Summary
Optogenetics is currently the state-of-the-art method for causal-oriented brain research. Despite an increasingly large number of invertebrate and rodent studies showing profound electrophysiological and behavioral effects induced by optogenetics [1,2], only two primate studies have reported modulation of local single-cell activity, but with no behavioral effects [3,4]. Here, we show that optogenetic stimulation of cortical neurons within rhesus monkey arcuate sulcus, during the execution of a visually-guided saccade task, evoked significant and reproducible changes in saccade latencies as a function of target position. Moreover, using concurrent optogenetic stimulation and functional magnetic resonance imaging (aka opto-fMRI, [5,6]) we observed optogenetically-induced changes in fMRI activity in specific functional cortical networks throughout the monkey brain. This is critical information for the advancement of optogenetic primate research models and for initiating the development of optogenetically-based cell-specific therapies with which to treat neurological diseases in humans.
Results
We tested whether optogenetic stimulation of neurons in the posterior and anterior bank of the arcuate sulcus alters behavioral performance of monkeys performing a cognitive task. We first identified cortical patches within the arcuate sulcus that were activated by the saccade task using functional Magnetic Resonance Imaging (fMRI) [7]. Then, using fMRI-guided neuronavigation, we transduced neurons in ventral premotor (F5) and prefrontal cortex (frontal eye fields, FEF) of two monkeys (M1, M2) with AAV5-CAG-ChR2-GFP (fig. 1) and stimulated these cortical patches with blue light. We stimulated with 2 optic fibers simultaneously to increase the number of neurons that were activated within a functional network relative to previous monkey optogenetic studies [3,4]. A visually-guided saccade task with peripheral go-cues, requiring divided attention across multiple peripheral visual field locations [8], was used to test for changes in behavioral performance (fig. 2, see Experimental Procedures for details). In short, this task started with a fixation period after which a green saccade target appeared either on the left or the right side of the fixation point together with four possible white go-cues. The latter were presented either on the vertical meridian (lower or upper visual field) or on the horizontal meridian (left or right). A luminance change of these peripheral go-cues indicated to the animal to make a saccade towards the green target in order to receive a reward.
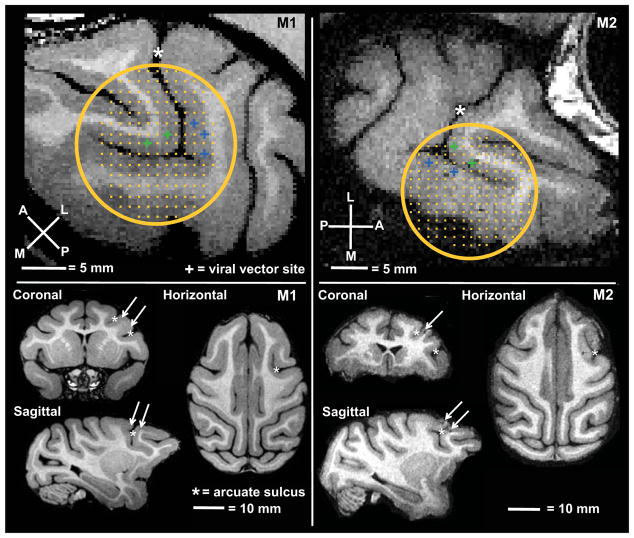
Figure 1. Injection and stimulation targets.
Position of chamber and grid relative to arcuate sulcus (*), stimulation sites in the anterior bank (FEF) are indicated in green, in the posterior bank (ventral premotor cortex) in blue. Arrows indicate optogenetic targets on orthogonal slices of monkey M1 and M2.
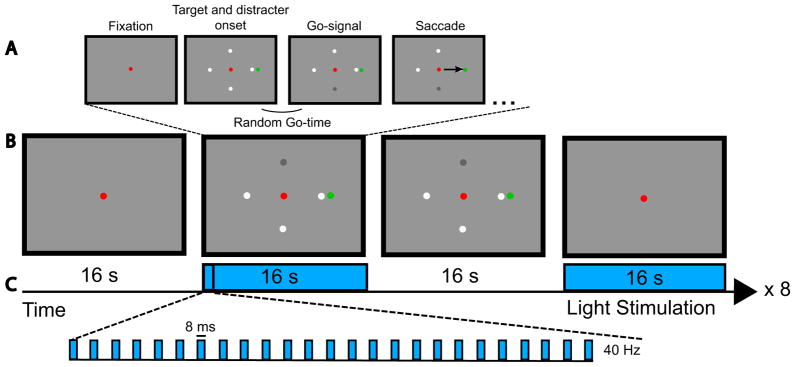
Figure 2. Visually-guided saccade task.
