Abstract
Myeloperoxidase (MPO) is a lysosomal enzyme that may be involved in oxidative stress-mediated kidney injury. Using a 2-step approach, we measured the association of 4 polymorphisms across the length of the MPO gene with systemic markers of oxidative stress; plasma MPO and urinary 15-F2t-isoprostane levels. Adverse outcomes were measured in a primary cohort of 262 adults hospitalized with acute kidney injury, and a secondary cohort of 277 adults undergoing cardiac surgery with cardiopulmonary bypass and at-risk for postoperative acute kidney injury. Dominant and haplotype multivariable logistic regression analyses found a genotype-phenotype association in the primary cohort between rs2243828, rs7208693, rs2071409, and rs2759 MPO polymorphisms and both markers of oxidative stress. In adjusted analyses, all 4 polymorphic allele groups had 2-3-fold higher odds for composite outcomes of dialysis or in-hospital death or a composite of dialysis, assisted mechanical ventilation or inhospital death. The MPO T-G-A-T haplotype copy-number was associated with lower plasma MPO levels and lower adjusted odds for the composite outcomes. Significant but less consistent associations were found in the secondary cohort. In summary, our 2-step genetic association study identified several polymorphisms spanning the entire MPO gene locus and a common haplotype marker for patients at-risk for acute kidney injury.
Keywords: acute kidney injury, cardiac surgery, dialysis, gene polymorphism, haplotype, mechanical ventilation, mortality, MPO
INTRODUCTION
Acute kidney injury (AKI) is a common occurrence in the hospital setting, and confers an increased risk of mortality, as well as heightened resource consumption including, prolonged hospital length of stay and increased costs1,2. Although many factors have been identified as predictors of development and evolution of AKI3-9, there remains significant unexplained variability in the prediction of adverse kidney-related outcomes10,11. In recent years, polymorphisms in several candidate genes have been explored as determinants of disease severity and adverse outcomes in patients with AKI12-19.
Myeloperoxidase (MPO) is a heme-containing enzyme belonging to the XPO subfamily of peroxidases. It is abundant in neutrophils and catalyzes the hydrogen peroxide dependent formation of hypochlorous acid, a potent oxidant20. Neutrophils store MPO in cytoplasmic azurophilic granules21. Upon cellular activation and degranulation, MPO is delivered into phagolysosomes where it is required for killing of phagocytosed bacteria22. Activated neutrophils also release granule contents in the extracellular space. Elevated plasma MPO levels have been associated with a variety of clinical conditions including ischemia-reperfusion injury, acute pancreatitis, vasculitis, atherosclerosis, cancer and neuro-degenerative disorders23-25.
The human MPO gene is located on chromosome 17q23.126. Several polymorphisms have been described in the MPO gene locus, some of which have been linked to systemic markers of oxidative stress in patients with chronic kidney disease27,28. Genetic variation in the expression of this key enzyme might thus account for the inter-individual variability observed in the severity and outcome of AKI29. In the present report, we first examine the association of 4 polymorphisms and haplotypes in the MPO gene locus with systemic markers of oxidative stress, measures of disease severity, and adverse clinical outcomes in hospitalized adults with an established diagnosis of AKI (primary cohort). Next, we examine the association of the same polymorphisms with adverse outcomes in a second cohort of adults at-risk for AKI undergoing cardiac surgery with cardiopulmonary bypass (secondary cohort).
RESULTS
Characteristics of Participants in the Primary and Secondary Cohorts
In the primary cohort, between November 2003 and February 2010, a total of 264 participants were enrolled of whom 2 were excluded due to study ineligibility and lack of genomic DNA sampling. The present analysis includes 262 participants who underwent genotyping analyses. At enrollment, mean age was 66 years, 91% were white, and 53% were men. Mean APACHE II score was 20, and 73% were in the intensive care unit. 137 (53%) subjects had stage-3 AKI, as defined by the AKI network criteria. 24% required mechanical ventilation, 39% required dialysis, 22% died in-hospital, and 47% experienced the composite outcome of dialysis requirement or in-hospital death.
In the secondary cohort, between January 2004 year and August 2011, a total of 277 study participants were enrolled. In brief, mean age was 68 years, 95% were white, 72% were men, 37% had a history of heart failure, and preoperative mean serum creatinine was 1.1 mg/dL. Thirty-one percent underwent elective cardiac surgery with 51% requiring valve replacement. Mean aortic cross-clamp and cardiopulmonary bypass (CPB) perfusion time were 91 and 118 minutes, respectively. On post-operative day 1, mean APACHE II score was 13, initial median mechanical ventilation was 9 hours, and stage-2 or 3 AKI developed in 7 subjects (2.5%).
Characteristics of the Primary and Secondary Cohorts Stratified by MPO Gene Polymorphisms
In both the primary and secondary cohorts, tests for Hardy-Weinberg equilibrium showed no deviation from expected frequencies (Table 1). All 4 MPO gene polymorphisms had minor allele frequencies of greater than 5%.
Table 1.
Observed and expected distribution of MPO gene polymorphisms in the primary and replication cohort
| Locus | RefSNP number (dbSNP) |
Single nucleotide polymorphism |
Domain | Number of samples successfully genotyped |
Allele frequencies |
Hardy Weinberg equilibrium |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||
| Primary cohort |
Secondary cohort |
Primary cohort |
Secondary cohort |
Primary cohort |
Secondary cohort |
||||||||
|
|
|||||||||||||
| Major | Minor | Major | Minor | χ 2 | P value |
χ 2 | P value |
||||||
| MPO | rs2243828 | −765 T to C | Promoter | 260 | 277 | T: 0.692 | C: 0.308 | T: 0.727 | C: 0.273 | 0.22 | 0.64 | 0.23 | 0.63 |
| MPO | rs7208693 | 157 G to T | Exon 2 (Val53Phe) |
262 | 277 | C: 0.874 | A: 0.126 | C: 0.884 | A: 0.116 | 3.14 | 0.08 | 2.51 | 0.11 |
| MPO | rs2071409 | 9890 A to C | Intron 11 | 260 | 275 | A: 0.771 | C: 0.229 | A: 0.798 | C: 0.202 | 1.41 | 0.23 | 2.02 | 0.16 |
| MPO | rs2759 | 2149 T to C | Exon 12 (Ile717Val) |
259 | 274 | T: 0.932 | C: 0.068 | T: 0.923 | C: 0.077 | 1.36 | 0.24 | 0.27 | 0.60 |
The characteristics of the primary cohort stratified by the 4 MPO gene polymorphisms are shown in Table 2. In brief, demographic characteristics, co-morbidities and disease severity measures did not significantly differ across genotypes, with a few notable exceptions. The MPO rs2243828 minor C-allele (TC/CC genotype) group had a higher mean APACHE II score (P=0.002) and lower mean urine output (P=0.05) compared with the TT genotype group; the MPO rs7208693 minor T-allele group (GT/TT genotype) had a higher prevalence of cirrhosis (P=0.02) and more advanced stages of AKI (P = 0.02), and a higher mean APACHE II score (P<0.001) compared with the GG genotype group; the MPO rs2071409 minor C-allele group (AC/CC genotype) had a higher prevalence of sepsis (P=0.01) and lower mean urine output (P=0.02) compared with the AA genotype group; the MPO rs2759 minor C-allele group (TC/CC genotype) had a lower prevalence of diabetes mellitus (P=0.02), higher prevalence of cirrhosis (P=0.02), sepsis (P=0.03), and shock (P=0.04), and a higher mean APACHE II score (P<0.001) and lower mean urine output (P<0.001) compared with the TT genotype group.
Table 2.
