Abstract
Many women with breast cancer, especially those treated with chemotherapy, experience cognitive decline due in part to neurotoxic brain injury. Recent neuroimaging studies suggest widespread brain structural abnormalities pointing to disruption of large-scale brain networks. We applied resting state functional magnetic resonance imaging and graph theoretical analysis to examine the connectome in breast cancer survivors treated with chemotherapy relative to healthy comparison women. Compared to healthy females, the breast cancer group displayed altered global brain network organization characterized by significantly decreased global clustering as well as disrupted regional network characteristics in frontal, striatal and temporal areas. Breast cancer survivors also showed significantly increased self-report of executive function and memory difficulties compared to healthy females. These results suggest that topological organization of both global and regional brain network properties may be disrupted following breast cancer and chemotherapy. This pattern of altered network organization is believed to result in reduced efficiency of parallel information transfer. This is the first report of alterations in large-scale functional brain networks in this population and contributes novel information regarding the neurobiologic mechanisms underlying breast cancer-related cognitive impairment.
Keywords: breast cancer, chemotherapy, cognition, brain, resting state fMRI, graph theory, connectome
Introduction
Cognitive deficit is a common complication following breast cancer chemotherapy with as many as 75% of patients experiencing significant deficits (Janelsins, Kohli et al., 2011). The most frequently observed impairments include executive functioning and memory deficits although difficulties in other cognitive domains have also been reported (Wefel, Saleeba et al., 2010; Janelsins et al., 2011). Chemotherapy-treated patients are up to 8 times more likely to experience cognitive deficit compared to non-chemotherapy treated patients (Schagen, Muller et al., 2006; Stewart, Collins et al., 2008). Although chemotherapeutic agents typically have restricted direct access to brain tissue due to the blood-brain barrier, animal studies indicate that even chemotherapeutic agents that are not known to readily cross the blood-brain barrier (e.g. doxorubicin) are associated with reduced neurogenesis (Janelsins, Roscoe et al., 2010). Both dividing and non-dividing neural and glial cells are significantly vulnerable to chemotherapies (Dietrich, Han et al., 2006; Winocur, Vardy et al., 2006; Seigers, Schagen et al., 2008; Seigers, Schagen et al., 2009; Dietrich, 2010) and even small amounts of chemotherapy in the brain may cause long-term damage (Dietrich, 2010).
Accordingly, emerging data suggest that breast cancer chemotherapy is associated with diffuse structural injury including widespread decreases in gray matter volume (McDonald, Conroy et al., 2010) and white matter integrity (Deprez, Amant et al., 2011). A pattern of diffuse damage is likely to disrupt overall brain organization, reducing efficiency of information transfer. Previous studies have suggested that breast cancer survivors show decreased efficiency of neural networks, requiring more functional activation across a range of brain regions to complete certain tasks compared to healthy women (Silverman, Dy et al., 2007; Kesler, Bennett et al., 2009; Cimprich, Reuter-Lorenz et al., 2010). The specific pattern of brain changes following breast cancer and chemotherapy, including global versus local deficits, would have important implications for developing the most effective interventions for breast cancer-related cognitive deficits. Elucidation of large-scale brain network configuration in breast cancer may therefore contribute important new information regarding the neurobiologic mechanisms of breast cancer-related cognitive impairment.
However, it is currently unknown whether breast cancer and/or its treatments disrupt the organization of whole-brain networks. Previous studies of healthy individuals as well as certain pathological conditions have consistently revealed that brain networks are organized in a small-world manner characterized by high local specialization combined with high capacity for global information transfer (Bullmore and Bassett, 2011; Sporns, 2011). This organizational scheme allows for high efficiency of parallel processes with low wiring or energy costs (Guye, Bettus et al., 2010). Evaluation of these network properties may provide a more ecologically valid assessment of the neural mechanisms of cognitive function and dysfunction compared to traditional approaches because network analysis is more sensitive to subtle or diffuse neurobiological changes (McIntosh, 2000; Sporns, Chialvo et al., 2004; Petrella, 2011). Furthermore, brain network abnormalities have been shown to precede other neurobiologic and even metabolic changes in neuropathologic disease (Sheline, Morris et al., 2010; Petrella, 2011). A multivariate mathematical framework known as graph theory analysis is the prominent method for measuring these brain network metrics (Bassett and Bullmore, 2006; Bullmore and Sporns, 2009; Rubinov and Sporns, 2010; Sporns, 2011).
Resting state functional magnetic resonance imaging (fMRI) is a reliable, non-invasive method for examining the intrinsic topology of large-scale brain networks (Wang, Zuo et al., 2010). Resting state fMRI networks are task-independent and thus less vulnerable to confounds due to performance variance. Resting state fMRI connectivity studies are sensitive to abnormal global network organization, revealing changes in several clinical populations with cognitive deficits including Alzheimer’s dementia (Sanz-Arigita, Schoonheim et al., 2010) schizophrenia (Lynall, Bassett et al., 2010), traumatic brain injury (Nakamura, Hillary et al., 2009) and attention-deficit hyperactivity disorder (Wang, Zhu et al., 2009). Resting state fMRI provides assessment of intrinsic connections that are believed to support cognitive functions and resting state networks have been shown to correlate with various cognitive skills (Church, Fair et al., 2009).
The present study examined changes in resting state functional connectivity networks utilizing graph analysis in women with breast cancer relative to healthy women matched for age, education and general intelligence. Our primary hypothesis was that the breast cancer group would show altered global and regional network organization as indicated by abnormal clustering, path length and/or nodal degree. We also explored potential associations between network properties and cognitive performance.
Methods
Participants
The present study included a total of 61 women aged 40.94 to 74.02 years. The participants were categorized into two groups; 34 women with a history of breast cancer, stages I-IV, all treated with chemotherapy, and 27 healthy comparison women. The women in the breast cancer group were, on average, 5.35 +/− 5.40 (median = 4.96; range = 28.75) years off-therapy and were free from disease or relapse as well as any gross neuropathology (e.g. brain metastases) at the time of evaluation. Individual chemotherapy treatment protocols included adriamycin/cytoxan/taxol or taxotere = 27, cytoxan/methotrexate/5- fluorouracil = 5 and adriamycin/cytoxan + cytoxan/methotrexate/5- fluorouracil = 2. Twenty-three women underwent radiation treatment and 19 received tamoxifen (5 were still taking tamoxifen at the time of assessment). The Stanford University Institutional Review Board approved this study. This study was conducted according to the principles expressed in the Declaration of Helsinki. All participants provided written informed consent.
There were no significant differences between the groups in age, education or general intelligence. However, significantly more women in the breast cancer group were post-menopausal, which was expected given that chemotherapy can induce early menopause (Mar Fan, Houédé-Tchen et al., 2010) and tamoxifen may also induce menopausal symptoms (Bakkum-Gamez, Laughlin et al., 2011). Therefore, we controlled for menopausal status in all analyses. Comparison women were excluded for any history of medical, neurologic or psychiatric conditions. Participants with breast cancer were excluded if they presented with history of psychiatric or neurologic conditions and/or significant concurrent medical diagnoses. Participants in both groups were excluded for any MRI contraindications. Breast cancer and comparison participants were recruited via the Army of Women (http://www.armyofwomen.org/), community flyer postings and advertisements. Breast cancer participants were also recruited via local support groups.
