Abstract
In our ongoing search toward identifying novel and synthetically simpler candidate vaccine adjuvants, we hypothesized that the imidazo[1,2-a]pyrazines, readily accessible via the Groebke-Blackburn-Bienaymé multicomponent reaction, would possess sufficient structural similarity with TLR7/8-agonistic imidazoquinolines. With pyridoxal as the aldehyde component, furo[2,3- c]pyridines, rather than the expected imidazo[1,2-a]pyridines were obtained, which were characterized by NMR spectroscopy and crystallography. Several analogues were found to activate TLR8-dependent NF-κB signaling. In a focused library of furo[2,3-c]pyridines, a distinct SAR was observed with varying substituents at C2. In human PBMCs, none of the furo[2,3-c]pyridines showed any proinflammatory cytokine induction, but upregulated several chemokine ligand genes. In immunization studies in rabbits, the most active compound showed prominent adjuvantic effects. The complete lack of proinflammatory cytokine induction coupled with strong adjuvantic activity of the novel furo[2,3-c]pyridines render this hitherto unknown chemotype an attractive class of compounds which are expected to be devoid of local or systemic reactogenicity.
Keywords: TLR8, TLR8 agonists, Vaccine adjuvants, Innate immunity, Furopyridines
Introduction
The innate and adaptive limbs of the immune system are activated in a highly regulated and coordinated manner to initiate host responses to invading pathogens. The innate immune system utilizes germline-encoded pattern recognition receptors (PRRs) to discern pathogen-associated molecular patterns (PAMPs) that are distinct to the pathogen.1–3 PRRs encompass diverse families of receptors4 that are secreted into the extracellular environment (such as the collectins,5 ficolins,6 pentraxins,7 alarmins8), exist in the cytosol (examples of which include the retinoic acid–inducible gene I–like receptors,9 and the nucleotide-binding domain and leucine-rich repeat–containing receptors10), or are present on membranes.
Important among the transmembrane PRRs include the Toll-like receptors11 (TLRs) which are either expressed on the plasma membrane or in the endolysosomal compartments.1 At least 10 functional TLRs are encoded in the human genome, each with an extracellular domain having leucine-rich repeats and a cytosolic domain called the Toll/IL-1 receptor domain.12 The ligands for these receptors are highly conserved microbial molecules such as lipopolysaccharides (LPS) (recognized by TLR4), lipopeptides (TLR2 in combination with TLR1 or TLR6), flagellin (TLR5), single stranded RNA (TLR7 and TLR8), double stranded RNA (TLR3), CpG motif-containing DNA (recognized by TLR9), and profilin present on uropathogenic bacteria (TLR11).13 TLR1, -2, -4, -5, and -6 recognize extracellular stimuli, while TLR3, -7, -8 and -9 function within the endolysosomal compartment.12 The activation of TLRs by their cognate ligands leads to production of inflammatory cytokines, and up-regulation of MHC molecules and co-stimulatory signals in antigen-presenting cells as well as activating natural killer cells (innate immune response), which leads to the priming and amplification of T-, and B-cell effector functions (adaptive immune responses).14–17
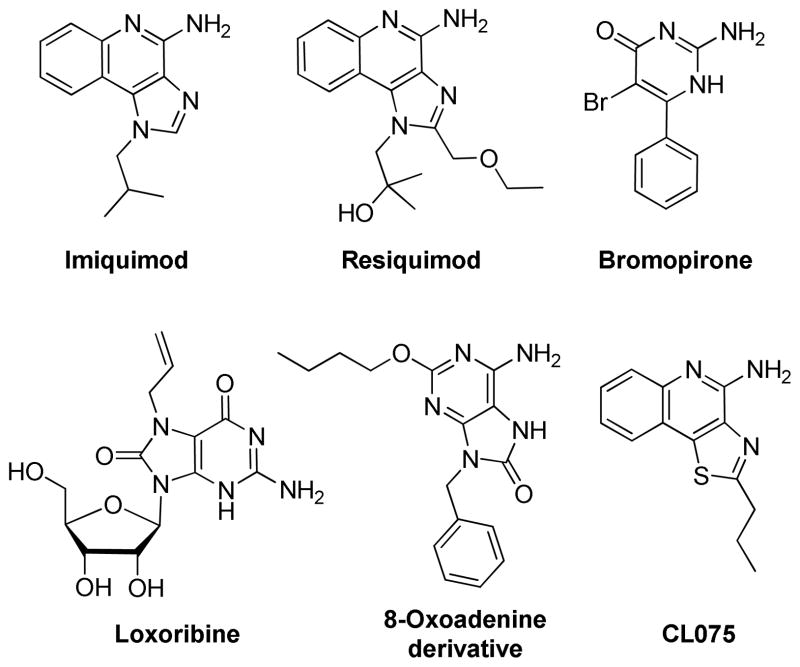
The discovery of TLRs has not only served to greatly accelerate our understanding of the interplay between the innate and adaptive immune systems, but is also catalyzing novel approaches to vaccine design and development. Several synthetic compounds have been identified as ligands and agonists of the TLRs, leading to detailed SAR studies on these chemotypes. For instance, the S-[2,3-bis(palmitoyloxy)-(2RS)-propyl]-R-cysteinyl-S-serine (PAM2CS) lipopeptides18 and simpler analogues19,20 activate TLR2. Polyriboinosinic polyribocytidylic acid (poly(I:C)) activates TLR3.21,22 Synthetic lipid A23 and various monosaccharide lipid A analogues24,25 have been reported to activate immune cells by utilizing TLR4. Imidazoquinolines such as imiquimod and resiquimod,26–28 2-amino-pyrimidines such as bromopirone,29,30 guanosine analogues such as loxoribine,31 and 8-hydroxyadenine derivatives32,33 are known to activate TLR7 and/or TLR8, while 2-alkylthiazolo[4,5-c]quinolin- 4-amine derivatives predominantly activate TLR8 (Figure 1).34,35
Figure 1.
Representative TLR7/8-agonistic heterocyclic small molecules.
Small molecule TLR7/8 activators constitute a small set of compounds occupying a very small chemical space, and are represented by substituted imidazo/thiazolo/oxazolo/selenazolo- [4,5-c]quinolines 1a-d, imidazo[4,5-c]pyridines 2, and a few purine and pyrimidine derivatives (Figures 1 and 2). The identification of simpler molecules as TLR7/8 agonists may pave the way for inexpensive vaccine constructs, and we are therefore keenly interested in exploring alternative chemotypes that are synthetically less complex. Unlike TLR2, TLR3, TLR4,36 and TLR537 for which crystal structures are available as complexes with their cognate ligands, a detailed structural characterization of the mode of binding of TLR7 ligands is not yet available to guide scaffold-hopping38,39 approaches. We speculated that 3,8-diamino-imidazo[1,2-a]pyrazines 4 (Figure 2) may bear sufficient structural similarities to the known TLR7/8 ligands (Figures 1 and 2). These molecules are, in principle, readily accessible in two steps (one-pot synthetic process) via the Groebke-Blackburn-Bienaymé multicomponent reaction,32,33 and we envisaged a rapid elaboration and screening of a library of compounds for TLR7/8 agonistic activities.
Figure 2.
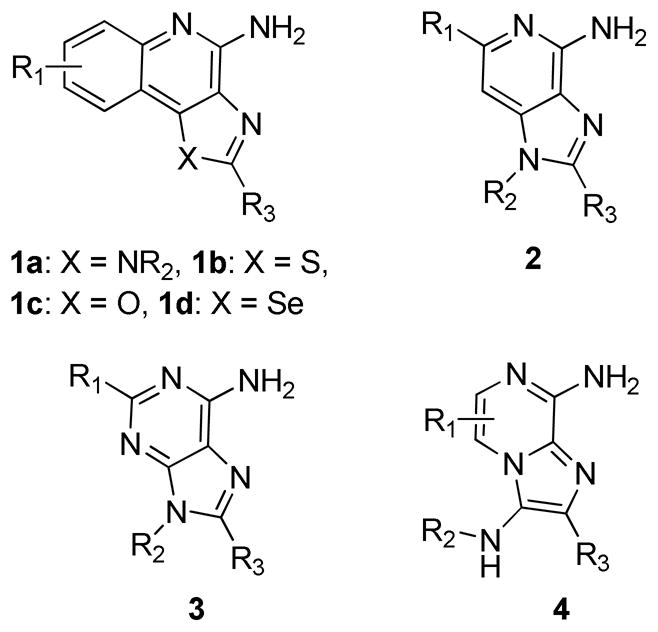
TLR7/8 agonistic scaffolds. R1 is typically alkyl or O-alkyl, R2 is alkyl or benzyl, and R3 is usually alkyl, and OH in oxoadenines.
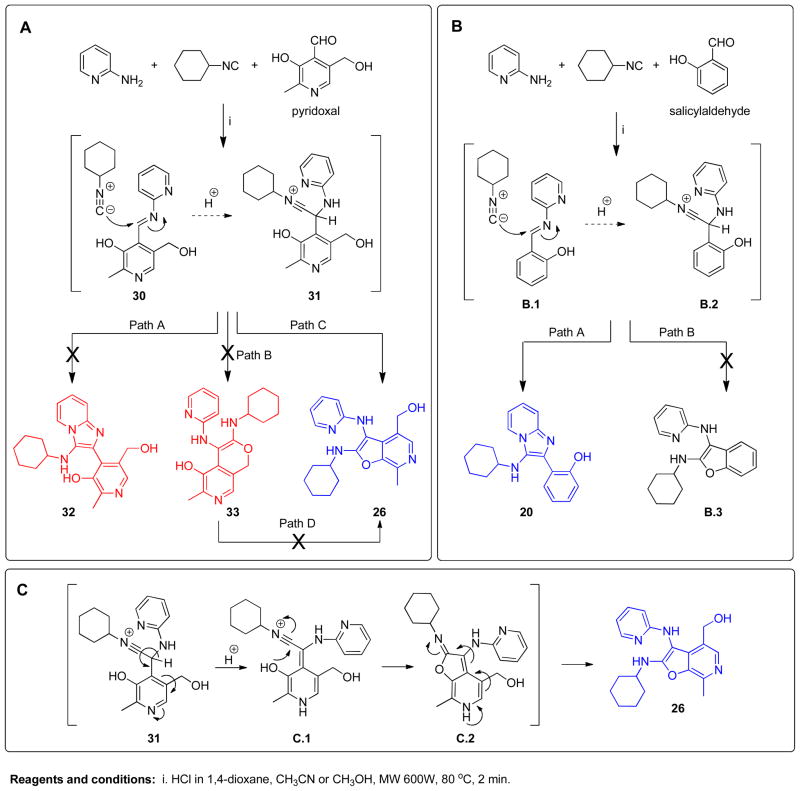
We began with the syntheses of small test-libraries of 3,8-diamino-imidazo[1,2- a]pyrazines as well as 3-amino-imidazo[1,2-a]pyridine/pyrazines (Schemes 1 and 2). Most of these compounds (5a–5d, 6–23) were inactive in NF-κB reporter gene assays specific for human TLR-3, -7, -8, and -9; however compounds 26–29 obtained with pyridoxal as the aldehyde component were found to specifically activate NF-κB signaling in TLR8-transfected HEK293 cells. Detailed spectroscopic analyses confirmed the formation of a hitherto unknown 2,3- diamino-furo[2,3-c]pyridine skeleton via a non-canonical pathway, which was unambiguously confirmed by single crystal X-ray analysis. The TLR8-specific agonistic properties of this novel and unexpected chemotype warranted a systematic SAR, which is presented herein.
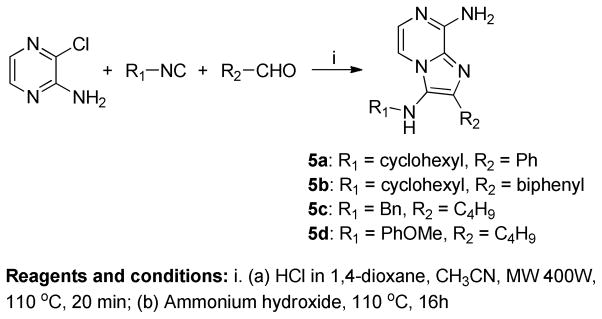
Scheme 1.
A two step one-pot synthetic process.
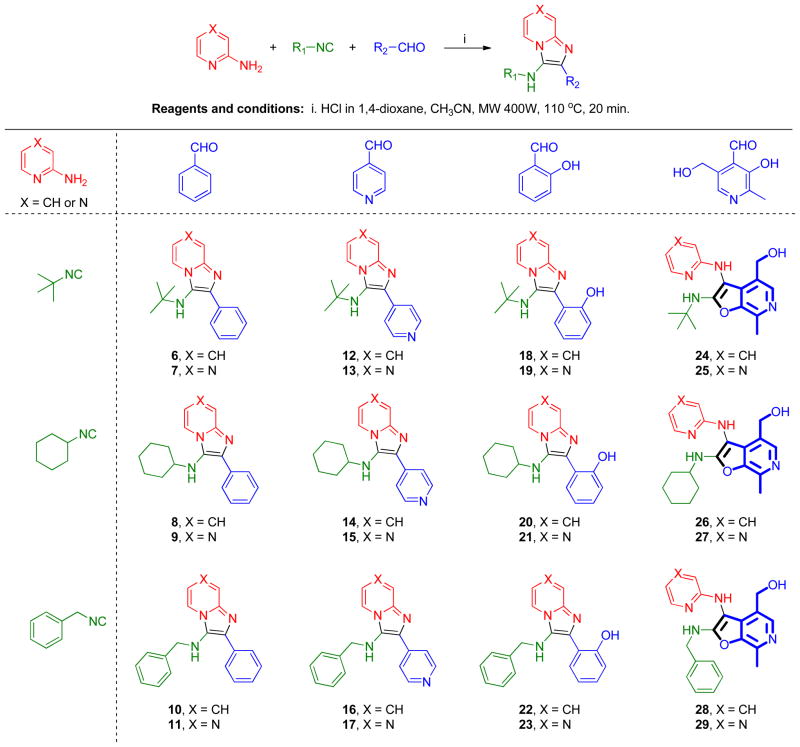
Scheme 2.
Twenty-four membered diverse test-library.
