Abstract
The Ca2+/Calmodulin(CaM)-dependent protein kinase II (CaMKII) is activated by Ca2+/CaM, but becomes partially autonomous (Ca2+-independent) upon autophosphorylation at T286. This hallmark feature of CaMKII regulation provides a form of molecular memory and is indeed important in long-term potentiation (LTP) of excitatory synapse strength and memory formation. However, emerging evidence supports a direct role in information processing, while storage of synaptic information may instead be mediated by regulated interaction of CaMKII with the NMDA receptor (NMDAR) complex. These and other CaMKII regulation mechanisms are discussed here in the context of the kinase structure and their impact on post-synaptic functions. Recent findings also implicate CaMKII in long-term depression (LTD), as well as functional roles at inhibitory synapses, lending renewed emphasis on better understanding the spatio-temporal control of CaMKII regulation.
Keywords: CaMKII, NMDA receptor, Autonomy, Long-term Potentiation, Long-term Depression, Learning and Memory
Introduction
CaMKII has sparked the imagination of neuroscientists since its discovery [1, 2]. It is highly expressed in brain and is further enriched at excitatory synapses and their post-synaptic densities (PSDs). Moreover, its stimulation by Ca2+/CaM can also generate Ca2+-independent autonomous activity that outlasts this initial stimulus [2–4]. Thus, CaMKII is ideally poised to mediate the induction and maintenance of synaptic plasticity underlying learning and memory. Indeed, CaMKII can mediate LTP of excitatory synapse strength [5–9], both by increasing the number of synaptic AMPA receptors (AMPARs) [10–12] and their conductance [13–16] (see also [17–21]). Furthermore, knockout mice of CaMKIIα, the major isoform in brain, were the first transgenic animals with a behavioral phenotype in learning and memory [22].
Over 25 years of research has firmly connected CaMKII with LTP of excitatory synapses (see Table 1), however, recent findings have expanded this traditional view: CaMKII is also required for post-synaptic mechanisms induced by excitatory LTD-stimuli, both for depressing excitatory synapses [23] and for potentiating inhibitory synapses [24, 25]. Further complicating the matter, at least some of the LTP- and LTD-mechanisms require CaMKII autophosphorylation at T286 [25–27]. Thus, the spatial and temporal control of CaMKII regulation in neurons has gained much renewed significance [28]. Despite recent advances on the regulation and structure of CaMKII [29–33], both areas still pose many unsolved riddles. Here, we discuss our current understanding of CaMKII regulation mechanisms in the context of the kinase structure and their role in synaptic functions, with a focus on the CaMKIIα isoform. While pre-synaptic [34], pathological [35], cerebellar [36], and non-neuronal [30] functions of CaMKII are emerging, our discussion here is largely restricted to post-synaptic CaMKII mechanisms in hippocampal pyramidal neurons (where the functions of specific CaMKII regulation mechanisms in information processing and storage are understood best).
Table 1.
Manipulations that affect CaMKII function and their effects on hippocampal LTP and learning
| (or) Protein/ residue |
Manipulation (or modification) |
Biochemical effect (of the manipulation or the native residue) |
Hippocampal LTP |
Spatial Learning |
Refs |
|---|---|---|---|---|---|
| CaMKIIα | Inhibition | no activity | impaired | impaired | [5, 27] |
| KO | no α isoform protein | impaired | impaired | [6, 22] | |
| K42 | Required for ATP binding | N/A | N/A | ||
| R or M | ATP binding impaired | impaired | impaired | [145] | |
| S314 | (Phosphorylation) | Phos together with T305/306, but unknown function | N/A | N/A | [70, 71] |
| T253 | (Phosphorylation) | Phos. enhances PSD association | N/A | N/A | [146] |
| T286 | (Phosphorylation) | phos. generates autonomy; fast reaction (~12 sec−1) | N/A | N/A | [2, 29][75] |
| A | no phos.; autonomy impaired | impaired | impaired | [26] | |
| D (regulated overexpression) | phos. mimic; constitutively autonomous | impaired | impaired | [147] | |
| T305/306 | (Phosphorylation) | Phos. prevents stimulation by CaM; slow reaction | N/A | N/A | [70–72] |
| A/V or A/A | no phos.; always stimulatable | facilitated | impaired “unlearning” | [127] | |
| D | phos mimic; no stimulation | impaired | impaired | [127] | |
| (β A303R) | prevents CaM binding; no stimulation | normal | normal | [89] | |
| CaMKIIβ | KO | no β isoform protein | impaired | impaired | [89] |
| GluN2B (T286) | L1298A/R1300Q or R1300Q/S1303D | impairs CaMKII binding to its major site on GluN2B | impaired | impaired | [93, 94] |
CaMKII isoforms, variants, subunits and holoenzymes
Four homologous CaMKII isoforms (α, β, γ, and δ) are encoded by separate genes, with alternative splicing in their variable linker domain generating additional diversity [2](Fig. 1a). Splicing can affect targeting and regulation. For instance, the minor splice variant αB contains a nuclear localization signal [37], and the developmental variant βe lacks the F-actin binding found for the major β isoform [38]. Additionally, variants with shorter linkers (such as the major α isoform) appear to be generally less sensitive to Ca2+-stimulation [31, 39, 40]. All isoforms are expressed in brain, and CaMKIIβ and δ are prominent in the cerebellum [36, 41]. However, α and β make up the vast majority of neuronal forebrain CaMKII, with an α:β ratio of ~3:1 and together constituting >1% of total protein [2–4].
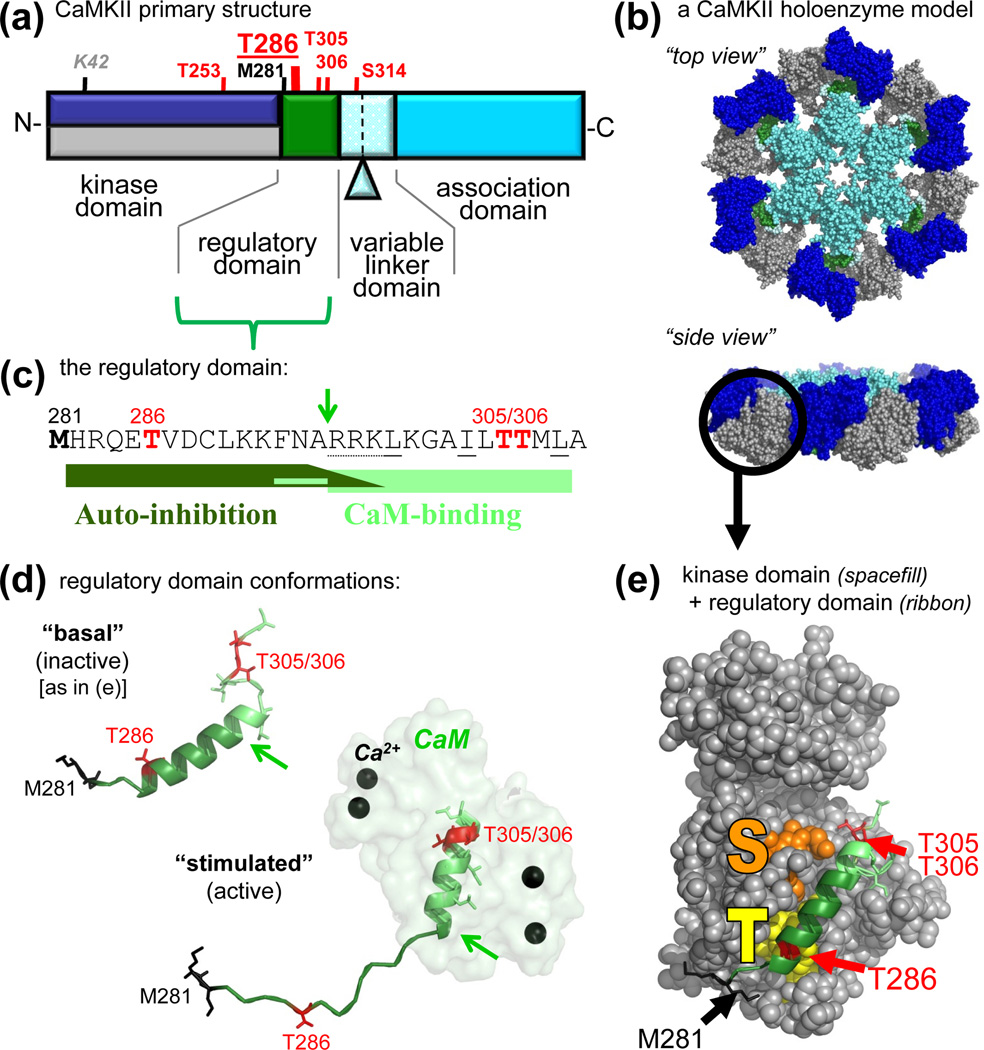
Figure 1.
CaMKII structure and regulation. (a) Primary structure of CaMKIIα, with an N-terminal kinase domain, followed by a CaM-binding regulatory domain, a variable linker domain (subject to alternative splicing, with the splice insert of αB indicated), and a C-terminal association domain. Phosphorylation sites (red) and other important residues are indicated (see also Table 1). The other isoforms (β, γ,δ) contain an additional Thr after the third residues, and the numbers of their homologous positions are accordingly higher by one. These other isoforms also show more extensive alternative splicing in the variable domain, which is typically longer. (b) A model of a 12meric CaMKII holoenzyme (in two different view angles), based on several partial crystal structures [44, 46, 59], with the association domains forming a central hub [44, 46], and the kinases domain [59] radiating outward. The 12meric nature, the particle size, and the principle arrangement is supported by EM studies [44, 45]; however, the precise positioning of the kinase domains in this holoenzyme conformation is unclear (but compare Fig. 4). The reconstruction was kindly provided by Drs. Chao and Kuriyan, and the image is adapted, with permission, from [35]. (c) Sequence of the regulatory domain. The autoinhibitory and CaM-binding regions are indicated. The arrow indicates the N-terminus of the core CaM-binding region (R296; also indicated by arrows in panel d); Ca2+/CaM trapping on the T286-phosphorylated form of the kinase also involves F293 [2]. Autoinhibition is maintained in a kinase 1-N294 truncation, but additional C-terminal residues participate in the substrate site block [2] (see also panel e). The oxidation (black) and phosphorylation (red) sites that generate autonomous activity (T286) [2, 29, 30] or inhibit CaM-binding (T306/306) [2, 70–72] are marked. (d) The regulatory domain in the basal (inactive) and stimulated (active) conformation, illustrating a structural transition of the different regions upon Ca2+/CaM binding [32]. The positioning relative to the kinase domain is known for the basal state (see panel e) but unclear for the active states. The region shown here for CaMKIIδ is identical in CaMKIIα. (e) Crystal structure of human CaMKIIδ in its basal state, with the regulatory domain (ribbon) held in place in part by interactions with the T286 binding T-site (yellow) and blocking access to the substrate binding S-site (orange). The structural representations in panels d and e are based on Protein Data Bank (PDB) files 2VN9 and 2WEL [32].