Cognitive Testing
General intelligence was measured using an abbreviated version of the Wechsler Adult Intelligence Scales Fourth Edition (Wechsler, 2008). Executive function was assessed using the Behavioral Rating Inventory of Executive Function (BRIEF) (Roth, Isquith et al., 2005), the Delis-Kaplan Executive Function System Letter Fluency subtest (Delis, Kaplan et al., 2001) the Neuropsychological Assessment Battery Categories test (Stern, 2003) and the Wisconsin Card Sorting test (WCST) (Heaton, 2004). Memory was assessed using the Hopkins Verbal Learning Test Revised (HVLT) which assesses immediate and delayed recall memory (Wefel, Vardy et al., 2011) and the Multifactorial Memory Questionnaire Ability Scale (MMQ), a self-rating of memory function (Troyer and Rich, 2002). Categories, WCST, HVLT and/or MMQ scores were missing for some participants (see Table 1). Although participants with psychiatric conditions were excluded, the CAD was used to measure symptoms including depression, anxiety and fatigue (Bracken, 2007). Women with breast cancer displayed elevated CAD score compared to the comparison group (p<0.001 for initial comparison and p<0.003 when menopausal status was included as a covariate) although, on average, scores were not clinically significant (mean T score <70). However, given that these symptoms may influence cognitive and brain functioning, this measure was used as a covariate (in additional to menopausal status) in all analyses.
Table 1.
Group differences in demographics and neuropsychological tests
| Measure | Group | N | Mean | Standard deviation |
P value |
|---|---|---|---|---|---|
| Age | Comparison | 27 | 55.08 | 9.12 | 0.971 |
| Breast cancer | 34 | 55.16 | 7.30 | ||
| Education | Comparison | 27 | 16.81 | 2.96 | 0.991 |
| Breast cancer | 34 | 16.82 | 2.75 | ||
| General Intelligence | Comparison | 27 | 113.03 | 13.46 | 0.694 |
| Breast cancer | 34 | 111.28 | 11.06 | ||
| Letter Fluency | Comparison | 27 | 12.78 | 3.76 | 0.966 |
| Breast cancer | 34 | 11.97 | 3.22 | ||
| NAB Categories | Comparison | 23 | 54.78 | 8.41 | 0.024* |
| Breast cancer | 32 | 50.78 | 7.42 | ||
| WCST | Comparison | 18 | 51.28 | 9.85 | 0.115 |
| Breast cancer | 32 | 46.72 | 9.31 | ||
| BRIEF | Comparison | 27 | 47.41 | 8.23 | 0.001** |
| Breast cancer | 34 | 62.35 | 11.60 | ||
| HVLT immediate recall | Comparison | 23 | 55.13 | 10.16 | 0.121 |
| Breast cancer | 32 | 51.29 | 7.43 | ||
| HVLT delayed recall | Comparison | 23 | 56.21 | 8.64 | 0.040* |
| Breast cancer | 32 | 50.10 | 6.89 | ||
| MMQ | Comparison | 25 | 59.22 | 7.04 | 0.0001** |
| Breast Cancer | 33 | 44.34 | 11.3 | ||
| CAD | Comparison | 27 | 42.63 | 7.104 | 0.003* |
| Breast cancer | 34 | 52.12 | 10.987 |
NAB = Neuropsychological Assessment Battery; WCST = Wisconsin Card Sort Task; BRIEF = The Behavioral Rating Inventory of Executive Function; HVLT = Hopkins Verbal Learning Test; MMQ = Multifactorial Memory Questionnaire; CAD = Clinical assessment of depression. Demographic variables (age and education) were compared with two sample T-tests and cognitive variables were compared using a general linear model framework with menopausal status and CAD depression score as covariates.
Significant at p<0.05 uncorrected
Significant at p<0.005 level indicated by Bonferroni correction for multiple comparisons.
Data normality was assessed and confirmed graphically using scatterplots in SPSS software (www.spss.com). A general linear model framework in SPSS was employed to evaluate group differences in performance on neuropsychological assessments, with Bonferroni correction for multiple comparisons.
MRI Acquisitions
MRI scanning was performed on a GE Discovery MR750 3.0 Tesla whole body scanner (GE Medical Systems, Milwaukee, WI). FMRI data were acquired while participants rested in the scanner with their eyes closed using a T2* weighted gradient echo spiral pulse sequence: relaxation time = 2000 msec, echo time = 30 msec, flip angle = 80° and 1 interleave, field of view = 220, matrix = 64×64, in-plane resolution = 3.125. Number of data frames collected was 216, thus total scan time was 7:12. An automated high-order shimming method based on spiral acquisitions was employed to reduce field heterogeneity (Glover and Lai, 1998). To coregister and normalize functional images with a standardized template, a high-resolution, 3 dimension inversion-recovery prepared fast spoiled gradient echo anatomical scan was acquired: relaxation time: minimum, echo time: minimum, flip: 11 degrees, inversion time: 300 msec, bandwidth: +/−31.25 kHz, field of view: 24cm, phase field of view: 0.75, slice thickness: 1.5mm, 125 slices, 256×256 at 1 excitation, scan time: 4:26. Two task based fMRI scans and a diffusion weighted scan were also acquired during the MRI session that are utilized in other analyses not reported here. However, the resting state scan was acquired prior to any other scans to reduce the effects of specific cognitive processes on the resting state networks.
Functional MRI preprocessing
Image preprocessing was performed using Statistical Parametric Mapping 8 (Wellcome Trust Centre, London, UK; http://www.fil.ion.ucl.ac.uk/spm/) as described in detail in our previous publications (Kesler et al., 2009; Kesler, Kent et al., 2011). We defined the regions involved in the functional resting state network using the Automated Anatomical Labeling atlas (Tzourio-Mazoyer, Landeau et al., 2002). We utilized the 90 regions representing cortical and subcortical structures in both hemispheres that have been employed in several previous studies of functional connectome (Lynall et al., 2010; Tian, Wang et al., 2011; Zhang, Wang et al., 2011). While the choice of parcellation scheme might affect the results of network analysis (Wang, Wang et al., 2009), recent evidence has shown that the between-group comparison results remain intact regardless of the chosen parcellation scheme (Zalesky, Fornito et al., 2010).
Further preprocessing of the functional volumes was performed using the Functional Connectivity Toolbox (http://web.mit.edu/swg/software.html). First, data were band pass filtered to 0.008 Hz – 0.09 Hz. Then, the CompCor method (Behzadi, Restom et al., 2007) was used to reduce physiological and other non-neuronal artifacts. This method involves extracting signal from white matter and cerebrospinal fluid regions using principal component analysis and then regressing these signals out of the total fMRI signal. Finally, temporal correlations between all possible pairs of regions were computed based on the corrected fMRI signal resulting in a 90×90 correlation matrix for each participant.
Graph Analysis
Network Measures
We defined global and regional network measures including clustering, path length, small-worldness and nodal degree as described in previous studies (Bassett and Bullmore, 2006; Bullmore and Sporns, 2009; Rubinov and Sporns, 2010; Sporns, 2011). Briefly, a network is defined as a set of regions called nodes and connections between those nodes are called edges. The clustering coefficient of a node is equal to the proportion of a node’s neighbors that are also neighbors with each other. The clustering coefficient of a network is equal to the average of clustering coefficients across nodes and is a measure of network segregation. Path length describes the minimum number of edges that separate pairs of nodes. The characteristic path length of a network is the average shortest path length between pairs of nodes in the network and it is the most commonly used measure of network integration. Small-worldness is a property of organization shared by brain networks and other large-scale complex biological networks that differentiates them from random networks. Random networks are networks that do not display any systematic organization of nodes and edges. Small-world networks display greater clustering but similar path length when compared to random networks and this organization is believed to facilitate an energy-efficient balance between network segregation and network integration. Nodal degree is defined as the number of connections that a node has with the rest of the network and is considered a measure of how interactive the node is within the network. To evaluate the topology of a brain network, we compared network measures from participant groups with the corresponding mean values of a random graph having the same number of nodes, total edges and degree distribution as described previously (Maslov and Sneppen, 2002; Milo, Shen-Orr et al., 2002).
Creation of Functional Correlation Networks
For each participant, the correlation matrix R was thresholded to create a binary adjacency matrix A where aij was retained as a connection, or edge (set equal to 1) if rij was greater than a threshold T, and aij was not retained as an edge (set equal to 0) if rij was less than T. Diagonal elements of the association matrix were set equal to 0. The adjacency matrix A represented a binary undirected graph G in which regions i and j were connected if gij was equal to 1. The correlation matrix provides an estimate of functional connectivity – a profile of the synchronization between brain regions, or nodes, during resting state. Graph G had a network degree of E equal to the number of edges, and a network density (cost) of D=E/[Nx(N-1)]/2 representing the ratio of existing edges relative to all possible edges.