Results and Discussion
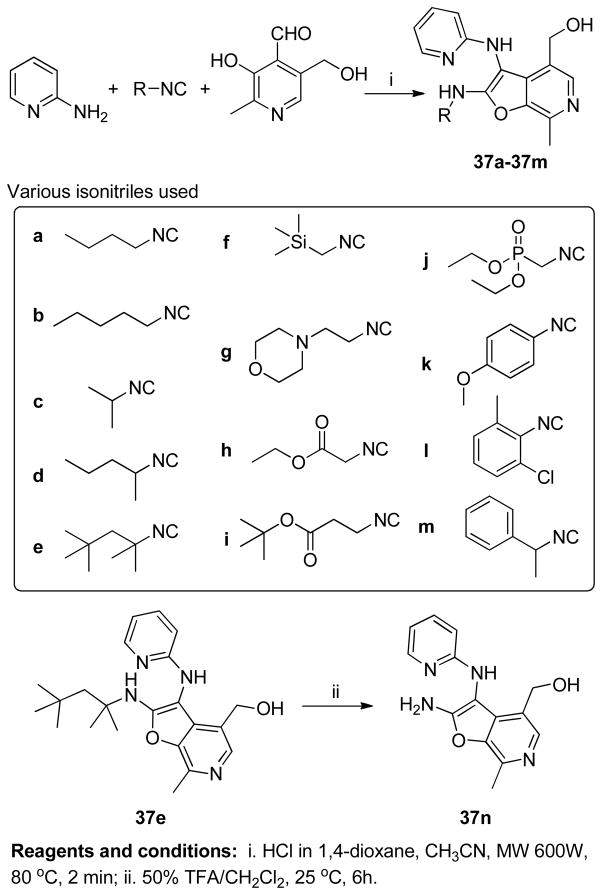
We have previously described extensive SAR on the 1,2-disubstituted-(1H-imidazo[4,5- c]quinoline-4-amines) class of compounds27,28,40,41 (1a, Figure 2) as TLR7/8 agonists, and their application in designing self-adjuvanting vaccine constructs.42 In our ongoing search toward identifying novel and synthetically simpler candidate vaccine adjuvants, we hypothesized that the imidazo[1,2-a]pyrazines 4 (Figure 2) would possess sufficient structural similarity with the known small molecule TLR7/8 ligands such as 1–3 (Figure 2). These molecules are readily accessible in a one-pot, two-step process using the Groebke-Blackburn-Bienaymé multicomponent reaction as a key step. An acid-catalyzed (HCl in dioxane), microwave-mediated (400W, 110 °C, 10 min) reaction using 2-amino-3-chloropyrazine (amidine component), isocyanocyclohexane (isonitrile component) and benzaldehyde (aldehyde component) resulted, as expected, in 8-chloro-N-cyclohexyl-2-phenylimidazo[1,2-a]pyrazin-3- amine (Scheme 1). Subsequent microwave-mediated ipso-chloro displacement using ammonium hydroxide was unsuccessful, but conventional heating in a sealed tube (110 °C, 16h) furnished the desired N3-cyclohexyl-2-phenylimidazo[1,2-a]pyrazine-3,8-diamine 5a (Scheme 1) in 30% yield over two steps. Using this one-pot process, a small set of 8-amino-imidazo[1,2-a]pyrazines 5b-5d was synthesized by varying the aldehyde and isonitrile components (Scheme 1).
Simultaneously, a diverse test-library comprising of twenty-four compounds was also synthesized (Scheme 2) using two amidines (2-aminopyridine and 2-aminopyrazine), three isonitriles (2-isocyano-2-methylpropane, isocyanocyclohexane, (isocyanomethyl)benzene), and four aldehydes (benzaldehyde, isonicotinaldehyde, salicylaldehyde and pyridoxal). The syntheses of 6–23 (Scheme 2) proceeded smoothly. The typical Groebke reaction, carried out at 100 °C for 20 min in CH3CN, was found to be excessively harsh for reactions using pyridoxal (24–29), leading to low yields and charring of reaction mixtures. Reactions for this subset of compounds progressed rapidly in 2 min under microwave conditions at 80 °C and 600W power in CH3CN or MeOH. We noticed that only compounds 24–29 (synthesized using pyridoxal) were fluorescent on TLC under long-wave ultraviolet radiation.
The compounds were screened in NF-κB reporter gene assays specific for human TLR-3, -4, -5, -7, -8, and -9. The 3,8-diamino-imidazo[1,2-a]pyrazines 5a-5d as well as 3-amino-imidazo[1,2-a]pyridine/pyrazine library members 6–23 did not display any activity in these assays up to concentrations of 250 μM (Supporting Information). However, compounds 26–29 (Scheme 2), obtained with the use of pyridoxal as the aldehyde component, were found to specifically activate NF-κB signaling in human TLR8-transfected HEK293 cells (Table 1, Figure 3), but not human TLR-3, -4, -5, -7, and -9. The NMR spectra of compounds 26–29 as well as their fluorescence properties alerted us to the possibility of the formation of a new chemical entity with a heterocyclic system other than the classical imidazo[1,2-a]pyridine/pyrazines during the Groebke-Blackburn-Bienaymé multicomponent reaction. In 1H NMR spectra, the aliphatic CH of the cyclohexyl group in compounds 8/9, 14/15, and 20/21 (Scheme 2) appeared in the range of 3.0 to 3.1 δ ppm, whereas a pronounced downfield shift of this CH proton (up to 3.85 δ ppm) was observed in compounds 26 and 27. Similar observations were noted in another set in which the aliphatic benzylic CH2 in compounds 10/11, 16/17, and 22/23 appeared in the range of 4.2 to 4.3 δ ppm, whereas a pronounced downfield shift of this CH2 (up to 4.76 δ ppm) was observed in compounds 28 and 29. 13C NMR spectra of compounds 24–29 showed an unusual upfield shift of one of the aromatic quaternary carbons in the region (90–96 δ ppm). These observations suggested a different heterocyclic system in 24–29. Initial efforts to crystallize these molecules were unsuccessful. Pending continuing crystallization efforts, we sought to elucidate the structures of these compounds via alternate routes.
Table 1.
EC50 values of compounds in human TLR8-specific reporter gene assay
| Structure | Compound Number | TLR8-Agonistic Activity (μM) |
|---|---|---|
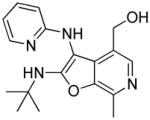
|
24 | Inactive |
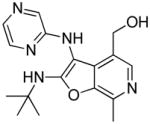
|
25 | Inactive |
|
26 | 6.93 |
|
27 | 10.58 |
|
28 | 4.91 |
|
29 | 9.79 |
|
35 | Inactive |
|
36 | 1.68 Very low AUC |
|
37a | 9.38 |
|
37b | 5.81 |
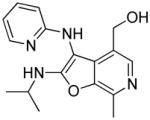
|
37c | Inactive |
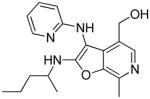
|
37d | 9.01 Low AUC |
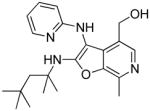
|
37e | Inactive |
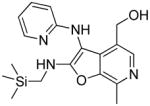
|
37f | 4.99 |
|
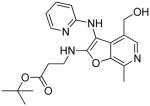
37g | Inactive |
|
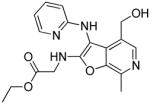
37h | Inactive |
|
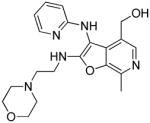
37i | 7.64 Low AUC |
|
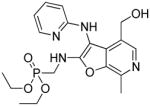
37j | Inactive |
|
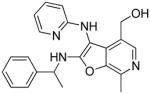
37k | Inactive |
|
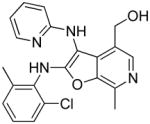
37l | Inactive |
|
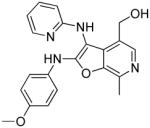
37m | 4.27 Very low AUC |
|
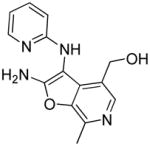
37n | Inactive |
|
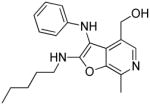
38a | 2.25 Low AUC |
|
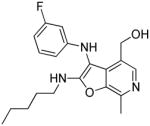
38b | 0.37 Low AUC |
|
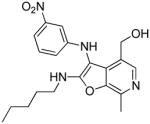
38c | 0.85 Low AUC |
|
39 | 3.37 |
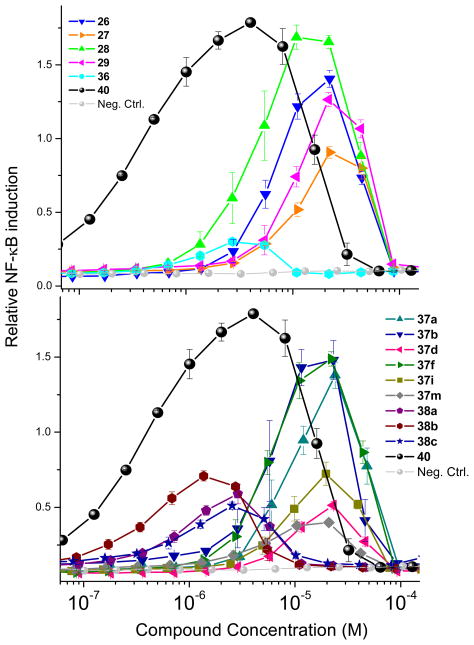
Figure 3.
Dose-response profiles of TLR8 agonism by select 2,3-diamino-furo[2,3-c]pyridines. Top: TLR8 agonism by compounds derived from Schemes 2 and 5. Bottom: TLR8 agonism by compounds derived from Schemes 6 and 7. Data points represent means and standard deviations obtained on quadruplicates.
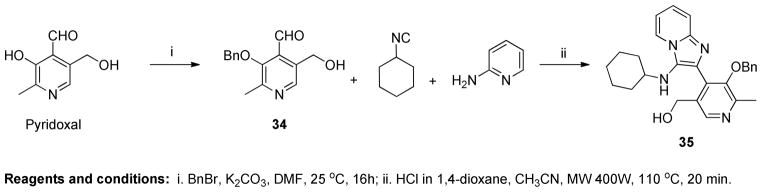
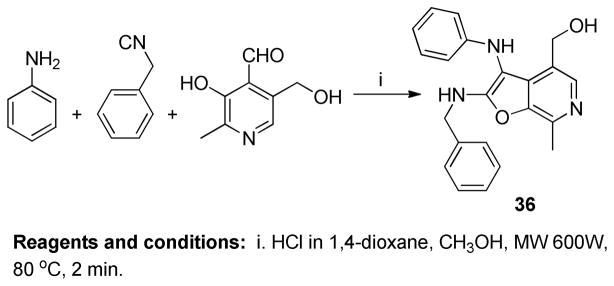
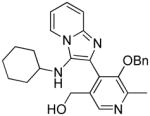
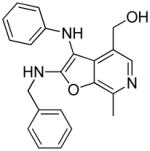
Three possible cyclization products appeared plausible in this acid-catalyzed multicomponent reaction (Panel A in Scheme 3). As mentioned earlier, NMR spectroscopic observations for compounds 24–29 were not congruent with the 4-(3- (cyclohexylamino)imidazo[1,2-a]pyridin-2-yl)-5-(hydroxymethyl)-2-methylpyridin-3-ol 32, the expected product via the canonical Groebke mechanism (‘Path A’, Panel A in Scheme 3). In order to test whether the phenolic or benzylic hydroxyl groups of pyridoxal may be involved in an alternate pathway of cyclization, we carried out a reaction using a pyridoxal derivative 34 with its phenolic hydroxyl protected with a benzyl group (Scheme 4). This resulted in a product with a spectral signature entirely consistent with the classical Groebke product (5-(benzyloxy)-4- (3-(cyclohexylamino)imidazo[1,2-a]pyridin-2-yl)-6-methylpyridin-3-yl)methanol 35, indirectly also ruling out the possibility of cyclization involving the benzylic hydroxyl group (‘Path B’, Panel A in Scheme 3). We reasoned that annulation via ‘Path C’ ought to lead to a furo[2,3- c]pyridine skeleton 26, and that if this were indeed the case, this cyclization could proceed even if the amidine were to be replaced with an aniline. We were gratified that a multicomponent reaction involving aniline, benzyl isonitrile and pyridoxal (Scheme 5) yielded the fluorescent compound 36, whose 1H and 13C NMR spectra resembled those of compounds 24–29. Interestingly, 36 was also found to be weakly active (EC50 = 1.68 μM) in primary TLR8 screens (Table 1, Figure 3). Our investigations thus far suggested the formation of a hitherto unknown furo[2,3- c]pyridine structure, exclusively when pyridoxal was used as the aldehyde component in the Groebke-Blackburn-Bienaymé multicomponent reaction.
Scheme 3.
Possible cyclization pathways and proposed mechanism.
Scheme 4.
Scheme 5.
Although a definitive elucidation of the reaction mechanism leading to the unexpected furo[2,3- c]pyridine was not an immediate goal, understanding plausible mechanisms was of interest, and was probed in some detail. Formation of the pyrano[3,4-c]pyridine 33 via nucleophilic attack of the benzylic hydroxyl group, and its subsequent rearrangement to the furo[2,3-c]pyridine 26 (Path D, Panel A in Scheme 3) appeared improbable. Salicylaldehyde, which lacks the bulky hydroxymethylene group, yielded the classical imidazo[1,2-a]pyridine 20 (confirmed by single crystal X-ray analysis, see below), rather than the benzofuran derivative B.3 (Panel B in Scheme 3). We reasoned, therefore, that the benzylic hydroxyl in the transition state 31 could assist cyclization via Path C (Panel A in Scheme 3) due to steric reasons, apposing the phenolic hydroxyl with the electrophilic carbon. In addition to the direct formation of the furo[2,3- c]pyridine 26 via path C, a plausible alternate mechanism for this unusual cyclization route, involving the pyridine ring system is proposed in Scheme 3 (Panel C).
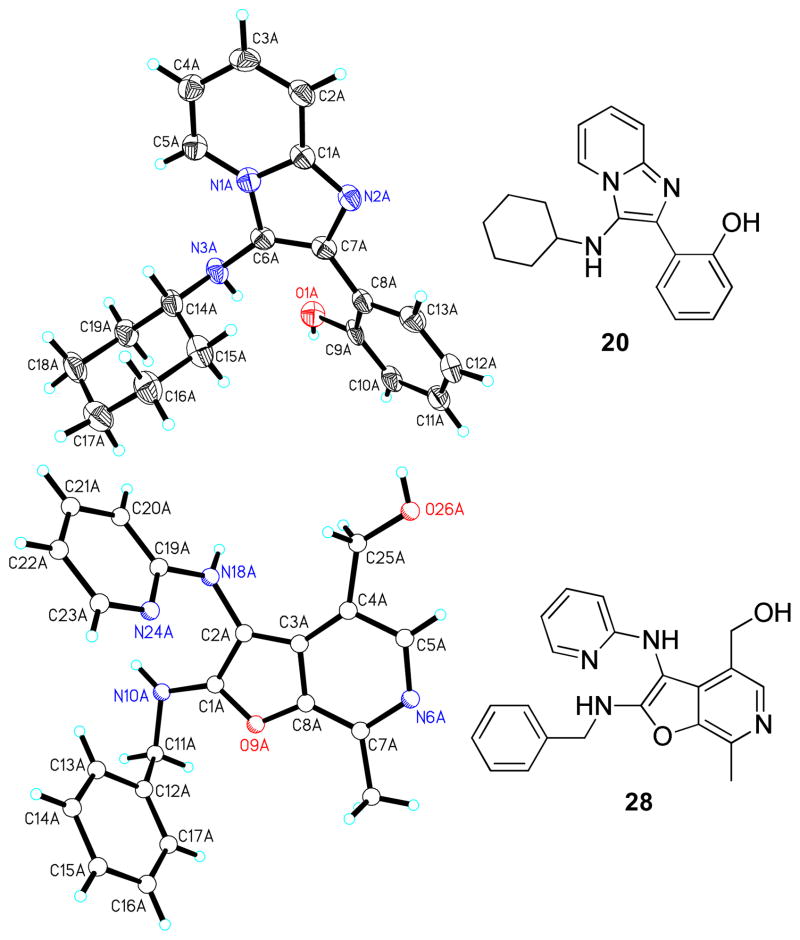
After many unsuccessful attempts, a hydrochloride salt of compound 28 was crystallized as multiply-twinned bundles in acetonitrile. A multi-domain specimen of 28 was cut from one bundle which gave a set of diffracted intensities, permitting a crystal structure solution (noncentrosymmetric, triclinic P1-C1 space group with eight crystallographically-independent molecules in the asymmetric unit), but not a satisfactory refinement (Figure 4). The structure of 28 unambiguously confirmed the furo[2,3-c]pyridine chemotype. The structure of compound 20 (obtained with salicylaldehyde, which also possesses a phenolic OH; Scheme 2) was also elucidated, which established the formation of a classic Groebke product 2-(3- (cyclohexylamino)imidazo[1,2-a]pyridin-2-yl)phenol (Figure 4). These observations clearly emphasize the significance of the additional substituents of pyridoxal, directing the unique cyclization route leading to the furo[2,3-c]pyridine scaffold.
Figure 4.