CaMKII holoenzymes are formed by 12 subunits via their C-terminal association domains, with the N-terminal kinase domains radiating outward (Fig. 1a,b). This principle arrangement (first visualized 30 years ago [42]) is now well supported by increasingly precise reconstructions based on electron microscopy (EM) and partial crystal structures [31, 32, 43–46]. Lack of any apparent isoform preference in this assembly should lead to mainly heteromeric holoenzymes, with accordingly mixed regulatory and targeting properties [39, 47]. However, formation of CaMKIIβ homomers is enabled in inhibitory hippocampal interneurons by lack of CaMKIIα expression [48, 49]. Formation of CaMKIIα homomers in excitatory hippocampal pyramidal neurons is enabled by dendritic localization and translation of only the CaMKIIα but not β mRNA [41, 50–52] and by the lack of CaMKIIβ in a subset of individual neurons [39]. Dendritic translation can be enhanced by neuronal stimulation [53], which may thus specifically increase CaMKIIα homomers. Once formed, CaMKII holoenzymes appear to be quite stable, however, subunit exchange has been observed - at least in vitro - after cleaving off the kinase domains [44]. Little is known about cellular CaMKII protein turnover and its regulation, but neuronal activity did not significantly affect the ~24 h CaMKII half-life measured in synaptosomes from cortical cultures [54]. However, CaMKII can affect degradation of other synaptic proteins by activating the proteasome and targeting it to spines [55, 56].
Structure and regulation of the CaMKII subunits
Stimulated and autonomous CaMKII activity
Activity of each CaMKII subunit is stimulated individually by direct binding of Ca2+/CaM to their regulatory domains (Fig. 1c–e; see also Fig. 3a)[1, 2]. Autophosphorylation at T286 within the regulatory domain transforms CaMKII from one of the lowest to one of the highest affinity CaM binders within the cell [57]. This autophosphorylation also generates autonomous (Ca2+-independent) kinase activity. However, contrary to common perception, such autonomous CaMKII is not fully active, but can instead be significantly further stimulated by Ca2+/CaM [29] (Fig. 2). Vice versa, T286 phosphorylation also further enhances Ca2+/CaM-stimulated activity, albeit to a much lesser extent [58] (Fig. 2). This mechanism still enables molecular memory of past stimulation by autonomous activity, but also prevents complete uncoupling from subsequent Ca2+-signaling. On a molecular level, this indicates a more extensive opening of an inhibitory gate by Ca2+/CaM-binding than by T286 phosphorylation alone. The main regulatory effect of the inhibitory gate of CaMKII is to restrict substrate access (although Ca2+/CaM also enhances ATP binding and vice versa) [2].
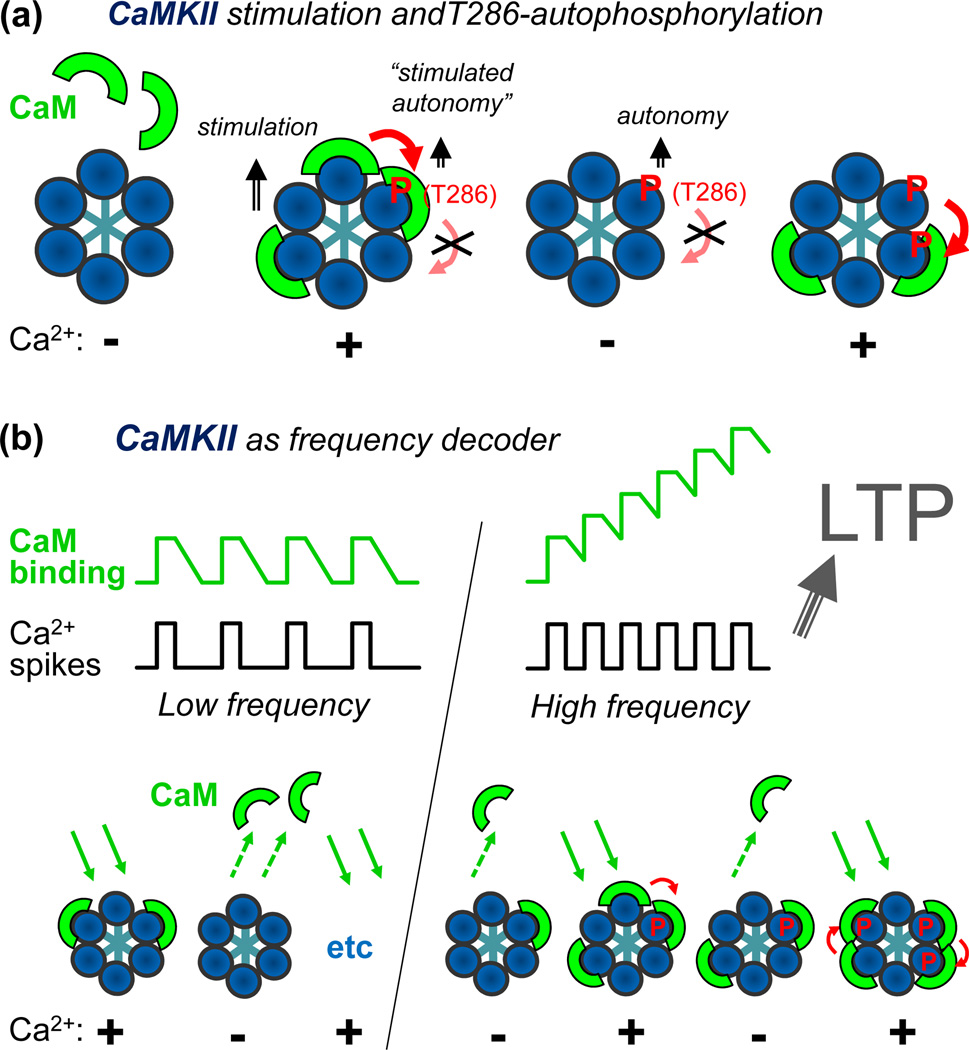
Figure 3.
Schematic representations of frequency detection by CaMKII autophosphorylation at T286. (a) Ca2+/CaM-binding separately stimulates each individual subunit of a CaMKII holoenzyme (only 6 of the 12 subunits shown in the schematic model). However, T286 autophosphorylation requires binding of two CaM molecules to two neighboring subunit, one to activate the subunit acting as kinase, the other to expose T286 for phosphorylation (see also Fig. 1e) on the neighboring subunit acting as substrate in this intra-holoenzyme inter-subunit reaction [82, 83]. Phospho-T286 generates Ca2+-independent “autonomous” activity (that can be further stimulated by Ca2+/CaM; see Fig. 2). It can likely also substitute for the kinase-directed function of CaM in further autophosphorylation, but it cannot substitute for the substrate-directed function of CaM [83]. (b) A simple model for frequency detection by T286 autophosphorylation. During submaximal Ca2+-spikes, some CaM molecules bind to some subunits of a CaMKII holoenzyme, and then dissociate during the spike interval, and so on. However, at higher frequencies, with the spike interval in the range of the CaM dissociation time, additional CaM molecules accumulate before all of the initial CaM molecules dissociate. This increases the chance of CaM binding to neighboring subunit and thus autophosphorylation at T286. Such a frequency-dependent response was found in biochemical studies [40, 47], and suggestively correlates with the requirements for LTP induction, which is favored by HFS and has een shown to depend on CaMKII autonomy [26, 27].
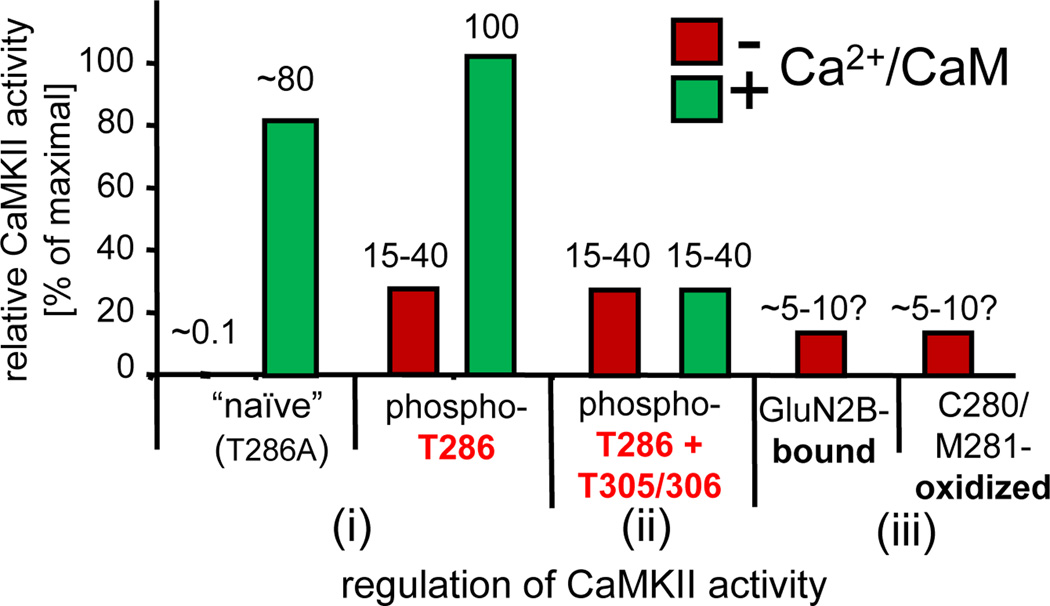
Figure 2.
Levels of CaMKII activity in response to different forms of regulation. (i) Naïve CaMKII shows only low basal activity (~0.1% of maximum), but is fully activated by Ca2+/CaM-stimulation [2]. This is accompanied by rapid T286-autophosphorylation; when T286-autophosphorylation is prevented by T286A mutation, the activity level remains slightly lower [58]. After T286-autophosphorylation, CaMKII remains partially active even after dissociation of Ca2+/CaM (autonomous), but this autonomous activity is significantly further stimulated by Ca2+/CaM [29]. (ii) T305/306-autophosphorylation of T286-phosphorylated CaMKII prevents Ca2+/CaM binding and thus further stimulation of the autonomous activity. (iii) Autonomous activity can also be induced by GluN2B binding [61] or by C280/M281 oxidation [30]; like T286-autophosphorylation, both mechanisms require an initial Ca2+/CaM-stimulus [30, 61]. The levels of “autonomy” (the ratio of autonomous over maximal CaM-stimulated activity) after GluN2B binding or oxidation are estimated adjustments (based on conditions in the original reports that overestimate autonomy, either by use of an autonomy-favoring substrate [29, 61] or by conditions resulting in unusually low rates of stimulated activity [30]). The activity estimates shown are based on an adapted compilation from [29, 30, 58, 61].