Network measures were computed over a range of thresholds T for each graph because thresholding at an absolute value would have resulted in different numbers of nodes and degrees across participants introducing a confound when measures were compared between groups (van Wijk, Stam et al., 2010). We therefore examined graphs over a range of connection densities for which graphs 1) were fully connected (each node had at least one connection with another node on the graph) and 2) displayed small-world properties (non-random graphs). For our data, connection densities ranging from 41% to 50% fit these criteria and we examined densities in steps equal to 1% (10 densities total). Below a connection density of 41% individual graphs began to fragment (i.e. some nodes were not connected to even one other node) and above a connection density of 50% graphs become increasingly random and do not display small-world properties (Humphries, Gurney et al., 2006). Furthermore, animal research indicates that connections at densities above 50% are likely non-biological (Kaiser and Hilgetag, 2006).
In addition to comparing networks at various densities, and to facilitate group comparisons of network measures, we employed a summary measure to integrate the values of network measures of interest over a range of densities. The summary measure employed utilizes and compares the areas under a curve (AUC) for each network measure (Bernhardt, Chen et al., 2011). For this purpose, the curves extracted from thresholding across a range of densities were used. Each of these curves depicts the changes in a specific network measure (for each group) as a function of network density. By performing AUC analysis, the comparison between network measures is less sensitive to the thresholding process. The AUC analysis was also performed within the 41% to 50% density range.
Network Analyses
To account for the effects of menopausal status and psychiatric symptoms, we performed a linear regression analysis across groups at every brain region. Menopausal status and CAD scores were used as covariates in the model and the residuals of the linear regression analysis, representing corrected resting state time series values, were used for comparison of group networks.
At minimum connection density of 41%, some individual graphs were fragmented and therefore were not included in those particular graph analyses. Specifically, there were 3 individuals (two breast cancer survivors and one comparison individual) with fragmented graphs at 41%. These subjects were excluded specifically because their networks were fragmented at 41% and the comparisons might be affected by group differences in the number of nodes and connections (van Wijk et al., 2010).
To facilitate group comparisons, the area under the curve of network measures (across the density range 41% to 50%) were used (Bernhardt et al., 2011). These AUC measures were used for all subsequent group comparisons and for within group correlations between network measures and cognitive measures. The Brain Connectivity Toolbox (Rubinov and Sporns, 2009) was utilized for quantification of network measures and our in-house software, Graph Analysis Toolbox (http://nnl.stanford.edu/tools.html) was used to compare functional networks and network measures between groups.
Network Hubs
The most important and most highly connected nodes in a brain network are called network hubs. Hub identification is a descriptive, qualitative assessment of network organization. Hubs are regions whose degree is higher than the average network degree (Bassett, Bullmore et al., 2008) and they facilitate efficient communication across the network. Hubs may also play an important role in resilience to injury (Rubinov and Sporns, 2009). A network node was considered to be a hub if its degree was at least 1 standard deviation above the mean network degree (Tian et al., 2011).
Comparison of Network Measures Between Groups
In order to test the statistical significance of the between-group differences in global and regional network measures, a non-parametric permutation test with 2000 repetitions was used (Lynall et al., 2010). In each repetition, the adjusted network measures of each participant were randomly reassigned to one of the two groups so that each randomized group had the same number of subjects as the original groups. The differences in network measures between randomized groups were then calculated resulting in a permutation distribution of difference under the null hypothesis. The actual between-group difference in network measures was then placed in the corresponding permutation distribution and a two-tailed p-value was calculated based on its percentile position (Bernhardt et al., 2011). The same permutation procedure was used to compare the AUC of network measures between groups.
Relationships Between Network Measures, Cognitive, Demographic and Disease Variables
The relationships between AUC values for network measures, cognitive, demographic (age, education level in years), disease (stage) and treatment (time since treatment in months, radiation, tamoxifen) were examined within each group using two-tailed Pearson or point biserial correlations as appropriate. Only those network and cognitive measures that differed between groups were examined.
Results
Cognitive Testing
Cognitive testing results are presented in Table 1. The breast cancer group showed significantly lower self-reported executive functioning as measured by the BRIEF (p=0.001) as well as reduced self-reported memory ability indicated by the MMQ (p<0.0001) after Bonferroni correction for multiple comparisons.
Network Topology
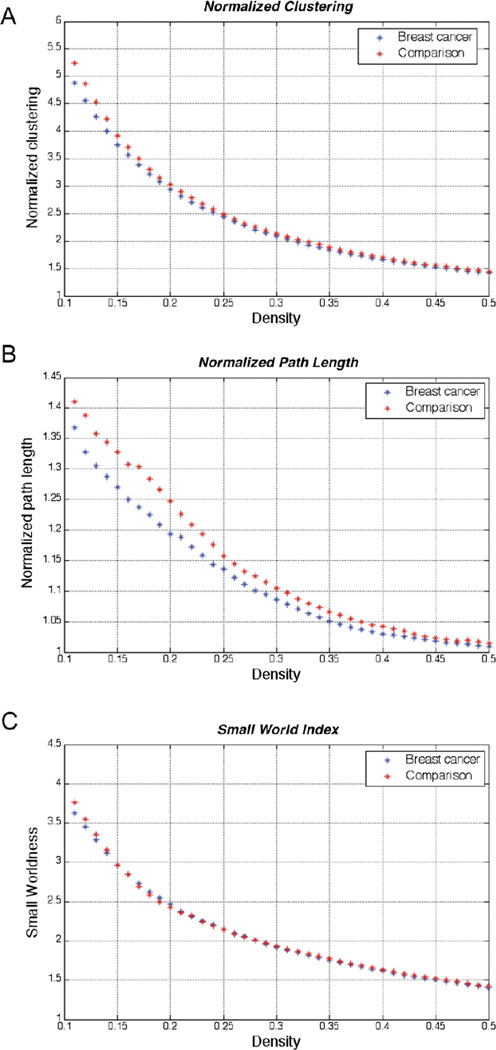
Topological metrics are illustrated in Figure 1. Measures are presented across a wide range of densities for illustrative purposes; and the densities which were used in subsequent analyses are highlighted. Functional correlation networks for both groups displayed normalized characteristic path length close to one (close to random networks’ path length) and normalized clustering coefficient greater than 1 (greater than random networks’ clustering), consistent with small-world organization. Below a network density of 41%, the individual networks in both groups began to fragment which results in different numbers of nodes for individual networks. This difference directly affects the values of network measures, as noted above. Thus, the observed between-group differences in network measures below a network density of 41% depend on the number of individual networks that fragment in each group and group comparisons below that density are not meaningful (van Wijk et al., 2010).
Figure 1. Global network topology.
A) normalized clustering, B) normalized path length, and C) small world index of breast cancer and comparison networks. The figures show that both networks display a small-world organization across a range of densities.