Crystal structures of the salicylaldehyde-derived classic Groebke product (imidazo[1,2-a]pyridine, 20), and a non-Groebke, pyridoxal-derived furo[2,3-c]pyridine, 28.
Thus, our initial attempts toward the synthesis of twenty-four membered imidazo[1,2- a]pyrazine/pyridine test-library unexpectedly resulted in the formation of densely substituted furo[2,3-c]pyridines 24–29. Pyridoxal was found to be an indispensable component for this cyclization reaction. Four of the six compounds obtained (26–29, Scheme 2) were found to be active in our primary screens using TLR8-transfected HEK293 cells, while compounds 24 and 25, synthesized using 2-isocyano-2-methylpropane as one of the components, were found to be inactive, warranting detailed structure based activity relationship investigations for this new chemotype.
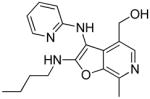
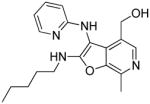
Among the active compounds 26–29, we observed that compounds 26 and 28 (derived from 2-aminopyridine), were more active than 27 and 29 (from 2-aminopyrazine; Table 1, Figure 3). We therefore selected 2-aminopyridine and pyridoxal as the invariant components and varied the isonitrile component. We explored thirteen different isonitriles (Scheme 6), including linear aliphatic (as in 37a and 37b), branched aliphatic (37c-37e), linear aliphatic with silyl (37f), heteroaromatic ring (37g), ester (37h, 37i), and phosphate ester (37j) termini, as well as aromatic substituents (37k-37m). Maximal activity was observed in 37b, with a pentylene substituent on the C2 amine (Scheme 6, Figure 3). Diminishing the chain length by one methylene unit (37a) decreased activity, and potency was further attenuated in compounds with alpha-branched substituents (37d). Compound 37n, with a free NH2 at C2, obtained by N-dealkylation of the tert-octylamine group43 of 37e with trifluoroacetic acid, as well as compounds with aromatic substituents (37k-37m) were devoid of TLR8-stimulatory activity (Table 1). Thus, a distinct dependence of the nature of the C2 amino substituent on the activity profiles was observed in the furo[2,3-c]pyridines, with the C2-N-pentyl (37b) and C2-N-(trimethylsilyl)methyl analogues (37f) displaying dominant TLR8 agonism.
Scheme 6.
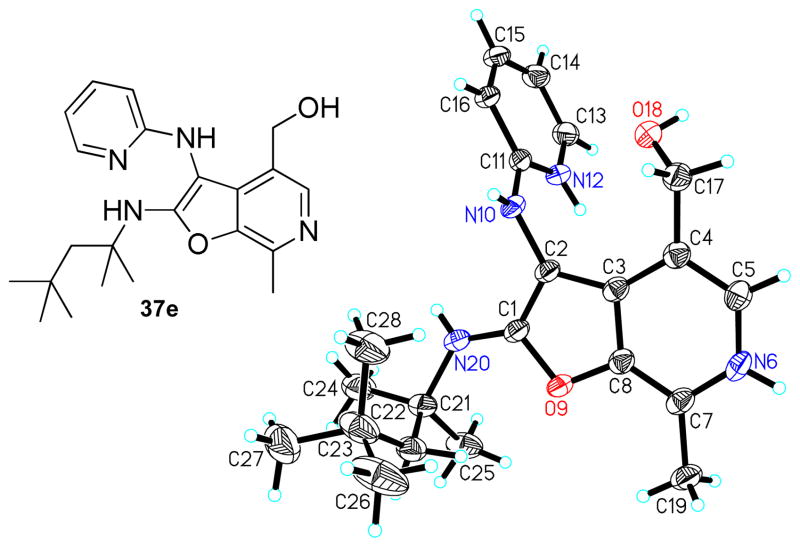
As mentioned earlier, our earlier efforts at unambiguously confirming the structure of 28 did not allow for satisfactory crystal structure refinement because of the intrinsic properties of the crystal space group. Having synthesized thirteen additional furo[2,3-c]pyridines (37a-37m) for purposes of delineating SAR, a parallel crystallization of these compounds was attempted using various solvents. Suitable crystals of compound 37e were obtained as pale yellow crystals by slow evaporation of a super-saturated solution of 37e in CH3CN/CH3OH mixtures at room temperature. A single-domain specimen was selected and the X-ray diffraction data was collected. The ORTEP diagram of 37e is shown in Figure 5, confirming the non-Groebke furo[2,3-c]pyridine.
Figure 5.
Crystal structure (ORTEP view) of the non-Groebke furo[2,3-c]pyridine, 37e, obtained with the use of pyridoxal as the aldehyde component.
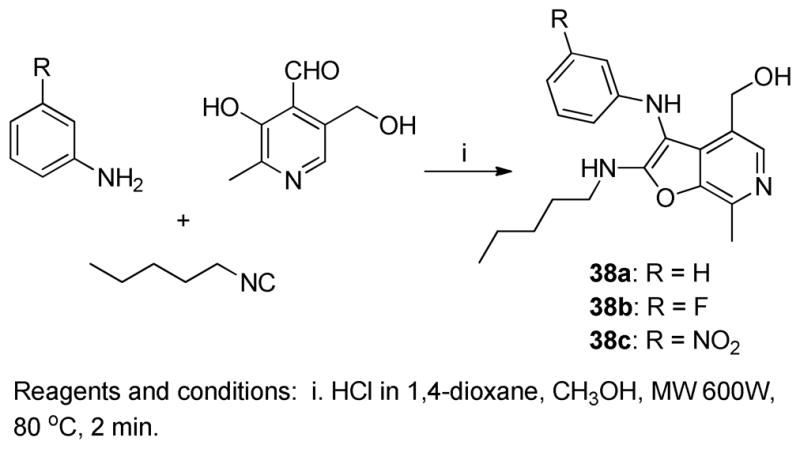
Previous mention was made that 36 (Scheme 5) was found to be weakly active (EC50 = 1.68 μM, Table 1, Figure 3B) relative to the lead compound 28, suggesting that the 2- aminopyridine core could be substituted by anilines in this multicomponent reaction. Having optimized the C2 group as pentylamine (derived from 1-isocyanopentane, Scheme 6), three more furo[2,3-c]pyridines were synthesized using aniline 38a, 3-fluoroaniline 38b and 3-nitroaniline 38c in combination with 1-isocyanopentane and pyridoxal (Scheme 7). The C2 N-pentyl analogue 38a was found to be more active than the C2 N-benzyl analogue 36 (Figure 3A and 3B, Table 1). The nitro derivative 38c was found to be as potent as the parent compound 38a, whereas substantial gain in TLR8 activity was noticed for the fluoro-substituted compound 38b in TLR8-specific functional assays. However, these compounds exhibited a poorer dose-response profile (lower area-under the-curve, Figure 3). It is pertinent to note that no stable product could be obtained by the replacement of 2-aminopyridine with aliphatic amines.
Scheme 7.
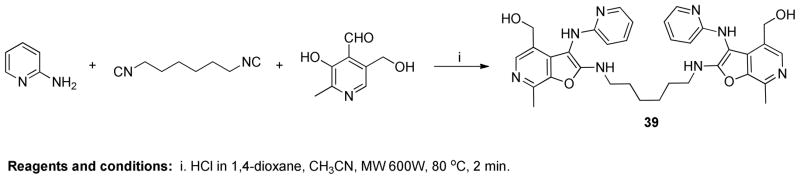
Several TLRs are thought to signal via ligand-induced dimerization,44 as evident in the crystal structures of TLR236,45 and TLR3.21 It is not yet understood, however, how TLR7 and TLR8, whose endogenous ligands are single-stranded viral RNA (ssRNA), recognize and transduce signals upon engagement by small, non-polymeric molecules such as the imidazoquinolines26,46 and the oxoadenines.47,48 In order to probe possible mechanisms of ligand recognition by TLR8, the dimeric compound 39 was synthesized using 1,6-diisocyanohexane (Scheme 8); the activity of this analogue was comparable in its TLR8-agonistic potency to the most active compounds, 28, 37a, 37b and 37f (Table 1).
Scheme 8.
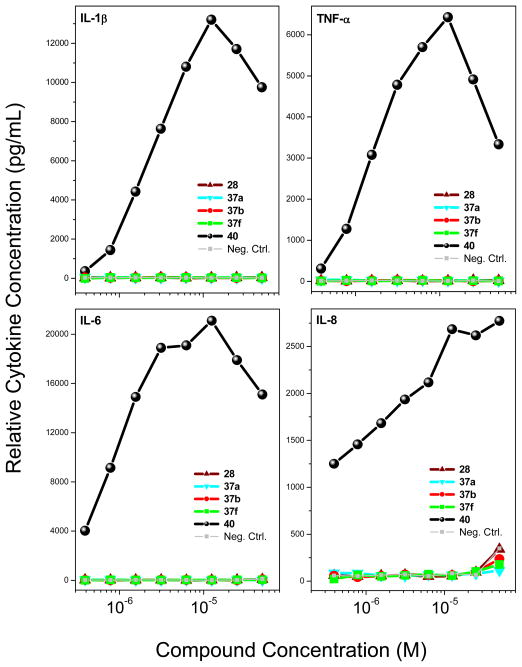
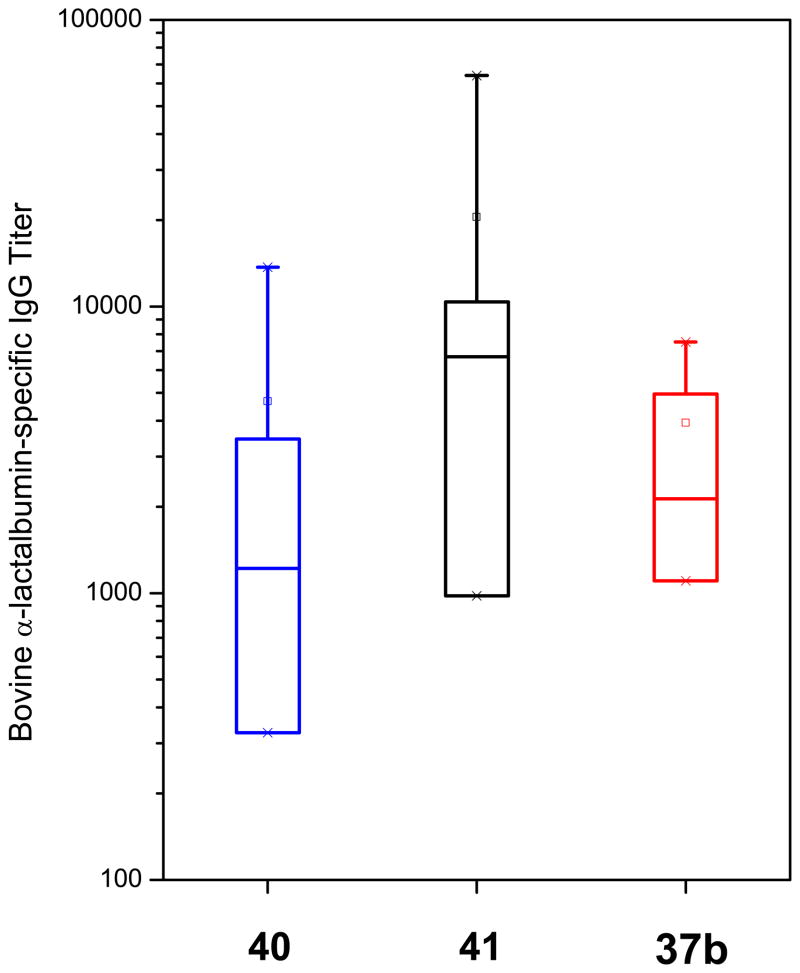
We examined the cytokine-inducing properties49,50 of a subset of compounds that were maximally active (28, 37a, 37b and 37f), all of which showed robust dose-response profiles in primary TLR8-agonistic screens (Figure 3). We used 2-propylthiazolo[4,5-c]quinolin-4-amine 40 (CL075) as a reference TLR8 agonist,34,35 which exhibited an EC50 of 1.32 μM (Figure 3). In human PBMCs, only the reference thiazoloquinoline 40, but none of the furo[2,3-c]pyridines showed any proinflammatory cytokine induction (Figure 6). We do not yet know if the dissociation between TLR8-specific NF-κB induction on the one hand, and lack of cytokine induction on the other is ascribable to a non-myeloid differentiation primary response gene 88 (MyD88)-independent mechanism.51,52 However, mindful of recent observations that proinflammatory activity is not an absolute prerequisite for adjuvantic properties,53 and because we had previously observed potent NF-κB transactivation in a bis-quinoline, 7-chloro-N-(4-(7- chloroquinolin-4-ylamino)butyl) quinolin-4-amine 41 (RE-660)54 unaccompanied by any proinflammatory cytokine induction, we decided to examine two representative compounds (37b and 37f) in transcriptomal profiling experiments. Consistent with the cytokine assays, there were no transcriptional signatures of inflammation; however, both compounds upregulated several chemokine ligand (both CXCL and CCL) genes (Supporting Information). Although entirely bereft of any proinflammatory activity, the bis-quinoline compound 41 was found to be a potent adjuvant which appears to be related to its functional agonism at CCR1.54 Given some similarities in activity profiles between the furo[2,3-c]pyridines and 41, we decided to evaluate and compare the adjuvantic activity of 37b alongside the reference compounds, 40 and 41. Rabbits were immunized using bovine α-lactalbumin as a model subunit antigen.42 Anti-α-lactalbumin IgG titers in immune sera clearly show an adjuvantic effect of 37b, with a rise-in-titer values of >1000, comparable to the adjuvantic activities of the reference compounds, 40 and 41 (Figure 7). The complete lack of proinflammatory cytokine induction coupled with strong adjuvantic activity of the novel furo[2,3-c]pyridines render this hitherto unknown chemotype an exceedingly attractive class of compounds which are expected to be devoid of local or systemic reactogenicity. Further structure-activity studies on the furopyridines and related chemotypes are currently in progress.
Figure 6.
Dose-response profiles of proinflammatory cytokine induction in hPBMCs by compounds 28, 37a, 37b and 37f. Representative data from three independent experiments are presented.
Figure 7.
Anti-bovine α-lactalbumin-specific IgG titers in rabbits adjuvanted with 37b, 40, and 41 (n=4, for each cohort). Box-plots of ratios of immune/pre-immune titers yielding absorbance values of 1.0 are shown for the individual samples. Means and medians of titers are represented by □ and − symbols within the box, respectively, and the X symbols indicate the 1% and 99% percentile values.
Experimental Section
Chemistry
All of the solvents and reagents used were obtained commercially and used as such unless noted otherwise. Moisture- or air-sensitive reactions were conducted under nitrogen atmosphere in oven-dried (120 °C) glass apparatus. The solvents were removed under reduced pressure using standard rotary evaporators. Flash column chromatography was carried out using RediSep Rf ‘Gold’ high performance silica columns on CombiFlash Rf instrument unless otherwise mentioned, while thin-layer chromatography was carried out on silica gel (200 μm) CCM pre-coated aluminum sheets. The purity of all final compounds was confirmed to be greater than 95% by HPLC-MS using a Zorbax Eclipse Plus 4.6 mm x 150 mm, 5 μm analytical reverse phase C18 column with either H2O-isopropanol or H2O-CH3CN gradients, a diode-array detector operating in 190–500 nm range (2 nm bandpass), and an Agilent ESI-TOF mass spectrometer (integration on total ion intensity counts, with a mass accuracy of 10 ppm) operating in the positive ion acquisition mode.