Indeed, the regulatory domain blocks the substrate binding site of the CaMKII kinase domain in crystal structures of its basal state [31, 32, 43]. The short regulatory domain is held in place in part through interaction of its N-terminal region around T286 (T286-region) with the T-site, which is located next to the substrate binding S-site on the kinase domain (Fig. 1e). Ca2+/CaM-binding to the C-terminal part (CaM-region) of the regulatory domain removes the S-site block, and T286 autophosphorylation prevents complete re-binding of the regulatory region even after dissociation of Ca2+/CaM. As indicated by an initial structure [59], any part of the regulatory domain can be in a helical conformation. However, recent evidence suggests that only the T286-region is helical in the basal state, while only the CaM-region is helical upon stimulation [32, 33, 60] (Fig. 1d). Such structural transition was also observed in solution [60] and may contribute to the tight regulation of CaMKII activity (~1000fold stimulation by Ca2+/CaM over basal activity; Fig. 2). In contrast to the basal state, much less structural information is available for activated CaMKII. One crystal structure of an activated CaMKII [32] provides information about the Ca2+/CaM-interaction, the T286 phosphorylation reaction (by a neighboring subunit, see below), and a structural shift of the first helix in the large lobe of the kinase domain (helix D). However, the principal positioning of the regulatory domain in stimulated or autonomous CaMKII remains unclear.
Two new mechanisms for generating autonomy
Two additional mechanisms can generate autonomous CaMKII activity, albeit to a lower level compared to T286 autophosphorylation: (i) oxidation of M281/M282 in CaMKIIδ (C280/M281 in CaMKIIα) [30] and CaMKII binding to the NMDAR subunit GluN2B [61]. M281/M282 are located at the hinge between the kinase and regulatory domains (Fig. 1c–e), and in the oxidized state appear to prevent complete closing of the inhibitory gate. CaMKII binds to GluN2B via its T-site [61, 62] (Fig. 1e), which keeps the regulatory domain displaced, but may also partially impede substrate access [61, 63]. Such a mechanism was described also for CaMKII binding to the neuronal connexin-36 [64] and proposed for binding to the kinesin KIF17 [65]. Both mechanisms (T-site binding and oxidation) require an initial Ca2+/CaM stimulus, likely in order to make the relevant residues accessible for modification or interaction. Thus, in the presence of ATP (~4 mM within cells), such Ca2+/CaM stimuli should simultaneously trigger generation of higher level autonomy by T286 autophosphorylation. However, the additional mechanisms may enable maintenance of lower level autonomy even after de-phosphorylation of T286.
Forms of completely Ca2+/CaM-independent activation
Without requirement of any Ca2+ or CaM, CaMKII can be directly activated by gangliosides (especially GT1b), Zn2+ [2], or α-actinin [66], at least in vitro. Interestingly, α-actinin selectivity stimulates phosphorylation of CaMKII substrates that bind to the T-site (demonstrated only for GluN2B), though not as effectively as does Ca2+/CaM [66]. Since α-actinin competes with Ca2+/CaM for binding to CaMKII [63], its net effect in presence of Ca2+/CaM is inhibitory for all substrates [63, 66], in addition to participating in mechanisms of substrate selection [29, 66].
Negative regulation of CaMKII activity
Negative regulation of CaMKII activity can be achieved by α-actinin (see above [63, 66]), by the inhibitory protein CaM-KIIN [67–69], or by T305/306 autophosphorylation [70–72]. Similar to GluN2B, binding of the CaM-KIINα or β isoforms (which share almost identical inhibitory regions [67–69]) to CaMKII involves the T-site of the kinase [33, 73], and thus, prior displacement of the CaMKII regulatory domain is required. However, in contrast to GluN2B, CaM-KIIN binding completely blocks substrate access [33, 69]. CaM-KIIN can be regulated by its expression level [74] and possibly by phosphorylation [69].
The T305/306 residues are located within the CaM-region of the regulatory domain, and a recent crystal structure [32] supports prior biochemical findings that T305/306 phosphorylation inhibits CaM-binding and the notion that, vice versa, CaM-binding inhibits T305/306 phosphorylation [2, 71, 72] (see Fig. 1d). As a result, efficient T305/306 autophosphorylation should require autonomous CaMKII with no bound CaM. Indeed, dissociating CaM from T286-phosphorylated CaMKII triggers T305/306 autophosphorylation [71, 72], however, with a reaction rate likely still >100-fold slower compared to the fast (~12 sec−1) autophosphorylation at T286 [75]. The resulting state of the kinase would be autonomous but without the ability to be further stimulated (Fig. 2). By contrast, making CaMKII autonomous by GluN2B binding inhibits T305/306 autophosphorylation, even after T286-phosphorylation and in absence of CaM [61] {whereas CaMKII binding to calcium/calmodulin-dependent serine protein kinase (Cask) instead promotes T305 phosphorylation [76]}. Protein phosphatases PP1, PP2A and PP2C can dephosphorylate CaMKII (including at T286 to reverse autonomy). This is likely dependent on the subcellular localization [77–80] and with GluN2B binding partially protecting T286, at least from PP1 [81]. As GluN2B-binding also directly increases CaM-affinity [61], the GluN2B-bound form may be the most readily Ca2+/CaM-stimulated pool of CaMKII within a neuron.
Regulation within the CaMKII holoenzyme
Frequency detection by T286 autophosphorylation
The holoenzyme assembly (Fig. 1b) has at least one profound effect on CaMKII regulation: T286 autophosphorylation occurs as an inter-subunit reaction within the holoenzyme, which makes it independent of the CaMKII concentration [2]. Importantly, Ca2+/CaM-binding has a dual role in this reaction: It is required not only for activation of the subunit acting as the kinase, but also for making T286 accessible on the neighboring subunit acting as the substrate [82, 83] (Fig. 3a). Indeed, this mechanism is supported by the CaMKII structure, which indicates inaccessibility of T286 in the basal state (see Fig. 1e). As a consequence, and in contrast to earlier models, the molecular memory provided by T286 phosphorylation should be relatively short-term: It is fully erased by phosphatases, as rephosphorylation even by a still autonomous neighboring subunit is possible only upon re-binding of Ca2+/CaM.
Another more intriguing consequence of this dual role of CaM is the decoding of stimulation frequencies by T286 autophosphorylation (Fig. 3b). During sub-maximal low frequency stimulation, some CaMKII subunits within a holoenzyme are activated by Ca2+/CaM-binding during the Ca2+-spikes, and inactivated by Ca2+/CaM-dissociation during the spike intervals. However, at higher frequencies (with the spike intervals within the range of the CaM dissociation time), Ca2+/CaM successively accumulates on the holoenzyme, which increases the chance of Ca2+/CaM-binding to neighboring subunits and in turn T286 autophosphorylation. Indeed, such frequency detection was measured in biochemical experiments with purified CaMKII [31, 40, 47]. Taken together, these mechanisms make T286 autophosphorylation well suited for temporal decoding and for short- but not long-term storage of information.
Two inactive conformations and other holoenzyme-specific effects
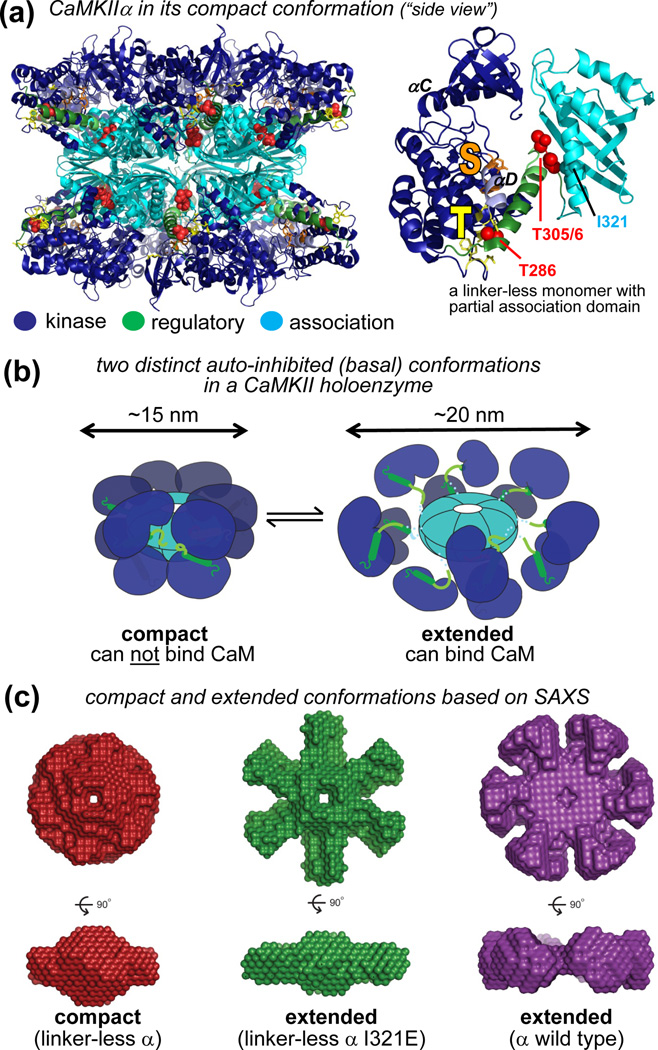
Within CaMKII holoenzymes, Ca2+/CaM-stimulation of the individual subunits is cooperative, and several potential underlying mechanisms have been proposed [31, 33, 43, 60]. Intriguingly, a recent holoenzyme crystal structure (for CaMKIIα without its 30 amino acid linker domain) shows a compact conformation in which each kinase domain folds back to directly interact with two association domains [31] (Fig. 4a). Clearly, this cannot be the only CaMKIIα holoenzyme conformation, because the size is significantly smaller than observed previously (15 vs ~20 nm diameter) [31, 44, 45] and because the CaM-binding region is completely inaccessible in this conformation [31]. However, a mutation that prevents the compact conformation reduced the CaM cooperativity not only for the linker-less form, but also for CaMKIIα wild type (at least under molecular crowding conditions that mimicked total cellular protein concentration)[31]. This indicates transition between two different inactive CaMKII holoenzyme conformations, one compact and one extended [31] (Fig. 4b,c). Only the extended conformation allows for Ca2+/CaM-binding, which in turn appears to promote also transition of neighboring subunits to this CaM-binding competent state [31]. Importantly, any mechanism that affects Ca2+/CaM-stimulation of a holoenzyme should contribute to shaping the frequency dependent response. Indeed, this notion is experimentally supported for factors related to the stimulus (spike amplitude and duration) or to the kinase (CaM affinity, autophosphorylation rate) [40, 47], and should also include factors regulating CaM cooperativity [31].
Figure 4.
Two distinct inactive conformations of the CaMKII holoenzyme: compact and extended. (a) The only current holoenzyme crystal structure (in a compact conformation) with accurate kinase domain positioning (left; PDB1 3SOA) [31] was derived from a monomeric linker-less CaMKIIα with a partial association domain (right; PDB 3SOA) [31], together with the known structure of the association domain assembly [32, 44, 46]. (b) In a compact conformation, the kinase domains fold back to the association domains, leaving the CaM-binding regulatory domain inaccessible to stimulation (as depicted in the crystal structure in panel a) [31]. In an extended conformation, the kinase domains protrude further outward, making the regulatory domains accessible (compare to the model depicted in Fig. 1b). CaM-binding to the extended conformation may, in turn, also favor transition of neighboring subunits to this CaM-binding competent extended conformation [31]. (c) Small-angle X-ray scattering (SAXS) analysis indicates that the I321E mutation in a linker-less CaMKIIα that interferes with the interaction of the kinase domain with the association domain causes a transition from a compact (i) to an extended conformation (ii) [31]. This mutation reduces cooperativity of activation by CaM not only for linker-less but also for full-length CaMKIIα (wild type, iii), at least under molecular crowding conditions that mimic cellular protein concentrations [31]. In SAXS analysis (without molecular crowding) the major CaMKIIα isoform was found in the extended conformation [31]. All panels are adapted, with permission, from [31].