Characteristics of the primary cohort according to the 4 MPO gene polymorphisms
| Characteristic | rs2243828 | genotypes | P value |
rs7208693 | genotypes | P value |
rs2071409 | genotypes | P value |
rs2759 | genotypes | P value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| TT (n=123) |
TC/CC (n=137) |
GG (n=197) |
GT/TT (n=65) |
AA (n=158) |
AC/CC (n=102) |
TT (n=224) |
TC/CC (n=35) |
|||||
| Age, years | 67 (16) | 65 (15) | 0.13 | 66 (16) | 66 (14) | 0.97 | 66 (16) | 66 (15) | 0.96 | 66 (16) | 65 (15) | 0.62 |
| Men, % | 54 | 52 | 0.77 | 56 | 43 | 0.07 | 55 | 49 | 0.34 | 53 | 49 | 0.62 |
| Race, % | 0.48 | 0.86 | 0.89 | 0.12 | ||||||||
| White | 93 | 88 | 90 | 91 | 90 | 91 | 91 | 86 | ||||
| Black | 5 | 9 | 7 | 8 | 8 | 6 | 6 | 14 | ||||
| Other | 2 | 3 | 3 | 1 | 2 | 3 | 3 | 0 | ||||
| Contributing cause of AKI, % | 0.57 | 0.21 | 0.79 | 0.20 | ||||||||
| Ischemic | 32 | 26 | 27 | 32 | 29 | 27 | 26 | 43 | ||||
| Nephrotoxic | 15 | 17 | 16 | 14 | 17 | 14 | 17 | 9 | ||||
| Septic | 4 | 7 | 9 | 11 | 9 | 10 | 10 | 3 | ||||
| Multifactorial/other | 32 | 35 | 37 | 23 | 34 | 34 | 34 | 34 | ||||
| Comorbid conditions, % | ||||||||||||
| Diabetes mellitus | 44 | 45 | 0.92 | 46 | 39 | 0.28 | 48 | 39 | 0.19 | 47 | 26 | 0.02 |
| Heart failure | 16 | 16 | 0.96 | 17 | 12 | 0.35 | 17 | 16 | 0.87 | 17 | 14 | 0.74 |
| Cirrhosis | 5 | 10 | 0.16 | 5 | 14 | 0.02 | 6 | 10 | 0.22 | 6 | 17 | 0.02 |
| Chronic lung disease | 18 | 18 | 0.94 | 19 | 17 | 0.74 | 18 | 18 | 0.99 | 17 | 20 | 0.71 |
| Chronic kidney disease | 73 | 65 | 0.17 | 71 | 60 | 0.13 | 66 | 71 | 0.43 | 69 | 58 | 0.19 |
| APACHE II score | 18 (5) | 21 (7) | 0.002 | 19 (6) | 23 (7) | <0.001 | 19 (6) | 20 (6) | 0.16 | 19 (6) | 25 (7) | <0.001 |
| Sepsis, % | 38 | 47 | 0.17 | 40 | 51 | 0.13 | 37 | 53 | 0.01 | 40 | 60 | 0.03 |
| Shock, % | 25 | 31 | 0.33 | 26 | 32 | 0.36 | 27 | 30 | 0.50 | 25 | 43 | 0.03 |
| Serum creatinine, mg/dl | ||||||||||||
| Baseline value | 1.5 (0.6) | 1.5 (0.8) | 0.92 | 1.6 (0.7) | 1.4 (0.6) | 0.11 | 1.5 (0.6) | 1.6 (0.9) | 0.35 | 1.5 (0.7) | 1.5 (0.6) | 0.57 |
| Enrollment value | 3.5 (1.8) | 3.6 (1.8) | 0.89 | 3.6 (1.8) | 3.5 (1.7) | 0.88 | 3.5 (1.6) | 3.7 (1.9) | 0.40 | 3.5 (1.8) | 3.7 (1.5) | 0.64 |
| Peak | 4.3 (2.4) | 4.4 (2.8) | 0.63 | 4.2 (2.2) | 4.7 (3.4) | 0.24 | 4.2 (2.1) | 4.6 (3.2) | 0.22 | 4.4 (2.7) | 4.2 (1.5) | 0.77 |
| Discharge | 2.6 (1.9) | 2.4 (1.4) | 0.21 | 2.5 (1.8) | 2.4 (1.4) | 0.78 | 2.5 (1.8) | 2.6 (1.5) | 0.63 | 2.5 (1.8) | 2.5 (1.2) | 0.93 |
| Urine output, L/day | 1.4 (1.1) | 1.1 (1.0) | 0.05 | 1.3 (1.1) | 1.1 (1.1) | 0.10 | 1.4 (1.2) | 1.1 (0.8) | 0.02 | 1.4 (1.1) | 0.7(0.7) | <0.001 |
| AKI stage at enrollment, % | 0.23 | 0.02 | 0.68 | 0.06 | ||||||||
| Stage 1 | 49 | 38 | 48 | 28 | 45 | 41 | 46 | 26 | ||||
| Stage 2 | 4 | 5 | 4 | 6 | 5 | 4 | 4 | 8 | ||||
| Stage 3 | 47 | 57 | 48 | 66 | 50 | 55 | 50 | 66 | ||||
| Oxidative stress markers | ||||||||||||
| Plasma nitrotyrosine, nM | 12 (9, 22) | 12 (10, 22) | 0.31 | 12 (9, 23) | 12 (9, 23) | 0.79 | 11 (9, 23) | 12 (10, 23) | 0.17 | 12 (9, 23) | 12 (11, 32) | 0.18 |
| Myeloperoxidase (ng/ml) | 176 (103,431) |
248 (117,674) |
0.04 | 191 (105,448) |
421 (131,1003) |
0.001 | 188 (105,444) |
301 (115,892) |
0.02 | 207 (105,501) |
390 (170,1208) |
0.03 |
| Urinary 15-F2t-isoprostane, ng/mg | 0.9 (0.5,1.7) |
1.3 (0.6,2.7) |
0.03 | 1.0 (0.6,2.0) |
1.7 (0.7,2.8) |
0.09 | 1.0 (0.5,2.1) |
1.4 (0.7,2.3) |
0.13 | 1.0 (0.5,2.1) |
1.8 (1.0,2.8) |
0.02 |
Abbreviations: AKI, acute kidney injury; APACHE, Acute Physiology and Chronic Health Evaluation.
Continuous variables are presented as mean (standard deviation) or median (25th, 75th percentile), and categorical variables as percentages.
Missing genotyping data on rs2243828 (n = 2), rs2071409 (n = 2), and rs2759 (n = 3)
Similarly, the characteristics of the secondary cohort stratified by the 4 MPO gene polymorphisms are shown in Table 3. In brief, the pre-operative, operative and post-operative variables did not significantly differ across genotypes, with a few exceptions. The MPO rs7208693 minor T-allele group (GT/TT genotype) had a higher prevalence of planned elective surgery (P=0.03) and valve replacement (P=0.04), a prolonged mean aortic cross clamp time (P=0.01) and CPB perfusion time (P<0.001), and a higher post-operative day-1 mean APACHE II score (P<0.001) compared with the GG genotype group; similarly, the MPO rs2071409 minor C-allele group (AC/CC genotype) had a higher prevalence of planned elective surgery (P=0.002), valve replacement (P=0.02), and dialysis requirement (P=0.001), and a higher median mechanical ventilation duration (P=0.007) and higher post-operative day-1 mean APACHE II score (P=0.01) compared with the AA genotype group; the MPO rs2759 minor C-allele group (TC/CC genotype) had a prolonged mean CPB perfusion time (P=0.05) compared with the TT genotype group.
Table 3.