General procedure for the syntheses of compounds 5a-5f
Synthesis of compound 5a: N3-cyclohexyl-2-phenylimidazo[1,2-a]pyrazine-3,8-diamine
To a solution of 2-amino-3-chloropyrazine (64 mg, 0.50 mmol) in anhydrous acetonitrile (1 mL), were added benzaldehyde (60μL, 0.60 mmol), 4N HCl/dioxane (10μL) and cyclohexylisonitrile (74 μL, 0.60 mmol). The reaction mixture was then heated under microwave conditions (400 W, 110 °C) in a sealed vial for 20 min. The reaction mixture was cooled to room temperature; ammonium hydroxide (NH3 content 28–30%, 0.5 mL) was added and further heated at 110 °C in a sealed vial for overnight. After cooling the reaction mixture to room temperature, the solvents were removed and the residue was purified using column chromatography to obtain the compound 5a (47 mg, 30%). 1H NMR (500 MHz, MeOD) δ 8.02 (dd, J = 8.2, 1.3 Hz, 2H), 7.64 (d, J = 4.8 Hz, 1H), 7.46 (dd, J= 10.9, 4.6 Hz, 2H), 7.39 – 7.28 (m, 1H), 7.21 (d, J = 4.8 Hz, 1H), 2.96 – 2.83 (m, 1H), 1.76 (d, J = 11.6 Hz, 2H), 1.71 – 1.62 (m, 2H), 1.57-1.51 (m, 1H), 1.31 – 1.19 (m, 2H), 1.19 – 1.03 (m, 3H).13C NMR (126 MHz, MeOD) δ 151.14, 136.46, 135.17, 129.95, 129.55, 129.44, 128.67, 128.24, 128.11, 109.02, 57.87, 35.05, 26.86, 25.99. MS (ESI) calcd for C18H21N5, m/z 307.1797, found 308.1923 (M+H)+.
Compounds 5b-5d were synthesized similarly as compound 5a.
5b: 2-([1,1′-biphenyl]-4-yl)-N3 -cyclohexylimidazo[1,2-a]pyrazine-3,8-diamine
(44 mg, 23%) 1H NMR (500 MHz, MeOD) δ 8.13 (d, J = 8.2 Hz, 2H), 7.72 (d, J = 8.3 Hz, 2H), 7.71 – 7.67 (m, 2H), 7.64 (d, J = 4.8 Hz, 1H), 7.45 (t, J = 7.7 Hz, 2H), 7.38 – 7.31 (m, 1H), 7.22 (d, J = 4.8 Hz, 1H), 3.02 – 2.84 (m, 1H), 1.80 (d, J = 12.2 Hz, 2H), 1.69 (dd, J = 8.2, 5.3 Hz, 2H), 1.55 (s, 1H), 1.35 – 1.23 (m, 2H), 1.24 – 1.01 (m, 3H). 13C NMR (126 MHz, MeOD) δ 151.16, 141.92, 141.53, 136.10, 134.18, 130.07, 129.93, 129.56, 128.57, 128.45, 128.13, 128.02, 127.86, 109.03, 57.99, 35.12, 26.88, 26.03. MS (ESI) calcd for C24H25N5, m/z 383.2110, found 384.2382 (M+H)+.
5c: N3-benzyl-2-butylimidazo[1,2-a]pyrazine-3,8-diamine
(10 mg, 7%) 1H NMR (500 MHz, MeOD) δ 7.44 (d, J = 4.8 Hz, 1H), 7.29 – 7.17 (m, 5H), 7.12 (d, J = 4.8 Hz, 1H), 4.13 (s, 2H), 2.51 – 2.42 (m, 2H), 1.54 – 1.42 (m, 2H), 1.31 (dq, J = 14.8, 7.4 Hz, 2H), 0.91 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, MeOD) δ 150.52, 141.10, 138.98, 130.14, 129.57, 129.49, 128.81, 128.40, 127.74, 108.93, 53.28, 32.76, 27.26, 23.73, 14.26. MS (ESI) calcd for C17H21N5, m/z 295.1797, found 296.1952 (M+H)+.
5d: 2-butyl-N3-(4-methoxyphenyl)imidazo[1,2-a]pyrazine-3,8-diamine
(36 mg, 23%) 1H NMR (500 MHz, MeOD) δ 7.26 (d, J = 4.7 Hz, 1H), 7.16 (d, J = 4.7 Hz, 1H), 6.80 – 6.69 (m, 2H), 6.49 – 6.38 (m, 2H), 3.70 (s, 3H), 2.67 (t, J = 7.6 Hz, 2H), 1.73 – 1.62 (m, 2H), 1.41 – 1.23 (m, 2H), 0.88 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, MeOD) δ 154.65, 150.75, 142.09, 140.98, 129.90, 128.28, 124.98, 115.97, 115.50, 109.10, 56.07, 32.40, 27.41, 23.46, 14.15. MS (ESI) calcd for C17H21N5O, m/z 311.1746, found 312.1905 (M+H)+.
General procedure for the syntheses of compounds 6–29
Synthesis of compound 6: N-(tert-butyl)-2-phenylimidazo[1,2-a]pyridin-3-amine
To a solution of 2-aminopyridine (24 mg, 0.25 mmol) in anhydrous acetonitrile, were added benzaldehyde (28 μL, 0.28 mmol), 4N HCl/dioxane (5 μL) and tert-butyl isonitrile (27 μL, 0.24 mmol). The reaction mixture was then heated under microwave conditions (400 W, 110 °C) in a sealed vial for 20 min. After cooling the reaction mixture to room temperature, the solvents were removed and the residue was purified using column chromatography to obtain compound 6 (44 mg, 70%). 1H NMR (400 MHz, CDCl3) δ 8.23 (dt, J = 6.9, 1.2 Hz, 1H), 7.90 (dt, J = 8.1, 1.6 Hz, 2H), 7.55 (dt, J = 9.0, 1.0 Hz, 1H), 7.43 (t, J = 7.6 Hz, 2H), 7.31 (t, J = 7.4 Hz, 1H), 7.13 (ddd, J = 9.0, 6.6, 1.3 Hz, 1H), 6.77 (td, J = 6.8, 1.1 Hz, 1H), 3.12 (s, 1H), 1.04 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 142.15, 135.42, 135.40, 128.43, 128.32, 127.52, 124.17, 123.64, 117.48, 111.46, 101.91, 56.59, 30.43. MS (ESI) calcd for C17H19N3, m/z 265.1579, found 266.1664 (M+H)+.
Compounds 7–29 were synthesized similarly as compound 6.
7: N-(tert-butyl)-2-phenylimidazo[1,2-a]pyrazin-3-amine
(47 mg, 74%) 1H NMR (400 MHz, CDCl3) δ 9.00 (d, J = 1.4 Hz, 1H), 8.14 (dd, J = 4.6, 1.5 Hz, 1H), 7.91 (dd, J = 8.3, 1.3 Hz, 2H), 7.86 (d, J = 4.6 Hz, 1H), 7.46 (t, J = 7.5 Hz, 2H), 7.37 (t, J = 7.4 Hz, 1H), 3.19 (s, 1H), 1.05 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 143.54, 142.40, 137.43, 134.38, 129.04, 128.66, 128.38, 128.33, 125.17, 116.49, 57.11, 30.45. MS (ESI) calcd for C16H18N4, m/z 266.1531, found 267.1588 (M+H)+.
8: N-cyclohexyl-2-phenylimidazo[1,2-a]pyridin-3-amine
(64 mg, 86%) 1H NMR (500 MHz, CDCl3) δ 8.16 (d, J = 6.8 Hz, 1H), 8.05 (dd, J = 8.3, 1.2 Hz, 2H), 7.62 (d, J = 9.0 Hz, 1H), 7.45 (t, J = 7.8 Hz, 2H), 7.32 (t, J = 7.4 Hz, 1H), 7.17 (ddd, J = 8.9, 6.7, 1.2 Hz, 1H), 6.82 (td, J = 6.8, 0.9 Hz, 1H), 3.32 (s, 1H), 3.07 – 2.85 (m, 1H), 1.81 (d, J = 13.1 Hz, 2H), 1.68 (dd, J = 9.1, 3.6 Hz, 2H), 1.60 – 1.54 (m, 1H), 1.28 – 1.12 (m, 5H). 13C NMR (126 MHz, CDCl3) δ 141.02, 135.43, 133.45, 128.74, 127.74, 127.20, 125.17, 125.04, 123.07, 116.94, 112.30, 57.02, 34.27, 25.81, 24.94. MS (ESI) calcd for C19H21N3, m/z 291.1735, found 292.1832 (M+H)+.
9: N-cyclohexyl-2-phenylimidazo[1,2-a]pyrazin-3-amine
(25 mg, 36%) 1H NMR (500 MHz, CDCl3) δ 8.99 (d, J = 1.4 Hz, 1H), 8.01 (ddd, J = 4.6, 3.2, 1.8 Hz, 3H), 7.85 (d, J = 4.6 Hz, 1H), 7.48 (t, J = 7.7 Hz, 2H), 7.38 (t, J = 7.4 Hz, 1H), 3.26 (s, 1H), 3.00 (m, 1H), 2.22 (s, 1H), 1.82 (dd, J = 6.6, 5.4 Hz, 2H), 1.70 (dd, J = 9.3, 3.3 Hz, 2H), 1.62 – 1.55 (m, 1H), 1.34 – 1.07 (m, 5H). 13C NMR (126 MHz, CDCl3) δ 143.37, 139.08, 136.82, 133.62, 129.01, 128.90, 128.30, 127.42, 126.70, 115.73, 57.02, 34.41, 25.69, 24.91. MS (ESI) calcd for C18H20N4, m/z 292.1688, found 293.1775 (M+H)+.
10: N-benzyl-2-phenylimidazo[1,2-a]pyridin-3-amine
(62 mg, 86%) 1H NMR (500 MHz, CDCl3) δ 7.98 (ddt, J = 3.7, 3.0, 1.6 Hz, 3H), 7.57 (dt, J = 9.0, 1.0 Hz, 1H), 7.45 (t, J = 7.7 Hz, 2H), 7.39 – 7.26 (m, 6H), 7.13 (ddd, J = 9.0, 6.7, 1.3 Hz, 1H), 6.74 (td, J = 6.8, 1.1 Hz, 1H), 4.20 (d, J = 6.1 Hz, 2H), 3.52 (t, J = 6.0 Hz, 1H), 2.45 (s, 1H). 13C NMR (126 MHz, CDCl3) δ 141.57, 139.06, 136.04, 134.13, 128.84, 128.30, 127.82, 127.65, 127.16, 125.77, 124.32, 122.50, 117.52, 111.94, 52.57. MS (ESI) calcd for C20H17N3, m/z 299.1422, found 300.1490 (M+H)+.
11: N-benzyl-2-phenylimidazo[1,2-a]pyrazin-3-amine
(45 mg, 62%) 1H NMR (500 MHz, CDCl3) δ 8.98 (d, J = 1.3 Hz, 1H), 7.94 (dt, J = 8.1, 1.6 Hz, 2H), 7.82 (dd, J = 4.6, 1.4 Hz, 1H), 7.77 (d, J = 4.6 Hz, 1H), 7.47 (t, J = 7.6 Hz, 2H), 7.39 (t, J = 7.4 Hz, 1H), 7.34 – 7.27 (m, 5H), 4.23 (d, J = 2.4 Hz, 2H), 3.66 (s, 1H), 2.20 (s, 1H). 13C NMR (126 MHz, CDCl3) δ 143.46, 138.76, 138.56, 136.79, 133.34, 129.06, 129.03, 128.99, 128.46, 128.24, 128.08, 127.42, 127.18, 115.38, 52.39. MS (ESI) calcd for C19H16N4, m/z 300.1375, found 301.1494 (M+H)+.
12: N-(tert-butyl)-2-(pyridin-4-yl)imidazo[1,2-a]pyridin-3-amine
(50 mg, 78%) 1H NMR (500 MHz, CDCl3) δ 8.65 (d, J = 5.9 Hz, 2H), 8.20 (dt, J = 6.9, 1.0 Hz, 1H), 7.96 (dd, J = 4.7, 1.4 Hz, 2H), 7.56 (d, J = 9.1 Hz, 1H), 7.18 (ddd, J = 9.0, 6.6, 1.2 Hz, 1H), 6.81 (td, J = 6.8, 1.0 Hz, 1H), 3.07 (s, 1H), 1.10 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 149.69, 142.82, 142.39, 136.46, 124.86, 124.77, 123.38, 122.17, 117.68, 111.82, 56.78, 30.38. MS (ESI) calcd for C16H18N4, m/z 266.1531, found 267.1747 (M+H)+.
13: N-(tert-butyl)-2-(pyridin-4-yl)imidazo[1,2-a]pyrazin-3-amine
(49 mg, 76%) 1H NMR (500 MHz, CDCl3) δ 9.02 (d, J = 1.4 Hz, 1H), 8.69 (dd, J = 4.5, 1.6 Hz, 2H), 8.11 (dd, J = 4.7, 1.5 Hz, 1H), 7.94 (dd, J = 4.5, 1.6 Hz, 2H), 7.88 (d, J = 4.7 Hz, 1H), 3.12 (s, 1H), 1.10 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 150.22, 144.25, 141.96, 139.25, 137.72, 129.39, 126.24, 122.42, 116.42, 57.46, 30.61. MS (ESI) calcd for C15H17N5, m/z 267.1484, found 268.1705 (M+H)+.
14: N-cyclohexyl-2-(pyridin-4-yl)imidazo[1,2-a]pyridin-3-amine
(69 mg, 98%) 1H NMR (500 MHz, CDCl3) δ 8.64 (dd, J = 4.6, 1.6 Hz, 2H), 8.07 (dt, J = 6.9, 1.2 Hz, 1H), 8.00 (dd, J = 4.6, 1.6 Hz, 2H), 7.54 (dt, J = 9.1, 1.0 Hz, 1H), 7.17 (ddd, J = 9.1, 6.6, 1.3 Hz, 1H), 6.81 (td, J = 6.8, 1.1 Hz, 1H), 3.11 (d, J = 3.9 Hz, 1H), 2.98 (td, J = 10.3, 4.1 Hz, 1H), 1.83 (d, J = 10.8 Hz, 2H), 1.71 (dd, J = 9.4, 3.3 Hz, 2H), 1.59 (dd, J = 7.3, 1.4 Hz, 1H), 1.32 – 1.09 (m, 5H). 13C NMR (126 MHz, CDCl3) δ 150.03, 142.26, 142.11, 133.78, 126.92, 124.92, 122.87, 121.21, 117.99, 112.31, 57.26, 34.42, 25.75, 24.97. MS (ESI) calcd for C18H20N4, m/z 292.1688, found 293.1854 (M+H)+.
15: N-cyclohexyl-2-(pyridin-4-yl)imidazo[1,2-a]pyrazin-3-amine
(56 mg, 80%) 1H NMR (500 MHz, CDCl3) δ 9.01 (d, J = 1.5 Hz, 1H), 8.70 (dd, J = 4.5, 1.6 Hz, 2H), 8.03 – 7.93 (m, 3H), 7.88 (d, J = 4.6 Hz, 1H), 3.21 (d, J = 5.9 Hz, 1H), 3.06 – 2.97 (m, 1H), 1.84 (d, J = 10.5 Hz, 2H), 1.72 (dd, J = 9.7, 2.6 Hz, 2H), 1.63 – 1.58 (m, 1H), 1.31 – 1.14 (m, 5H). 13C NMR (126 MHz, CDCl3) δ 150.42, 144.32, 141.25, 137.17, 135.99, 129.45, 128.03, 121.37, 115.69, 57.35, 34.55, 25.62, 24.93.MS (ESI) calcd for C17H19N5, m/z 293.1640, found 294.1894 (M+H)+.