Beyond regulation of CaMKII activity, the various mono-valent protein-protein interactions of individual CaMKII subunits [79, 84] can become multi-valent interactions for CaMKII holoenzymes. Indeed, this appears to promote synaptic localization [62] and to enable crosslinking of binding partners [38, 85, 86], which in turn may contribute to non-catalytic structural functions of CaMKII [34, 87–89], as discussed later.
Function of T286 autophosphorylation in processing of information
A long-standing hypothesis proposed a function for CaMKII stimulation in LTP induction and for CaMKII autonomy in LTP maintenance. Indeed, maintaining the kinase activity that induced LTP [5, 6] should then also be sufficient to maintain the potentiated state, and this intuitive view is supported by experiments that introduced active CaMKII into postsynaptic neurons [90] and by T286A mutant mice [26]. However, recent studies reported that autonomous CaMKII is not fully active [29] and that CaMKII activation after LTP is quickly reversed (within ~l min) [28]. Furthermore, tatCN21 (a CaM-KIIN derived peptide that inhibits stimulated and autonomous CaMKII activity with equal potency [27, 73]) did not affect LTP maintenance or memory storage/retrieval at concentrations sufficient to inhibit LTP induction and memory formation (5 µM) [27]. Previous studies are consistent with this conclusion, but used inhibitors that affect autonomous activity less potently [5, 91]. In combination with studies using T286A mutant mice, which clearly indicated a role in LTP and learning/memory [26], the inhibitor studies strongly support a function of T286 autophosphorylation in mediating LTP induction and memory formation rather than LTP maintenance and memory storage. Such a role of CaMKII autonomy in processing rather than storage of synaptic information correlates very suggestively with the biochemistry of its regulation (see previous section): Like LTP, T286 autophosphorylation is more readily induced by high frequency stimulation (HFS) [40, 47]. However, T286 autophosphorylation is important also in other synaptic processes, and storage functions related to LTD and/or cross-synaptic communication remain to be elucidated (see below).
The CaMKII/NMDAR complex in storage of synaptic information
The tatCN21 inhibitor interferes not only with CaMKII activity but also with binding to the NMDAR subunit GluN2B [73]. However, in contrast to inhibition of phosphorylation of regular substrates, inhibition of GluN2B binding is competitive and likely occurs with a higher IC50 [73]. Indeed, transient tatCN21 application persistently disrupts the CaMKII/NMDAR complex in hippocampal slices, but only at higher concentrations (20 µM) and not at the lower concentrations (5 µM) sufficient to block LTP induction [27, 92]. Importantly, only the treatment disrupting the CaMKII/NMDAR complex also persistently reduced synaptic strength [27, 92], indicating a direct function of the complex in storage of synaptic information. Only pharmacological treatments allow the temporal resolution required for distinguishing between induction and maintenance effects, but they also raise questions regarding target specificity. However, plausibility of a role for the CaMKII/NMDAR complex in synaptic information storage is supported by independent evidence. Specifically, mutating GluN2B to prevent its interaction with CaMKII impairs enhancement of synaptic strength [93, 94], and the amount of CaMKII localized at individual synapses directly correlates with their strength [95]. Furthermore, while synaptic localization and NMDAR binding of CaMKII are further increased by LTP-stimuli, basal levels of both (localization and binding) are also supported by basal neuronal activity [62, 96, 97]. Accordingly, disrupting the CaMKII/NMDAR complex reduced both potentiated and basal transmission [92]. Thus, while this type of synaptic information storage has a clear and direct link to LTP, it is not restricted to this form of plasticity.
NMDARs are composed of two GluN1 and two GluN2 (or GluN3) subunits. A partial developmental switch from GluN2B to GluN2A is known to be important in synapse maturation [98]. However, even in mature hippocampus, the vast majority of receptors appear to still contain a GluN2B subunit (in tri-heteromeric complexes of GluN1/GluN2A/GluN2B)[99]. CaMKII can bind to GluN1, GluN2A and GluN2B, but the interaction with GluN2B is by far the strongest, followed by the interaction with GluN1 [100–102]. Notably, while the cytoplasmic C-termini of GluN2B and 2A are homologous, their CaMKII binding-sites are not [79, 84]. The major CaMKII binding site on GluN2B (around S1303) [61, 100] is lacking in GluN2A, and this site appears to confer much of the subtype-specific functions [93, 98]. Additionally, CaMKII can bind to several other proteins within the NMDAR complex, including α-actinin, Synaptic Ras GTPase activating protein β (SynGAPβ) and synapse-associated protein 97 (SAP97) [79, 84, 103]. It is presently unclear if and how tatCN21 may interfere with these additional CaMKII interactions, but mutating the major binding site on GluN2B was sufficient to reduce CaMKII association with the NMDAR complex (and LTP) to a similar extent as seen with 20 µM tatCN21 [92, 94]. This indicates that GluN2B is indeed the functionally relevant CaMKII binding partner for maintenance of synaptic strength.
CaMKII, the synaptic tag, and other structural functions related to LTP
As LTP maintenance requires synthesis of new proteins that selectively strengthen only activated synapses but not their neighbors [21], some synaptic tag must enable differentiation between these synapses. Strong evidence indicates that CaMKII activation is required for this tagging [104–106]. CaMKII may induce structural changes that create the tag, be part of the tag itself, or both. CaMKII can induce structural changes by regulating the synaptic scaffolding proteins PSD95 [107, 108] and SAP97 [103, 109] and possibly directly by its interaction with the NMDAR complex (see above) and/or by aggregation of CaMKII holoenzymes into clusters [80, 110–112]. Another attractive structural tagging mechanism is by CaMKII-regulated changes in the spine actin cytoskeleton that indeed result in increased spine size after LTP [113, 114]. CaMKII regulation of actin can occur through regulation of small GTPases [43, 115–118] and by direct actin-interactions of the CaMKIIβ isoform [38, 87, 119, 120].
At least some of the structural effects of CaMKIIβ on spine actin and spine size are independent of its kinase activity [87], and so are some effects of CaMKIIα (which does not bind actin) on spine size [88]. Furthermore, hippocampal LTP is normal in mice with activation-incompetent CaMKIIβ, but impaired in mice that lack CaMKIIβ (or α) completely [89]. Activation-incompetent CaMKIIβ can still target α/β-heteromeric holoenzymes to the F-actin cytoskeleton [121], and only the mice with complete CaMKIIβ loss showed aberrant targeting of the α isoform [89]. Together, this evidence suggest a structural function of heteromeric CaMKIIα/β holoenzymes in plasticity, with a physical link to the actin-cytoskeleton provided by the β-subunits and a physical link to PSD-proteins provided largely by the α-subunits.
CaMKIIα can interact with a variety of proteins in the PSD, including various proteins in the NMDAR complex (as discussed above) and densin-180 [79, 84]. While GluN2B is currently the only known binding partner that is sufficient to mediate activation-induced synaptic translocation of CaMKII [61, 62], the additional interactions are likely important in shaping structure and function of the PSD protein assembly. Indeed, GluN2B binding alone does not appear sufficient to explain the CaMKIIα-specific functions, as this binding is seen for all isoforms [62]. Other CaMKII interactions with the PSD may be α-specific, however, CaMKIIβ binding has been formally ruled out only for one binding site on densin-180 [86, 122]. While only GluN2B null mice, but not densin-180 null mice, show reduced general synaptic CaMKIIα localization, both are impaired in CaMKII-mediated synaptic functions [94, 123].
Taken together, there is now strong evidence for both catalytic and non-catalytic functions of CaMKII in regulating synaptic plasticity. However, catalytic and non-catalytic functions are not as easily distinguished as it may seem at first glance: Some essentially purely structural functions can still require regulation by autophosphorylation and thus kinase activity [88]. Even autophosphorylation-independent structural functions can be regulated by Ca2+/CaM [38, 61, 87, 119] and thus be affected by inhibitors like KN93 or by mutations that prevent Ca2+/CaM binding. Inhibitors like AC3I or CN peptides directly block not only activity but also CaMKII binding to GluN2B or densin-180 [62, 73, 122]. Even the kinase dead CaMKII K42M mutant (nucleotide binding-incompetent) can report structural rather than catalytic functions, as nucleotide binding is not only required for activity but can also regulate CaMKII protein interactions, for instance with Cask, GluN2B and between CaMKII holoenzymes [76, 112, 124].
Beyond LTP: CaMKII functions in LTD-related mechanisms
Glutamatergic LTD-stimuli cause depression of excitatory glutamatergic synapses and potentiation of inhibitory GABAergic synapses in the same post-synaptic neurons, and CaMKII has recently been implicated in the regulation of both events [23, 24]. The specific CaMKII-mediated downstream signaling steps are currently unclear, but phosphorylation of the AMPAR subunit GluA1 at S567 is an attractive candidate for depressing glutamatergic synapses: Phosphorylation of S567 in loop 1 directly reduces spine localization of the channel [125] (in contrast to LTP-induced phosphorylation at S831 in the C-tail, which enhances channel conductance [14, 15]).
Both metabotropic glutatamate receptor (mGluR)- and NMDAR- dependent LTD-stimuli trigger CaMKII autophosphorylation at T286 [23, 25]. The latter was shown to be necessary and sufficient for the NMDAR dependent potentiation of inhibitory synapses [25]. If T286 phosphorylation mediates both LTD- and LTP-mechanisms, then what determines the opposing effects on synaptic strength? Such differentiation mechanisms could include selective inhibitory phosphorylation at T305/306 during LTD but not LTP. Indeed, overexpressing a constitutively autonomous CaMKIIα mutant (T286D) directly induced either synaptic depression or potentiation, depending on if T305/306 phosphorylation was either mimicked (T305/306D) or prevented (T305/306A) by additional mutation [126]. Differential T305/306 phosphorylation would allow further stimulation and full activation of autonomous CaMKII only during LTP, and restrict CaMKII activity to the lower autonomous level during LTD [29] (see Fig. 2). Additionally, it may contribute to the regulation of CaMKII localization [102]. Intriguingly, while T286 autophosphorylation (like LTP) is promoted by HFS (see Fig. 3), T305/306 autophosphorylation (like LTD) should be promoted by low frequency stimulation with longer spike intervals that promote full dissociation of Ca2+/CaM, and thus, accessibility of T305/306. Notably, medium frequency stimulations (10 Hz) that did not change synaptic strength in wild type mice caused LTP in T305/306VA-mutant mice and LTD in T305/306D-mutant mice [127]. Additionally, while CaMKII functions in synaptic potentiation involve binding to GluN2B [92–94], functions in synaptic depression may instead require binding to densin-180, as mice lacking this protein are impaired for hippocampal LTD [123].