The monkey fixates upon a red fixation point until the four possible go-cues and the green target appear. The change in luminance of one of the four possible go-cues indicates to the monkey to initiate a saccade to the target (A).
Task paradigm and optical stimulation paradigm. We alternated between a fixation epoch, a saccade epoch with stimulation, a saccade epoch without stimulation, and a fixation epoch with stimulation. This sequence was repeated 8 times within a run. Each epoch lasted 16 s (B). During the stimulation epochs (blue) we stimulated at 40 Hz with 8 ms long pulses (C).
We compared behavioral performance during visually-guided saccade trials with and without optical stimulation at cortical sites in the posterior and anterior bank of the arcuate sulcus that were previously injected with AAV-ChR2 (stimulation sessions) (fig. 1). In addition, we also compared the monkey’s behavioral performance between stimulated and non-stimulated trials of control sessions during which the optical fibers were inserted in prefrontal sites that were not previously injected with the viral vector construct -but otherwise using exactly the same experimental paradigm as during the stimulation sessions (see Experimental Procedures). Finally, brain-wide optogenetically-induced functional changes were probed using opto-fMRI [5,6], analogous to previous experiments combining monkey fMRI with electrical stimulation [9].
Behavioral results
In total, we performed 5 stimulation sessions and 4 control sessions in each animal (excluding the lentiviral experiments in M1, see Experimental procedures). Optogenetic stimulation experiments targeting the AAV sites started 130 and 122 days after injection of the viral vectors in M1 and M2, respectively. We obtained 32 and 31 runs from M1 and 52 and 57 runs from M2, during stimulation and control sessions respectively. For the statistical analysis we included only trials from runs in which the monkeys were able to correctly complete > 60% of the trials within the time windows as listed in the Experimental Procedures. Furthermore, we analyzed only trials with saccadic latencies > 50 ms and < 400 ms. This resulted in a total of 1961 and 1680 analyzed trials from M1 and M2, respectively. M1 successfully completed 83.9 ± 1.5 and 81.1 ± 1.9% of the fixation trials during the stimulation and control sessions, respectively. For M2 these figures were 85.6 ± 1.0; 81.6 ± 1.3%. No statistical difference in fixation performance was observed between stimulated and non-stimulated fixation conditions (t-test; p >0.5 for both animals).
Saccade latencies
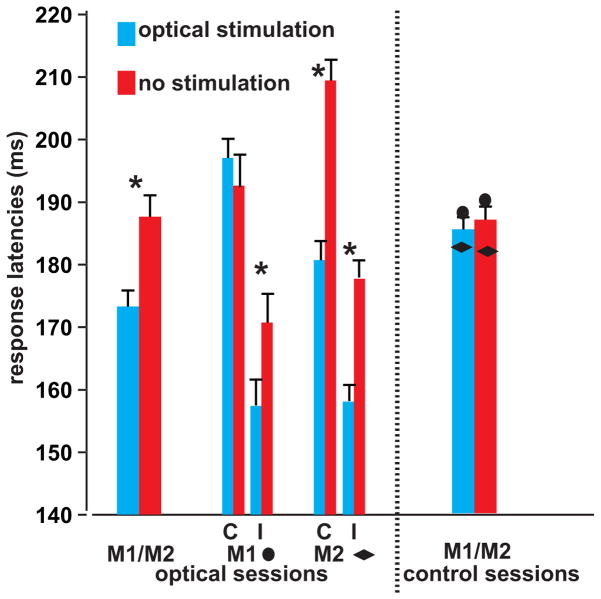
In both animals there was a significant interaction between target position and stimulation (2-way ANOVA, F = 7.92, P = 0.005 for M1 and F = 5.76; p = 0.016 for M2), but not during the control sessions (F = 2.7; p = 0.1 for M1 and F = 3.7 p = 0.06 for M2) (fig. 3). In M1, saccade latencies in stimulated versus non-stimulated trials were shorter for ipsilateral (median of 14 ms, t-test, p = 0.016) but not contralateral targets (median of −4.42 ms, t-test, p = 0.27). For M2, faster saccade latencies were observed for targets presented in both hemifields, although the effect was larger for contralateral saccades. Median differences in saccade latencies measured 20 and 28 ms in M2 for ipsi- and contralateral targets, respectively (t-test, p < 10 e−6 for both targets). A significant interaction for saccade latencies was observed between cue position and stimulation for M1 (F = 6.3, p = 0.01), but not for M2 (F = 1.3, p = 0.25). One could argue that the fixed order of conditions may have contributed to the observed saccade latency effects. This is very unlikely, however, since we used exactly the same order of conditions during the stimulation and control sessions, and there was a highly significant interaction between the type of session (stimulation/control) and trial type (stimulation/no stimulation) (F = 54,9; p = 1.5 e −13, see also fig. 3).