Characteristics of the secondary cohort according to the 4 MPO gene polymorphisms
| Characteristic | rs2243828 | genotypes | P value |
rs7208693 | genotypes | P value |
rs2071409 | genotypes | P value |
rs2759 | genotypes | P value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| TT (n=145) |
TC/CC (n=132) |
GG (n=214) |
GT/TT (n=63) |
AA (n=179) |
AC/CC (n=96) |
TT (n=233) |
TC/CC (n=41) |
|||||
| Preoperative variables | ||||||||||||
| Age, years | 67 (12) | 68 (11) | 0.55 | 67 (11) | 70 (12) | 0.04 | 68 (12) | 68 (11) | 0.59 | 67 (12) | 69 (11) | 0.29 |
| Men, % | 77 | 67 | 0.09 | 73 | 70 | 0.63 | 73 | 71 | 0.68 | 74 | 61 | 0.08 |
| White ethnicity, % | 96 | 94 | 0.69 | 95 | 95 | 0.91 | 95 | 96 | 0.76 | 95 | 93 | 0.45 |
| Hypertension, % | 84 | 85 | 0.89 | 85 | 83 | 0.64 | 87 | 81 | 0.25 | 85 | 83 | 0.75 |
| Diabetes mellitus, % | 34 | 32 | 0.76 | 32 | 37 | 0.50 | 30 | 39 | 0.17 | 34 | 27 | 0.36 |
| Heart failure, % | 33 | 41 | 0.20 | 35 | 43 | 0.26 | 33 | 44 | 0.08 | 37 | 39 | 0.76 |
| Chronic lung disease, % | 28 | 33 | 0.36 | 30 | 30 | 0.99 | 28 | 34 | 0.29 | 28 | 44 | 0.16 |
| Chronic kidney disease, % | 18 | 27 | 0.15 | 21 | 32 | 0.22 | 21 | 25 | 0.53 | 22 | 27 | 0.59 |
| Peripheral vascular disease, % | 17 | 18 | 0.70 | 16 | 22 | 0.25 | 13 | 26 | 0.01 | 17 | 17 | 0.98 |
| Cerebrovascular disease, % | 11 | 11 | 0.93 | 11 | 11 | 0.98 | 11 | 12 | 0.94 | 12 | 7 | 0.38 |
| 3-vessel coronary artery disease, % | 49 | 44 | 0.44 | 48 | 44 | 0.57 | 48 | 44 | 0.46 | 45 | 54 | 0.31 |
| Left main coronary artery disease, % | 21 | 29 | 0.11 | 26 | 21 | 0.43 | 24 | 25 | 0.86 | 23 | 30 | 0.37 |
| Serum creatinine, mg/dL | 1.1 (1.0) | 1.1 (0.4) | 0.45 | 1.1 (0.9) | 1.1 (0.3) | 0.50 | 1.1 (0.9) | 1.1 (0.3) | 0.95 | 1.1 (0.8) | 1.1 (0.3) | 0.62 |
| Plasma myeloperoxidase, ng/mL | 180 (87, 924) |
180 (75, 949) |
0.71 | 121 (74, 381) |
1379 (802, 1721) |
<0.001 | 115 (76, 527) |
664 (124, 1513) |
<0.001 | 179 (82, 834) |
170 (67, 1213) |
0.91 |
| Operative variables | ||||||||||||
| Elective surgery, % | 26 | 37 | 0.06 | 28 | 43 | 0.03 | 25 | 43 | 0.002 | 29 | 43 | 0.09 |
| Valve replacement, % | 45 | 57 | 0.05 | 47 | 62 | 0.04 | 46 | 60 | 0.02 | 50 | 55 | 0.53 |
| Aortic cross clamp time, min | 87 (40) | 95 (43) | 0.16 | 87 (37) | 104 (52) | 0.01 | 88 (39) | 96 (45) | 0.13 | 89 (41) | 96 (45) | 0.38 |
| CPB perfusion time, min | 113 (48) | 124 (54) | 0.07 | 111 (45) | 142 (62) | <0.001 | 111 (49) | 131 (54) | 0.002 | 115 (51) | 132 (53) | 0.05 |
| Postoperative variables | ||||||||||||
| Initial mechanical ventilation duration, hours | 13 (23) | 25 (98) | 0.14 | 16 (47) | 30 (117) | 0.16 | 11 (12) | 34 (116) | 0.007 | 15 (45) | 43 (146) | 0.02 |
| Day-1 APACHE II score | 13 (7) | 14 (7) | 0.22 | 12 (7) | 18 (6) | <0.001 | 13 (7) | 15 (7) | 0.01 | 13 (7) | 14 (6) | 0.54 |
| Day-1 urine output, L/day | 2.6 (1.0) | 2.7 (1.0) | 0.72 | 2.5 (1.0) | 2.9 (1.0) | 0.01 | 2.5 (1.0) | 2.8 (1.1) | 0.06 | 2.6 (1.0) | 2.7 (1.3) | 0.62 |
| Stage-2 or 3 AKI*, % | 2 | 3 | 0.58 | 3 | 0 | 0.51 | 1 | 5 | 0.07 | 2 | 7 | 0.05 |
| Dialysis requirement, % | 2 | 2 | 0.99 | 2 | 3 | 0.53 | 0 | 6 | 0.001 | 2 | 5 | 0.20 |
CPB denotes cardiopulmonary bypass; APACHE, Acute Physiology and Chronic Health Evaluation; and AKI, acute kidney injury.
Continuous variables are presented as mean (standard deviation) or median (25th, 75th percentile), and categorical variables as percentages.
> 2-fold increase in serum creatinine over the first 3 postoperative days. Missing genotyping data on rs2071409 (n = 2), and rs2759 (n = 3)
Association of MPO Genotypes with Plasma MPO and Urinary 15-F2t-Isoprostane Levels
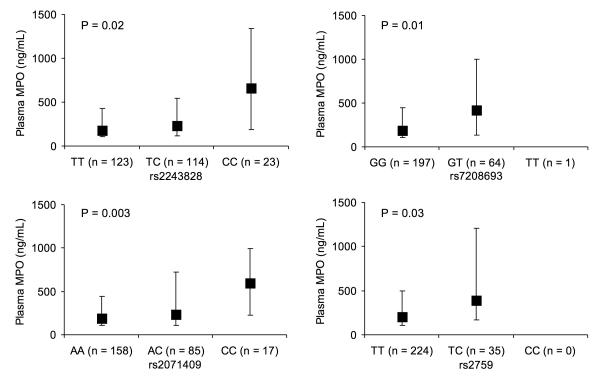
In the primary cohort, there was a strong genotype-phenotype association (Figure 1), as evidenced by the presence of higher plasma MPO levels among the MPO rs2243828 (P=0.02), rs7208693 (P=0.01), rs2071409 (P=0.003), and rs2759 (P=0.03) minor-allele carriers. When the heterozygous and homozygous minor-allele genotypes were combined, the MPO rs2243825 (P=0.04), rs7208693 (P=0.001), rs2071409 (P=0.02), and rs2759 (P=0.03), minor-allele carriers remained significantly associated with higher plasma MPO levels (Table 2). To a lesser degree, there was a similar but less robust association between higher urinary 15-F2t-isoprostane levels and the MPO rs2243828 (P=0.03), rs7208693 (P=0.09), and rs2759 (P=0.02) minor-allele genotypes (Table 2).
Figure 1. Plasma levels of myeloperoxidase (MPO) in the primary cohort stratified by the MPO genotypes.
The data are represented as median (25th and 75th percentile) values. The P values are derived from by Wilcoxon signed-rank test.
In the secondary cohort, there was a similar but less global genotype-phenotype association (Table 3), as evidenced by higher pre-operative plasma MPO levels among minor allele genotype carriers for 2 of the 4 the MPO polymorphisms, rs7208693 (P<0.001) and rs2071409 (P<0.001).
Association of MPO Gene Polymorphisms and Adverse Outcomes
For the primary cohort, the results of the multivariable genetic dominant models are displayed in Table 4, showing a robust association between the individual MPO polymorphic minor alleles and the outcome of dialysis requirement or in-hospital death, after adjustment for sex, race, and APACHE II score. Compared with the TT genotype group, the MPO rs2243828 C-allele group had an adjusted OR of 2.19 (95% CI 1.25, 3.82) for this composite outcome. A similar association was observed for the MPO rs7208693 T-allele group compared with the GG genotype group (adjusted OR 2.73; 95% CI 1.38, 5.38); the MPO rs2071409 C-allele group compared with the AA genotype (adjusted OR 2.09; 95% CI 1.18, 3.69); and the MPO rs2759 C-allele group compared with the TT genotype group (adjusted OR 3.15; 95% CI 1.22, 8.12). In all the models, plasma MPO level was independently associated with the 2 composite outcomes (Table 5), and after adding this covariate to the models (model 3), the associations of the MPO gene polymorphisms with the composite outcome persisted but were attenuated (Table 4). Of note, in the primary cohort, there was no association between urinary 15-F2t-isoprostane level and adverse outcomes (data not shown). The associations between the individual MPO polymorphic minor alleles and the composite of dialysis requirement, assisted mechanical ventilation or in-hospital death were less global, displaying significance on adjusted analyses for 2 of the 4 (rs2243828 and rs7208693) polymorphisms (Table 4).
Table 4.