A pre-synaptic involvement of CaMKII in LTD has been described over 15 years ago [128]. By contrast, and despite several exciting hints described above, CaMKII in post-synaptic LTD is just emerging as a field of study.
CaMKII in global communication between different synapses and receptor systems
CaMKII can communicate the input information of glutamatergic LTD-stimuli to inhibitory synapses, both by increased CaMKII localization to these synapses and by increased GABAA receptor surface expression [25]. CaMKII can also communicate input information by serotonin, dopamine, and Wnt signaling pathways to glutamatergic synapses. Specifically, CaMKII can mediate 5-HT1A receptor induced suppression of NMDAR function [129], D4-type dopamine receptor-induced bidirectional regulation of AMPARs [127, 130], and Wnt7a-induced increase in spine size [131].
Additional mechanisms involving indirect synapse to synapse communication may occur by more global CaMKII regulation of neuronal functions. For instance, effects on dendritic shape and arborization have been observed that are mediated by CaMKIIβ - both by its association with F-actin [119] and the centrosome [132], although it should be noted that CaMKIIβ knock-out mice showed no impairments in dendritic arborization [89]. Additionally, CaMKII can regulate transcription and translation of a variety of genes [133, 134]. For excitation/transcription coupling, Ca2+-entry through L-type voltage-dependent Ca2+-channels is of special importance [135], and CaMKII can bind and modulate these channels [136, 137]. Finally, while CaMKII translocation to glutamatergic synapses can occur in an input-specific manner [97], a global spread of translocation after regional stimulation has also been observed [138]. Many of the regulatory mechanisms underlying such global effects and their functional consequences await further elucidation. However, CaMKII clearly plays an important role not only in local signaling at individual synapses, but also in more global neuronal signal processing and in synapse to synapse communication.
Directing neuronal plasticity by spatio-temporal regulation of CaMKII?
Traditionally, significant CaMKII T286 phosphorylation (and thus autonomy) was thought to be induced specifically by LTP-stimuli, and then to persist for hours (Fig. 5). Such a view is supported by experimental evidence [139–141], however, may need to be reassessed in light of recent studies. For instance, a live-imaging study [28] that used a Fluorescence resonance energy transfer (FRET)-based sensor [142] to visualize CaMKII activity in individual spines found that LTP-stimuli induced a sharp rise in CaMKII activation that quickly decayed (with two time-constants of inactivation: 6 sec and 45 sec) [28]. Notably, a previous biochemical study has described a similar decay of autonomous CaMKII activity (Fig. 5), accompanied by decay of T286 phosphorylation [143]. While phospho-T286 immunodetection appeared to remain slightly elevated [143], the difference was not highly significant [143]. While autonomy appeared to decay faster than phospho-T286 immunodetection [143], a direct comparison would require quantification of the phospho-T286 levels [144], ideally at the same time points and in the same samples. Thus, while there is some indication for uncoupling of T286 phosphorylation from autonomous activity after LTP, this still requires further testing. Mechanistically, such uncoupling could be achieved by CaMKII binding to CaM-KIIN, which would block CaMKII activity even if T286 remains phosphorylated [27, 67]. However, this mechanism would not provide reconciliation: Inhibition by CaM-KIIN would still keep the CaMKII autoinhibitory domain displaced [33, 73] and the CaMKII FRET-sensor used in the imaging study [28, 142] should thus still report an active conformation. Reconciliation may be provided instead by LTP-induced increase of both autonomy (or T286 phosphorylation) and total CaMKII activity (or total CaMKII protein) found in biochemical [139] or histological [140]) studies. The ratio of phopho-T286 over total CaMKII was found to remain persistently increased only in the soma, but not in dendrites [140], consistent with the fast reversal of CaMKII activity in spines seen by the recent imaging study [28].
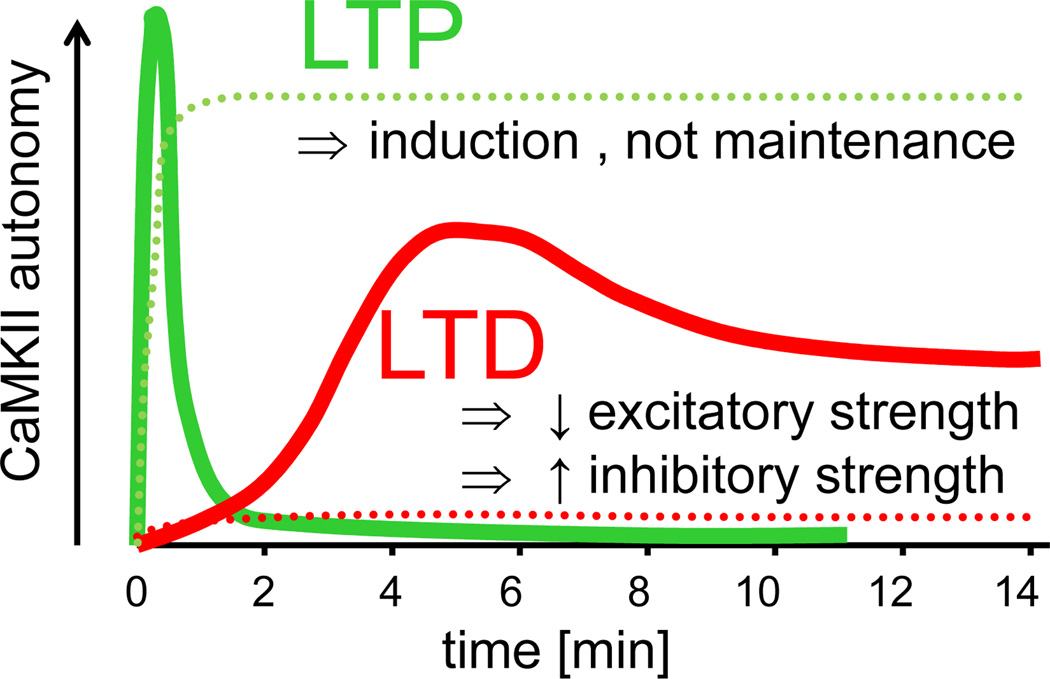
Figure 5.
Schematic diagram illustrating the time course of CaMKII autonomy after LTP (green) versus LTD (red) stimuli. The time course of CaMKII autonomy according to recent studies (solid lines) [23, 25, 28, 143] differs from the “traditional” perception (dotted lines) [139], but is consistent with a role of CaMKII T286 phosphorylation in LTP induction rather than maintenance [27], as well as with newly discovered functions in mechanisms induced by excitatory LTD-stimuli in hippocampal pyramidal neurons (depression of excitatory synapses [23] and potentiation of inhibitory synapses [25]). After LTP, the rapid activation of CaMKII in spines was found to decay with two time-constants (6 sec and 45 sec) [28], with the slower time-constant that is consistent with the decay of autonomy found in a previous biochemical study [143] illustrated here. The recent LTD-related studies assessed T286 phosphorylation after mGluR [23] or NMDAR [25] dependent LTD-stimuli, but found very similar time courses (with a peak at 5 min followed by persistence at a lower level, as illustrated here). The illustration represents an adapted compilation of the results of several studies [23, 25, 28, 139, 143]. The similarities between the two more recent studies related to LTP [28, 143] and LTD [23, 25] provide good estimates of the respective time course (as shown), however, a direct comparison between the absolute levels of peak autonomy after LTP versus LTD has not yet been made (and the relative peak levels shown are arbitrary).
By contrast, LTD-stimuli were traditionally thought to cause no significant CaMKII activation [139]. However, recent studies found a slow increase in T286 phosphorylation that peaked ~5 min after LTD-stimuli and then persisted at a lower level [23, 25](Fig. 5). Thus, while both LTP- and LTD-stimuli induce generation of autonomous CaMKII activity, the time course differs significantly (Fig. 5). As CaMKII autonomy is required in both LTP- and LTD-mechanisms [25–27], the opposing outcome on synaptic strength appears to be encoded by such temporal differences. The downstream mechanisms are unclear, but likely include secondary effects on CaMKII regulation, such as differential targeting and/or inhibitory autophosphorylation at T305/306.
Concluding remarks
While we now know many details about CaMKII, both regarding its complex regulation and its various neuronal functions, there is undoubtedly still much to be learned in both areas (Box 1). The most significant advances will likely come from tying together specific regulation mechanisms with specific signaling functions. This will require further elucidating the specific aspects of the complex regulation of CaMKII activity and localization that are engaged in response to different neuronal stimuli. Such information is bound to greatly enhance our understanding of the diverse and distinct functions of CaMKII in neuronal information processing and storage.
Box 1. Outstanding Questions.
What is the CaMKII holoenzyme structure? Despite significant recent progress [31, 32], the CaMKII holoenzyme structure is known only for one of its inactive conformations. Structures of the activated states of CaMKII will be most meaningful within the holenzyme structure, which is likely to determine degree of accessibility of the catalytic site.
What are the differences in spatio-temporal CaMKII regulation after LTP versus LTD stimuli? Recent studies have clearly challenged the traditional view of CaMKII activation after LTP [28, 143] and LTD [23, 25]. However, more work is needed to support this new view, which will also impact our general understanding of the emerging functions of CaMKII beyond LTP at excitatory synapses.
What mechanisms direct potentiation of excitatory versus inhibitory synapses by autonomous CaMKII? CaMKII functions in potentiating inhibitory synapses also require the T286 autophosphorylation [25], traditionally linked to potentiation of excitatory synapses. Both the differentiating mechanism and the principle downstream mechanisms causing inhibitory synapse potentiation are still unclear.
What are the mechanisms for depression of excitatory synapses by CaMKII? Arguably, even the mechanisms for potentiation are still incompletely understood. The mechanisms for the new role of CaMKII in depression of excitatory synapses [23] are currently largely speculative.
-
Does CaMKII also mediate depression of inhibitory synapses? As glutamatergic LTD-stimuli result in CaMKII-mediated depression of excitatory synapses [23] and potentiation of inhibitory synapses [25], then glutamatergic LTP-stimuli may also require CaMKII not only for potentiation of excitatory synapses but also for depression of inhibitory synapses.
Does T286 autophosphorylation store synaptic information related to LTD? In LTP, CaMKII autonomy appears to have largely an information processing function. However, storage functions in other situations, including in LTD, are unknown.
Are there functional mechanisms to uncouple T286 autophosphorylation from autonomous activity? One study has suggested such an uncoupling after LTP [143], but this needs to be investigated in more detail. Such uncoupling could be mediated by CaM-KIIN, but the functions of this CaMKII-inhibitory protein are still unclear.
What are the functions of T286-independent autonomy? CaMKII interaction with the NMDAR is important in regulating plasticity [92–94], however, it is still not clear if this involves the resulting autonomous activity [61] or if it is mediated solely by CaMKII targeting. Oxidation-induced autonomy [30] has yet to be studied in neurons.