16: N-benzyl-2-(pyridin-4-yl)imidazo[1,2-a]pyridin-3-amine
(54 mg, 75%) 1H NMR (500 MHz, CDCl3) δ 8.62 (dd, J = 4.6, 1.6 Hz, 2H), 7.94 (dt, J = 6.9, 1.1 Hz, 1H), 7.90 (dd, J = 4.6, 1.6 Hz, 2H), 7.55 (dt, J = 9.1, 1.0 Hz, 1H), 7.34 – 7.27 (m, 5H), 7.17 (ddd, J = 9.1, 6.6, 1.3 Hz, 1H), 6.76 (td, J = 6.8, 1.1 Hz, 1H), 4.22 (d, J = 6.1 Hz, 2H), 3.52 (t, J = 6.1 Hz, 1H). 13C NMR (126 MHz, CDCl3) δ 150.12, 142.11, 141.94, 138.64, 133.41, 128.98, 128.31, 128.06, 127.46, 125.05, 122.50, 121.13, 118.06, 112.42, 52.64. MS (ESI) calcd for C19H16N4, m/z 300.1375, found 301.1599 (M+H)+.
17: N-benzyl-2-(pyridin-4-yl)imidazo[1,2-a]pyrazin-3-amine
(47 mg, 65%) 1H NMR (500 MHz, CDCl3) δ 9.01 (d, J = 1.4 Hz, 1H), 8.67 (dd, J = 4.5, 1.6 Hz, 2H), 7.87 (dd, J = 4.5, 1.6 Hz, 2H), 7.78 (dt, J = 4.6, 3.0 Hz, 2H), 7.30 (dd, J = 5.1, 1.9 Hz, 3H), 7.24 (dd, J = 6.9, 2.6 Hz, 2H), 4.25 (d, J = 6.2 Hz, 2H), 3.66 (t, J = 6.2 Hz, 1H). 13C NMR (126 MHz, CDCl3) δ 150.46, 144.33, 140.94, 138.23, 137.12, 135.70, 129.45, 129.14, 128.45, 128.32, 128.25, 121.34, 115.35, 52.59. MS (ESI) calcd for C18H15N5, m/z 301.1327, found 302.1474 (M+H)+.
18: 2-(3-(tert-butylamino)imidazo[1,2-a]pyridin-2-yl)phenol
(20 mg, 30%) 1H NMR (500 MHz, CDCl3) δ 12.47 (bs, 1H), 8.19 (d, J = 6.9 Hz, 1H), 8.12 (dd, J = 7.8, 1.6 Hz, 1H), 7.51 (d, J = 9.0 Hz, 1H), 7.24 – 7.17 (m, 2H), 7.07 – 6.97 (m, 1H), 6.95 – 6.87 (m, 1H), 6.83 (td, J = 6.8, 0.9 Hz, 1H), 3.16 (bs, 1H), 1.16 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 157.07, 148.51, 140.76, 138.42, 134.51, 131.35, 129.43, 128.13, 124.82, 123.01, 118.69, 117.76, 116.96, 112.09, 109.94, 57.22, 30.50. MS (ESI) calcd for C17H19N3O, m/z 281.1528, found 282.1770 (M+H)+.
19: 2-(3-(tert-butylamino)imidazo[1,2-a]pyrazin-2-yl)phenol
(40 mg, 59%) 1H NMR (500 MHz, CDCl3) δ 11.87 (s, 1H), 8.98 (d, J = 1.4 Hz, 1H), 8.16 – 8.08 (m, 2H), 7.92 (d, J = 4.6 Hz, 1H), 7.31 – 7.24 (m, 1H), 7.05 (dd, J = 8.2, 1.0 Hz, 1H), 6.93 (td, J = 7.9, 1.2 Hz, 1H), 3.22 (bs, 1H), 1.17 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 157.08, 142.79, 140.85, 135.93, 130.39, 129.72, 128.31, 123.47, 119.03, 118.04, 117.65, 115.85, 57.73, 30.54. MS (ESI) calcd for C16H18N4O, m/z 282.1481, found 283.1690 (M+H)+.
20: 2-(3-(cyclohexylamino)imidazo[1,2-a]pyridin-2-yl)phenol
(30 mg, 41%) 1H NMR (500 MHz, CDCl3) δ 13.16 (s, 1H), 8.17 (d, J = 6.8 Hz, 1H), 8.04 (dd, J = 7.8, 1.3 Hz, 1H), 7.50 (dt, J = 9.0, 0.9 Hz, 1H), 7.25 – 7.17 (m, 2H), 7.04 (dd, J = 8.2, 1.1 Hz, 1H), 6.95 – 6.89 (m, 1H), 6.87 (td, J = 6.8, 1.0 Hz, 1H), 3.17 – 2.99 (m, 2H), 1.83 (d, J = 12.5 Hz, 2H), 1.72 (dd, J = 9.5, 3.1 Hz, 2H), 1.64 – 1.57 (m, 1H), 1.32 (dd, J = 22.7, 11.7 Hz, 2H), 1.26 – 1.13 (m, 3H). 13C NMR (126 MHz, CDCl3) δ 157.67, 139.51, 136.00, 129.25, 126.41, 124.88, 123.55, 122.62, 118.82, 117.84, 117.40, 116.64, 112.46, 57.08, 34.19, 25.83, 24.96. MS (ESI) calcd for C19H21N3O, m/z 307.1685, found 308.1995 (M+H)+.
21: 2-(3-(cyclohexylamino)imidazo[1,2-a]pyrazin-2-yl)phenol
(54 mg, 73%) 1H NMR (500 MHz, CDCl3) δ 12.52 (bs, 1H), 8.96 (d, J = 1.4 Hz, 1H), 8.05 (ddd, J = 9.5, 6.2, 1.5 Hz, 2H), 7.94 (d, J = 4.6 Hz, 1H), 7.30 – 7.26 (m, 1H), 7.07 (dd, J = 8.2, 1.1 Hz, 1H), 6.94 (td, J = 8.0, 1.2 Hz, 1H), 3.31 – 2.99 (m, 2H), 1.83 (d, J = 12.3 Hz, 2H), 1.77 – 1.70 (m, 2H), 1.62 (dd, J = 7.5, 2.3 Hz, 1H), 1.33 (dd, J = 21.3, 10.5 Hz, 2H), 1.27 – 1.15 (m, 3H). 13C NMR (126 MHz, CDCl3) δ 157.70, 142.41, 138.53, 134.72, 130.19, 129.97, 126.50, 125.10, 119.13, 118.09, 116.60, 115.35, 57.17, 34.34, 25.69, 24.92.MS (ESI) calcd for C18H20N4O, m/z 308.1637, found 309.1856 (M+H)+.
22: 2-(3-(benzylamino)imidazo[1,2-a]pyridin-2-yl)phenol
(10 mg, 13%) 1H NMR (500 MHz, CDCl3) δ 13.02 (s, 1H), 8.06 – 7.95 (m, 2H), 7.51 (d, J = 9.0 Hz, 1H), 7.42 – 7.29 (m, 5H), 7.25 – 7.17 (m, 2H), 7.06 (dd, J = 8.2, 1.0 Hz, 1H), 6.95 – 6.89 (m, 1H), 6.80 (td, J = 6.8, 0.9 Hz, 1H), 4.24 (bs, 2H), 3.41 (s, 1H).13C NMR (126 MHz, CDCl3) δ 157.75, 139.49, 138.91, 135.58, 129.38, 128.96, 128.43, 127.97, 125.95, 124.95, 124.41, 122.19, 119.04, 117.88, 117.21, 116.74, 112.51, 52.43. MS (ESI) calcd for C20H17N3O, m/z 315.1372, found 316.1611 (M+H)+.
23: 2-(3-(benzylamino)imidazo[1,2-a]pyrazin-2-yl)phenol
(43 mg, 57%) 1H NMR (500 MHz, CDCl3) δ 12.40 (bs, 1H), 8.95 (d, J = 1.4 Hz, 1H), 8.02 (dd, J = 7.9, 1.6 Hz, 1H), 7.82 (d, J = 4.6 Hz, 1H), 7.78 (dd, J = 4.6, 1.4 Hz, 1H), 7.37 – 7.27 (m, 6H), 7.09 (dd, J = 8.3, 1.2 Hz, 1H), 6.94 (ddd, J = 7.9, 7.3, 1.2 Hz, 1H), 4.26 (d, J = 5.1 Hz, 2H), 3.53 (s, 1H). 13C NMR (126 MHz, CDCl3) δ 157.70, 142.41, 138.44, 138.07, 134.64, 130.35, 129.95, 129.12, 128.39, 128.24, 126.21, 125.76, 119.36, 118.13, 116.33, 114.97, 52.35. MS (ESI) calcd for C19H16N4O, m/z 316.1324, found 317.1545 (M+H)+.
24: (2-(tert-butylamino)-7-methyl-3-(pyridin-2-ylamino)furo[2,3-c]pyridin-4-yl)methanol
(13 mg, 17%) 1H NMR (400 MHz, MeOD) δ 7.97 (d, J = 4.1 Hz, 1H), 7.87 (s, 1H), 7.59 (ddd, J = 8.7, 7.2, 1.8 Hz, 1H), 6.75 (ddd, J = 7.0, 5.2, 0.7 Hz, 1H), 6.67 (d, J = 8.4 Hz, 1H), 4.61 (s, 2H), 2.72 (s, 3H), 1.53 (s, 9H). 13C NMR (126 MHz, MeOD) δ 165.65, 159.92, 147.97, 143.43, 140.55, 139.89, 132.15, 131.86, 125.53, 115.57, 110.65, 96.72, 59.04, 55.33, 30.04, 13.08. MS (ESI) calcd for C18H22N4O2, m/z 326.1743, found 327.1782 (M+H)+.
25: (2-(tert-butylamino)-7-methyl-3-(pyrazin-2-ylamino)furo[2,3-c]pyridin-4-yl)methanol
(14 mg, 18%) 1H NMR (500 MHz, MeOD) δ 8.01 (d, J = 1.1 Hz, 1H), 7.88 (dd, J = 2.8, 1.4 Hz, 1H), 7.80 (s, 1H), 7.77 (d, J = 2.8 Hz, 1H), 4.56 (s, 2H), 2.65 (s, 3H), 1.43 (s, 9H). 13C NMR (126 MHz, MeOD) δ 165.60, 156.50, 143.30, 143.00, 140.79, 134.76, 134.39, 132.05, 131.74, 125.39, 95.43, 58.99, 55.50, 29.98, 12.92. MS (ESI) calcd for C17H21N5O2, m/z 327.1695, found 328.1836 (M+H)+.
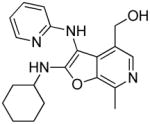
26: (2-(cyclohexylamino)-7-methyl-3-(pyridin-2-ylamino)furo[2,3-c]pyridin-4-yl)methanol
(19 mg, 22%) 1H NMR (500 MHz, MeOD) δ 8.07 (t, J = 7.4 Hz, 1H), 7.96 (s, 1H), 7.90 (d, J = 5.9 Hz, 1H), 7.29 (d, J = 8.4 Hz, 1H), 7.07 (t, J = 6.7 Hz, 1H), 4.64 (s, 2H), 3.91 – 3.79 (m, 1H), 2.75 (s, 3H), 2.05 (d, J = 8.8 Hz, 2H), 1.87 – 1.77 (m, 2H), 1.68 (d, J = 13.1 Hz, 1H), 1.48 – 1.33 (m, 4H), 1.21 (dd, J = 12.1, 9.2 Hz, 1H). 13C NMR (126 MHz, MeOD) δ 164.86, 155.98, 145.65, 143.70, 142.10, 137.34, 133.72, 132.85, 124.81, 115.77, 115.45, 89.79, 59.50, 54.25, 34.33, 26.28, 26.16, 12.97. MS (ESI) calcd for C20H24N4O2, m/z 352.1899, found 353.2023 (M+H)+.
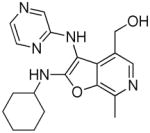
27: (2-(cyclohexylamino)-7-methyl-3-(pyrazin-2-ylamino)furo[2,3-c]pyridin-4-yl)methanol
(24 mg, 28%) 1H NMR (500 MHz, MeOD) δ 8.10 (s, 1H), 7.97 (d, J = 1.4 Hz, 1H), 7.86 (s, 2H), 4.64 (s, 2H), 3.86 – 3.78 (m, 1H), 2.71 (s, 3H), 2.03 – 1.96 (m, 2H), 1.80 (dd, J = 5.3, 3.1 Hz, 2H), 1.66 (d, J = 13.0 Hz, 1H), 1.38 (t, J = 9.6 Hz, 4H), 1.23 – 1.15 (m, 1H). 13C NMR (126 MHz, MeOD) δ 165.01, 156.57, 143.09, 142.98, 141.82, 134.49, 132.10, 131.50, 125.12, 94.07, 58.98, 53.94, 34.42, 26.31, 26.20, 12.80. MS (ESI) calcd for C19H23N5O2, m/z 353.1852, found 354.1902 (M+H)+.
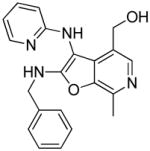
28: (2-(benzylamino)-7-methyl-3-(pyridin-2-ylamino)furo[2,3-c]pyridin-4-yl)methanol. (13
mg, 15%) 1H NMR (500 MHz, MeOD) δ 8.06 – 8.01 (m, 1H), 8.00 (s, 1H), 7.89 (d, J = 5.9 Hz, 1H), 7.44 – 7.39 (m, 2H), 7.34 (t, J = 7.5 Hz, 2H), 7.28 (t, J = 7.3 Hz, 1H), 7.23 (d, J = 8.6 Hz, 1H), 7.05 (t, J = 6.7 Hz, 1H), 4.76 (s, 2H), 4.65 (s, 2H), 2.77 (s, 3H). 13C NMR (126 MHz, MeOD) δ 165.45, 156.13, 145.31, 143.75, 142.15, 138.61, 138.18, 133.67, 133.22, 130.23, 130.00, 129.91, 128.97, 128.77, 125.32, 115.82, 115.01, 90.66, 59.42, 47.33, 12.98. MS (ESI) calcd for C21H20N4O2, m/z 360.1586, found 361.1830 (M+H)+.
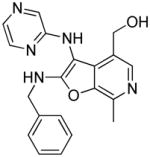
29: (2-(benzylamino)-7-methyl-3-(pyrazin-2-ylamino)furo[2,3-c]pyridin-4-yl)methanol. (15
mg, 16%) 1H NMR (500 MHz, MeOD) δ 7.99 (s, 1H), 7.94 (dd, J = 2.8, 1.5 Hz, 1H), 7.87 (s, 1H), 7.81 (d, J = 2.8 Hz, 1H), 7.37 – 7.32 (m, 2H), 7.29 (dd, J = 10.3, 5.0 Hz, 2H), 7.21 (t, J = 7.3 Hz, 1H), 4.64 (s, 2H), 4.61 (s, 2H), 2.61 (s, 3H). 13C NMR (126 MHz, MeOD) δ 163.44, 156.59, 143.82, 143.17, 139.78, 139.64, 135.93, 134.61, 134.23, 134.16, 129.68, 128.54, 128.38, 125.19, 93.30, 59.41, 47.06, 14.34. MS (ESI) calcd for C20H19N5O2, m/z 361.1539, found 362.1805 (M+H)+.