Group Differences In Global Network Measures
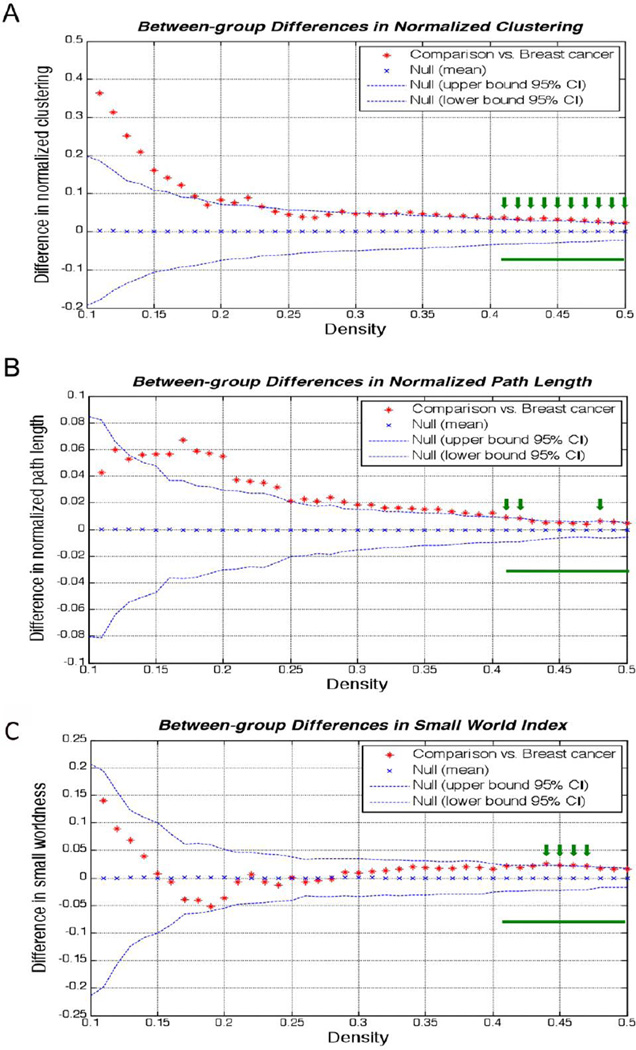
Group differences in global network measures are displayed in Figure 2. The normalized clustering coefficient (p=0.029) was significantly lower in the breast cancer network compared to healthy females. Although the small-worldness (p = 0.061) and characteristic path length (p=0.054) were higher in the comparison network than in the breast cancer network, the differences were only marginally significant.
Figure 2. Between-group differences in network measures.
The breast cancer group showed lower A) normalized clustering, B) normalized path length, and C) small world index compared to healthy controls although only clustering was significant. The green bar indicates range of densities for which we compared network values and the green arrows indicate individual densities, within the compared range, for which the group difference was significant.
Group Differences In Regional Network Measures (Nodal Degree)
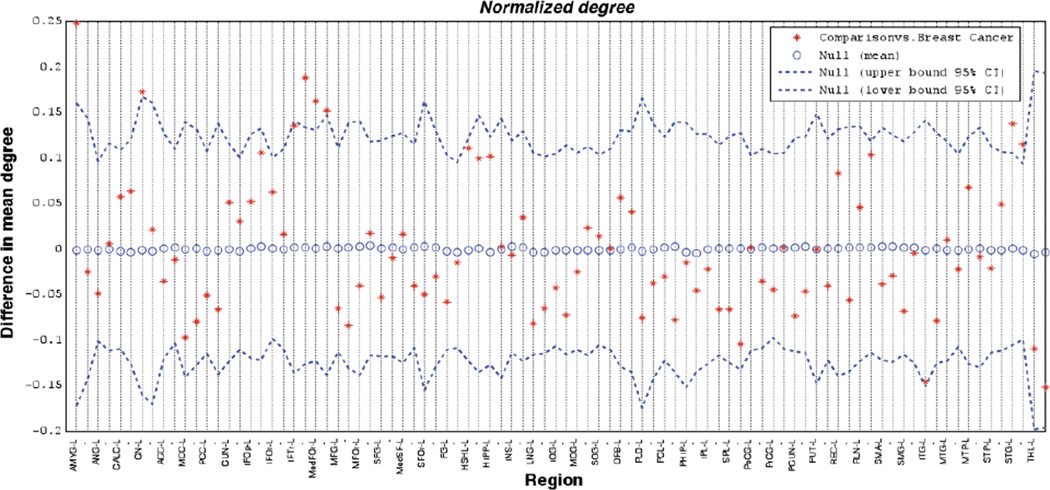
The results of regional node characteristic comparisons are presented in Figures 3 and 4. Nodal degree was significantly lower (p<0.05) in the breast cancer group relative to the comparison group for the left amygdala, left caudate, right inferior frontal gyrus, bilateral medial orbital frontal gyri, and bilateral superior temporal gyri. There were no regions for which degree was significantly higher in the breast cancer group relative to the comparison group.
Figure 3. Group differences nodal degree.
Group differences are displayed relative to random networks (null mean). Names of brain regions are given for the left hemisphere and the following region is the corresponding right hemisphere homologue denoted with a –. Abbreviations are used as follows: AMYG=Amygdala, ANG=Angular gyrus, CALC=Calcarine sulcus, CN=Caudate, ACC=Anterior cingulate cortex, MCC=Middle cingulate cortex, PCC=Posterior cingulate cortex, CUN=Cuneus, IFOp=Inferior frontal gyrus, opercular, IFOr=Inferior frontal gyrus, orbital, IFTr=Inferior frontal gyrus, triangular, MedFOr=Medial frontal gyrus, orbital, MFG=Middle frontal gyrus, MFOr=Middle frontal gyrus, orbital, SFG=Superior frontal gyrus, MedSFG=Superior frontal gyrus medial, SFOr=Superior frontal gyrus, orbital, FG=Fusiform gyrus, HSHL=Heschel's gyrus, HIPP=Hippocampus, INS=Insula, LNG=Lingual gyrus, IOG=Inferior occipital gyrus, MOG=Middle occipital gyrus, SOG=Superior occipital gyrus, OFB=Olfactory bulb, PLD=Pallidum, PCL=Paracentral lobule, PHIP=Parahippocampal gyrus, IPL=Inferior parietal lobule, SPL=Superior parietal lobule, PoCG=Postcentral gyrus, PrCG=Precentral gyrus, PCUN=Precuneus, PUT=Putamen, REC=Gyrus recuts, RLN=Rolandic opercular, SMA=Supplementary motor area, SMG=Supramarginal gyrus, ITG=Inferior temporal gyrus, MTG=Middle temporal gyrus, MTP=Middle temporal pole, STP=Superior temporal pole, STG=Superior temporal gyrus, THL=Thalamus.
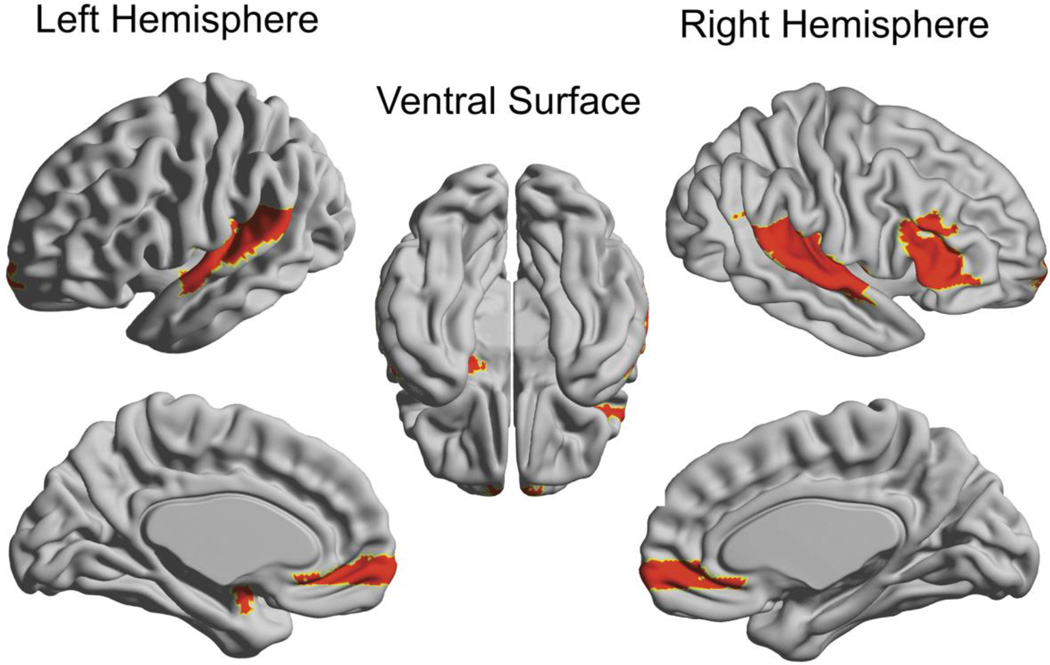
Figure 4. Brain map of regional group differences in nodal degree.