Acknowledgement
We thank Drs. Howard Schulman and Matt Kennedy for critical reading of the manuscript and helpful comments; and Drs. Howard Schulman, Luke Chao, and John Kuriyan for providing CaMKII holoenzyme models. Our research was supported by a National Institutes of Health grant (R01 NS052644).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: The University of Colorado is currently seeking patent protection for tatCN21, its derivatives, and uses.
References
- 1.Schulman H, Greengard P. Stimulation of brain membrane protein phosphorylation by calcium and an endogenous heat-stable protein. Nature. 1978;271:478–479. doi: 10.1038/271478a0. [DOI] [PubMed] [Google Scholar]
- 2.Hudmon A, Schulman H. Neuronal CA2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu Rev Biochem. 2002;71:473–510. doi: 10.1146/annurev.biochem.71.110601.135410. [DOI] [PubMed] [Google Scholar]
- 3.Feng B, et al. Quantitative estimates of the cytoplasmic PSD, and NMDAR-bound pools of CaMKII in dendritic spines. Brain Res. 2011;1419:46–52. doi: 10.1016/j.brainres.2011.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erondu NE, Kennedy MB. Regional distribution of type II Ca2+/calmodulin-dependent protein kinase in rat brain. J Neurosci. 1985;5:3270–3277. doi: 10.1523/JNEUROSCI.05-12-03270.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malinow R, et al. Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science. 1989;245:862–866. doi: 10.1126/science.2549638. [DOI] [PubMed] [Google Scholar]
- 6.Silva AJ, et al. Deficient hippocampal long-term potentiation in a-calcium-calmodulin kinase II mutant mice. Science. 1992;257:201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- 7.Hvalby O, et al. Specificity of protein kinase inhibitor peptides and induction of long-term potentiation. Proc Natl Acad Sci U S A. 1994;91:4761–4765. doi: 10.1073/pnas.91.11.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hinds HL, et al. CA1 long-term potentiation is diminished but present in hippocampal slices from alpha-CaMKII mutant mice. Learn Mem. 1998;5:344–354. [PMC free article] [PubMed] [Google Scholar]
- 9.Barria A, et al. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276:2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi Y, et al. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- 11.Opazo P, et al. CaMKII triggers the diffusional trapping of surface AMPARs through phosphorylation of stargazin. Neuron. 2010;67:239–252. doi: 10.1016/j.neuron.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Correia SS, et al. Motor protein-dependent transport of AMPA receptors into spines during long-term potentiation. Nat Neurosci. 2008;11:457–466. doi: 10.1038/nn2063. [DOI] [PubMed] [Google Scholar]
- 13.Benke TA, et al. Modulation of AMPA receptor unitary conductance by synaptic activity. Nature. 1998;393:793–797. doi: 10.1038/31709. [DOI] [PubMed] [Google Scholar]
- 14.Derkach V, et al. Ca2+/calmodulin-kinase II enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc Natl Acad Sci U S A. 1999;96:3269–3274. doi: 10.1073/pnas.96.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kristensen AS, et al. Mechanism of Ca2+/calmodulin-dependent kinase II regulation of AMPA receptor gating. Nat Neurosci. 2011;14:727–735. doi: 10.1038/nn.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee HK, et al. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000;405:955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- 17.Lu W, Roche KW. Posttranslational regulation of AMPA receptor trafficking and function. Curr Opin Neurobiol. 2011 doi: 10.1016/j.conb.2011.09.008. http://dx.doi.org/10.1016/j.conb.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson AC, Nicoll RA. The expanding social network of ionotropic glutamate receptors: TARPs and other transmembrane auxiliary subunits. Neuron. 2011;70:178–199. doi: 10.1016/j.neuron.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anggono V, Huganir RL. Regulation of AMPA receptor trafficking and synaptic plasticity. Curr Opin Neurobiol. 2012 doi: 10.1016/j.conb.2011.12.006. http://dx.doi.org/10.1016/j.conb.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murakoshi H, Yasuda R. Postsynaptic signaling during plasticity of dendritic spines. Trends Neurosci. 2012;35:135–143. doi: 10.1016/j.tins.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lisman J, et al. Mechanisms of CaMKII action in long-term potentiation. Nat Rev Neurosci. 2012;13:169–182. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva AJ, et al. Impaired spatial learning in a-calcium-calmodulin kinase II mutant mice. Science. 1992;257:206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- 23.Mockett BG, et al. Calcium/calmodulin-dependent protein kinase II mediates group I metabotropic glutamate receptor-dependent protein synthesis and long-term depression in rat hippocampus. J Neurosci. 2011;31:7380–7391. doi: 10.1523/JNEUROSCI.6656-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marsden KC, et al. NMDA receptor activation potentiates inhibitory transmission through GABA receptor-associated protein-dependent exocytosis of GABA(A) receptors. J Neurosci. 2007;27:14326–14337. doi: 10.1523/JNEUROSCI.4433-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marsden KC, et al. Selective translocation of Ca2+/calmodulin protein kinase IIalpha (CaMKIIalpha) to inhibitory synapses. Proc Natl Acad Sci U S A. 2010;107:20559–20564. doi: 10.1073/pnas.1010346107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giese KP, et al. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- 27.Buard I, et al. CaMKII "autonomy" is required for initiating but not for maintaining neuronal long-term information storage. J Neurosci. 2010;30:8214–8220. doi: 10.1523/JNEUROSCI.1469-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SJ, et al. Activation of CaMKII in single dendritic spines during long-term potentiation. Nature. 2009;458:299–304. doi: 10.1038/nature07842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coultrap SJ, et al. CaMKII autonomy is substrate-dependent and further stimulated by Ca2+/calmodulin. J Biol Chem. 2010;285:17930–17937. doi: 10.1074/jbc.M109.069351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erickson JR, et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chao LH, et al. A mechanism for tunable autoinhibition in the structure of a human Ca2+/calmodulin- dependent kinase II holoenzyme. Cell. 2011;146:732–745. doi: 10.1016/j.cell.2011.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rellos P, et al. Structure of the CaMKIIdelta/calmodulin complex reveals the molecular mechanism of CaMKII kinase activation. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000426. e1000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chao LH, et al. Intersubunit capture of regulatory segments is a component of cooperative CaMKII activation. Nat Struct Mol Biol. 2010;17:264–272. doi: 10.1038/nsmb.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hojjati MR, et al. Kinase activity is not required for alphaCaMKII-dependent presynaptic plasticity at CA3-CA1 synapses. Nat Neurosci. 2007;10:1125–1127. doi: 10.1038/nn1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coultrap SJ, et al. CaMKII in cerebral ischemia. Acta Pharmacol Sin. 2011;32:861–872. doi: 10.1038/aps.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Woerden GM, et al. betaCaMKII controls the direction of plasticity at parallel fiber-Purkinje cell synapses. Nat Neurosci. 2009;12:823–825. doi: 10.1038/nn.2329. [DOI] [PubMed] [Google Scholar]
- 37.Brocke L, et al. Developmental and regional expression of multifunctional Ca2+/calmodulin-dependent protein kinase isoforms in rat brain. J Neurosci. 1995;15:6797–6808. doi: 10.1523/JNEUROSCI.15-10-06797.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Leary H, et al. CaMKIIbeta association with the actin cytoskeleton is regulated by alternative splicing. Mol Biol Cell. 2006;17:4656–4665. doi: 10.1091/mbc.E06-03-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brocke L, et al. Functional implications of the subunit composition of neuronal CaM kinase II. J Biol Chem. 1999;274:22713–22722. doi: 10.1074/jbc.274.32.22713. [DOI] [PubMed] [Google Scholar]
- 40.Bayer KU, et al. Alternative splicing modulates the frequency-dependent response of CaMKII to Ca(2+) oscillations. EMBO J. 2002;21:3590–3597. doi: 10.1093/emboj/cdf360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bayer KU, et al. Developmental expression of the CaM kinase II isoforms: ubiquitous gamma-and delta-CaM kinase II are the early isoforms and most abundant in the developing nervous system. Brain Res Mol Brain Res. 1999;70:147–154. doi: 10.1016/s0169-328x(99)00131-x. [DOI] [PubMed] [Google Scholar]
- 42.Woodgett JR, et al. The calmodulin-dependent glycogen synthase kinase from rabbit skeletal muscle. Purification, subunit structure and substrate specificity. Eur J Biochem. 1983;136:481–487. doi: 10.1111/j.1432-1033.1983.tb07766.x. [DOI] [PubMed] [Google Scholar]
- 43.Krapivinsky G, et al. SynGAP-MUPP1-CaMKII synaptic complexes regulate p38 MAP kinase activity and NMDA receptor-dependent synaptic AMPA receptor potentiation. Neuron. 2004;43:563–574. doi: 10.1016/j.neuron.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Rosenberg OS, et al. Oligomerization states of the association domain and the holoenyzme of Ca2+/CaM kinase II. FEBS J. 2006;273:682–694. doi: 10.1111/j.1742-4658.2005.05088.x. [DOI] [PubMed] [Google Scholar]
- 45.Morris EP, Torok K. Oligomeric structure of alpha-calmodulin-dependent protein kinase II. J Mol Biol. 2001;308:1–8. doi: 10.1006/jmbi.2001.4584. [DOI] [PubMed] [Google Scholar]
- 46.Hoelz A, et al. Crystal structure of a tetradecameric assembly of the association domain of Ca2+/calmodulin-dependent kinase II. Mol Cell. 2003;11:1241–1251. doi: 10.1016/s1097-2765(03)00171-0. [DOI] [PubMed] [Google Scholar]
- 47.De Koninck P, Schulman H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science. 1998;279:227–230. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- 48.Sik A, et al. The absence of a major Ca2+ signaling pathway in GABAergic neurons of the hippocampus. Proc Natl Acad Sci U S A. 1998;95:3245–3250. doi: 10.1073/pnas.95.6.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lamsa K, et al. NMDA receptor-dependent long-term potentiation in mouse hippocampal interneurons shows a unique dependence on Ca(2+)/calmodulin-dependent kinases. J Physiol. 2007;584:885–894. doi: 10.1113/jphysiol.2007.137380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller S, et al. Disruption of dendritic translation of CaMKIIalpha impairs stabilization of synaptic plasticity and memory consolidation. Neuron. 2002;36:507–519. doi: 10.1016/s0896-6273(02)00978-9. [DOI] [PubMed] [Google Scholar]
- 51.Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127:49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 52.Wang DO, et al. Spatially restricting gene expression by local translation at synapses. Trends Neurosci. 2010;33:173–182. doi: 10.1016/j.tins.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aakalu G, et al. Dynamic visualization of local protein synthesis in hippocampal neurons. Neuron. 2001;30:489–502. doi: 10.1016/s0896-6273(01)00295-1. [DOI] [PubMed] [Google Scholar]