Synthesis of compound 34: (2-(benzylamino)-7-methyl-3-(phenylamino)furo[2,3-c]pyridin- 4-yl)methanol
To a solution of aniline (45 μL, 0.5 mmol) in anhydrous methanol, were added pyridoxal hydrochloride (112 mg, 0.55 mmol), 4N HCl/dioxane (10 μL) and benzyl isonitrile (68 μL, 0.55 mmol). The reaction mixture was then heated under microwave conditions (600 W, 80 °C) in a sealed vial for 2 min. After cooling the reaction mixture to room temperature, the solvents were removed and the residue was purified using column chromatography to obtain compound 34 (84 mg, 47%).1H NMR (500 MHz, MeOD) δ 7.88 (s, 1H), 7.34 (d, J = 7.8 Hz, 2H), 7.29 (t, J = 7.6 Hz, 2H), 7.21 (t, J = 7.2 Hz, 1H), 7.09 (t, J = 7.9 Hz, 2H), 6.66 (td, J = 7.3, 0.9 Hz, 1H), 6.58 – 6.51 (m, 2H), 4.57 (s, 2H), 4.52 (s, 2H), 2.54 (s, 3H). 13C NMR (126 MHz, MeOD) δ 160.73, 150.14, 141.35, 141.10, 138.40, 137.10, 130.29, 129.51, 128.35, 128.23, 119.02, 114.15, 94.89, 60.27, 47.02, 16.66. MS (ESI) calcd for C22H21N3O2, m/z 359.1634, found 360.1832 (M+H)+.
Synthesis of compound 35: 3-(benzyloxy)-5-(hydroxymethyl)-2-methylisonicotinaldehyde
To a solution of pyridoxalhydrochloride (500 mg, 2.45 mmol) in anhydrous DMF, were added potassium carbonate (408 mg, 2.95 mmol), and benzyl bromide (0.35 mL, 2.95 mmol). The reaction mixture was stirred at room temperature for overnight. After the completion of reaction, water (20 mL) was added and the crude product obtained was extracted in ethyl acetate. The organic layer was washed with water, dried over anhydrous sodium sulfate and evaporated. The residue was purified using column chromatography to obtain compound 35 (100 mg, 15%) 1H NMR (500 MHz, CDCl3) δ 8.05 (s, 1H), 7.45 – 7.32 (m, 5H), 6.64 (d, J = 1.7 Hz, 1H), 5.34 (d, J = 11.2 Hz, 1H), 5.22 (d, J = 12.7 Hz, 1H), 5.19 (d, J = 11.2 Hz, 1H), 4.99 (d, J = 12.7 Hz, 1H), 2.50 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 150.88, 148.63, 136.84, 136.17, 135.53, 135.42, 128.80, 128.47, 127.74, 100.10, 74.13, 69.95, 19.38. MS (ESI) calcd for C15H15NO3, m/z 257.1052, found 258.1279 (M+H)+.
Synthesis of compound 36: (5-(benzyloxy)-4-(3-(cyclohexylamino)imidazo[1,2-a]pyridin-2- yl)-6-methylpyridin-3-yl)methanol
To a solution of 2-aminopyridine (24 mg, 0.25 mmol) in anhydrous acetonitrile, were added 3-(benzyloxy)-5-(hydroxymethyl)-2-methylisonicotinaldehyde 34 (68 mg, 0.28 mmol), 4N HCl/dioxane (5 μL) and cyclohexylisonitrile (30 μL, 0.24 mmol). The reaction mixture was then heated under microwave conditions (400 W, 110 °C) in a sealed vial for 20 min. After cooling the reaction mixture to room temperature, solvents were removed and the residue was purified using column chromatography to obtain compound 36 (19 mg, 17%) 1H NMR (500 MHz, CDCl3) δ 8.41 (s, 1H), 8.07 (d, J = 6.9 Hz, 1H), 7.54 (d, J = 9.1 Hz, 1H), 7.26 – 7.15 (m, 4H), 7.02 – 6.97 (m, 2H), 6.90 (td, J = 6.8, 1.0 Hz, 1H), 4.59 (bs, 1H), 4.43 (s, 2H), 4.36 (bs, 1H), 3.88 (d, J = 7.9 Hz, 1H), 2.61 (bs, 1H), 2.58 (s, 3H), 1.85-1.75 (m, 1H), 1.70-1.65 (m, 1H), 1.50-1.40 (m, 2H), 1.37-1.28 (m, 1H), 1.22-1.05(s, 2H), 1.05-0.85 (m, 2H), 0.60 (bs, 1H). 13C NMR (126 MHz, CDCl3) δ 152.95, 150.53, 145.20, 141.29, 135.58, 129.62, 129.25, 128.76, 128.58, 128.57, 128.40, 126.28, 125.05, 123.16, 117.47, 112.53, 76.73, 61.64, 56.36, 34.32, 33.74, 25.60, 25.03, 24.53, 19.58. MS (ESI) calcd for C27H30N4O2, m/z 442.2369, found 443.2410 (M+H)+.
General procedure for the syntheses of compounds 37a-37m
Synthesis of compound 37a: (2-(butylamino)-7-methyl-3-(pyridin-2-ylamino)furo[2,3- c]pyridin-4-yl)methanol
To a solution of 2-aminopyridine (24 mg, 0.25 mmol) in anhydrous acetonitrile, were added pyridoxal hydrochloride (56 mg, 0.28 mmol), 4N HCl/dioxane (5 μL) and n-butyl isonitrile (25 μL, 0.24 mmol). The reaction mixture was then heated under microwave conditions (600 W, 80 °C) in a sealed vial for 2 min. After cooling the reaction mixture to room temperature, solvents were removed and the residue was purified using column chromatography to obtain compound 37a (32 mg, 41%) 1H NMR (500 MHz, MeOD) δ 8.06 (t, J = 8.0 Hz, 1H), 7.96 (s, 1H), 7.90 (d, J = 5.8 Hz, 1H), 7.29 (d, J = 8.5 Hz, 1H), 7.07 (t, J = 6.6 Hz, 1H), 4.65 (s, 2H), 3.56 (t, J = 7.1 Hz, 2H), 2.75 (s, 3H), 1.75 – 1.61 (m, 2H), 1.49 – 1.35 (m, 2H), 0.96 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, MeOD) δ 165.68, 155.98, 145.61, 143.66, 142.08, 137.58, 133.67, 132.87, 124.84, 115.82, 115.31, 90.01, 59.50, 43.64, 32.79, 20.93, 13.99, 12.96. MS (ESI) calcd for C18H22N4O2, m/z 326.1743, found 327.1925 (M+H)+.
Compounds 37b-37mwere synthesized similarly as compound 37a.
37b: (7-methyl-2-(pentylamino)-3-(pyridin-2-ylamino)furo[2,3-c]pyridin-4-yl)methanol
(27 mg, 33%) 1H NMR (500 MHz, MeOD) δ 8.06 (t, J = 8.0 Hz, 1H), 7.96 (s, 1H), 7.91 (d, J = 5.9 Hz, 1H), 7.28 (d, J = 8.5 Hz, 1H), 7.07 (t, J = 6.6 Hz, 1H), 4.65 (s, 2H), 3.56 (t, J = 7.1 Hz, 2H), 2.75 (s, 3H), 1.75 – 1.64 (m, 2H), 1.38 (d, J = 3.6 Hz, 4H), 0.92 (t, J = 6.9 Hz, 3H). 13C NMR (126 MHz, MeOD) δ 165.68, 156.04, 145.55, 143.66, 142.09, 137.74, 133.66, 132.85, 124.85, 115.82, 115.25, 90.09, 59.49, 43.90, 30.42, 29.98, 23.31, 14.32, 12.95.MS (ESI) calcd for C19H24N4O2, m/z 340.1899, found 341.2058 (M+H)+.
37c: (2-(isopropylamino)-7-methyl-3-(pyridin-2-ylamino)furo[2,3-c]pyridin-4-yl)methanol
(20 mg, 27%) 1H NMR (500 MHz, MeOD) δ 8.05 (t, J = 8.0 Hz, 1H), 7.95 (s, 1H), 7.90 (d, J = 5.9 Hz, 1H), 7.26 (d, J = 8.6 Hz, 1H), 7.05 (t, J = 6.7 Hz, 1H), 4.63 (s, 2H), 4.25 (dt, J = 13.0, 6.5 Hz, 1H), 2.74 (s, 3H), 1.33 (d, J = 6.5 Hz, 6H). 13C NMR (126 MHz, MeOD) δ 164.96, 156.16, 145.41, 143.72, 142.06, 137.79, 133.63, 132.82, 124.86, 115.77, 115.24, 90.04, 59.48, 47.16, 23.12, 12.94. MS (ESI) calcd for C17H20N4O2, m/z 312.1586, found 313.1740 (M+H)+.
37d: (7-methyl-2-(pentan-2-ylamino)-3-(pyridin-2-ylamino)furo[2,3-c]pyridin-4- yl)methanol
(34 mg, 42%) 1H NMR (500 MHz, MeOD) δ 7.99 (t, J = 8.0 Hz, 1H), 7.88 (s, 1H), 7.83 (d, J = 5.2 Hz, 1H), 7.20 (d, J = 7.3 Hz, 1H), 7.00 (t, J = 6.7 Hz, 1H), 4.56 (s, 2H), 4.03 (dq, J = 12.9, 6.5 Hz, 1H), 2.66 (s, 3H), 1.60 – 1.41 (m, 2H), 1.38 – 1.26 (m, 2H), 1.23 (d, J = 6.6 Hz, 3H), 0.86 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, MeOD) δ 165.14, 155.98, 145.72, 143.66, 142.17, 137.38, 133.77, 132.84, 124.79, 115.82, 115.41, 89.73, 59.52, 51.18, 39.97, 21.53, 20.53, 14.15, 12.94. MS (ESI) calcd for C19H24N4O2, m/z 340.1899, found 341.2077 (M+H)+.
37e: (7-methyl-3-(pyridin-2-ylamino)-2-((2,4,4-trimethylpentan-2-yl)amino)furo[2,3- c]pyridin-4-yl)methanol
(33 mg, 36%) 1H NMR (500 MHz, MeOD) δ 8.08 (t, J = 8.0 Hz, 1H), 7.99 (s, 1H), 7.90 (s, 1H), 7.27 (s, 1H), 7.08 (dd, J = 7.0, 6.4 Hz, 1H), 4.63 (s, 2H), 2.78 (s, 3H), 1.95 (d, J = 12.9 Hz, 2H), 1.60 (s, 6H), 1.00 (s, 9H). 13C NMR (126 MHz, MeOD) δ 165.37, 156.14, 145.64, 143.96, 141.40, 137.05, 133.87, 132.93, 124.92, 115.77, 115.62, 90.82, 59.88, 59.51, 53.31, 32.62, 31.82, 30.73, 13.06.MS (ESI) calcd for C22H30N4O2, m/z 382.2369, found 383.2541 (M+H)+.
37f: (7-methyl-3-(pyridin-2-ylamino)-2-(((trimethylsilyl)methyl)amino)furo[2,3-c]pyridin-4- yl)methanol
(24 mg, 28%) 1H NMR (500 MHz, MeOD) δ 8.03 (t, J = 8.0 Hz, 1H), 7.91-7.90 (m, 2H), 7.24 (d, J = 8.7 Hz, 1H), 7.04 (t, J = 6.6 Hz, 1H), 4.62 (s, 2H), 3.11 (s, 2H), 2.72 (s, 3H), 0.12 (s, 9H). 13C NMR (126 MHz, MeOD) δ 165.66, 156.25, 145.27, 143.32, 142.02, 138.19, 133.57, 132.16, 124.28, 115.77, 115.00, 90.25, 59.52, 34.64, 12.94, -2.60. MS (ESI) calcd for C18H24N4O2Si, m/z 356.1669, found 357.1815 (M+H)+.
37g: (7-methyl-2-((2-morpholinoethyl)amino)-3-(pyridin-2-ylamino)furo[2,3-c]pyridin-4- yl)methanol
(9 mg, 10%) 1H NMR (500 MHz, MeOD) δ 8.03 (s, 2H), 7.93 (d, J = 5.8 Hz, 1H), 7.27 (d, J = 7.3 Hz, 1H), 7.05 (t, J = 6.5 Hz, 1H), 4.66 (s, 2H), 4.06-3.95 (m, 6H), 3.54 (bs, 2H), 3.42 (bs, 4H), 2.80 (s, 3H). 13C NMR (126 MHz, MeOD) δ 165.51, 156.26, 145.10, 143.92, 141.95, 138.27, 134.09, 133.62, 126.01, 115.97, 115.07, 90.35, 64.80, 59.39, 57.37, 53.45, 37.84, 13.21. MS (ESI) calcd for C20H25N5O3, m/z 383.1957, found 384.2132 (M+H)+.
37h: Ethyl 2-((4-(hydroxymethyl)-7-methyl-3-(pyridin-2-ylamino)furo[2,3-c]pyridin-2- yl)amino)acetate
(21 mg, 25%) 1H NMR (500 MHz, MeOD) δ 8.07 (t, J = 8.0 Hz, 1H), 8.03 (s, 1H), 7.91 (d, J = 6.1 Hz, 1H), 7.29 (d, J = 8.9 Hz, 1H), 7.08 (t, J = 6.7 Hz, 1H), 4.66 (s, 2H), 4.36 (s, 2H), 4.18 (q, J = 7.1 Hz, 2H), 2.74 (s, 3H), 1.25 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, MeOD) δ 170.68, 165.42, 155.75, 145.81, 143.87, 142.15, 137.71, 133.94, 133.89, 125.93, 115.98, 115.25, 91.02, 62.95, 59.42, 44.55, 14.48, 13.03. MS (ESI) calcd for C18H20N4O4, m/z 356.1485, found 357.1738 (M+H)+.
37i: tert-butyl 3-((4-(hydroxymethyl)-7-methyl-3-(pyridin-2-ylamino)furo[2,3-c]pyridin-2- yl)amino)propanoate
(20 mg, 21%) 1H NMR (500 MHz, MeOD) δ 8.08 (t, J = 8.0 Hz, 1H), 8.00 (s, 1H), 7.91 (d, J = 5.8 Hz, 1H), 7.29 (d, J = 7.8 Hz, 1H), 7.08 (t, J = 6.7 Hz, 1H), 4.66 (s, 2H), 3.80 (t, J = 6.4 Hz, 2H), 2.78 (s, 3H), 2.67 (t, J = 6.7 Hz, 2H), 1.42 (s, 9H). 13C NMR (126 MHz, MeOD) δ 171.99, 165.49, 155.86, 145.77, 143.76, 142.08, 137.49, 133.79, 133.30, 125.17, 115.86, 115.36, 90.25, 82.28, 59.49, 39.67, 36.03, 28.31, 13.02. MS (ESI) calcd for C21H26N4O4, m/z 398.1954, found 399.2128 (M+H)+.
37j: Diethyl (((4-(hydroxymethyl)-7-methyl-3-(pyridin-2-ylamino)furo[2,3-c]pyridin-2- yl)amino)methyl)phosphonate
(13 mg, 13%) 1H NMR (500 MHz, MeOD) δ 8.08 – 8.00 (m, 2H), 7.95 (d, J = 5.7 Hz, 1H), 7.23 (d, J = 4.9 Hz, 1H), 7.06 (t, J = 6.5 Hz, 1H), 4.69 (s, 2H), 4.24 – 4.16 (m, 4H), 4.09 (d, J = 10.6 Hz, 2H), 2.81 (s, 3H), 1.33 (t, J = 7.0 Hz, 6H). 13C NMR (126 MHz, MeOD) δ 164.98, 156.41, 145.04, 143.68, 142.06, 139.12, 133.83, 133.71, 125.96, 115.97, 114.67, 91.93, 64.56 (d, J = 6.9 Hz), 59.37, 39.02 (d, J = 158.9 Hz), 16.78 (d, J = 5.6 Hz), 13.09. MS (ESI) calcd for C19H25N4O5P, m/z 420.1563, found 421.1672 (M+H)+.