Significant group differences are displayed on an inflated brain (left hemisphere, right hemisphere and ventral surface as indicated). Colored regions indicate lower degree for breast cancer networks relative to the healthy comparison group’s networks. There were no areas of greater regional differences in the breast cancer group compared to healthy females. This Figure was created using the Brain Net Viewer (http://www.nitrc.org/projects/bnv/).
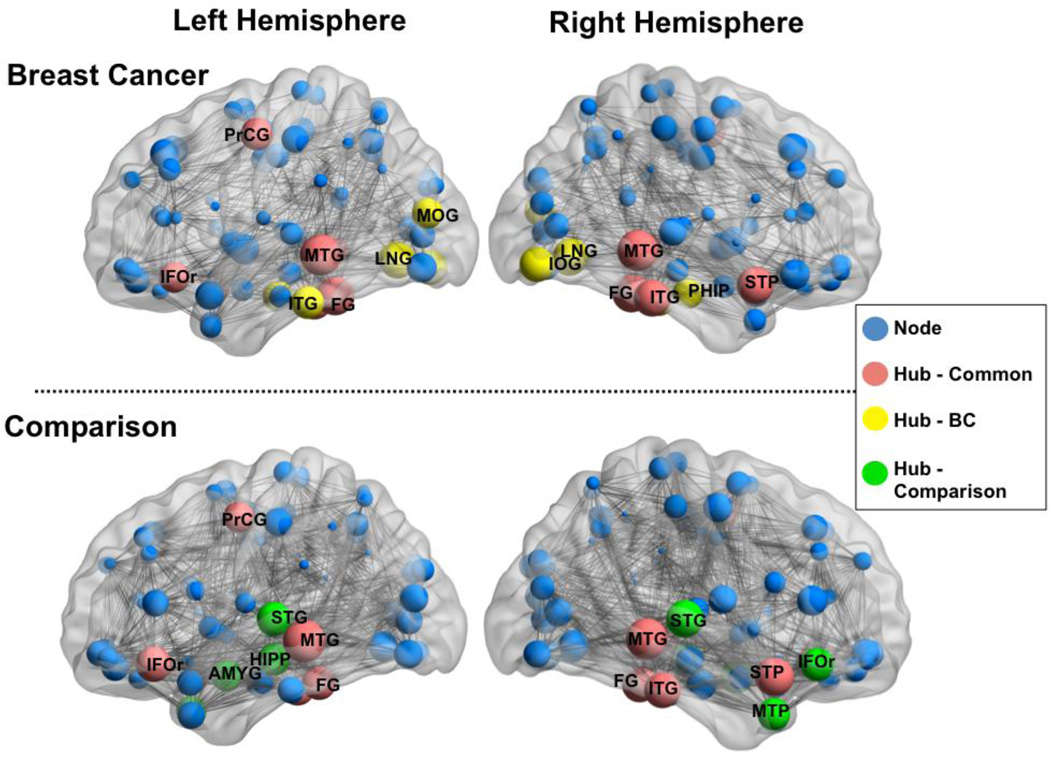
Network Hubs
Network hubs for each group are presented in Table 2 and Figure 5. There was some similarity between breast cancer and comparison groups in terms of network hub locations. Hubs were identified for both groups in frontal and temporal regions, although the comparison group had additional hubs in right inferior frontal gyrus, bilateral superior temporal gyri, left hippocampus and left amygdala. Additional hubs were identified for the breast cancer group in bilateral lingual and occipital gyri, right parahippocampus and left inferior temporal gyrus.
Table 2.
Network Hubs
| Hubs present in networks for both groups of women |
Hubs present in breast cancer group only |
Hubs present in healthy comparison group only |
|---|---|---|
| Left inferior frontal gyrus, orbital |
Left lingual gyrus | Left amygdala |
| Left fusiform gyrus | Right lingual gyrus | Right inferior frontal gyrus, orbital |
| Right fusiform gyrus | Right inferior occipital gyrus |
Left hippocampus |
| Left precentral gyrus | Left middle occipital gyrus |
Left middle temporal pole |
| Right inferior temporal gyrus | Right parahippocampal gyrus |
Left superior temporal gyrus |
| Left middle temporal gyrus | Left inferior temporal gyrus |
Right superior temporal gyrus |
| Right middle temporal gyrus | ||
| Right superior temporal pole |
Figure 5. Network layout and hubs.
Functional correlation network and hubs for each hemisphere superimposed on inflated standard brains for breast cancer and healthy comparison groups (left hemisphere and right hemisphere as indicated). Grey lines indicate edges or functional connections. Blue spheres represent nodes with the size of the sphere being proportional to the degree of the node. Red spheres indicate nodes classified as hubs common between both groups. Green spheres indicate hubs unique to the healthy comparison group and yellow spheres are hubs unique to the breast cancer group. Hubs for each group are labeled with the following abbreviations AMYG=Amygdala, IFOr=Inferior frontal gyrus, FG=Fusiform gyrus, LNG=Lingual gyrus, ITG=Inferior temporal gyrus, IOC = Inferior occipital cortex, MOG=Middle occipital gyrus, MTG=Middle temporal gyrus, MTP=Middle temporal pole, STP=Superior temporal pole, STG=Superior temporal gyrus, HIPP = Hippocampus, PHIP = Parahippocampal gyrus. This Figure was created using the Brain Net Viewer (http://www.nitrc.org/projects/bnv/).
Relationships Between Network Measures, Cognitive, Demographic and Disease Variables
Within the breast cancer group there were no significant correlations between global network measures and cognitive tests, demographic or disease variables (p>0.10). There were significant correlations between regional degree in left hippocampus and time since treatment (r = −0.45, p = 0.009) and breast cancer stage (rs = −0.38, p = 0.033). There was also a significant correlation between regional degree in right parahippocampal gyrus and time since treatment (r = −0.45, p = 0.009) and age (r = −0.41, p = 0.020). There were no significant correlations between regional degree and cognitive test scores, tamoxifen or radiation treatment (p>0.10).
There were no significant correlations in the comparison group (p>0.10).
Discussion
The present study examined functional brain network topology in breast cancer survivors treated with chemotherapy relative to healthy comparison women using resting state fMRI and graph analysis. Both groups of women displayed connectomes with the small-world properties characteristic of complex networks when compared to random networks. However, the breast cancer group displayed altered network organization including significantly decreased global clustering values when compared to healthy comparison females as well as marginally decreased path length and small-worldness. The breast cancer group also displayed reduced nodal degree and number of hubs in areas that are implicated in executive control, memory and emotion regulation networks. This is the first report to date showing alterations in large-scale functional brain networks of women with breast cancer.
Our results indicate that breast cancer and/or chemotherapy may impact cognitive function via disrupted coordination among various brain regions in the global functional brain network. We noted impaired interaction within discrete brain regions as evidenced by reduced clustering coefficient. Clustering indicates the quantity of connections between a region and its neighboring regions and therefore reflects local connectivity or the brain network’s capacity for functional specialization (Rubinov and Sporns, 2009). We also report marginally significantly reduced path length and small-world index in the breast cancer group. Path length is considered a measure of the functional integration of segregated resources (Rubinov and Sporns, 2009). It has been suggested that shorter path lengths in clinical populations may result from increased long-range functional connections between spatially distant regions that increase the cost of the network (Zhang et al., 2011). Complex networks like the brain must be economical by minimizing wiring cost (i.e. many short-range but fewer long-range connections) while optimizing global efficiency (Achard and Bullmore, 2007).
Our findings indicate that the brain network’s ability to coordinate parallel processes that support various cognitive capacities is disrupted following breast cancer and chemotherapy. This reduction of network efficiency is consistent with previous reports suggesting more effortful processing for chemotherapy-treated breast cancer survivors demonstrated by diffuse neural over-activation (Ferguson, McDonald et al., 2007; Silverman et al., 2007; Kesler et al., 2009; Cimprich et al., 2010). Normal aging has also been associated with reduced path length (Wu, Taki et al., 2011), reduced connectivity in executive control regions, reduced functional segregation (Chen, He et al., 2011) and increased network cost (Gong, Rosa-Neto et al., 2009) suggesting that breast cancer and/or chemotherapy may accelerate the aging process.