- 54.Ehlers MD. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat Neurosci. 2003;6:231–242. doi: 10.1038/nn1013. [DOI] [PubMed] [Google Scholar]
- 55.Bingol B, et al. Autophosphorylated CaMKIIalpha acts as a scaffold to recruit proteasomes to dendritic spines. Cell. 2010;140:567–578. doi: 10.1016/j.cell.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 56.Djakovic SN, et al. Regulation of the proteasome by neuronal activity and calcium/calmodulin-dependent protein kinase II. J Biol Chem. 2009;284:26655–26665. doi: 10.1074/jbc.M109.021956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meyer T, et al. Calmodulin trapping by calcium-calmodulin-dependent protein kinase. Science. 1992;256:1199–1202. doi: 10.1126/science.256.5060.1199. [DOI] [PubMed] [Google Scholar]
- 58.Coultrap SJ, et al. A significant but rather mild contribution of T286 autophosphorylation to Ca2+/CaM-stimulated CaMKII activity. PLoS ONE. 2012 doi: 10.1371/journal.pone.0037176. http://dx.plos.org/10.1371/journal.pone.0037176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosenberg OS, et al. Structure of the autoinhibited kinase domain of CaMKII and SAXS analysis of the holoenzyme. Cell. 2005;123:849–860. doi: 10.1016/j.cell.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 60.Hoffman L, et al. Conformational changes underlying calcium/calmodulin-dependent protein kinase II activation. EMBO J. 2011;30:1251–1262. doi: 10.1038/emboj.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bayer KU, et al. Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature. 2001;411:801–805. doi: 10.1038/35081080. [DOI] [PubMed] [Google Scholar]
- 62.Bayer KU, et al. Transition from reversible to persistent binding of CaMKII to postsynaptic sites and NR2B. J Neurosci. 2006;26:1164–1174. doi: 10.1523/JNEUROSCI.3116-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robison AJ, et al. Differential modulation of Ca2+/calmodulin-dependent protein kinase II activity by regulated interactions with N-methyl-D-aspartate receptor NR2B subunits and alpha-actinin. J Biol Chem. 2005;280:39316–39323. doi: 10.1074/jbc.M508189200. [DOI] [PubMed] [Google Scholar]
- 64.Alev C, et al. The neuronal connexin36 interacts with and is phosphorylated by CaMKII in a way similar to CaMKII interaction with glutamate receptors. Proc Natl Acad Sci U S A. 2008;105:20964–20969. doi: 10.1073/pnas.0805408105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guillaud L, et al. Disruption of KIF17-Mint1 interaction by CaMKII-dependent phosphorylation: a molecular model of kinesin-cargo release. Nat Cell Biol. 2008;10:19–29. doi: 10.1038/ncb1665. [DOI] [PubMed] [Google Scholar]
- 66.Jalan-Sakrikar N, et al. Substrate-selective and calcium-independent activation of CaMKII by alpha-actinin. J Biol Chem. 2012 doi: 10.1074/jbc.M112.351817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chang BH, et al. Characterization of a calmodulin kinase II inhibitor protein in brain. Proc Natl Acad Sci U S A. 1998;95:10890–10895. doi: 10.1073/pnas.95.18.10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chang BH, et al. Calcium/calmodulin-dependent protein kinase II inhibitor protein: localization of isoforms in rat brain. Neuroscience. 2001;102:767–777. doi: 10.1016/s0306-4522(00)00520-0. [DOI] [PubMed] [Google Scholar]
- 69.Coultrap SJ, Bayer KU. Improving a Natural CaMKII Inhibitor by Random and Rational Design. PLoS One. 2011;6:e25245. doi: 10.1371/journal.pone.0025245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Colbran RJ, Soderling TR. Calcium/calmodulin-independent autophosphorylation sites of calcium/calmodulin-dependent protein kinase II. Studies on the effect of phosphorylation of threonine 305/306 and serine 314 on calmodulin binding using synthetic peptides. J Biol Chem. 1990;265:11213–11219. [PubMed] [Google Scholar]
- 71.Hanson PI, Schulman H. Inhibitory autophosphorylation of multifunctional Ca2+/calmodulin-dependent protein kinase analyzed by site-directed mutagenesis. J Biol Chem. 1992;267:17216–17224. [PubMed] [Google Scholar]
- 72.Colbran RJ. Inactivation of Ca2+/calmodulin-dependent protein kinase II by basal autophosphorylation. J Biol Chem. 1993;268:7163–7170. [PubMed] [Google Scholar]
- 73.Vest RS, et al. Dual Mechanism of a Natural CaMKII Inhibitor. Mol Biol Cell. 2007;18:5024–5033. doi: 10.1091/mbc.E07-02-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Radwanska K, et al. Differential regulation of CaMKII inhibitor beta protein expression after exposure to a novel context and during contextual fear memory formation. Genes Brain Behav. 2010;9:648–657. doi: 10.1111/j.1601-183X.2010.00595.x. [DOI] [PubMed] [Google Scholar]
- 75.Bradshaw JM, et al. Chemical quenched flow kinetic studies indicate an intraholoenzyme autophosphorylation mechanism for Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 2002;277:20991–20998. doi: 10.1074/jbc.M202154200. [DOI] [PubMed] [Google Scholar]
- 76.Lu CS, et al. Regulation of the Ca2+/CaM-responsive pool of CaMKII by scaffold-dependent autophosphorylation. Neuron. 2003;40:1185–1197. doi: 10.1016/s0896-6273(03)00786-4. [DOI] [PubMed] [Google Scholar]
- 77.Strack S, et al. Differential inactivation of postsynaptic density-associated and soluble Ca2+/calmodulin-dependent protein kinase II by protein phosphatases 1 and 2A. J Neurochem. 1997;68:2119–2128. doi: 10.1046/j.1471-4159.1997.68052119.x. [DOI] [PubMed] [Google Scholar]
- 78.Mullasseril P, et al. A structural mechanism for maintaining the 'on-state' of the CaMKII memory switch in the post-synaptic density. J Neurochem. 2007;103:357–364. doi: 10.1111/j.1471-4159.2007.04744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Colbran RJ. Targeting of calcium/calmodulin-dependent protein kinase II. Biochem J. 2004;378:1–16. doi: 10.1042/BJ20031547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tao-Cheng JH, et al. Inhibition of phosphatase activity facilitates the formation and maintenance of NMDA-induced calcium/calmodulin-dependent protein kinase II clusters in hippocampal neurons. Neuroscience. 2005;130:651–656. doi: 10.1016/j.neuroscience.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 81.Cheriyan J, et al. Calcium/calmodulin dependent protein kinase II bound to NMDA receptor 2B subunit exhibits increased ATP affinity and attenuated dephosphorylation. PLoS One. 2011;6:e16495. doi: 10.1371/journal.pone.0016495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hanson PI, et al. Dual role of calmodulin in autophosphorylation of multifunctional CaM kinase may underlie decoding of calcium signals. Neuron. 1994;12:943–956. doi: 10.1016/0896-6273(94)90306-9. [DOI] [PubMed] [Google Scholar]
- 83.Rich RC, Schulman H. Substrate-directed function of calmodulin in autophosphorylation of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 1998;273:28424–28429. doi: 10.1074/jbc.273.43.28424. [DOI] [PubMed] [Google Scholar]
- 84.Merrill MA, et al. Activity-driven postsynaptic translocation of CaMKII. Trends Pharmacol Sci. 2005;26:645–653. doi: 10.1016/j.tips.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 85.Robison AJ, et al. Multivalent interactions of calcium/calmodulin-dependent protein kinase II with the postsynaptic density proteins NR2B, densin-180, and alpha-actinin-2. J Biol Chem. 2005;280:35329–35336. doi: 10.1074/jbc.M502191200. [DOI] [PubMed] [Google Scholar]
- 86.Walikonis RS, et al. Densin-180 forms a ternary complex with the (alpha)-subunit of Ca2+/calmodulin-dependent protein kinase II and (alpha)-actinin. J Neurosci. 2001;21:423–433. doi: 10.1523/JNEUROSCI.21-02-00423.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Okamoto K, et al. The role of CaMKII as an F-actin-bundling protein crucial for maintenance of dendritic spine structure. Proc Natl Acad Sci U S A. 2007;104:6418–6423. doi: 10.1073/pnas.0701656104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pi HJ, et al. CaMKII control of spine size and synaptic strength: role of phosphorylation states and nonenzymatic action. Proc Natl Acad Sci U S A. 2010;107:14437–14442. doi: 10.1073/pnas.1009268107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Borgesius NZ, et al. betaCaMKII plays a nonenzymatic role in hippocampal synaptic plasticity and learning by targeting alphaCaMKII to synapses. J Neurosci. 2011;31:10141–10148. doi: 10.1523/JNEUROSCI.5105-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lledo PM, et al. Calcium/calmodulin-dependent kinase II and long-term potentiation enhance synaptic transmission by the same mechanism. Proc Natl Acad Sci U S A. 1995;92:11175–11179. doi: 10.1073/pnas.92.24.11175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen HX, et al. Is persistent activity of calcium/calmodulin-dependent kinase required for the maintenance of LTP? J Neurophysiol. 2001;85:1368–1376. doi: 10.1152/jn.2001.85.4.1368. [DOI] [PubMed] [Google Scholar]
- 92.Sanhueza M, et al. Role of the CaMKII/NMDA receptor complex in the maintenance of synaptic strength. J Neurosci. 2011;31:9170–9178. doi: 10.1523/JNEUROSCI.1250-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 94.Halt AR, et al. CaMKII binding to GluN2B is critical during memory consolidation. EMBO J. 2012;31:1203–1216. doi: 10.1038/emboj.2011.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Asrican B, et al. Synaptic strength of individual spines correlates with bound Ca2+-calmodulin-dependent kinase II. J Neurosci. 2007;27:14007–14011. doi: 10.1523/JNEUROSCI.3587-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Otmakhov N, et al. Persistent accumulation of calcium/calmodulin-dependent protein kinase II in dendritic spines after induction of NMDA receptor-dependent chemical long-term potentiation. J Neurosci. 2004;24:9324–9331. doi: 10.1523/JNEUROSCI.2350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang YP, et al. Optical induction of plasticity at single synapses reveals input-specific accumulation of alphaCaMKII. Proc Natl Acad Sci U S A. 2008;105:12039–12044. doi: 10.1073/pnas.0802940105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gambrill AC, Barria A. NMDA receptor subunit composition controls synaptogenesis and synapse stabilization. Proc Natl Acad Sci U S A. 2011;108:5855–5860. doi: 10.1073/pnas.1012676108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rauner C, Kohr G. Triheteromeric NR1/NR2A/NR2B receptors constitute the major N-methyl-D-aspartate receptor population in adult hippocampal synapses. J Biol Chem. 2011;286:7558–7566. doi: 10.1074/jbc.M110.182600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Strack S, et al. Mechanism and regulation of calcium/calmodulin-dependent protein kinase II targeting to the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 2000;275:23798–23806. doi: 10.1074/jbc.M001471200. [DOI] [PubMed] [Google Scholar]
- 101.Gardoni F, et al. Hippocampal synaptic plasticity involves competition between Ca2+/calmodulin-dependent protein kinase II and postsynaptic density 95 for binding to the NR2A subunit of the NMDA receptor. J Neurosci. 2001;21:1501–1509. doi: 10.1523/JNEUROSCI.21-05-01501.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Leonard AS, et al. Regulation of calcium/calmodulin-dependent protein kinase II docking to N-methyl-D-aspartate receptors by calcium/calmodulin and alpha-actinin. J Biol Chem. 2002;277:48441–48448. doi: 10.1074/jbc.M205164200. [DOI] [PubMed] [Google Scholar]