Figure 3. Optogenetically-induced changes in behavior.
Saccadic reaction times of optogenetically-stimulated (blue) and non-stimulated (red) trials during optical stimulation (left) and control (right) sessions. The first 2 bars show the median change in saccadic latencies of the two monkeys for the stimulation sessions. The two groups of four bars show the individual data per monkey and per target location (C = contralateral relative to stimulated hemisphere, I = ipsilateral). The last two bars show the median saccade latencies acquired during the control sessions (single-subject data are indicated by the diamond and circles) (Error bars represent SEM across trials; t-test, * = p < 0.05).
Target detection accuracy and saccade metrics
Accuracy for target detection by the two monkeys was unaltered by light stimulation both during stimulation (median correctly-executed trials across conditions = 77%; with less than 3% difference in accuracy between stimulation and control trials, t-test, p > 0.43 for both animals), and control sessions (p > 0.24 for both animals). Finally, no changes were observed between saccade endpoints of stimulation and control trials (t-test, p > 0.35 for both monkeys), nor were there any differences in the number of saccades (t-test, p > 0.11 for either monkey) or eye blinks (t-test, p > 0.24 for both monkeys) during stimulation and control. Thus, activation of light-sensitive depolarizing ion channels in transduced neurons of the arcuate sulcus results in faster saccadic reaction times as a function of target position.
Functional MRI results
In a case where focal perturbation of brain activity is causally linked to behavior, one expects not only local, but also more extensive network-specific changes in function. To test this prediction, we measured fMRI signals during epochs with and without optogenetic stimulation [5,6] of the foci in the anterior or posterior bank of the arcuate sulcus (fig. 1). Significant optogenetically-induced activations were observed near the site of stimulation in both monkeys (white arrows, fig. 4A). Moreover, clearly different, largely bilateral, functional networks were activated by stimulation of each of these two injection sites. Optical stimulation of control sites, not previously injected with AAV-ChR2, revealed virtually no local or remote fMRI activations (fig. 4A).
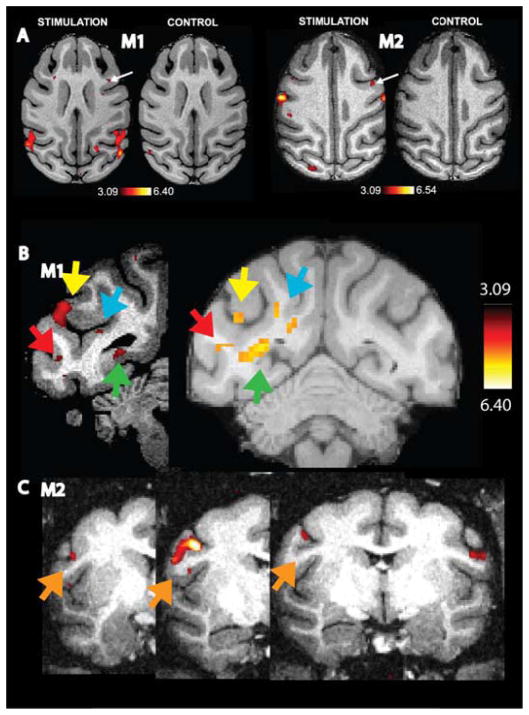
Figure 4. Optogenetically-induced changes in fMRI activity.
t-score maps overlying horizontal T1-weighted images (optical stimulation versus non-stimulation, p < 0.001, uncorrected) after stimulating the anterior (FEF) or posterior bank (F5) of the arcuate sulcus of monkeys M1 and M2. The control panels represent fMRI data after optogenetic stimulation (with exactly the same stimulation parameters, see text) of nearby cortical sites that were not transduced. Only 1 small focus within the entire brain showed an effect at p < 0.001 (uncorrected), which is most likely a false positive result. (A). Coronal T1-weighted images with t-score maps showing reproducible activations from different sessions in visual cortex of M1 (red arrow = area V4; green arrow = peripheral area V1; blue arrow = MSTv; yellow arrow = MSTd) (B) and postcentral sulcus (orange arrow) from different sessions of M2 (C).