Alterations in large scale functional brain networks have been revealed in several clinical populations with cognitive dysfunction including Alzheimer’s dementia (Sanz-Arigita et al., 2010) schizophrenia (Lynall et al., 2010), traumatic brain injury (Nakamura et al., 2009) and attention-deficit hyperactivity disorder (Wang et al., 2009). Damage to large-scale brain networks is thought to be indicative of a “disconnection syndrome” where there is diffuse injury to white matter pathways (Petrella, 2011). Accordingly, recent studies demonstrate widespread reductions in white matter microstructure following breast cancer chemotherapy (Deprez et al., 2011; Deprez, Amant et al., 2012). Animal models indicate that systemically delivered chemotherapies can destroy white matter progenitor cells, causing loss of self-renewal ability even after initial doses (Dietrich et al., 2006; Dietrich, 2010). From a network perspective, functional integration is dynamic; functional networks are temporarily reconfigured to meet various task demands (McIntosh, 2000; Bassett and Bullmore, 2006). This coordinated, dynamic response depends on stable structural networks (Sporns, 2011). Thus, the disruption of functional network organization noted here may reflect a loss of dynamic response plasticity. Altered network properties were not associated with other breast cancer treatments including radiation or tamoxifen. However, longitudinal studies are required to determine the disease- and treatment-specific effects on network connectivity and function in breast cancer.
Group differences in nodal degree and network hubs help identify specific regions that show altered integration within the network and therefore which specific neural circuits may be at highest risk for loss of response plasticity. Breast cancer survivors displayed reduced nodal degree and number of hubs within dorsolateral prefrontal, orbitofrontal and mesial temporal regions which may correspond to the deficits in executive function, memory and learning and emotion regulation that are often noted among these patients. Accordingly, the breast cancer group showed significantly reduced executive and memory function as well as elevated psychiatric distress. This included only subjective measures after correction for multiple comparisons. Post hoc analysis indicated no significant correlations between objective and subjective measures (p < 0.15) which is consistent with previous studies of cognition in breast cancer (Castellon and Ganz, 2009; Vardy, 2009). Self-report measures may have greater sensitivity to the subtle nature of chemotherapy-related brain injury due to their higher ecological, or real-world validity (Castellon and Ganz, 2009; Vardy, 2009; Kesler et al., 2011). However, there were no significant associations between network properties and subjective cognitive measures in the present sample indicating that further research is required to determine how altered connectome relates to cognitive outcome in breast cancer survivors.
The breast cancer group showed increased hubs in posterior regions including occipital and inferior temporal unimodal association cortex and paralimbic areas compared to healthy females. Highly similar findings have been noted among individuals with Alzheimer’s dementia (He, Chen et al., 2008). These regions may represent areas of compensatory coordination for supporting cognitive processes in the context of abnormalities in other hub groups (He et al., 2008). Our findings suggest that these particular regions may show increased robustness to neurologic injury across conditions. Given the debated link between breast cancer and dementia (Du, Xia et al., 2010) and the promising use of network analysis to classify neurodegenerative disorders (Supekar, Menon et al., 2008; Chen, Ward et al., 2011), it will be important to identify further similarities and differences in the specific type of damage to large-scale brain networks associated with breast cancer, particularly in terms of default mode network.
There was a significant negative correlation between time since treatment and degree in left hippocampus and right parahippocampal gyrus suggesting that the effects of breast cancer treatments on the connectivity of these regions are persistent and may potentially worsen over time. This is consistent with a previous study demonstrating that subgroups of patients showed persistent memory deficits or new onset of previously non-existent memory deficits (Wefel et al., 2010). Additionally, breast cancer disease stage was negatively related to regional degree in left hippocampus indicating that increased disease severity is associated with lessening of this region’s interactivity within the network. Also noted was a negative correlation between age and degree in right parahippocampal gyrus, potentially indicating that breast cancer and/or treatment effects are more severe in older women. This is consistent with previous findings of increased vulnerability to cognitive decline in women diagnosed with breast cancer at an older age and suggests that there may be an interaction between age and breast cancer related cognitive decline (Ahles, Saykin et al., 2010; Kesler et al., 2011).
We noted increased psychiatric symptoms in the breast cancer group. Depression has been associated with alterations in functional whole-brain network topology, including reduced path length, increased nodal centralities in default mode network regions and decreased nodal centralities in occipital, frontal and temporal regions (Leistedt, Coumans et al., 2009; Zhang et al., 2011). Our results showed a primary abnormality in clustering and a very different profile of disrupted regional node characteristics. Our analyses were controlled for self-reported psychiatric distress and it was noted that this was not a significant covariate in the statistical models. Although psychiatric distress likely contributes to cognitive dysfunction following breast cancer, our results provide further evidence that psychiatric distress is not a sufficient explanation for breast cancer-related cognitive deficits. Additionally, disease and/or treatment related disruptions in regional network characteristics among areas involved in emotion regulation as noted above could make breast cancer survivors more vulnerable to psychiatric distress. Longitudinal studies are required to determine when in the disease and treatment course network disruption is most likely to occur.
The most common finding across previous neuroimaging studies of breast cancer, irrespective of imaging modality, is prefrontal cortex abnormality (Inagaki, Yoshikawa et al., 2007; Silverman et al., 2007; Kesler et al., 2009; de Ruiter, Reneman et al., 2011; Deprez et al., 2011; Kesler et al., 2011). While many disorders are associated with similar prefrontal deficits, the characterization of multivariate neurobiologic patterns as noted here may help distinguish the particular neural effects of breast cancer chemotherapy. For example, depression, dementia and breast cancer chemotherapy share a common mechanistic pathway for brain injury, namely, inflammation (Janelsins et al., 2011; Maes, Mihaylova et al., 2011; Sardi, Fassina et al., 2011) yet have distinct profiles of brain network abnormalities, as noted above. Thus, targeting common pathways may have limited effectiveness for reducing neural effects of breast cancer and its treatments. Further specifying the unique profile of the brain network disruption in breast cancer could help elucidate other possible mechanisms of brain changes in this population to provide breast cancer-specific targets for intervention. Additionally, network disruption may be present prior to other neurobiologic or even molecular changes associated with disease onset or progression in several conditions (Petrella, 2011). Network dysfunction could thus potentially be used as an indicator of disease progression in breast cancer, such as brain metastases, for example.
The present study has several limitations. As with most studies of breast cancer survivors, the sample was very heterogeneous in terms of treatment regimens, time off-therapy and disease characteristics. For example, we were unable to examine the relationship between individual chemotherapy regimens and functional networks. The cross-sectional design presents several obstacles for examining the specific effects of disease versus treatment variables on network topology. Longitudinal research will be critical to our understanding of the effects of specific chemotherapy regimens on neurobiologic status, although randomized groups are not feasible due to the ethical ramifications of denying patients potentially life-saving treatments.
It was also not possible to determine if our findings were specific to breast cancer. The aforementioned animal literature (see Dietrich, 2010 for a review) suggests that the effects of chemotherapy on the brain are likely common among cancers. However, other factors such as hormonal involvement, age and cognitive reserve may contribute to an individual’s vulnerability to the effects of chemotherapy (Ahles et al., 2010; Kesler et al., 2011). Other cancers that involve chemotherapy treatment, including leukemia and lymphoma have been associated with white matter abnormalities and cognitive effects (Syrjala, Artherholt et al., 2011; Correa, Shi et al., 2012) indicating the potential for altered large scale functional brain networks. However, neuroimaging studies have been very limited in other adult cancers to date. Participants were excluded for Axis I diagnoses such as depression or anxiety, and analyses were controlled for self-reported psychiatric symptoms, which were not clinically significant on average. However, a more comprehensive assessment of psychiatric conditions may be required to determine the impact of these symptoms on network topology in breast cancer. FMRI methods are limited in general by their indirect measure of neural functioning.and signal to noise ratio. Other neuroimaging techniques that measure neural signals more directly (e.g. EEG, PET) and/or alternative data analysis methods that are not dependent on parcellation scheme (e.g. independent components analysis) may be useful in further elucidating aberrant network topology in breast cancer.