Association of the 4 MPO gene polymorphisms with adverse outcomes in the primary cohort
|
MPO gene polymorphism |
Dialysis requirement or in-hospital death |
Dialysis requirement, mechanical ventilation, or in-hospital death |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Dominant model | ||||||
| rs2243828 (C-allele vs. T/T) | ||||||
| - Unadjusted | 2.53 | 1.53, 4.19 | <0.001 | 2.31 | 1.40, 3.80 | 0.001 |
| - Model 1 | 2.65 | 1.59, 4.41 | <0.001 | 2.37 | 1.43, 3.92 | 0.001 |
| - Model 2 | 2.19 | 1.25, 3.82 | 0.006 | 2.01 | 1.13, 3.57 | 0.017 |
| - Model 3 | 2.12 | 1.20, 3.75 | 0.010 | 1.98 | 1.10, 3.57 | 0.023 |
| rs7208693 (T-allele vs. G/G) | ||||||
| - Unadjusted | 3.77 | 2.06, 6.92 | <0.001 | 3.79 | 2.02, 7.13 | <0.001 |
| - Model 1 | 4.04 | 2.18, 7.50 | <0.001 | 3.91 | 2.07, 7.38 | <0.001 |
| - Model 2 | 2.73 | 1.38, 5.38 | 0.004 | 2.52 | 1.22, 5.18 | 0.012 |
| - Model 3 | 2.66 | 1.31, 5.41 | 0.007 | 2.54 | 1.19, 5.41 | 0.016 |
| rs2071409 (C-allele vs. A/A) | ||||||
| - Unadjusted | 2.07 | 1.25, 3.43 | 0.005 | 1.65 | 0.99, 2.73 | 0.053 |
| - Model 1 | 2.11 | 1.27, 3.51 | 0.004 | 1.65 | 0.99, 2.73 | 0.054 |
| - Model 2 | 2.09 | 1.18, 3.69 | 0.012 | 1.52 | 0.84, 2.73 | 0.164 |
| - Model 3 | 1.88 | 1.05, 3.38 | 0.035 | 1.34 | 0.73, 2.46 | 0.342 |
| rs2759 (C-allele vs. T/T) | ||||||
| - Unadjusted | 5.63 | 2.36, 13.45 | <0.001 | 5.48 | 2.19, 13.71 | <0.001 |
| - Model 1 | 6.02 | 2.49, 14.55 | <0.001 | 5.71 | 2.26, 14.40 | <0.001 |
| - Model 2 | 3.15 | 1.22, 8.12 | 0.018 | 2.66 | 0.96, 7.37 | 0.059 |
| - Model 3 | 3.13 | 1.17, 8.40 | 0.024 | 2.68 | 0.93, 7.76 | 0.069 |
| Additive model | ||||||
| rs2243828 (per C-allele copy increase) | ||||||
| - Unadjusted | 2.46 | 1.63, 3.72 | <0.001 | 2.22 | 1.48, 3.35 | <0.001 |
| - Model 1 | 2.54 | 1.67, 3.86 | <0.001 | 2.25 | 1.49, 3.41 | <0.001 |
| - Model 2 | 2.13 | 1.35, 3.37 | 0.001 | 1.92 | 1.20, 3.09 | 0.007 |
| - Model 3 | 2.07 | 1.29, 3.33 | 0.003 | 1.89 | 1.15, 3.09 | 0.012 |
| rs7208693(per T-allele copy increase) | ||||||
| - Unadjusted | 3.75 | 2.06, 6.85 | <0.001 | 3.77 | 2.02, 7.05 | <0.001 |
| - Model 1 | 4.02 | 2.17, 7.42 | <0.001 | 3.88 | 2.06, 7.31 | <0.001 |
| - Model 2 | 2.72 | 1.39, 5.35 | 0.004 | 2.51 | 1.22, 5.16 | 0.012 |
| - Model 3 | 2.66 | 1.31, 5.39 | 0.007 | 2.53 | 1.19, 5.39 | 0.016 |
| rs2071409 (per C-allele copy increase) | ||||||
| - Unadjusted | 2.26 | 1.48, 3.45 | <0.001 | 1.87 | 1.23, 2.85 | 0.003 |
| - Model 1 | 2.28 | 1.49, 3.50 | <0.001 | 1.87 | 1.23, 2.85 | 0.004 |
| - Model 2 | 2.26 | 1.40, 3.64 | 0.001 | 1.77 | 1.09, 2.86 | 0.021 |
| - Model 3 | 2.07 | 1.27, 3.37 | 0.004 | 1.59 | 0.97, 2.62 | 0.067 |
| T-G-A-T haplotype * | ||||||
| - Unadjusted | ||||||
| 1 copy (vs. none) | 0.15 | 0.05, 0.40 | <0.001 | 0.18 | 0.07, 0.50 | 0.001 |
| 2 copies (vs. none) | 0.10 | 0.04, 0.30 | <0.001 | 0.14 | 0.05, 0.41 | <0.001 |
| - Model 1 | ||||||
| 1 copy (vs. none) | 0.15 | 0.05, 0.41 | <0.001 | 0.18 | 0.07, 0.51 | 0.001 |
| 2 copies (vs. none) | 0.10 | 0.03, 0.29 | <0.001 | 0.14 | 0.05, 0.40 | <0.001 |
| - Model 2 | ||||||
| 1 copy (vs. none) | 0.16 | 0.06, 0.49 | 0.001 | 0.20 | 0.07, 0.62 | 0.005 |
| 2 copies (vs. none) | 0.13 | 0.04, 0.42 | 0.001 | 0.20 | 0.06, 0.63 | 0.006 |
| - Model 3 | ||||||
| 1 copy (vs. none) | 0.18 | 0.06, 0.55 | 0.003 | 0.22 | 0.07, 0.71 | 0.011 |
| 2 copies (vs. none) | 0.15 | 0.05, 0.50 | 0.002 | 0.23 | 0.07, 0.77 | 0.017 |
95% CI denotes 95% confidence interval for the odds ratio; model 1 is adjusted for sex, race, and age; model 2 is adjusted for sex, race, and APACHE II score; model 3 is adjusted for sex, race, APACHE II score, and plasma MPO level.
The haplotype was generated from the MPO rs2243828, rs7208693, rs2071409 and rs2759 polymorphisms.
Table 5.
Association of plasma MPO levels with adverse clinical outcomes in the primary cohort
| Outcome variable | Plasma log-MPO level (per 1-SD increase) |
P value | C statistic |
|---|---|---|---|
| Dialysis requirement or in-hospital death | |||
| Unadjusted | 1.96 (1.48, 2.58) | < 0.001 | 0.68 |
| Adjusted for sex, race, APACHE II score, and rs2243828* | 1.61 (1.18, 2.19) | 0.002 | 0.79 |
| Adjusted for sex, race, APACHE II score, and rs7208693* | 1.61 (1.18, 2.20) | 0.003 | 0.79 |
| Adjusted for sex, race, APACHE II score, and rs2071409* | 1.58 (1.16, 2.16) | 0.004 | 0.79 |
| Adjusted for sex, race, APACHE II score, and rs2759* | 1.61 (1.18, 2.21) | 0.003 | 0.79 |
| Adjusted for sex, race, APACHE II score, and T-G-A-T haplotype | 1.55 (1.13, 2.14) | 0.007 | 0.80 |
| Mechanical ventilation, dialysis requirement or in-hospital death | |||
| Unadjusted | 2.00 (1.51, 2.65) | < 0.001 | 0.68 |
| Adjusted for sex, race, APACHE II score, and rs2243828* | 1.62 (1.18, 2.23) | 0.003 | 0.81 |
| Adjusted for sex, race, APACHE II score, and rs7208693* | 1.63 (1.17, 2.26) | 0.004 | 0.82 |
| Adjusted for sex, race, APACHE II score, and rs2071409* | 1.60 (1.16, 2.22) | 0.004 | 0.81 |
| Adjusted for sex, race, APACHE II score, and rs2759* | 1.67 (1.20, 2.31) | 0.002 | 0.82 |
| Adjusted for sex, race, APACHE II score, and T-G-A-T haplotype | 1.57 (1.13, 2.19) | 0.007 | 0.82 |
95% CI denotes the 95% confidence interval for the odds ratio (OR); MPO, myeloperoxidase; and SD, standard deviation
the analysis refers to the dominant models.
For the secondary cohort, the results of the multivariable genetic dominant models are displayed in Table 6, showing a strong association between 2 of the 4 MPO polymorphic minor alleles and the composite outcome of stage-2 AKI, dialysis requirement, prolonged mechanical ventilation or in-hospital death, after adjustment for sex, race, age, heart failure, surgery status, and CPB time. Indeed, compared with the AA genotype group, the MPO rs2071409 C-allele group had an adjusted OR of 2.82 (95% CI 1.22, 6.50) for this composite outcome. A similar association was observed for the MPO rs2759 C-allele group compared with the TT genotype group (adjusted OR 3.56; 95% CI 1.44, 8.82). Of note, in the secondary cohort, there was no association between plasma MPO level and the composite outcome (OR 0.89; 95% CI 0.62, 1.27; P = 0.514).
Table 6.
