Abstract
Background
CXCR4 and CXCR7 are seven transmembrane chemokine receptors of the stroma-derived factor (SDF-1). CXCR4, but not CXCR7, has been examined in bladder cancer (BCa). We examined the functional and clinical significance of CXCR7 in BCa.
Methods
CXCR4 and CXCR7 levels were measured in BCa cell lines, tissues (normal = 25; BCa = 44) and urine specimens (n=186) by quantitative PCR and/or immunohistochemistry. CXCR7 function in BCa cells were examined by transient transfections using a CXCR7 expression vector or siRNA.
Results
In BCa cell lines CXCR7 mRNA levels were 5–37-fold higher than CXCR4 levels. Transient overexpression of CXCR7 in BCa cell lines promoted growth and chemotactic motility. CXCR7 co-localized and formed a functional complex with EGF-receptor, PI3-kinase/Akt, Erk and src and induced their phosphorylation. CXCR7 also induced upregulation of cyclin-D1 and bcl-2. Suppression of CXCR7 expression reversed these effects and induced apoptosis. CXCR7 mRNA levels and CXCR7 staining scores were significantly (5–10-fold) higher in BCa tissues than in normal tissues (P<0.001). CXCR7 expression independently associated with metastasis (P=0.019) and disease specific mortality (P=0.03). CXCR7 was highly expressed in endothelial cells in high-grade BCa tissues when compare to low-grade BCa and normal bladder. CXCR7 levels were elevated in exfoliated urothelial cells from high-grade BCa patients (P=0.0001; 90% sensitivity; 75% specificity); CXCR4 levels were unaltered.
Conclusion
CXCR7 promotes BCa cell proliferation and motility plausibly through EGF-receptor and Akt-signaling. CXCR7 expression is elevated in BCa tissues and exfoliated cells and is associated with high-grade and metastasis.
Keywords: CXCR7, bladder cancer, molecular marker, metastasis, CXCR4
INTRODUCTION
Tumor heterogeneity in terms of metastasis and frequent recurrence make the clinical management of BCa (BCa) challenging (1). While several determinants of growth and metastasis have been characterized in BCa, the functional and clinical significance of chemokine receptor CXCR7 has not been evaluated. CXCR7/RDC-1 binds chemokines CXCL11/I-TAC and CXCL12 (2–5). Both of these are cysteine-X-cysteine chemokines that lack the glutamine-leucine-arginine (or ELR) motif (2). CXCL12 or stromal cell derived growth factor-1 (SDF-1) promotes angiogenesis and metastasis by binding to another chemokine receptor, CXCR4 (2–5).
Both CXCR7 and CXCR4 are seven-span transmembrane G-protein coupled receptors (2–4). Once CXCL12 binds CXCR4, the receptor complexes with the Gαi subunit of G-protein. This results in the inhibition of cAMP production and mobilization of intracellular calcium. The dissociation of Gαi subunit from Gβγ activates, among others, MAP-kinase and Akt signaling (6). CXCR4 expression either alone or together with CXCL12 or CXCR7 correlates with metastasis in breast and renal carcinomas (7–9). CXCR4 expression is increased in bladder tumor tissues; however, the expression does not correlate with prognosis (10–12). A peptide antagonist of CXCR4 has been shown to aid in fluorescent imaging of exfoliated cells in the urine of patients with invasive BCa (13).
CXCR7 binds to CXCL12 with high affinity, but it has low affinity for CXCL11. It does not activate the Gi pathway and consequently does not induce calcium mobilization (4). CXCR7 expression is elevated in renal and lung tumors and correlates with metastasis (14–15). In breast, prostate and lung xenografts models, CXCR7 expression stimulates proliferation, invasion and motility, and promotes tumor growth and angiogenesis (16–19). In prostate cancer cells, IL-8 expression increases CXCR7 expression by 4–14-fold (19).
In this study, we found that CXCR7 expression in BCa cells regulated proliferation, chemotactic motility and a complex formation between signaling molecules, EGF-receptor, PI3-kinase and src. CXCR7 expression was upregulated in bladder tumor tissues and exfoliated urothelial cells present in the urine of patients with BCa, and associated with prognosis.
MATERIALS AND METHODS
Cell culture
BCa cells were obtained from ATCC, except, 253J-Lung cells were provided by Dr. Colin Dinney, MD Anderson Cancer Center, TX. All BCa cell lines except the 253J-Lung cell line were isolated from primary high-grade muscle invasive tumors. 253J-Lung cells metastasize to lung when injected orthotopically in athymic mice (20 and ATCC website). All BCa cells were cultured in RPMI1640 + 10% fetal bovine serum + gentamicin. Cell lines were authenticated by Genetica® DNA Laboratories Inc (Cincinnati OH).
Antibodies
All antibodies are described previously (21), except the following: CXCR7: GeneTex (Irvine, CA), phospho-src (Y-416), src: Cell Signaling Technology (Danvers MA), EGFR, CXCR4: Sigma Aldrich (St. Luis, MO), phospho-EGFR (Y-1173): Epitomics (Burlingame, CA).