Although slightly different locations were stimulated during the various sessions, we observed reproducible activation patterns across sessions of the same subject. For example, fig. 4B shows optogenetically-induced activity in several visual areas across two different sessions in M1 (e.g. MSTd (yellow arrow), MSTv (blue arrow), V4 (red arrow) and peripheral V1 (green arrow). In M2, to take another example, we observed reproducible patterns of optogenetic induced activity across 3 sessions in the post central sulcus (orange arrow, fig. 4C).
Discussion
Optogenetics is a recently-developed method to increase and decrease the activities of specific neurons with high temporal resolution in order to relate their function to behavior, and to make causal inferences about the role they play, both within local micro-circuitry and across macroscopic functional brain networks [1,2]. Critical with regard to future translational purposes, optogenetics presents a promising method for manipulating activity in distinct cell types that are relevant to specific neurological diseases [10,11]. This novel method has been shown to evoke clear behavioral and neuronal effects in invertebrates [12] and rodents [13,14]. However, despite some evidence that optogenetics can also alter single unit activity in macaques [3,4], no study has reported optogenetically-induced behavioral or functional network changes in primates –which is of critical importance for translational purposes. Possibly the number of optically-stimulated neurons needs to be substantially greater in primates than in animals with simpler brains to evoke any behavioral effects. Alternatively, previous primate optogenetic studies may have failed to reveal psychophysical or motor changes, because behavioral tests used thus far have been insufficiently sensitive.
In this study, we attempted to overcome these potential issues by injecting a viral vector construct under the control of a cell-type non-specific CAG promoter in order to stimulate as many neurons as possible. Additionally, we made an effort to stimulate larger pools of neurons within a specific functional network by using neuronavigation to target the viral injections based on task-related fMRI data, and by using 2 optical fibers simultaneously.
In both monkeys, we found significant optogenetically-induced decreases in saccadic latencies as a function of target position. Target detection accuracy and eye-movement metrics were unaffected by optogenetic stimulation of neurons in the right arcuate sulcus. Although the sign of the behavioral effects (shortening of saccadic latencies) was the same for both subjects, the lateralization of the effect was not identical across animals. We suspect this may be due to slightly different positions of the optic fiber pairs in the two animals. Alternatively, it may be related to the first series of lentiviral experiments performed in M1 which could have damaged cortex in the contralateral arcuate sulcus, and which may have triggered compensatory mechanisms in M1. It is noteworthy that after the reported series of sessions during which the 200 μm optical fibers were inserted in the brain (~ 20 times), we were no longer able to reproduce behavioral or functional effects (in 2 final stimulation sessions, not included in the present results), which we attribute to local cortical damage. Therefore, to improve the efficacy of the optogenetic technique in monkeys, we suggest that future studies should use chronically implanted rather than acute optical fibers or optrodes.
Since we observed a systematic interaction between target position and stimulation but not between cue position and stimulation, and because no changes in target detection accuracy and eye movement metrics were measured, it is tempting to speculate that mechanisms related to saccade planning rather than attentional or saccade execution mechanisms were those affected by the optogenetic stimulation.
We conclude that optogenetics can be used to alter not only focal but also more global network functions in primates, which is presumably critical for eliciting changes in behavioral performance. The evidence that optogenetics can alter behavior in monkeys opens exciting possibilities for dissecting causal relationship between neural circuits and behavior in primates using cell-specific promoters and to develop optogenetically-based therapies for treating neurological diseases in humans.