Despite these limitations, these findings extend our understanding regarding breast cancer-related neurologic injury. We show evidence of an altered connectome following breast cancer and chemotherapy pointing to reduced efficiency of parallel information processing. Data from continued studies of network dysfunction in breast cancer could potentially be utilized to develop indicators of disease progression and develop interventions for cognitive decline by emphasizing treatments that target integrated neural processes.
Highlights.
Global brain network organization is disrupted following breast cancer chemotherapy
Regional network characteristics are altered in frontal, striatal and temporal areas
Breast cancer survivors demonstrate increased report of executive and memory deficits
Acknowledgments
This research was supported by the NIH Director’s New Innovator Award (1 DP2 OD004445-01 to SK)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achard S, Bullmore E. Efficiency and cost of economical brain functional networks. PLoS Computational Biology. 2007;3:e17. doi: 10.1371/journal.pcbi.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ, et al. Longitudinal Assessment of Cognitive Changes Associated With Adjuvant Treatment for Breast Cancer: Impact of Age and Cognitive Reserve. Journal of Clinical Oncology. 2010;28:4434–4440. doi: 10.1200/JCO.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkum-Gamez JN, Laughlin SK, et al. Challenges in the gynecologic care of premenopausal women with breast cancer. Mayo Clinic proceedings. Mayo Clinic. 2011;86:229–240. doi: 10.4065/mcp.2010.0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E. Small-world brain networks. The neuroscientist. 2006;12:512–523. doi: 10.1177/1073858406293182. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E, et al. Hierarchical organization of human cortical networks in health and schizophrenia. J Neurosci. 2008;28:9239–9248. doi: 10.1523/JNEUROSCI.1929-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, et al. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt BC, Chen Z, et al. Graph-Theoretical Analysis Reveals Disrupted Small- World Organization of Cortical Thickness Correlation Networks in Temporal Lobe Epilepsy. Cerebral cortex. 2011;21:2147–2157. doi: 10.1093/cercor/bhq291. [DOI] [PubMed] [Google Scholar]
- Bracken BA, Howell K. Test Review: Clinical Assessment of Depression. Odessa, Fl: Psychological Assessment Resources; 2007. [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Bassett DS. Brain graphs: graphical models of the human brain connectome. Annu Rev Clin Psychol. 2011;7:113–140. doi: 10.1146/annurev-clinpsy-040510-143934. [DOI] [PubMed] [Google Scholar]
- Castellon S, Ganz PA. Neuropsychological studies in breast cancer: in search of chemobrain. Breast Cancer Res Treat. 2009;116:125–127. doi: 10.1007/s10549-008-0211-2. [DOI] [PubMed] [Google Scholar]
- Chen G, Ward BD, et al. Classification of Alzheimer disease, mild cognitive impairment, and normal cognitive status with large-scale network analysis based on resting-state functional MR imaging. Radiology. 2011;259:213–221. doi: 10.1148/radiol.10100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZJ, He Y, et al. Age-related alterations in the modular organization of structural cortical network by using cortical thickness from MRI. Neuroimage. 2011;56:235–245. doi: 10.1016/j.neuroimage.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Church JA, Fair DA, et al. Control networks in paediatric Tourette syndrome show immature and anomalous patterns of functional connectivity. Brain. 2009;132:225–238. doi: 10.1093/brain/awn223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimprich B, Reuter-Lorenz P, et al. Prechemotherapy alterations in brain function in women with breast cancer. J Clin Exp Neuropsychol. 2010;32:324–331. doi: 10.1080/13803390903032537. [DOI] [PubMed] [Google Scholar]
- Correa DD, Shi W, et al. Cognitive functions in primary CNS lymphoma after single or combined modality regimens. Neuro Oncol. 2012;14:101–108. doi: 10.1093/neuonc/nor186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruiter MB, Reneman L, et al. Cerebral hyporesponsiveness and cognitive impairment 10 years after chemotherapy for breast cancer. Hum Brain Mapp. 2011;32:1206–1219. doi: 10.1002/hbm.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis D, Kaplan E, et al. Delis-Kaplan executive function system. New York: Psychological Corporation; 2001. [Google Scholar]
- Deprez S, Amant F, et al. Longitudinal Assessment of Chemotherapy-Induced Structural Changes in Cerebral White Matter and Its Correlation With Impaired Cognitive Functioning. J Clin Oncol. 2012;30:274–281. doi: 10.1200/JCO.2011.36.8571. [DOI] [PubMed] [Google Scholar]
- Deprez S, Amant F, et al. Chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning in breast cancer patients. Human Brain Mapping. 2011;32:480–493. doi: 10.1002/hbm.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich J. Chemotherapy associated central nervous system damage. Advances in experimental medicine and biology. 2010;678:77–85. doi: 10.1007/978-1-4419-6306-2_11. [DOI] [PubMed] [Google Scholar]
- Dietrich J, Han R, et al. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J Biol. 2006;5:22. doi: 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du XL, Xia R, et al. Relationship between chemotherapy use and cognitive impairments in older women with breast cancer: findings from a large population-based cohort. American journal of clinical oncology. 2010;33:533–543. doi: 10.1097/COC.0b013e3181b9cf1b. [DOI] [PubMed] [Google Scholar]
- Ferguson RJ, McDonald BC, et al. Brain structure and function differences in monozygotic twins: possible effects of breast cancer chemotherapy. J Clin Oncol. 2007;25:3866–3870. doi: 10.1200/JCO.2007.10.8639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH, Lai S. Self-navigated spiral fMRI: interleaved versus single-shot. Magn Reson Med. 1998;39:361–368. doi: 10.1002/mrm.1910390305. [DOI] [PubMed] [Google Scholar]
- Gong G, Rosa-Neto P, et al. Age- and gender-related differences in the cortical anatomical network. The Journal of neuroscience. 2009;29:15684–15693. doi: 10.1523/JNEUROSCI.2308-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guye M, Bettus G, et al. Graph theoretical analysis of structural and functional connectivity MRI in normal and pathological brain networks. MAGMA. 2010;23:409–421. doi: 10.1007/s10334-010-0205-z. [DOI] [PubMed] [Google Scholar]
- He Y, Chen Z, et al. Structural insights into aberrant topological patterns of largescale cortical networks in Alzheimer's disease. J Neurosci. 2008;28:4756–4766. doi: 10.1523/JNEUROSCI.0141-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton R. Wisconsin card sorting test computer version 4 [WCST: CV4] Odessa, FL: Psychological Assessment Resources; 2004. [Google Scholar]
- Humphries M, Gurney K, et al. The brainstem reticular formation is a small-world, not scale-free, network. Proceedings of the Royal Society of Biological Sciences. 2006;273:503–511. doi: 10.1098/rspb.2005.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki M, Yoshikawa E, et al. Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer. 2007;109:146–156. doi: 10.1002/cncr.22368. [DOI] [PubMed] [Google Scholar]