- 103.Nikandrova YA, et al. Ca2+/calmodulin-dependent protein kinase II binds to and phosphorylates a specific SAP97 splice variant to disrupt association with AKAP79/150 and modulate alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-type glutamate receptor (AMPAR) activity. J Biol Chem. 2010;285:923–934. doi: 10.1074/jbc.M109.033985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Redondo RL, et al. Synaptic tagging and capture: differential role of distinct calcium/calmodulin kinases in protein synthesis-dependent long-term potentiation. J Neurosci. 2010;30:4981–4989. doi: 10.1523/JNEUROSCI.3140-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sajikumar S, et al. Identification of compartment- and process-specific molecules required for "synaptic tagging" during long-term potentiation and long-term depression in hippocampal CA1. J Neurosci. 2007;27:5068–5080. doi: 10.1523/JNEUROSCI.4940-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moncada D, et al. Identification of transmitter systems and learning tag molecules involved in behavioral tagging during memory formation. Proc Natl Acad Sci U S A. 2011;108:12931–12936. doi: 10.1073/pnas.1104495108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Steiner P, et al. Destabilization of the postsynaptic density by PSD-95 serine 73 phosphorylation inhibits spine growth and synaptic plasticity. Neuron. 2008;60:788–802. doi: 10.1016/j.neuron.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gardoni F, et al. Calcium-calmodulin-dependent protein kinase II phosphorylation modulates PSD-95 binding to NMDA receptors. Eur J Neurosci. 2006;24:2694–2704. doi: 10.1111/j.1460-9568.2006.05140.x. [DOI] [PubMed] [Google Scholar]
- 109.Mauceri D, et al. Dual role of CaMKII-dependent SAP97 phosphorylation in mediating trafficking and insertion of NMDA receptor subunit NR2A. J Neurochem. 2007;100:1032–1046. doi: 10.1111/j.1471-4159.2006.04267.x. [DOI] [PubMed] [Google Scholar]
- 110.Hudmon A, et al. A mechanism for Ca2+/calmodulin-dependent protein kinase II clustering at synaptic and nonsynaptic sites based on self-association. J Neurosci. 2005;25:6971–6983. doi: 10.1523/JNEUROSCI.4698-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dosemeci A, et al. Glutamate-induced transient modification of the postsynaptic density. Proc Natl Acad Sci U S A. 2001;98:10428–10432. doi: 10.1073/pnas.181336998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vest RS, et al. Differential regulation by ATP versus ADP further links CaMKII aggregation to ischemic conditions. FEBS Lett. 2009;583:3577–3581. doi: 10.1016/j.febslet.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ramachandran B, Frey JU. Interfering with the actin network and its effect on long-term potentiation and synaptic tagging in hippocampal CA1 neurons in slices in vitro. J Neurosci. 2009;29:12167–12173. doi: 10.1523/JNEUROSCI.2045-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Okamoto K, et al. The roles of CaMKII and F-actin in the structural plasticity of dendritic spines: a potential molecular identity of a synaptic tag? Physiology (Bethesda) 2009;24:357–366. doi: 10.1152/physiol.00029.2009. [DOI] [PubMed] [Google Scholar]
- 115.Kim JH, et al. SynGAP: a synaptic RasGAP that associates with the PSD-95/SAP90 protein family. Neuron. 1998;20:683–691. doi: 10.1016/s0896-6273(00)81008-9. [DOI] [PubMed] [Google Scholar]
- 116.Chen HJ, et al. A synaptic Ras-GTPase activating protein (p135 SynGAP) inhibited by CaM kinase II. Neuron. 1998;20:895–904. doi: 10.1016/s0896-6273(00)80471-7. [DOI] [PubMed] [Google Scholar]
- 117.Xie Z, et al. Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron. 2007;56:640–656. doi: 10.1016/j.neuron.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Murakoshi H, et al. Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature. 2011;472:100–104. doi: 10.1038/nature09823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fink CC, et al. Selective regulation of neurite extension and synapse formation by the beta but not the alpha isoform of CaMKII. Neuron. 2003;39:283–297. doi: 10.1016/s0896-6273(03)00428-8. [DOI] [PubMed] [Google Scholar]
- 120.Sanabria H, et al. {beta}CaMKII regulates actin assembly and structure. J Biol Chem. 2009;284:9770–9780. doi: 10.1074/jbc.M809518200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shen K, et al. CaMKIIbeta functions as an F-actin targeting module that localizes CaMKIIalpha/beta heterooligomers to dendritic spines. Neuron. 1998;21:593–606. doi: 10.1016/s0896-6273(00)80569-3. [DOI] [PubMed] [Google Scholar]
- 122.Jiao Y, et al. Characterization of a central Ca2+/calmodulin-dependent protein kinase IIalpha/beta binding domain in densin that selectively modulates glutamate receptor subunit phosphorylation. J Biol Chem. 2011;286:24806–24818. doi: 10.1074/jbc.M110.216010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Carlisle HJ, et al. Deletion of Densin-180 Results in Abnormal Behaviors Associated with Mental Illness and Reduces mGluR5 and DISC1 in the Postsynaptic Density Fraction. J Neurosci. 2011;31:16194–16207. doi: 10.1523/JNEUROSCI.5877-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.O'Leary H, et al. Nucleotides and phosphorylation bi-directionally modulate Ca2+/calmodulin-dependent protein kinase II (CaMKII) binding to the N-methyl-D-aspartate (NMDA) receptor subunit GluN2B. J Biol Chem. 2011;286:31272–31281. doi: 10.1074/jbc.M111.233668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lu W, et al. Synaptic targeting of AMPA receptors is regulated by a CaMKII site in the first intracellular loop of GluA1. Proc Natl Acad Sci U S A. 2010;107:22266–22271. doi: 10.1073/pnas.1016289107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pi HJ, et al. Autonomous CaMKII can promote either long-term potentiation or long-term depression, depending on the state of T305/T306 phosphorylation. J Neurosci. 2010;30:8704–8709. doi: 10.1523/JNEUROSCI.0133-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yuen EY, et al. Homeostatic regulation of glutamatergic transmission by dopamine D4 receptors. Proc Natl Acad Sci U S A. 2010;107:22308–22313. doi: 10.1073/pnas.1010025108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stanton PK, Gage AT. Distinct synaptic loci of Ca2+/calmodulin-dependent protein kinase II necessary for long-term potentiation and depression. J Neurophysiol. 1996;76:2097–2101. doi: 10.1152/jn.1996.76.3.2097. [DOI] [PubMed] [Google Scholar]
- 129.Yuen EY, et al. Serotonin 5-HT1A receptors regulate NMDA receptor channels through a microtubule-dependent mechanism. J Neurosci. 2005;25:5488–5501. doi: 10.1523/JNEUROSCI.1187-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yuen EY, Yan Z. Cellular mechanisms for dopamine D4 receptor-induced homeostatic regulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors. J Biol Chem. 2011;286:24957–24965. doi: 10.1074/jbc.M111.221416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ciani L, et al. Wnt7a signaling promotes dendritic spine growth and synaptic strength through Ca(2)/Calmodulin-dependent protein kinase II. Proc Natl Acad Sci U S A. 2011;108:10732–10737. doi: 10.1073/pnas.1018132108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Puram SV, et al. A CaMKIIbeta signaling pathway at the centrosome regulates dendrite patterning in the brain. Nat Neurosci. 2011;14:973–983. doi: 10.1038/nn.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wheeler DG, et al. CaMKII locally encodes L-type channel activity to signal to nuclear CREB in excitation-transcription coupling. J Cell Biol. 2008;183:849–863. doi: 10.1083/jcb.200805048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Atkins CM, et al. Bidirectional regulation of cytoplasmic polyadenylation element-binding protein phosphorylation by Ca2+/calmodulin-dependent protein kinase II and protein phosphatase 1 during hippocampal long-term potentiation. J Neurosci. 2005;25:5604–5610. doi: 10.1523/JNEUROSCI.5051-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Greer PL, Greenberg ME. From synapse to nucleus: calcium-dependent gene transcription in the control of synapse development and function. Neuron. 2008;59:846–860. doi: 10.1016/j.neuron.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 136.Hudmon A, et al. CaMKII tethers to L-type Ca2+ channels, establishing a local and dedicated integrator of Ca2+ signals for facilitation. J Cell Biol. 2005;171:537–547. doi: 10.1083/jcb.200505155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Grueter CE, et al. L-type Ca2+ channel facilitation mediated by phosphorylation of the beta subunit by CaMKII. Mol Cell. 2006;23:641–650. doi: 10.1016/j.molcel.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 138.Rose J, et al. Heterosynaptic molecular dynamics: locally induced propagating synaptic accumulation of CaM kinase II. Neuron. 2009;61:351–358. doi: 10.1016/j.neuron.2008.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fukunaga K, et al. Long-term potentiation is associated with an increased activity of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 1993;268:7863–7867. [PubMed] [Google Scholar]
- 140.Ouyang Y, et al. Visualization of the distribution of autophosphorylated calcium/calmodulin-dependent protein kinase II after tetanic stimulation in the CA1 area of the hippocampus. J Neurosci. 1997;17:5416–5427. doi: 10.1523/JNEUROSCI.17-14-05416.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Makhinson M, et al. Adenylyl cyclase activation modulates activity-dependent changes in synaptic strength and Ca2+/calmodulin-dependent kinase II autophosphorylation. J Neurosci. 1999;19:2500–2510. doi: 10.1523/JNEUROSCI.19-07-02500.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Takao K, et al. Visualization of synaptic Ca2+ /calmodulin-dependent protein kinase II activity in living neurons. J Neurosci. 2005;25:3107–3112. doi: 10.1523/JNEUROSCI.0085-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lengyel I, et al. Autonomous activity of CaMKII is only transiently increased following the induction of long-term potentiation in the rat hippocampus. Eur J Neurosci. 2004;20:3063–3072. doi: 10.1111/j.1460-9568.2004.03748.x. [DOI] [PubMed] [Google Scholar]
- 144.Coultrap SJ, Bayer KU. Ca2+/Calmodulin-Dependent Protein Kinase II (CaMKII) In: Mukai H, editor. Neuromethods: Protein Kinase Technologies. Humana Press; 2012. in press. [Google Scholar]
- 145.Yamagata Y, et al. Kinase-dead knock-in mouse reveals an essential role of kinase activity of Ca2+/calmodulin-dependent protein kinase IIalpha in dendritic spine enlargement, long-term potentiation, and learning. J Neurosci. 2009;29:7607–7618. doi: 10.1523/JNEUROSCI.0707-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Migues PV, et al. Phosphorylation of CaMKII at Thr253 occurs in vivo and enhances binding to isolated postsynaptic densities. J Neurochem. 2006;98:289–299. doi: 10.1111/j.1471-4159.2006.03876.x. [DOI] [PubMed] [Google Scholar]
- 147.Mayford M, et al. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]