37k: (2-((4-methoxyphenyl)amino)-7-methyl-3-(pyridin-2-ylamino)furo[2,3-c]pyridin-4- yl)methanol
(13 mg, 14%) 1H NMR (500 MHz, MeOD) δ 7.99 – 7.87 (m, 2H), 7.62 (t, J = 7.8 Hz, 1H), 7.33 (d, J = 8.8 Hz, 2H), 6.90 (d, J = 8.8 Hz, 2H), 6.77 (t, J = 6.3 Hz, 2H), 4.66 (s, 2H), 3.77 (s, 3H), 2.71 (s, 3H). 13C NMR (126 MHz, MeOD) δ 162.79, 159.21, 158.92, 146.57, 143.34, 141.31, 140.61, 132.84, 132.22, 130.80, 126.51, 124.33, 115.64, 115.43, 111.46, 96.53, 62.30, 58.97, 55.97, 13.06. MS (ESI) calcd for C21H20N4O3, m/z 376.1535, found 377.1694 (M+H)+.
37l: (2-((2-chloro-6-methylphenyl)amino)-7-methyl-3-(pyridin-2-ylamino)furo[2,3- c]pyridin-4-yl)methanol
(27 mg, 28%) 1H NMR (500 MHz, MeOD) δ 8.10 (s, 1H), 7.90 (dd, J = 20.4, 6.6 Hz, 2H), 7.28 – 7.15 (m, 3H), 7.03 (t, J = 6.6 Hz, 1H), 6.95 (s, 1H), 4.70 (s, 2H), 2.75 (s, 3H), 2.32 (s, 3H). 13C NMR (126 MHz, MeOD) δ 162.57, 155.17, 145.80, 144.21, 142.68, 139.93, 136.98, 134.45, 133.96, 133.78, 132.14, 130.74, 128.65, 126.80, 115.93, 114.82, 91.45, 59.30, 18.71, 13.11. MS (ESI) calcd for C21H19ClN4O2, m/z 394.1197, found 395.1349 (M+H)+.
37m: (S)-(7-methyl-2-((1-phenylethyl)amino)-3-(pyridin-2-ylamino)furo[2,3-c]pyridin-4- yl)methanol
(28 mg, 31%) 1H NMR (500 MHz, MeOD) δ 7.92 (s, 1H), 7.89 (t, J = 8.0 Hz, 1H), 7.90 – 7.81 (m, 1H), 7.38 (d, J = 7.6 Hz, 2H), 7.29 (t, J = 7.4 Hz, 2H), 7.21 (t, J = 7.2 Hz, 1H), 7.05 (d,J = 6.1 Hz, 1H), 6.94 (t, J = 6.4 Hz, 1H), 5.20 (q, J = 6.8 Hz, 1H), 4.58 (s, 2H), 2.68 (s, 3H), 1.60 (d, J = 6.9 Hz, 3H). 13C NMR (126 MHz, MeOD) δ 164.81, 157.13, 144.72, 143.97, 143.61, 142.11, 140.61, 133.20, 132.80, 129.92, 128.71, 126.98, 125.52, 115.70, 113.93, 91.91, 59.24, 54.49, 23.31, 12.88. MS (ESI) calcd for C22H22N4O2, m/z 374.1743, found 375.1931 (M+H)+.
Synthesis of compound (37n): ((2-amino-7-methyl-3-(pyridin-2-ylamino)furo[2,3-c]pyridin- 4-yl)methanol
The solution of compound 37e (95 mg, 0.25 mmol) in TFA/CH2Cl2 (50%, 4 mL) was stirred at room temperature for 6 h. The solvents were removed and the residue obtained was column purified to afford compound 37n (56 mg, 84%).1H NMR (500 MHz, MeOD) δ 7.91 – 7.86 (m, 2H), 7.81 (t, J = 7.9 Hz, 1H), 6.96 (d, J = 8.6 Hz, 1H), 6.87 (t, J = 6.5 Hz, 1H), 4.61 (s, 2H), 2.66 (s, 3H). 13C NMR (126 MHz, MeOD) δ 167.39, 157.68, 143.36, 143.14, 142.12, 132.82, 132.44, 125.47, 115.59, 113.10, 92.54, 59.18, 12.80. MS (ESI) calcd for C14H14N4O2, m/z 270.1117, found 271.1310 (M+H)+.
Compounds 38a-38cwere synthesized similarly as compound 34.
38a: (7-methyl-2-(pentylamino)-3-(phenylamino)furo[2,3-c]pyridin-4-yl)methanol
(117 mg, 70%) 1H NMR (500 MHz, MeOD) δ 7.85 (s, 1H), 7.14 (dd, J = 8.6, 7.4 Hz, 2H), 6.71 (tt, J = 7.4, 1.0 Hz, 1H), 6.60 (dd, J = 8.6, 1.0 Hz, 2H), 4.65 – 4.59 (m, 2H), 3.51 (t, J = 7.1 Hz, 2H), 2.68 (s, 3H), 1.72 – 1.57 (m, 2H), 1.41 – 1.28 (m, 4H), 0.95 – 0.84 (m, 3H). 13C NMR (126 MHz, MeOD) δ 165.54, 149.56, 143.22, 141.35, 133.46, 132.44, 130.44, 125.33, 119.45, 114.08, 96.74, 59.31, 43.42, 31.00, 29.94, 23.33, 14.34, 13.48. MS (ESI) calcd for C20H25N3O2, m/z 339.1947, found 340.2087 (M+H)+.
38b: (3-((3-fluorophenyl)amino)-7-methyl-2-(pentylamino)furo[2,3-c]pyridin-4-yl) methanol
(70 mg, 82%) 1H NMR (500 MHz, MeOD) δ 7.81 (s, 1H), 7.06 (td, J = 8.2, 6.7 Hz, 1H), 6.42 – 6.32 (m, 2H), 6.23 (d, J = 11.5 Hz, 1H), 4.60 (s, 2H), 3.46 (t, J = 7.1 Hz, 2H), 2.65 (s, 3H), 1.59 (p, J = 7.1 Hz, 2H), 1.31 – 1.23 (m, 4H), 0.83 (t, J = 7.0 Hz, 3H). 13C NMR (126 MHz, MeOD) 166.34, 165.62 (d, J = 242.1 Hz), 151.55 (d, J = 10.4 Hz), 142.79, 142.04, 131.84 (d, J = 10.1 Hz), 131.65, 131.28, 125.42, 110.01,105.58 (d, J = 21.8 Hz), 100.74 (d, J = 25.8 Hz), 96.47, 58.95, 43.43, 30.83, 29.93, 23.33, 14.35, 12.79. MS (ESI) calcd for C20H24FN3O2, m/z 357.1853, found 358.2013 (M+H)+.
38c: (7-methyl-3-((3-nitrophenyl)amino)-2-(pentylamino)furo[2,3-c]pyridin-4-yl)methanol
(34 mg, 37%) 1H NMR (500 MHz, MeOD) δ 7.90 (s, 1H), 7.49 (dd, J = 8.1, 2.2 Hz, 1H), 7.34 (s, 1H), 7.31 (t, J = 8.1 Hz, 1H), 6.95 (d, J = 8.0 Hz, 1H), 4.54 (s, 2H), 3.41 (t, J = 7.0 Hz, 2H), 2.56 (s, 3H), 1.65 – 1.52 (m, 2H), 1.30 (dd, J = 8.5, 4.8 Hz, 4H), 0.86 (t, J = 6.5 Hz, 3H). 13C NMR (126 MHz, MeOD) δ 160.86, 151.59, 150.89, 144.93, 141.84, 138.47, 136.71, 131.12, 124.51, 120.06, 113.05, 107.81, 92.23, 60.13, 43.34, 31.50, 30.02, 23.40, 16.73, 14.35. MS (ESI) calcd for C20H24N4O4, m/z 384.1798, found 385.1950 (M+H)+.
Synthesis of compound (39): 2,2′-(hexane-1,6-diylbis(azanediyl))bis(7-methyl-3-(pyridin-2- ylamino)furo[2,3-c]pyridine-4,2-diyl))dimethanol
To a solution of 2-aminopyridine (48 mg, 0.50 mmol) in anhydrous acetonitrile, were added pyridoxalhydrochloride (112 mg, 0.56 mmol), 4N HCl/dioxane (10 μL) and 1,6-diisocyanohexane (36 μL, 0.24 mmol). The reaction mixture was then heated under microwave conditions (600 W, 80 °C) in a sealed vial for 2 min. After cooling the reaction mixture to room temperature, solvent was removed and the residue was purified using column chromatography to obtain compound 39 (21 mg, 7%). 1H NMR (500 MHz, MeOD) δ 8.07 (t, J = 8.0 Hz, 2H), 7.97 (s, 2H), 7.90 (d, J = 5.6 Hz, 2H), 7.31 (d, J = 5.6 Hz, 2H), 7.07 (t, J = 6.7 Hz, 2H), 4.64 (s, 4H), 3.57 (t, J = 7.0 Hz, 4H), 2.75 (s, 6H), 1.78 – 1.65 (m, 4H), 1.46 (s, 4H). 13C NMR (126 MHz, MeOD) δ 165.67, 155.95, 145.66, 143.71, 142.10, 137.48, 133.70, 132.91, 124.87, 115.83, 115.43, 90.05, 59.50, 43.86, 30.65, 27.48, 13.02. MS (ESI) calcd for C34H38N8O4, m/z 622.3016, found 623.3187 (M+H)+.
X-ray crystallographic structural studies of 20, 28, and 37e
Crystals for compound 28 utilize the non-centrosymmetric triclinic space group P1-C1 with eight crystallographically-independent molecules in the asymmetric unit. They also invariably form multiply-twinned bundles. After many unsuccessful attempts, a multi-domain specimen of 28 was cut from one of these bundles that gave a set of diffracted intensities that permitted a crystal structure solution but not a satisfactory refinement. Full sets of unique diffracted intensities were measured for single-domain specimens of compounds 20 and 37e using monochromated CuKα radiation (λ= 1.54178 Å) on a Bruker Proteum Single Crystal Diffraction System equipped with Helios multilayer optics, an APEX II CCD detector and a Bruker MicroStar microfocus rotating anode x-ray source operating at 45kV and 60mA. Diffracted intensities were obtained with the Bruker program SAINT and the structures were solved using “direct methods” techniques incorporated into the Bruker SHELXTL Version 2010.3-0 software package. All stages of weighted full-matrix least-squares refinement were performed using the SHELXTL software with Fo2 data. The final structural model for compounds 20 and 37e incorporated anisotropic thermal parameters for all non-hydrogen atoms and isotropic thermal parameters for all hydrogen atoms. The respective asymmetric units consist of four molecules of 20; one molecule of 37e. All hydrogen atoms for 37e and hydrogen atoms of 20 that are bonded to amine nitrogens (one in each molecule) were located in a difference Fourier map and included in the structural model as independent isotropic atoms whose parameters were allowed to vary in least-squares refinement cycles. All hydroxyl hydrogens for 20 were placed at idealized sp3-hybridized positions with an O-H bond length of 0.84 Å; the hydroxyl group was then allowed to rotate about its O-C bond during least squares refinement cycles. The remaining hydrogen atoms for 20 were included in the structural model as idealized atoms (assuming sp2- or sp3-hybridization of the carbon or nitrogen atoms and C-H bond lengths of 0.95 to 1.00 Å or N-H bond lengths of 0.88 Å). The isotropic thermal parameters of all idealized hydrogen atoms for 20 were fixed at values 1.2 (nonmethyl) or 1.5 (methyl) times the equivalent isotropic thermal parameter of the carbon, nitrogen or oxygen atom to which they are covalently bonded.
Human TLR-3/-4/-5/-7/-8/-9 Reporter Gene assays (NF-κB induction)
The induction of NF- κB was quantified using human TLR-3/-4/-5/-7/-8/-9-specific HEK-Blue™ reporter gene assays as previously described by us.27,40,49 HEK293 cells stably co-transfected with the appropriate hTLR and secreted alkaline phosphatase (sAP) were maintained in HEK-Blue™ Selection medium containing zeocin and normocin. Stable expression of secreted alkaline phosphatase (sAP) under control of NF-κB/AP-1 promoters is inducible by appropriate TLR agonists, and extracellular sAP in the supernatant is proportional to NF-κB induction. HEK-Blue™ cells were incubated at a density of ~105 cells/mL in a volume of 80 WL/well, in 384-well, flat-bottomed, cell culture-treated microtiter plates until confluency was achieved, and subsequently stimulated with graded concentrations of stimuli. sAP was assayed spectrophotometrically using an alkaline phosphatase-specific chromogen (present in HEK-detection medium as supplied by the vendor) at 620 nm.
Immunoassays for cytokines
Fresh human peripheral blood mononuclear cells (hPBMC) were isolated from human blood obtained by venipuncture with informed consent and as per institutional guidelines on Ficoll-Hypaque gradients as described elsewhere.55 Aliquots of PBMCs (105 cells in 100 μL/well) were stimulated for 12 h with graded concentrations of test compounds. Supernatants were isolated by centrifugation, and were assayed in triplicates using analyte-specific multiplexed cytokine/chemokine bead array assays as reported by us previously.56
Transcriptomal profiling in human PBMCs
Detailed procedures for transcriptomal profiling have been described by us previously.49 Briefly, fresh human PBMC samples were stimulated with 10 μg/mL of 37b and 37f for two hours, and total RNA was extracted from treated and negative control blood samples with QIAamp RNA Blood Mini Kit (Qiagen). Subsequently, 160 ng of each of the RNA samples was used. The Human Genome GeneChip U133 plus 2.0 oligonucleotide array (Affymetrix, Santa Clara, CA) was employed. Established standard protocols at the KU Genomics Facility were performed on cRNA target preparation, array hybridization, washing, staining and image scanning. The microarray data was first subjected to quality assessment using the Affymetrix GeneChip Operating Software (GCOS). QC criteria included low background, low noise, detection of positive controls, and a 5′/3′ ratio of < 3.0. To facilitate direct comparison of gene expression data between different samples, the GeneChip data were first subjected to preprocessing. This step involved scaling (in GCOS) data from all chips to a target intensity value of 500, and further normalizations steps in GeneSpring GX (Agilent Technologies, Santa Clara, CA). Prior to identifying target genes, genes that were detected as non-expressed in all samples, i.e., those with absence calls, were filtered out. To identify genes whose expression was changed by our compounds, a fold change threshold of 2.0 between the compound treatment and the negative control was used.
Rabbit immunization and antigen-specific ELISA
All experiments were performed at Harlan Laboratories (Indianapolis, IN) in accordance with institutional guidelines (University of Kansas IACUC permit # 119-06). Cohorts of adult female New Zealand White rabbits (n = 4 per cohort) were immunized intramuscularly in the flank region with either 100 μg of bovine α-lactalbumin in 0.2 mL saline (unadjuvanted control), or 100 μg of bovine α-lactalbumin plus 100 μg of 37b, 40, or 41 in 0.2 mL saline. Pre-immune test-bleeds were first obtained via venipuncture of the marginal vein of the ear. Animals were immunized on Days 1, 15 and 28. A final test-bleed was performed via the marginal vein of the ear on Day 38. Sera were stored at -80 °C until used. Bovine α-lactalbumin-specific ELISAs were performed in 384-well format using automated liquid handling methods as described by us.42
Supplementary Material
Acknowledgments
This work was supported by NIH/NIAID contract HSN272200900033C. We gratefully acknowledge NSF-MRI grant CHE-0923449 which supported the purchase of a Bruker MicroStar Cu rotating anode diffractometer at the University of Kansas Small-Molecule X-Ray Crystallography Laboratory.
Abbreviations used
- dsRNA
double-stranded RNA
- EC50
Half-maximal effective concentration
- ESI-TOF
Electrospray ionization-time of flight
- HEK
Human embryonic kidney
- IL
Interleukin
- LPS
Lipopolysaccharides
- MHC
Major histocompatibility complex
- MyD88
myeloid differentiation primary response gene 88
- NF-κB
Nuclear factor-κB
- PAMPs
Pathogen associated molecular patterns
- PBMCs
Peripheral blood mononuclear cells
- PRRs
Pattern recognition receptors
- SAP
Secreted alkaline phosphatase
- SAR
Structure activity relationship
- ssRNA
singlestranded RNA
- TLR
Toll like receptor
- TNF-α
Tumor necrosis factor-α
Footnotes
PDB ID Codes: Crystal structures for Compounds 20 and 37e have been deposited in the Cambridge Crystallographic Data Centre (CCDC). Deposition numbers are 893565 and 893566.