Tissue specimens
All specimens were obtained based on their availability for research purpose and under a protocol approved by University of Miami’s Institutional Review Board (Table 1). Normal bladder tissues (NBL; n=25) were obtained either from organ donors or from patients who underwent cystectomy. A portion of each BCa (n=44) and NBL tissue was paraffin embedded and the other was flash frozen. Total RNA was isolated from tissues (~ 30 mg) using the RNeasy Mini kit.
Table 1. Specimen and patient characteristics.
Characteristics of bladder tissue and urine specimens are shown. BGU: patients with benign genitourinary conditions. HX: history of
| Tissue specimens n= 69 (NBL = 25) Organ donors (NBL-O) = 17 BCa patients undergoing cystectomy (NBL-B) = 8 |
Urine Specimens (n = 186) | ||
|---|---|---|---|
|
| |||
| BCa = 44; Transurethral resection (TURBT) = 11 Cystectomy = 33 |
Normal | n = 27 Group 1: n = 17; Age: 38.3 ±13.9; Median: 30.5 yrs Gender: Female = 6; Male = 12 Group 2: n = 10 Age: 63.2 ± 10.1; Median: 61 yrs Gender: Female = 6; Male = 4 |
|
|
| |||
| Gender | Female n = 9 Male, n = 35 |
BCa | n = 57 LG = 17; HG = 40 Stage: Ta = 19; CIS = 1; T1 = 15; T2 = 13; T3 = 8; T4 = 1 Age: 68.9 ± 9.2; 69 Gender: Female = 20; Male = 37 |
|
| |||
| Smoker | +) = 25 (−): 2 Unknown: 17 |
BGU | n = 55 Urinary tract infection = 8 Benign prostatic hyperplasia = 7 Hematuria = 10 Hydronephrosis = 1 Nephrolithiasis = 13 Prostatitis = 5 Hydrocele = 1 Dysuria = 1 Urethral stricture = 4 Impotence = 1 Renal cyst = 3 Adrenal mass = 1 |
| Age = 54.3 ± 12.7; 55 Gender: Female = 23; Male = 32 |
|||
|
| |||
| Grade | LG = 7 (all, stage Ta) HG = 37 |
HXBCa | n = 30 Age: 65.1 ± 7.6; 63.5 Gender: Female = 7; Male = 23 |
|
| |||
| HX other cancers | n = 8 Hx Renal cancer n = 2 Hx prostate cancer = 6 Age: 62.9 ± 12.9; 62.5 Gender: Female = 3; Male = 5 |
||
|
| |||
| Stage | Ta = 8; T1 = 3 T2 = 12; T3 = 16; T4 = 5 Concomitant CIS was present in 4 patients |
Other cancers | N = 9 Prostate cancer n = 7 Rectal cancer metastatic to bladder = 1 |
| Cervical cancer = 1 Age: 65.7 ± 9.6; 64 Gender: Female = 2; Male = 7 |
|||
|
| |||
| LN | (+) = 11 (−) = 26 Unknown =7 |
||
|
|
|||
| Metastasis (all BCa patients) | (−) = 20 (+) = 16 Unknown = 8 |
||
| Metastasis in patients with stage ≥ T2 tumor | (−) = 9 (+) = 16 Unknown = 7 |
||
|
|
|||
| Age | Metastasis (+): 65.7 ± 12.1yrs Median: 68 yrs |
||
| Metastasis (−): 64.1 ± 10.9 yrs Median 63 yrs |
|||
|
|
|||
| Neoadjuvant Chemotherapy | (+) = 8 (−) = 24 Unknown = 12 |
||
|
|
|||
| Adjuvant Chemotherapy | (+) = 6 (−) = 22 Unknown = 16 |
||
|
|
|||
| Radiation | (+) = 6 (−) = 19 Unknown = 19 |
||
|
|
|||
| Death | (−) = 23 (+) = 18; BCa specific = 16 Unknown = 3 |
||
|
|
|||
| Mean Follow-up | 26.8 ± 4.4; median = 20.5 (1* – 120 months) | ||
| For patients with stage ≥ T2 tumor | 19.1 ± 2.2; median = 17.5 (1*–41) | ||
Note:
All stage T4 patients were considered as positive for metastasis at the time of diagnosis.
Urine specimens
Urine specimens were collected from 186 individuals (Table 1). Clinical follow-up was collected on patients with HxBCa. All urine specimens were brought to the laboratory within two hours of collection and processed for total RNA isolation using the ZR urine isolation kit™ (22).
Transfection of BCa cells with CXCR7 plasmid and CXCR7 siRNA
HT1376 and 253J-Lung cells were transiently transfected with a full length CXCR7 cDNA construct (Origene) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). 5637 cells were transfected with CXCR7 siRNA or control siRNA (100 nM; Dharmacon-RNAi Technologies; Thermo Scientific). 72-hours following transfection, the transfectants were counted and apoptosis was measured by Cell Death ELISA Plus kit. Apoptosis index was calculated as O.D 450 nm per 20,000 cells. Chemotactic motility assays was carried out using the Boyden chamber (8-μM inserts), as described previously (21). Percent motility was calculated as (O.D. bottom chamber ÷ O.D. (top + bottom chambers)) ×100. Cell lysates were analyzed by immunoblotting.