Association of the 4 MPO gene polymorphisms with > 2-fold increase in serum creatinine, dialysis requirement, prolonged initial mechanical ventilation (>24 hours) or in-hospital death in the secondary cohort
| MPO gene polymorphism | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Dominant model | |||
| rs2243828 (C-allele vs. T/T) | |||
| - Unadjusted | 1.38 | 0.68, 2.81 | 0.375 |
| - Model 1 | 1.27 | 0.55, 2.91 | 0.571 |
| - Model 2 | 1.20 | 0.52, 2.78 | 0.666 |
| rs7208693 (T-allele vs. G/G) | |||
| - Unadjusted | 1.41 | 0.64, 3.12 | 0.395 |
| - Model 1 | 1.05 | 0.42, 2.62 | 0.911 |
| - Model 2 | 1.14 | 0.45, 2.87 | 0.780 |
| rs2071409 (C-allele vs. A/A) | |||
| - Unadjusted | 2.90 | 1.41, 5.97 | 0.004 |
| - Model 1 | 2.86 | 1.24, 6.57 | 0.013 |
| - Model 2 | 2.82 | 1.22, 6.50 | 0.015 |
| rs2759 (C-allele vs. T/T) | |||
| - Unadjusted | 3.29 | 1.46, 7.41 | 0.004 |
| - Model 1 | 3.50 | 1.42, 8.58 | 0.006 |
| - Model 2 | 3.56 | 1.44, 8.82 | 0.006 |
| Additive model | |||
| rs2243828 (per C-allele copy increase) | |||
| - Unadjusted | 1.62 | 0.94, 2.78 | 0.082 |
| - Model 1 | 1.52 | 0.82, 2.81 | 0.179 |
| - Model 2 | 1.47 | 0.79, 2.74 | 0.227 |
| rs7208693(per T-allele copy increase) | |||
| - Unadjusted | 1.36 | 0.63, 2.94 | 0.438 |
| - Model 1 | 1.04 | 0.42. 2.54 | 0.939 |
| - Model 2 | 1.12 | 0.45, 2.80 | 0.801 |
| rs2071409 (per C-allele copy increase) | |||
| - Unadjusted | 2.22 | 1.30, 3.78 | 0.003 |
| - Model 1 | 1.94 | 1.06, 3.57 | 0.033 |
| - Model 2 | 1.91 | 1.04, 3.51 | 0.037 |
| T-G-A-T haplotype * | |||
| - Unadjusted | |||
| 1 copy (vs. none) | 0.37 | 0.15, 0.92 | 0.032 |
| 2 copies (vs. none) | 0.24 | 0.08, 0.71 | 0.010 |
| - Model 1 | |||
| 1 copy (vs. none) | 0.46 | 0.17, 1.27 | 0.134 |
| 2 copies (vs. none) | 0.30 | 0.08, 1.16 | 0.080 |
| - Model 2 | |||
| 1 copy (vs. none) | 0.49 | 0.18, 1.36 | 0.173 |
| 2 copies (vs. none) | 0.32 | 0.08, 1.28 | 0.108 |
95% CI denotes 95% confidence interval for the odds ratio; model 1 is adjusted for age, sex, race and cardiopulmonary bypass time; model 2 is adjusted for age, sex, race, heart failure, surgery status, and cardiopulmonary bypass time.
The haplotype was generated from the MPO rs2243828, rs7208693, rs2071409 and rs2759 polymorphisms.
Sensitivity Analyses
In sensitivity analyses performed in the primary cohort using additive genetic models (Table 4), each copy of the MPO rs2243828 C-allele, rs7208693 T-allele, and rs2071409 C-allele was associated with 2.13- (95% CI 1.35, 3.37), 2.72- (95% CI 1.39, 5.35) and 2.26- (95% CI 1.40, 3.64) fold higher adjusted odds for dialysis requirement or in-hospital death, respectively. Using additive genetic models in the secondary cohort, each copy of the MPO rs2071409 C-allele was associated with 1.91- (95% CI 1.04, 3.51) fold higher adjusted odds for the composite of stage-2 AKI, dialysis requirement, prolonged mechanical ventilation or in-hospital death (Table 6).
Sensitivity analyses restricted to white subjects did not affect any of the adjusted analyses in both the primary and secondary cohort (Supplemental Table 1).
Association of MPO Haplotypes and Adverse Outcomes
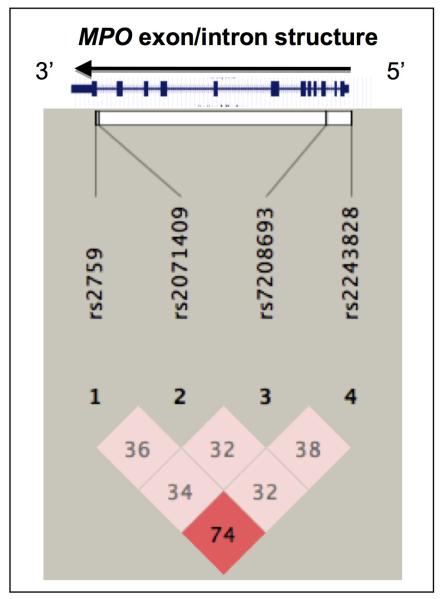
We next generated a Haploview plot on the 4 MPO polymorphisms. Figure 2 displays the linkage disequilibrium (LD) plot at the MPO gene locus with representation of the 4 variants analyzed in the primary cohort. The LD plot revealed substantial marker-on-marker correlation (as defined by the D’ parameter) across the ~11 kbp human MPO locus, with the rs2243828 and rs2759 polymorphisms displaying the highest correlation, as evidenced by a D’ parameter of 74, on a scale of 0 to 100.
Figure 2. Haploview plot of the MPO gene locus in the primary cohort.
The plot illustrates pair-wise linkage disequilibrium (LD) between the 4 MPO polymorphisms, based on the D’ parameter value (scaled from 0-100). The darker a rhombus appears, the higher the LD is between the respective polymorphisms.
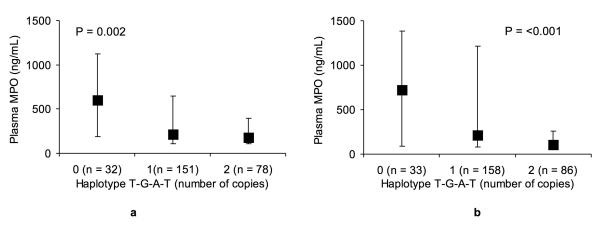
We then conducted haplotype analyses. The most frequent haplotype identified in the primary cohort across the 4 MPO polymorphisms (rs2243828, rs7208693, rs2071409 and rs2759) was T-G-A-T, which was estimated on 308 of the 524 chromosomes with a frequency of 59%, followed by C-G-A-T (10%) and C-G-C-T (9%). As shown in Figure 3A, patients with a higher copy-number of the T-G-A-T haplotype had significantly lower plasma MPO levels (P=0.002). This association translated into a protective effect. Indeed, as shown in Table 4, the presence of one copy or 2 copies of the T-G-A-T haplotype was associated with a respective OR of 0.16 (95% CI 0.06, 0.49) and 0.13 (95% CI 0.04, 0.42) for the outcome of dialysis requirement on in-hospital, after adjustment for sex, race, and APACHE II score. A similar independent protective effect was observed for the composite of dialysis requirement, assisted mechanical ventilation, or in-hospital death (Table 4). In the secondary cohort, the most frequent haplotype identified across the 4 MPO polymorphisms was also T-G-A-T, which was estimated on 330 of the 554 chromosomes with a similar frequency of 60%, followed by C-G-A-T (13%) and C-G-C-T (5%). A higher copy-number of the T-G-A-T haplotype was also associated with lower plasma MPO levels (P<0.001; Figure 3B), and a similar but less global protective effect of the T-G-A-T haplotype was observed (Table 6).
Figure 3. Plasma levels of myeloperoxidase (MPO) in the (3A) primary and (3B) secondary cohort stratified by the MPO haplotype T-G-A-T copy number (0, 1, 2 copies per diploid individual).
The data are represented as median (25th and 75th percentile) values. The P values are derived from by Wilcoxon signed-rank test.
Using the SNP Spectral Decomposition (SNPSpD) method to account for multiple testing, the experiment-wide significance threshold required to maintain a type-I error rate at less than 5% was 0.013, reflecting linkage disequilibrium within this block. In the primary cohort, this threshold (P < 0.013) was exceeded by markers rs2243828 and rs7208693 for the outcome of dialysis requirement or in-hospital death in the adjusted dominant-model analyses (model 3). The threshold was also exceeded by markers rs2243828, rs7208693, and rs2071409 in the adjusted additive-model analyses. Using this method, the T-G-A-T haplotype also remained associated with dialysis requirement or in-hospital death. The association between the 4 MPO SNPs and the composite outcome of dialysis requirement, assisted mechanical ventilation or in-hospital death became less robust after applying this method. Similarly, in the secondary cohort, the only marker that remained associated with the composite outcome was rs2759 in the adjusted dominant-model analyses.