Supporting Information: Characterization data (1H, 13C, mass spectra), LC-MS analyses of final compounds, and summary crystallographic data of compounds 20, and 37e. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 3.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 4.Lee MS, Kim YJ. Pattern-recognition receptor signaling initiated from extracellular, membrane, and cytoplasmic space. Mol Cells. 2007;23:1–10. [PubMed] [Google Scholar]
- 5.Seaton BA, Crouch EC, McCormack FX, Head JF, Hartshorn KL, Mendelsohn R. Review: Structural determinants of pattern recognition by lung collectins. Innate Immun. 2010;16:143–150. doi: 10.1177/1753425910368716. [DOI] [PubMed] [Google Scholar]
- 6.Runza VL, Schwaeble W, Mannel DN. Ficolins: novel pattern recognition molecules of the innate immune response. Immunobiol. 2008;213:297–306. doi: 10.1016/j.imbio.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Agrawal A, Singh PP, Bottazzi B, Garlanda C, Mantovani A. Pattern recognition by pentraxins. Adv Exp Med Biol. 2009;653:98–116. doi: 10.1007/978-1-4419-0901-5_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 9.Yoneyama M, Fujita T. Function of RIG-I-like receptors in antiviral innate immunity. J Biol Chem. 2007;282:15315–15318. doi: 10.1074/jbc.R700007200. [DOI] [PubMed] [Google Scholar]
- 10.Shaw MH, Reimer T, Kim YG, Nunez G. NOD-like receptors (NLRs): bona fide intracellular microbial sensors. Curr Opin Immunol. 2008;20:377–382. doi: 10.1016/j.coi.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 12.Kumagai Y, Takeuchi O, Akira S. Pathogen recognition by innate receptors. J Infect Chemother. 2008;14:86–92. doi: 10.1007/s10156-008-0596-1. [DOI] [PubMed] [Google Scholar]
- 13.Takeda K, Akira S. Toll-like receptors. Curr Protoc Immunol. 2007;Chapter 14(Unit) doi: 10.1002/0471142735.im1412s77. [DOI] [PubMed] [Google Scholar]
- 14.Akira S. Toll-like receptors and innate immunity. Adv Immunol. 1902;78:1–56. doi: 10.1016/s0065-2776(01)78001-7. [DOI] [PubMed] [Google Scholar]
- 15.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nature Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 16.Cottalorda A, Verschelde C, Marcais A, Tomkowiak M, Musette P, Uematsu S, Akira S, Marvel J, Bonnefoy-Berard N. TLR2 engagement on CD8 T cells lowers the threshold for optimal antigen-induced T cell activation. Eur J Immunol. 2006;36:1684–1693. doi: 10.1002/eji.200636181. [DOI] [PubMed] [Google Scholar]
- 17.Kaisho T, Akira S. Toll-like receptors as adjuvant receptors. Biochim Biophys Acta. 2002;1589:1–13. doi: 10.1016/s0167-4889(01)00182-3. [DOI] [PubMed] [Google Scholar]
- 18.Wu W, Li R, Malladi SS, Warshakoon HJ, Kimbrell MR, Amolins MW, Ukani R, Datta A, David SA. Structure-activity relationships in toll-like receptor-2 agonistic diacylthioglycerol lipopeptides. J Med Chem. 2010;53:3198– 3213. doi: 10.1021/jm901839g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agnihotri G, Crall BM, Lewis TC, Day TP, Balakrishna R, Warshakoon HJ, Malladi SS, David SA. Structure-activity relationships in toll-like receptor 2-agonists leading to simplified monoacyl lipopeptides. J Med Chem. 2011;54:8148–8160. doi: 10.1021/jm201071e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salunke DB, Shukla NM, Yoo E, Crall BM, Balakrishna R, Malladi SS, David SA. Structure-Activity Relationships in Human Toll-like Receptor 2- Specific Monoacyl Lipopeptides. J Med Chem. 2012;55:3353–3363. doi: 10.1021/jm3000533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu L, Botos I, Wang Y, Leonard JN, Shiloach J, Segal DM, Davies DR. Structural basis of toll-like receptor 3 signaling with double-stranded RNA. Science. 2008;320:379–381. doi: 10.1126/science.1155406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uematsu S, Akira S. Toll-like receptors and Type I interferons. J Biol Chem. 2007;282:15319–15323. doi: 10.1074/jbc.R700009200. [DOI] [PubMed] [Google Scholar]
- 23.Rietschel ET, Brade H, Brade L, Brandenburg K, Schade UF, Seydel U, Z„hringer U, Galanos C, Lüderitz O, Westphal O, Labischinski H, Kusumoto S, Shiba T. Lipid A, the endotoxic center of bacterial lipopolysaccharides: Relation of chemical structure to biological activity. Prog Clin Biol Res. 1987;231:25–53. [PubMed] [Google Scholar]
- 24.Bowen WS, Minns LA, Johnson DA, Mitchell TC, Hutton MM, Evans JT. Selective TRIF-dependent signaling by a synthetic toll-like receptor 4 agonist. Sci Signal. 2012;5:ra13. doi: 10.1126/scisignal.2001963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stover AG, Da Silva CJ, Evans JT, Cluff CW, Elliott MW, Jeffery EW, Johnson DA, Lacy MJ, Baldridge JR, Probst P, Ulevitch RJ, Persing DH, Hershberg RM. Structure-activity relationship of synthetic toll-like receptor 4 agonists. J Biol Chem. 2004;279:4440–4449. doi: 10.1074/jbc.M310760200. [DOI] [PubMed] [Google Scholar]
- 26.Gerster JF, Lindstrom KJ, Miller RL, Tomai MA, Birmachu W, Bomersine SN, Gibson SJ, Imbertson LM, Jacobson JR, Knafla RT, Maye PV, Nikolaides N, Oneyemi FY, Parkhurst GJ, Pecore SE, Reiter MJ, Scribner LS, Testerman TL, Thompson NJ, Wagner TL, Weeks CE, Andre JD, Lagain D, Bastard Y, Lupu M. Synthesis and structureactivity- relationships of 1H-imidazo[4,5-c]quinolines that induce interferon production. J Med Chem. 2005;48:3481–3491. doi: 10.1021/jm049211v. [DOI] [PubMed] [Google Scholar]
- 27.Shukla NM, Malladi SS, Mutz CA, Balakrishna R, David SA. Structureactivity relationships in human toll-like receptor 7-active imidazoquinoline analogues. J Med Chem. 2010;53:4450–4465. doi: 10.1021/jm100358c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shukla NM, Mutz CA, Malladi SS, Warshakoon HJ, Balakrishna R, David SA. Toll-Like Receptor (TLR)-7 and -8 Modulatory Activities of Dimeric Imidazoquinolines. J Med Chem. 2012;55:1106–1116. doi: 10.1021/jm2010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamilton RD, Wynalda MA, Fitzpatrick FA, Teagarden DL, Hamdy AH, Snider BG, Weed SD, Stringfellow DA. Comparison between circulating interferon and drug levels following administration of 2-amino-5- bromo-6-phenyl-4(3H)-pyrimidinone (ABPP) to different animal species. J Interferon Res. 1982;2:317–327. doi: 10.1089/jir.1982.2.317. [DOI] [PubMed] [Google Scholar]
- 30.Stringfellow DA. Comparation interferon- inducing and antiviral properties of 2-amino- 5-bromo-6-methyl-4-pyrimidinol (U-25,166), tilorone hydrochloride, and polyinosinic-polycytidylic acid. Antimicrob Agents Chemother. 1977;11:984–992. doi: 10.1128/aac.11.6.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J, Chuang TH, Redecke V, She L, Pitha PM, Carson DA, Raz E, Cottam HB. Molecular basis for the immunostimulatory activity of guanine nucleoside analogs: activation of Toll-like receptor 7. Proc Natl Acad Sci U S A. 2003;100:6646–6651. doi: 10.1073/pnas.0631696100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirota K, Kazaoka K, Niimoto I, Kumihara H, Sajiki H, Isobe Y, Takaku H, Tobe M, Ogita H, Ogino T, Ichii S, Kurimoto A, Kawakami H. Discovery of 8-hydroxyadenines as a novel type of interferon inducer. J Med Chem. 2002;45:5419–5422. doi: 10.1021/jm0203581. [DOI] [PubMed] [Google Scholar]
- 33.Isobe Y, Tobe M, Ogita H, Kurimoto A, Ogino T, Kawakami H, Takaku H, Sajiki H, Hirota K, Hayashi H. Synthesis and structure-activity relationships of 2-substituted-8-hydroxyadenine derivatives as orally available interferon inducers without emetic side effects. Bioorg Med Chem. 2003;11:3641–3647. doi: 10.1016/s0968-0896(03)00369-9. [DOI] [PubMed] [Google Scholar]
- 34.Gorden KB, Gorski KS, Gibson SJ, Kedl RM, Kieper WC, Qiu X, Tomai MA, Alkan SS, Vasilakos JP. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J Immunol. 2005;174:1259–1268. doi: 10.4049/jimmunol.174.3.1259. [DOI] [PubMed] [Google Scholar]
- 35.Gorski KS, Waller EL, Bjornton-Severson J, Hanten JA, Riter CL, Kieper WC, Gorden KB, Miller JS, Vasilakos JP, Tomai MA, Alkan SS. Distinct indirect pathways govern human NK-cell activation by TLR-7 and TLR-8 agonists. Int Immunol. 2006;18:1115–1126. doi: 10.1093/intimm/dxl046. [DOI] [PubMed] [Google Scholar]
- 36.Jin MS, Lee JO. Structures of TLR-ligand complexes. Curr Opin Immunol. 2008;20:414–419. doi: 10.1016/j.coi.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Yoon SI, Kurnasov O, Natarajan V, Hong M, Gudkov AV, Osterman AL, Wilson IA. Structural basis of TLR5-flagellin recognition and signaling. Science. 2012;335:859–864. doi: 10.1126/science.1215584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petrone PM, Simms B, Nigsch F, Lounkine E, Kutchukian P, Cornett A, Deng Z, Davies JW, Jenkins JL, Glick M. Rethinking Molecular Similarity: Comparing Compounds on the Basis of Biological Activity. ACS Chem Biol. 2012 doi: 10.1021/cb3001028. [DOI] [PubMed] [Google Scholar]
- 39.Tsunoyama K, Amini A, Sternberg MJ, Muggleton SH. Scaffold hopping in drug discovery using inductive logic programming. J Chem Inf Model. 2008;48:949–957. doi: 10.1021/ci700418f. [DOI] [PubMed] [Google Scholar]
- 40.Shukla NM, Kimbrell MR, Malladi SS, David SA. Regioisomerismdependent TLR7 agonism and antagonism in an imidazoquinoline. Bioorg Med Chem Lett. 2009;19:2211–2214. doi: 10.1016/j.bmcl.2009.02.100. [DOI] [PubMed] [Google Scholar]
- 41.Shukla NM, Mutz CA, Ukani R, Warshakoon HJ, Moore DS, David SA. Syntheses of fluorescent imidazoquinoline conjugates as probes of Toll-like receptor 7. Bioorg Med Chem Lett. 2010;20:6384–6386. doi: 10.1016/j.bmcl.2010.09.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shukla NM, Lewis TC, Day TP, Mutz CA, Ukani R, Hamilton CD, Balakrishna R, David SA. Toward self-adjuvanting subunit vaccines: model peptide and protein antigens incorporating covalently bound toll-like receptor-7 agonistic imidazoquinolines. Bioorg Med Chem Lett. 2011;21:3232–3236. doi: 10.1016/j.bmcl.2011.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baviskar AT, Madaan C, Preet R, Mohapatra P, Jain V, Agarwal A, Guchhait SK, Kundu CN, Banerjee UC, Bharatam PV. N-fused imidazoles as novel anticancer agents that inhibit catalytic activity of topoisomerase IIalpha and induce apoptosis in G1/S phase. J Med Chem. 2011;54:5013–5030. doi: 10.1021/jm200235u. [DOI] [PubMed] [Google Scholar]
- 44.Botos I, Segal DM, Davies DR. The structural biology of Toll-like receptors. Structure. 2011;19:447–459. doi: 10.1016/j.str.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin MS, Kim SE, Heo JY, Lee ME, Kim HM, Paik SG, Lee H, Lee JO. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a triacylated lipopeptide. Cell. 2007;130:1071–1082. doi: 10.1016/j.cell.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 46.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, Akira S. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 47.Weterings JJ, Khan S, van der Heden van Noort GJ, Melief CJ, Overkleeft HS, van der Burg SH, Ossendorp F, Van der Marel GA, Filippov DV. 2- Azidoalkoxy-7-hydro-8-oxoadenine derivatives as TLR7 agonists inducing dendritic cell maturation. Bioorg Med Chem Lett. 2009;19:2249–2251. doi: 10.1016/j.bmcl.2009.02.095. [DOI] [PubMed] [Google Scholar]
- 48.Kurimoto A, Hashimoto K, Nakamura T, Norimura K, Ogita H, Takaku H, Bonnert R, McInally T, Wada H, Isobe Y. Synthesis and biological evaluation of 8-oxoadenine derivatives as toll-like receptor 7 agonists introducing the antedrug concept. J Med Chem. 2010;53:2964–2972. doi: 10.1021/jm100070n. [DOI] [PubMed] [Google Scholar]
- 49.Hood JD, Warshakoon HJ, Kimbrell MR, Shukla NM, Malladi S, Wang X, David SA. Immunoprofiling toll-like receptor ligands: Comparison of immunostimulatory and proinflammatory profiles in ex vivo human blood models. Hum Vaccin. 2010;6:1–14. doi: 10.4161/hv.6.4.10866. [DOI] [PubMed] [Google Scholar]
- 50.Warshakoon HJ, Hood JD, Kimbrell MR, Malladi S, Wu WY, Shukla NM, Agnihotri G, Sil D, David SA. Potential adjuvantic properties of innate immune stimuli. Hum Vaccin. 2009;5:381–394. doi: 10.4161/hv.5.6.8175. [DOI] [PubMed] [Google Scholar]
- 51.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 52.Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 53.Smirnov D, Schmidt JJ, Capecchi JT, Wightman PD. Vaccine adjuvant activity of 3M-052: An imidazoquinoline designed for local activity without systemic cytokine induction. Vaccine. 2011;29:5434–5442. doi: 10.1016/j.vaccine.2011.05.061. [DOI] [PubMed] [Google Scholar]
- 54.Ukani R, Lewis TC, Day TP, Wu W, Malladi SS, Warshakoon HJ, David SA. Potent adjuvantic activity of a CCR1-agonistic bis-quinoline. Bioorg Med Chem Lett. 2012;22:293–295. doi: 10.1016/j.bmcl.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.David SA, Smith MS, Lopez G, Mukherjee S, Buch S, Narayan O. Selective transmission of R5-tropic HIV-1 from dendritic cells to resting CD4+ T cells. AIDS Res Human Retrovir. 2001;17:59–68. doi: 10.1089/088922201750056799. [DOI] [PubMed] [Google Scholar]
- 56.Kimbrell MR, Warshakoon H, Cromer JR, Malladi S, Hood JD, Balakrishna R, Scholdberg TA, David SA. Comparison of the immunostimulatory and proinflammatory activities of candidate Gram-positive endotoxins, lipoteichoic acid, peptidoglycan, and lipopeptides, in murine and human cells. Immunol Lett. 2008;118:132–141. doi: 10.1016/j.imlet.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.