- Janelsins MC, Kohli S, et al. An update on cancer- and chemotherapy-related cognitive dysfunction: current status. Seminars in oncology. 2011;38:431–438. doi: 10.1053/j.seminoncol.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelsins MC, Roscoe JA, et al. IGF-1 partially restores chemotherapy-induced reductions in neural cell proliferation in adult C57BL/6 mice. Cancer Invest. 2010;28:544–553. doi: 10.3109/07357900903405942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser M, Hilgetag CC. Nonoptimal component placement, but short processing paths, due to long-distance projections in neural systems. PLoS computational biology. 2006;2:e95. doi: 10.1371/journal.pcbi.0020095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Bennett FC, et al. Regional brain activation during verbal declarative memory in metastatic breast cancer. Clin Cancer Res. 2009;15:6665–6673. doi: 10.1158/1078-0432.CCR-09-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Kent JS, et al. Prefrontal Cortex and Executive Function Impairments in Primary Breast Cancer. Arch Neurol. 2011;68:1447–1453. doi: 10.1001/archneurol.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leistedt SJ, Coumans N, et al. Altered sleep brain functional connectivity in acutely depressed patients. Hum Brain Mapp. 2009;30:2207–2219. doi: 10.1002/hbm.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynall ME, Bassett DS, et al. Functional connectivity and brain networks in schizophrenia. The Journal of neuroscience. 2010;30:9477–9487. doi: 10.1523/JNEUROSCI.0333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Mihaylova I, et al. Activation of cell-mediated immunity in depression: Association with inflammation, melancholia, clinical staging and the fatigue and somatic symptom cluster of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;36:169–175. doi: 10.1016/j.pnpbp.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Mar Fan H, Houédé-Tchen N, et al. Menopausal symptoms in women undergoing chemotherapy-induced and natural menopause: a prospective controlled study. Annals of Oncology. 2010;21:983–987. doi: 10.1093/annonc/mdp394. [DOI] [PubMed] [Google Scholar]
- Maslov S, Sneppen K. Specificity and stability in topology of protein networks. Science. 2002;296:910–913. doi: 10.1126/science.1065103. [DOI] [PubMed] [Google Scholar]
- McDonald BC, Conroy SK, et al. Gray matter reduction associated with systemic chemotherapy for breast cancer: a prospective MRI study. Breast Cancer Research and Treatment. 2010;123:819–828. doi: 10.1007/s10549-010-1088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AR. Towards a network theory of cognition. Neural Networks. 2000;13:861–870. doi: 10.1016/s0893-6080(00)00059-9. [DOI] [PubMed] [Google Scholar]
- Milo R, Shen-Orr S, et al. Network Motifs: Simple Building Blocks of Complex Networks. Science. 2002;298:824–827. doi: 10.1126/science.298.5594.824. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Hillary FG, et al. Resting network plasticity following brain injury. PloS one. 2009;4:e8220. doi: 10.1371/journal.pone.0008220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrella JR. Use of graph theory to evaluate brain networks: a clinical tool for a small world? Radiology. 2011;259:317–320. doi: 10.1148/radiol.11110380. [DOI] [PubMed] [Google Scholar]
- Roth RM, Isquith PK, et al. Behavioral Rating Inventory of Executive Function - Adult Version. Lutz, FL: Psychological Assessment Resources; 2005. [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Sanz-Arigita EJ, Schoonheim MM, et al. Loss of 'small-world' networks in Alzheimer's disease: graph analysis of FMRI resting-state functional connectivity. PloS one. 2010;5:e13788. doi: 10.1371/journal.pone.0013788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardi F, Fassina L, et al. Alzheimer's disease, autoimmunity and inflammation. The good, the bad and the ugly. Autoimmun Rev. 2011 doi: 10.1016/j.autrev.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Schagen SB, Muller MJ, et al. Change in cognitive function after chemotherapy: a prospective longitudinal study in breast cancer patients. Journal of the National Cancer Institute. 2006;98:1742–1745. doi: 10.1093/jnci/djj470. [DOI] [PubMed] [Google Scholar]
- Seigers R, Schagen SB, et al. Long-lasting suppression of hippocampal cell proliferation and impaired cognitive performance by methotrexate in the rat. Behav Brain Res. 2008;186:168–175. doi: 10.1016/j.bbr.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Seigers R, Schagen SB, et al. Methotrexate decreases hippocampal cell proliferation and induces memory deficits in rats. Behav Brain Res. 2009;201:279–284. doi: 10.1016/j.bbr.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Morris JC, et al. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Abeta42. J Neurosci. 2010;30:17035–17040. doi: 10.1523/JNEUROSCI.3987-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman DH, Dy CJ, et al. Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5–10 years after chemotherapy. Breast Cancer Res Treat. 2007;103:303–311. doi: 10.1007/s10549-006-9380-z. [DOI] [PubMed] [Google Scholar]
- Sporns O. The human connectome: a complex network. Ann N Y Acad Sci. 2011;1224:109–125. doi: 10.1111/j.1749-6632.2010.05888.x. [DOI] [PubMed] [Google Scholar]
- Sporns O, Chialvo DR, et al. Organization, development and function of complex brain networks. Trends Cogn Sci. 2004;8:418–425. doi: 10.1016/j.tics.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Stern R, W T. Neuropsychological Assessment Battery. Lutz, FL: Psychological Assessment Resources, Inc; 2003. [Google Scholar]
- Stewart A, Collins B, et al. The cognitive effects of adjuvant chemotherapy in early stage breast cancer: a prospective study. Psychooncology. 2008;17:122–130. doi: 10.1002/pon.1210. [DOI] [PubMed] [Google Scholar]
- Supekar K, Menon V, et al. Network analysis of intrinsic functional brain connectivity in Alzheimer's disease. PLoS Computational Biology. 2008;4:e1000100. doi: 10.1371/journal.pcbi.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syrjala KL, Artherholt SB, et al. Prospective neurocognitive function over 5 years after allogeneic hematopoietic cell transplantation for cancer survivors compared with matched controls at 5 years. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:2397–2404. doi: 10.1200/JCO.2010.33.9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Wang J, et al. Hemisphere- and gender-related differences in small-world brain networks: a resting-state functional MRI study. NeuroImage. 2011;54:191–202. doi: 10.1016/j.neuroimage.2010.07.066. [DOI] [PubMed] [Google Scholar]
- Troyer AK, Rich JB. Psychometric Properties of a New Metamemory Questionnaire for Older Adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2002;57:P19–P27. doi: 10.1093/geronb/57.1.p19. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van Wijk BC, Stam CJ, et al. Comparing brain networks of different size and connectivity density using graph theory. PloS one. 2010;5:e13701. doi: 10.1371/journal.pone.0013701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardy J. Cognitive function in breast cancer survivors. Cancer Treat Res. 2009;151:387–419. doi: 10.1007/978-0-387-75115-3_24. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang L, et al. Parcellation-dependent small-world brain functional networks: a resting-state fMRI study. Hum Brain Mapp. 2009;30:1511–1523. doi: 10.1002/hbm.20623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zuo X, et al. Graph-based network analysis of resting-state functional MRI. Frontiers in systems neuroscience. 2010;4:16. doi: 10.3389/fnsys.2010.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhu C, et al. Altered small-world brain functional networks in children with attention-deficit/hyperactivity disorder. Hum Brain Mapp. 2009;30:638–649. doi: 10.1002/hbm.20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale Fourth Edition. San Antonio, TX: The Psychological Corporation; 2008. [Google Scholar]
- Wefel JS, Saleeba AK, et al. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer. 2010;116:3348–3356. doi: 10.1002/cncr.25098. [DOI] [PubMed] [Google Scholar]
- Wefel JS, Vardy J, et al. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. The lancet oncology. 2011;12:703–708. doi: 10.1016/S1470-2045(10)70294-1. [DOI] [PubMed] [Google Scholar]
- Winocur G, Vardy J, et al. The effects of the anti-cancer drugs, methotrexate and 5- fluorouracil, on cognitive function in mice. Pharmacology, biochemistry, and behavior. 2006;85:66–75. doi: 10.1016/j.pbb.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Wu K, Taki Y, et al. Age-related changes in topological organization of structural brain networks in healthy individuals. Hum Brain Mapp. 2011;33:552–568. doi: 10.1002/hbm.21232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, et al. Whole-brain anatomical networks: does the choice of nodes matter? Neuroimage. 2010;50:970–983. doi: 10.1016/j.neuroimage.2009.12.027. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang J, et al. Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biol Psychiatry. 2011;70:334–342. doi: 10.1016/j.biopsych.2011.05.018. [DOI] [PubMed] [Google Scholar]