DISCUSSION
In the present 2-step genetic study, we first examined the relationship of 4 polymorphisms in the MPO gene locus located in the promoter (rs2243828), coding (rs7208693 and rs2759), and non-coding (rs2071409) regions, with plasma MPO and urinary 15-F2t-isoprostane levels, disease severity measures, and adverse clinical outcomes in a large cohort of hospitalized adults with a diagnosis of AKI, which constituted our primary cohort. The observed and expected genotype frequencies were not significantly different, and fulfilled Hardy-Weinberg equilibrium. Minor allele frequencies ranged from 7 to 31% for all 4 polymorphisms. The overall population was 91% white, and there were no race, sex, or age differences within genotype groups. Strong genotype-phenotype associations were observed between the 4 MPO gene polymorphisms and the two selected markers of oxidative stress, plasma MPO (all 4 SNPs) and urinary 15-F2t-isoprostane (SNPs rs2243828 and rs2759) levels. All 4 polymorphisms were independently associated with 2- to 3-fold higher odds for the composite outcome of dialysis requirement or in-hospital death. A similar but less robust association was observed for the composite outcome of dialysis requirement, assisted mechanical ventilation or in-hospital death. Finally, we identified a common haplotype (T-G-A-T) whereby the number of copies was associated with lower plasma MPO level and lower odds for adverse outcomes. The results remained robust across several sensitivity analyses including the use of additive models, restriction to white subjects only, and correction for multiple testing using the SNPSpD method. We next examined similar associations in a secondary cohort consisting of adults at-risk for AKI undergoing cardiac surgery with cardiopulmonary bypass, and observed a significant though less global genotype-phenotype association between 2 of the 4 MPO gene polymorphisms and the outcome of stage-2 AKI, dialysis requirement, prolonged mechanical ventilation or in-hospital death.
Myeloperoxidase is an essential enzyme involved in oxidative responses to a variety of stimuli. Following release from neutrophils and monocytes through degranulation, MPO reacts with hydrogen peroxide formed by the respiratory burst to form a complex that can oxidize various compounds including chloride, which is oxidized to hypochlorous acid leading to the formation of chlorine and chloramine. These end-products are powerful oxidants that can exert biological effects in the kidney30. Indeed, in situ MPO activity has been used as an index of neutrophil kidney parenchymal infiltration in experimental models of ischemia-reperfusion injury31-35 and nephrotoxicity36,37.
Given the importance of MPO in mediating oxidative cellular injury in response to ischemic and toxic kidney injury, one might anticipate that polymorphisms disrupting or enhancing the function or expression of MPO would alter oxidative stress–mediated cellular responses in AKI. Several polymorphisms in the MPO gene locus have been described and variably linked to measures of oxidative stress, including rs7208693 (Val53Phe in exon-2 and examined in our study) associated with higher MPO activity38, and rs2333227 (located in the promoter region), associated with lower MPO transcriptional activation23.
In the primary cohort, the potential mechanisms underlying the protective association of the common MPO T-G-A-T haplotype with the composite endpoint of dialysis requirement or in-hospital death are not completely understood, but we propose that the improved outcomes observed among carriers of this haplotype might in part be due to lower generation or release of MPO from neutrophils, resulting in less extensive organ injury. This hypothesis is supported by the observed lower plasma MPO levels with a higher copy-number of the T-G-A-T haplotype, which is driven by the major rather than minor alleles of the individual MPO SNPs.
To our knowledge, this is the first study to test the hypothesis of whether representative polymorphisms across the MPO gene locus associate with not only intermediate phenotypes, i.e., plasma and urinary markers of oxidative stress, but also adverse clinical outcomes in 2 separate cohorts of adults with a diagnosis of AKI (primary cohort) as well as adults at-risk for AKI (secondary cohort). We used overlapping composite outcome measures to allow meaningful comparisons between the 2 cohorts. The demonstrable MPO genotype-phenotype associations, whereby all 4 polymorphic alleles were associated with higher plasma MPO levels, strengthen the credibility of the observed association between the various polymorphic alleles and adverse outcomes. We also used clinically relevant covariates in the multivariable models. These include the APACHE II score in the primary cohort, which captures severity of the acute illness, and heart failure, surgery status, and CPB time in the secondary cohort, which capture known risk factors for post-cardiac surgery AKI. Our composite outcomes included in-hospital death, which accounted for survival bias when assessing endpoints of artificial organ support (i.e., dialysis requirement or assisted/prolonged mechanical ventilation). Finally, the results observed on the secondary cohort support and validate our primary findings.
There are several study limitations that should be noted. The heterogeneity of the primary cohort was offset by the selective inclusion of subjects with more advanced stages of AKI requiring nephrology consultation. Although the 2 cohorts were relatively small for genetic epidemiological analyses, there was no deviation from expected genotype frequencies, and the ~91-95% majority of white subjects likely minimized the potential influence of race on genotype frequencies. Although the observed genetic associations might reflect some degree of genetic admixture, the association of higher plasma MPO level with increased odds for adverse outcomes in the primary cohort argues for the suitability of this oxidative stress marker as a downstream effector of polymorphisms in the MPO gene locus. The attenuation of the point estimates in the genetic markers after adding plasma MPO levels to the multivariable models supports this hypothesis. Multiple testing, which is an important limitation of the analysis, was addressed by applying the SNPSpD method to derive stricter P values to define significance. Finally, within the associated MPO haplotype block, several potentially functional genetic variants lie, but the biological mechanism by which MPO haplotypes might influence renal traits remains uncertain.
In conclusion, in the present 2-step genetic study, we provide evidence that 4 polymorphisms in the MPO gene locus and a common corresponding haplotype influence the risk of adverse outcomes in hospitalized adults with or at-risk for AKI. Although this effect seems to be mediated by modulation of the extracellular release of MPO, additional studies are needed to establish the mechanisms underlying the influence of the identified genotypes on disease traits.
METHODS
Study Design and Participants
The primary cohort was derived from a prospective cohort study conducted at two acute care hospitals where adults with AKI, in whom nephrology consultation was requested, were eligible for enrollment. Acute kidney injury was defined as a rise in serum creatinine by 0.5, 1.0, or 1.5 mg/dl from a baseline level of ≤ 1.9, 2.0-4.9, or ≥ 5.0 mg/dL, respectively39. Although this definition was adopted prior to the development of the AKI network consensus definition40, we used this classification to categorize stages of AKI. Exclusion criteria were age < 18 years, pregnancy, chronic dialysis, organ transplantation within the prior year, and urinary obstruction.
The secondary cohort was derived from a prospective cohort study of patients at-risk for AKI following cardiac surgery, conducted at four acute care hospitals, where adults scheduled to undergo on-pump cardiac surgery were eligible for enrollment. Exclusion criteria were age < 18 years, off-pump surgery, pregnancy, acute or chronic dialysis dependence, and organ transplantation within the prior year.
Institutional review board approval was granted, and written informed consent was obtained from all participants or next of kin.
Data Collection
Data collection for the primary cohort included baseline demographic characteristics, comorbid conditions, 24-hour urine output at enrollment, and serial serum creatinine values. Sepsis was ascertained using the systemic inflammatory response syndrome criteria41, and two severity-of-illness scores were calculated, the Acute Physiology and Chronic Health Evaluation (APACHE) II score42, and the Multiple Organ Failure (MOF) score43. Pre-existing chronic kidney disease was defined on the basis of a baseline estimated glomerular filtration rate of less than 60 ml/min/1.73 m2, which was calculated using the Modification of Diet in Renal Disease (MDRD) study equation44.
Data collection for the secondary cohort, included baseline demographic characteristics, pre-operative comorbid conditions and laboratory variables, intra-operative variables (surgery electivity and type, CPB perfusion time and aortic cross clamp time), and several post-operative variables including, day-1 APACHE II score and urine output, initial hours of assisted mechanical ventilation, and serial serum creatinine values over the first 3 days.
Blood and Urine Sampling
At enrollment, EDTA-anticoagulated whole blood and urine were collected. Plasma was separated, and the remaining blood was aliquoted for subsequent DNA extraction. Urine samples were obtained from a fresh void or the access port of a Foley catheter, kept on ice, centrifuged (within 30 minutes) for 10 min at 3,000 rpm, treated with a protease inhibitor cocktail (Complete Mini, Roche Diagnostics, Mannheim, Germany), aliquoted in cryotubes and stored at −80°C.