Experimental Procedures
Viral vector injections
In M1 two different constructs were injected which both contained the reporter gene GFP and the membrane channel channelrhodopsin-2 (ChR2), but under the control of different promoters. The first was a lentiviral (LVV) construct consisting of a specific CaMKII promoter to target only excitatory cells, as used previously in monkeys by Han and colleagues [3]. This LVV construct was injected into the posterior and anterior bank of the left arcuate sulcus. The second adeno-associated viral vector construct (AAV) serotype 5 under control of the non-specific CAG promoter [4] was injected into the right frontal cortex (fig. 1). No behavioral or functional MRI effects were obtained after stimulation of the targeted LVV sites in the left arcuate sulcus of M1 (5 stimulation sessions). Therefore, we injected only the AAV-construct in the right frontal cortex of M2 and we focused exclusively on results obtained after stimulating the sites injected with the AAV construct.
All injections were made through a grid placed in an implanted chamber over the right arcuate sulcus (Crist Instrument) (fig. 1). The 3-dimensional coordinates of the injection targets were determined using Brainsight neuronavigation and were based on prior fMRI activations for the visually-guided saccade task. We injected only in cortical sites activated by the saccade task relative to the fixation task (i.e. in cortical tissue corresponding to voxels with p< 0.001, uncorrected). In the target sites, a total of 1 μl (per site) of the viral vector solution (1.1 × 109 genome copies per μl) was slowly delivered with a 5 μl Hamilton syringe at a rate of 0.2 μl every 2 minutes at 2 cortical depths per site.
Behavioral tasks
In the visually guided saccade task with multiple possible go-cues, the animals had to maintain fixation in a virtual window of maximum 2 × 2 degrees around a small red spot in the center of the display for a fixed duration of 700–1400 ms. Thereafter, a single green saccade target and four possible white go-cues appeared (fig. 2). Target and go-cues were equal in size (0.14°) and luminance (6 cd/m2). The saccade target appeared either on the left or right side of the fixation point at 10 degrees eccentricity along the horizontal meridian. The possible go-cues appeared at 8 degrees eccentricity, either on the vertical meridian (lower or upper visual field) or on the horizontal meridian (left or right). After a variable delay of 700 to 1400 ms, one of the randomly-selected go-cues would turn grey. This change was the go-signal indicating to the animal to make a saccade towards the green target. Note that all four peripheral go-cues had to be attended by the animal to detect the go-signal as quickly as possible. The monkey was rewarded for making a saccade towards the green target within 700 ms after the go-signal and for maintaining fixation within a 3–4 degree window around the target for 200 ms. To encourage rapid responses, reward size varied as an exponential function of reaction time (RT) between 150 and 700 ms after the go-signal. The time between target onset and the go-signal was a random variable drawn from a unimodal Weibull distribution delayed by 500 ms [15]. Saccades were detected using a computer algorithm that searched first for significantly elevated velocity (>30°/s). Saccade i nitiation and termination were then defined as the beginning and end of the monotonic change in eye position before and after the high-velocity gaze shift.
During the fixation task the monkeys were rewarded with juice for fixating upon a red fixation point in the center of an otherwise empty screen within a 2 X 2 degree window. The average duration of the fixation trials was the same as the saccade trials.
Stimulation paradigm
Stimulation sessions utilized two fibers simultaneously in the posterior and anterior banks of the arcuate sulcus, at sites were viral injections had been made. Light pulses (40 Hz, 8 ms pulses) were administered with two 473 nm blue lasers (Shanghai Laser & Optics Century), coupled to two optical fibers 200 μm in diameter. The light intensity at the fiber tip ranged from 80–300 mW/mm2, as measured before and after each session with a PM 100D power meter (Thorlabs). We alternated between a fixation epoch without stimulation, a saccade epoch with stimulation, a saccade epoch without stimulation and a fixation epoch with stimulation. This sequence was repeated 8 times within a given 512 s run. In each 16 s task epoch both monkeys were able to complete 4 ± 1 saccade trials.
During control sessions, we stimulated with fibers inserted at nearby prefrontal locations (3.5–8.5 mm removed from the previous sites, mainly in the anterior portion of the superior branch of the arcuate sulcus and in the principal sulcus) but without prior viral vector injections. We used exactly the same experimental paradigm as described above for the stimulation sessions and monkeys performed the same number of trials per task epoch as in the stimulation sessions (4 ± 1 trials/epoch).
fMRI data acquisition
Contrast-agent-enhanced functional images were acquired using a four-channel phased array coil (GRAPPA, acceleration factor 2) on a 3 Tesla TIM-Trio scanner (Siemens) with AC88 gradient coil, using a gradient-echo T2*-weighted echo-planar sequence (62 and 64 sagittal slices for M1 and M2, respectively; 102 × 106 matrix, TR = 2 s, TE = 17 ms, 1 mm isotropic voxels) [7]. We performed single-subject analyses and compared the stimulation condition to non-stimulation (p < 0.001 uncorrected, t > 3.09).
