10.1007/978-1-60327-530-9_16. [DOI] [PubMed] [Google Scholar]
- 75.Fishman AP. Pulmonary hypertension—beyond vasodilator therapy. N Eng J Med. 1998;338:321–322. doi: 10.1056/NEJM199801293380609. [DOI] [PubMed] [Google Scholar]
- 76.Okano Y, et al. Orally active prostacyclin analogue in primary pulmonary hypertension. Lancet. 1997;349:1365. doi: 10.1016/S0140-6736(97)24019-5. [DOI] [PubMed] [Google Scholar]
- 77.White WB. Cardiovascular effects of the cyclooxygenase inhibitors. Hypertension. 2007;49:408–418. doi: 10.1161/01.HYP.0000258106.74139.25. [DOI] [PubMed] [Google Scholar]
- 78.Grosser T, et al. Emotion recollected in tranquility: lessons learned from the COX-2 saga. Annu Rev Med. 2010;61:17–33. doi: 10.1146/annurev-med-011209-153129. [DOI] [PubMed] [Google Scholar]
- 79.Liou JY, et al. Nonsteroidal anti-inflammatory drugs induced endothelial apoptosis by perturbing peroxisome proliferator-activated receptor-δ transcriptional pathway. Molec Pharmacol. 2008;74:1399–1406. doi: 10.1124/mol.108.049569. [DOI] [PubMed] [Google Scholar]
- 80.Nilsson E, et al. Regulation of skeletal muscle PPAR delta mRNA expression in twins. J Physiol. 2007;584:1011–1017. doi: 10.1113/jphysiol.2007.140673. [DOI] [PMC free article] [PubMed] [Google Scholar]