Selection Criteria for Genetic Variants
To obtain comprehensive representation of the genetic variability in the MPO gene locus (reference sequence GeneBank accession number, NM 000250.1), we selected tag-SNPs using the HapMap genome browser for European populations (http://hapmap.ncbi.nlm.nih.gov/) with the tagger pair-wise method, a r2 cutoff of 0.8, and a minor allele frequency of at least 10%. These genetic variants were also selected on the basis of published reports on investigated clinical and functional associations. Through this selection process, we identified 4 MPO polymorphisms of interest spanning the gene (5′ to 3′ direction) (Table 1), located in the promoter (−765 T to C, RefSNP rs2243828), exon 2 (+157 G to T, RefSNP rs7208693), intron 11 (+9890 A to C, RefSNP rs2071409), and exon 12 (+2149 T to C, Ile717Val, RefSNP rs2759).
TaqMan Genotyping and Haplotype Analyses
Genomic DNA was prepared from whole blood leukocytes using the QiAmp DNA blood kit (Qiagen, Valencia, CA). Using the Applied Biosystems 7900HT fast real-time PCR system (Applied Biosystems, Foster City, CA), DNA samples were genotyped by combining the TaqMan universal PCR master mix with TaqMan SNP genotyping assay into a 384-well plate according to a standardized protocol. Resulting sequences were analyzed by the SNP software (SDS version 2.2), which utilizes an advanced multi-component algorithm to calculate distinct allele/marker signal contributions from fluorescence measurements for each sample-well during the assay plate read with the SNP auto-caller. The SNP auto-caller automatically determines sample genotypes and generates a cluster plot to better visualize data across samples.
Pair-wise patterns of LD across the MPO gene locus were visualized by Haploview, plotting LD as the D’ parameter (scaled from 0-100). Haplotypes were imputed from the MPO polymorphism diploid genotypes using PLINK version 1.07, a toolset for whole-genome association and population-based linkage analysis45 (http://pngu.mgh.harvard.edu/~purcell/plink/). The most frequent haplotype (T-G-A-T) identified across the 4 (5′ to 3′ direction) MPO polymorphisms (rs2243828, rs7208693, rs2071409 and rs2759) was expressed as number of chromosomal copies (0, 1, or 2 haplotypes) per diploid individual.
Measurement of Markers of Oxidative Stress
To evaluate whether the MPO gene polymorphisms were associated with markers of oxidative stress, we measured plasma levels of MPO, the MPO gene product, and urinary levels of 15-F2t-isoprostane, a marker of lipid peroxidation. Plasma MPO was measured by solid-phase ELISA (R&D Systems, Minneapolis, MN), results reported as ng/ml. The intra- and inter-assay coefficient of variation is 7.3% and 8.1%, respectively. Urinary 15-F2t-isoprostane was measured by competitive ELISA (Northwest Life Science Specialties, LLC, Vancouver, WA), results were normalized to urinary creatinine and expressed as ng/mg of creatinine. The intra- and inter-assay coefficient of variation is 5% and 10%, respectively. All measurements were performed in duplicate.
Outcome Measures
In the primary cohort, the main outcomes of interest were the composite of dialysis requirement or in-hospital death as well as dialysis requirement, assisted mechanical ventilation, or in-hospital death. In the secondary cohort, the main outcome measure was the composite of stage-2 or 3 AKI according to the AKI network consensus definition (>2-fold increase in serum creatinine over the first 3 post-operative days)40, dialysis requirement, prolonged (>24 hours) mechanical ventilation according to definition by the Society of Thoracic Surgeons46, or inhospital death.
Statistical Analyses
The genotype frequencies were tested for Hardy-Weinberg equilibrium using a standard Chi-square test for any deviation of the observed frequencies. Comparisons between genotype groups were made by the t-test and the two-tailed Mann Whitney test for continuous variables, and by Chi square or Fisher exact test for categorical variables. Continuous variables are reported as mean (with standard deviation) or median (with 25th and 75th percentile) according to their distribution. Binary variables are reported as count (with percentage).
Multivariable logistic regression analyses were performed to examine the association of each MPO gene polymorphism with the afore-mentioned outcomes, using dominant genetic models (i.e., one or two copies of the minor allele). In the primary cohort, the models were adjusted for sex, race, and age (model 1); sex, race, and APACHE II score (model 2); or sex, race, APACHE II score, and log-transformed plasma MPO level (model 3). In the secondary cohort, the models were adjusted for sex, race, and age (model 1); or sex, race, age, heart failure, surgery status, and CPB time (model 2).
Similar multivariable logistic regression analyses were performed for the association of the MPO T-G-A-T, haplotype and the outcomes of interest. Several sensitivity analyses were performed, including the use of additive genetic models and restriction of the cohort to white subjects only. The SNPSpD method47 was used to correct for multiple testing for SNPs that were in LD with each other, on the basis of the spectral decomposition of matrices of pairwise LD between SNPs displayed in the Haploview plot of the MPO gene locus (Figure 2). In brief, this method estimates within a haplotype block, the experiment-wide significance threshold required to maintain the type-1 error rate at 5% or less.
The results of the logistic regression analyses are displayed as odds ratios (OR) with 95% confidence interval (CI). All statistical analyses were performed using the SAS software (version 9.2, SAS Institute, Cary, NC). Differences were considered statistically significant at a P value of less than 0.05.
Supplementary Material
ACKNOWLEDGMENTS
This study was funded by a grant from the National Institutes of Health (DK083428 to Mary C. Perianayagam, PhD). The authors thank Gordon S. Huggins, MD (Molecular Cardiology Research Institute, Tufts Medical Center, Boston, MA) for providing access to the Applied Biosystems 7900HT fast real-time PCR system (RR023387) for genotyping analyses. The authors also acknowledge the UAB-UCSD O’Brien Core Center for Acute Kidney Injury Research (DK079337) for assistance in the haplotype analyses.
Footnotes
DISCLOSURE None.