Highlights.
Optogenetics induces behavioral changes in monkeys
Optogenetics evokes fMRI signal changes in functional networks of macaques
Saccade latencies decrease by optogenetic stimulation of the arcuate sulcus
Acknowledgments
We thank S Verstraeten, H Deng, M Depaep, E Premereur, M. Deforche, S. Raiguel, B Verhoef, S Rozzi and G. Luppino for their help and comments. This work was supported by Inter-University Attraction Pole 7/21, Programme Financing PF/10/008, Geconcerteerde Onderzoeks Actie 10/19, Hercules foundation, Fulbright foundation, FWO G062208N10 and G043912N, and IWT- SBO 110068. The Martinos Center is supported by National Center for Research Resources grant P41RR14075. ESB is a shareholder of Eos Neuroscience.
Footnotes
All other authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Deisseroth K. Optogenetics. Nat Methods. 2011;8:26–29. doi: 10.1038/nmeth.f.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71:9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Han X, Qian X, Bernstein JG, Zhou HH, Franzesi GT, Stern P, Bronson RT, Graybiel AM, Desimone R, Boyden ES. Millisecond-timescale optical control of neural dynamics in the nonhuman primate brain. Neuron. 2009;62:191–198. doi: 10.1016/j.neuron.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diester I, Kaufman MT, Mogri M, Pashaie R, Goo W, Yizhar O, Ramakrishnan C, Deisseroth K, Shenoy KV. An optogenetic toolbox designed for primates. Nat Neurosci. 2011;14:387–397. doi: 10.1038/nn.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JH, Durand R, Gradinaru V, Zhang F, Goshen I, Kim DS, Fenno LE, Ramakrishnan C, Deisseroth K. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature. 2010;465:788–792. doi: 10.1038/nature09108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahn I, Desai M, Knoblich U, Bernstein J, Henninger M, Graybiel AM, Boyden ES, Buckner RL, Moore CI. Characterization of the functional MRI response temporal linearity via optical control of neocortical pyramidal neurons. J Neurosci. 2011;31:15086–15091. doi: 10.1523/JNEUROSCI.0007-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vanduffel W, Fize D, Mandeville JB, Nelissen K, Van HP, Rosen BR, Tootell RB, Orban GA. Visual motion processing investigated using contrast agent-enhanced fMRI in awake behaving monkeys. Neuron. 2001;32:565–577. doi: 10.1016/s0896-6273(01)00502-5. [DOI] [PubMed] [Google Scholar]
- 8.Premereur E, Vanduffel W, Janssen P. Functional heterogeneity of macaque lateral intraparietal neurons. J Neurosci. 2011;31:12307–12317. doi: 10.1523/JNEUROSCI.2241-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ekstrom LB, Roelfsema PR, Arsenault JT, Bonmassar G, Vanduffel W. Bottom-up dependent gating of frontal signals in early visual cortex. Science. 2008;321:414–417. doi: 10.1126/science.1153276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deisseroth K. Optogenetics and psychiatry: applications, challenges, and opportunities. Biol Psychiatry. 2012;71:1030–1032. doi: 10.1016/j.biopsych.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 11.Karayiorgou M, Flint J, Gogos JA, Malenka RC, Bargmann CI, Boyden ES, Bullmore ET, Chan AW, Davis M, Deisseroth K, et al. The best of times, the worst of times for psychiatric disease. Nat Neurosci. 2012;15:811–812. doi: 10.1038/nn.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagel G, Brauner M, Liewald JF, Adeishvili N, Bamberg E, Gottschalk A. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr Biol. 2005;15:2279–2284. doi: 10.1016/j.cub.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 13.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 14.Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de LL, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janssen P, Shadlen MN. A representation of the hazard rate of elapsed time in macaque area LIP. Nat Neurosci. 2005;8:234–241. doi: 10.1038/nn1386. [DOI] [PubMed] [Google Scholar]