REFERENCES
- 1.Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 2.Liangos O, Wald R, O’Bell JW, et al. Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol. 2006;1:43–51. doi: 10.2215/CJN.00220605. [DOI] [PubMed] [Google Scholar]
- 3.Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol. 2003;14:2199–2210. doi: 10.1097/01.asn.0000079785.13922.f6. [DOI] [PubMed] [Google Scholar]
- 4.Molitoris BA, Sutton TA. Endothelial injury and dysfunction: role in the extension phase of acute renal failure. Kidney Int. 2004;66:496–499. doi: 10.1111/j.1523-1755.2004.761_5.x. [DOI] [PubMed] [Google Scholar]
- 5.Kusaka J, Koga H, Hagiwara S, et al. Age-Dependent Responses to Renal Ischemia-Reperfusion Injury. J Surg Res. 2012;172:153–158. doi: 10.1016/j.jss.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka R, Fujita M, Tsuruta R, et al. Urinary trypsin inhibitor suppresses excessive generation of superoxide anion radical, systemic inflammation, oxidative stress, and endothelial injury in endotoxemic rats. Inflamm Res. 2010;59:597–606. doi: 10.1007/s00011-010-0166-8. [DOI] [PubMed] [Google Scholar]
- 7.Kfouri F, de Castro I, Testagrossa L, et al. Role of p21 and oxidative stress on renal tubular resistance after acute ischaemic injury. Nephrol Dial Transplant. 2010;25:1795–1803. doi: 10.1093/ndt/gfp719. [DOI] [PubMed] [Google Scholar]
- 8.Billings FT, 4th, Ball SK, Roberts LJ, 2nd, et al. Postoperative acute kidney injury is associated with hemoglobinemia and an enhanced oxidative stress response. Free Radic Biol Med. 2011;11:1480–1487. doi: 10.1016/j.freeradbiomed.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Homsi E, Mota da Silva S, Jr., Machado de Brito S, et al. p53-Mediated oxidative stress and tubular injury in rats with glycerol-induced acute kidney injury. Am J Nephrol. 2011;33:49–59. doi: 10.1159/000322836. [DOI] [PubMed] [Google Scholar]
- 10.Jaber BL, Rao M, Guo D, et al. Cytokine gene promoter polymorphisms and mortality in acute renal failure. Cytokine. 2004;25:212–219. doi: 10.1016/j.cyto.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Lu JC, Coca SG, Patel UD, et al. Searching for genes that matter in acute kidney injury: a systematic review. Clin J Am Soc Nephrol. 2009;4:1020–1031. doi: 10.2215/CJN.05411008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stafford-Smith M, Podgoreanu M, Swaminathan M, et al. Association of genetic polymorphisms with risk of renal injury after coronary bypass graft surgery. Am J Kidney Dis. 2005;45:519–530. doi: 10.1053/j.ajkd.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 13.Perianayagam MC, Liangos O, Kolyada AY, et al. NADPH oxidase p22phox and catalase gene variants are associated with biomarkers of oxidative stress and adverse outcomes in acute renal failure. J Am Soc Nephrol. 2007;18:255–263. doi: 10.1681/ASN.2006070806. [DOI] [PubMed] [Google Scholar]
- 14.du Cheyron D, Fradin S, Ramakers M, et al. Angiotensin converting enzyme insertion/deletion genetic polymorphism: its impact on renal function in critically ill patients. Crit Care Med. 2008;36:3178–3183. doi: 10.1097/CCM.0b013e318186a299. [DOI] [PubMed] [Google Scholar]
- 15.Haase-Fielitz A, Haase M, Bellomo R, et al. Decreased catecholamine degradation associates with shock and kidney injury after cardiac surgery. J Am Soc Nephrol. 2009;20:1393–1403. doi: 10.1681/ASN.2008080915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Popov AF, Hinz J, Schulz EG, et al. The eNOS 786C/T polymorphism in cardiac surgical patients with cardiopulmonary bypass is associated with renal dysfunction. Eur J Cardiothorac Surg. 2009;36:651–656. doi: 10.1016/j.ejcts.2009.04.049. [DOI] [PubMed] [Google Scholar]
- 17.Alam A, O’Connor DT, Perianayagam MC, et al. Phenylethanolamine N-methyltransferase gene polymorphisms and adverse outcomes in acute kidney injury. Nephron Clin Pract. 2010;114:c253–259. doi: 10.1159/000276577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Popov AF, Schulz EG, Schmitto JD, et al. Relation between renal dysfunction requiring renal replacement therapy and promoter polymorphism of the erythropoietin gene in cardiac surgery. Artif Organs. 2010;34:961–968. doi: 10.1111/j.1525-1594.2010.01108.x. [DOI] [PubMed] [Google Scholar]
- 19.Perianayagam MC, Tighiouart H, Nievergelt CM, et al. CYBA gene polymorphisms and adverse outcomes in acute kidney injury: a prospective cohort study. Nephron Extra. 2011;1:112–123. doi: 10.1159/000333017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 21.Cramer E, Pryzwansky KB, Villeval JL, et al. Ultrastructural localization of lactoferrin and myeloperoxidase in human neutrophils by immunogold. Blood. 1985;65:423–432. [PubMed] [Google Scholar]
- 22.Nauseef WM. Contributions of myeloperoxidase to proinflammatory events: more than an antimicrobial system. Int J Hematol. 2001;74:125–133. doi: 10.1007/BF02981994. [DOI] [PubMed] [Google Scholar]
- 23.Piedrafita FJ, Molander RB, Vansant G, et al. An Alu element in the myeloperoxidase promoter contains a composite SP1-thyroid hormone-retinoic acid response element. J Biol Chem. 1996;271:14412–14420. doi: 10.1074/jbc.271.24.14412. [DOI] [PubMed] [Google Scholar]
- 24.Babior BM. Phagocytes and oxidative stress. Am J Med. 2000;109:33–44. doi: 10.1016/s0002-9343(00)00481-2. [DOI] [PubMed] [Google Scholar]
- 25.Chooklin S, Pereyaslov A, Bihalskyy I. Pathogenic role of myeloperoxidase in acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2009;8:627–631. [PubMed] [Google Scholar]
- 26.Morishita K, Tsuchiya M, Asano S, et al. Chromosomal gene structure of human myeloperoxidase and regulation of its expression by granulocyte colony-stimulating factor. J Biol Chem. 1987;262:15208–15213. [PubMed] [Google Scholar]
- 27.Pecoits-Filho R, Stenvinkel P, Marchlewska A, et al. A functional variant of the myeloperoxidase gene is associated with cardiovascular disease in end-stage renal disease patients. Kidney Int Suppl. 2003;63:S172–176. doi: 10.1046/j.1523-1755.63.s84.32.x. [DOI] [PubMed] [Google Scholar]
- 28.Doi K, Noiri E, Maeda R, et al. Functional polymorphism of the myeloperoxidase gene in hypertensive nephrosclerosis dialysis patients. Hypertens Res. 2007;30:1193–1198. doi: 10.1291/hypres.30.1193. [DOI] [PubMed] [Google Scholar]
- 29.Jaber BL, Pereira BJ, Bonventre JV, et al. Polymorphism of host response genes: implications in the pathogenesis and treatment of acute renal failure. Kidney Int. 2005;67:14–33. doi: 10.1111/j.1523-1755.2005.00051.x. [DOI] [PubMed] [Google Scholar]
- 30.Malle E, Buch T, Grone HJ. Myeloperoxidase in kidney disease. Kidney Int. 2003;64:1956–1967. doi: 10.1046/j.1523-1755.2003.00336.x. [DOI] [PubMed] [Google Scholar]
- 31.Chatterjee PK, Brown PA, Cuzzocrea S, et al. Calpain inhibitor-1 reduces renal ischemia/reperfusion injury in the rat. Kidney Int. 2001;59:2073–2083. doi: 10.1046/j.1523-1755.2001.00722.x. [DOI] [PubMed] [Google Scholar]
- 32.Burne-Taney MJ, Kofler J, Yokota N, et al. Acute renal failure after whole body ischemia is characterized by inflammation and T cell-mediated injury. Am J Physiol Renal Physiol. 2003;285:F87–94. doi: 10.1152/ajprenal.00026.2003. [DOI] [PubMed] [Google Scholar]
- 33.Molinas SM, Cortes-Gonzalez C, Gonzalez-Bobadilla Y, et al. Effects of losartan pretreatment in an experimental model of ischemic acute kidney injury. Nephron Exp Nephrol. 2009;112:e10–19. doi: 10.1159/000210574. [DOI] [PubMed] [Google Scholar]
- 34.Kim T, Harman PK, Lyons R, et al. Brain natriuretic peptide is not reno-protective during renal ischemia-reperfusion injury in the rat. J Surg Res. 2010;164:e13–19. doi: 10.1016/j.jss.2010.06.041. [DOI] [PubMed] [Google Scholar]
- 35.Kong HY, Zhu SM, Wang LQ, et al. Sevoflurane protects against acute kidney injury in a small-size liver transplantation model. Am J Nephrol. 2010;32:347–355. doi: 10.1159/000319623. [DOI] [PubMed] [Google Scholar]
- 36.Gulec M, Iraz M, Yilmaz HR, et al. The effects of ginkgo biloba extract on tissue adenosine deaminase, xanthine oxidase, myeloperoxidase, malondialdehyde, and nitric oxide in cisplatin-induced nephrotoxicity. Toxicol Ind Health. 2006;22:125–130. doi: 10.1191/0748233705th255oa. [DOI] [PubMed] [Google Scholar]
- 37.Zhao ZG, Niu CY, Zhang YP, et al. The mechanism of myocardium and pancreas injury in rabbits with acute renal failure might be related to myeloperoxidase and membrane pump activities. Ren Fail. 2010;32:1216–1222. doi: 10.3109/0886022X.2010.517351. [DOI] [PubMed] [Google Scholar]
- 38.Chevrier I, Tregouet DA, Massonnet-Castel S, et al. Myeloperoxidase genetic polymorphisms modulate human neutrophil enzyme activity: genetic determinants for atherosclerosis? Atherosclerosis. 2006;188:150–154. doi: 10.1016/j.atherosclerosis.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 39.Hou SH, Bushinsky DA, Wish JB, et al. Hospital-acquired renal insufficiency: a prospective study. Am J Med. 1983;74:243–248. doi: 10.1016/0002-9343(83)90618-6. [DOI] [PubMed] [Google Scholar]
- 40.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 42.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 43.Knaus WA, Wagner DP. Multiple systems organ failure: epidemiology and prognosis. Crit Care Clin. 1989;5:221–232. [PubMed] [Google Scholar]
- 44.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 45.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. http://www.sts.org/sites/default/files/documents/pdf/trainingmanuals/Tab15-SectionOPOSTOPERATIVE.pdf:
- 47.Nyholt DR. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet. 2004;74:765–769. doi: 10.1086/383251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.