Co-immunoprecipitation
Vector and CXCR7 transient transfectants of 253J-Lung cells (2×106 cells) were immunoprecipitated using a rabbit anti-CXCR7 antibody and protein A-agarose beads (19). The immunoprecipitates were subjected to immunoblotting using anti-CXCR7, anti-src antibodies under non-reduced conditions and anti-EGFR, anti-85 PI3-kinase antibodies under reduced conditions.
Q-PCR
Total RNA isolated from transfectants, tissues or exfoliated cells was subjected to Q-PCR in a Bio-Rad iCycler iQ real time PCR system (22) using the following primers: CXCR4: Forward: 5′TCATCAAGCAAGGGTGTG3′; Reverse: 5′GGCTCCAAGGAAAGCATAGA3′; CXCR7: Forward: 5′TACCCCGAGCACAGCATCAA3′; Reverse: 5′TGGAGAAGGGAACGGCAA AG3′; SDF1-α: Forward: 5′CACAGAAGGTCCTGGTGG3′; SFD1-β: Forward: 5′CGCCTTTCCC AGGTGCTACA3′; Reverse: 5′TGGTCTGCTTAGGGGATTTGGA3′. Each cDNA sample was simultaneously subjected to β-actin (for tissues and transfectants) or 18S (urine specimens) Q-PCR and the normalized transcript levels for each gene were calculated as (1/2Δct × 100); ΔCt = Ct (transcript) – Ct (β-actin).
In-cell Co-IP
Transient transfectants of 253J-Lung cells were fixed in 4% paraformaldehyde at 4°C for 15 minutes and then subjected to the “In-cell co-IP”, a fluorescence-PCR-based antibody binding technique, using rabbit anti-CXCR7 (1:500), mouse anti-EGFR (1:500) antibodies and the Duolink®II kit, as per the manufacturer’s instructions (Olink-Bioscience, Uppsala, Sweden). The slides were observed under a Zeiss LSM700 Confocal microscope equipped with multi-variant fluorescence filters in two channels (red and blue) under a 40× oil-immersion lens.
Immunohistochemistry
Five-micron sections of paraffin-fixed bladder tissues on positively charged slides were sequentially deparaffinized, rehydrated and subjected to antigen retrieval (22). The slides were incubated at 37°C and room temperature for 1 hour each, with a rabbit anti-CXCR7 (1:4000) or a rabbit anti-CXCR4 (1:1750) antibody. Immunohistochemistry procedure and all the controls were done as described previously (22). The same batch of antibodies and commercial reagents were used in all experiments. The specificity of the antibodies was determined by downregulation of CXCR7 and CXCR4 by siRNA, followed by immunoblotting.
Stained slides were graded by two individuals in a blinded fashion. To account for the heterogeneity in staining, each specimen was graded for staining intensity (0 to 3 +) and then multiplied by the area in the specimen showing that staining intensity. The intensity scores in all areas within a specimen were added to obtain the staining score for the specimen. Therefore, each specimen received a staining score between 0 and 300 (22). The intensity scores of the two readers were then averaged to obtain the final score.
The In-cell co-IP was performed on paraffin fixed bladder tissues using the same procedure as the immunohistochemistry up to the step of antigen retrieval. The specimens were stained using the rabbit anti-CXCR7 (1:300) and mouse anti-CD31 (GeneTex; 1:300) antibodies at 37°C for 1 hour and the Duolink®II kit, as per the manufacturer’s instructions (Olink-Bioscience, Uppsala, Sweden). The slides were observed by confocal microscopy.
Statistical analyses
Differences in proliferation, apoptosis and motility among transfectants were compared using the unpaired t-test. CXCR7 and CXCR4 levels among bladder tissues (e.g., NBL versus low-grade, NBL versus high-grade) and among various categories of urine specimens were compared using the Mann-Whitney U-test, because the data showed a non-normal distribution. All of the P-values reported in this study are two-tailed. Logistic regression model (univariate analysis) was used to determine: 1. the association of clinical parameters, and CXCR7 levels (i.e., transcript levels or staining scores) with metastasis and disease-specific survival; 2. the association of urinary CXCR7 levels with BCa. Cox-proportional hazards model (multivariate analysis) was used to determine which of the pre- and post-operative parameters and/or tissue CXCR levels predict metastasis and disease-specific mortality.
Cut-off values were selected from the ROC curve for calculating sensitivity and specificity of CXCR7 to predict metastasis, disease-specific survival (tissue specimens) and the presence of BCa (urine specimens). A biomarker level that yielded the highest efficacy (i.e., sensitivity – (1-specificity)) was selected by the statistical program as the cut-off limit. JMP® Software Program (SAS Institute, Cary, NC) was used for statistical analyses.
RESULTS
Expression of CXCR7 and CXCR4 in BCa cell lines
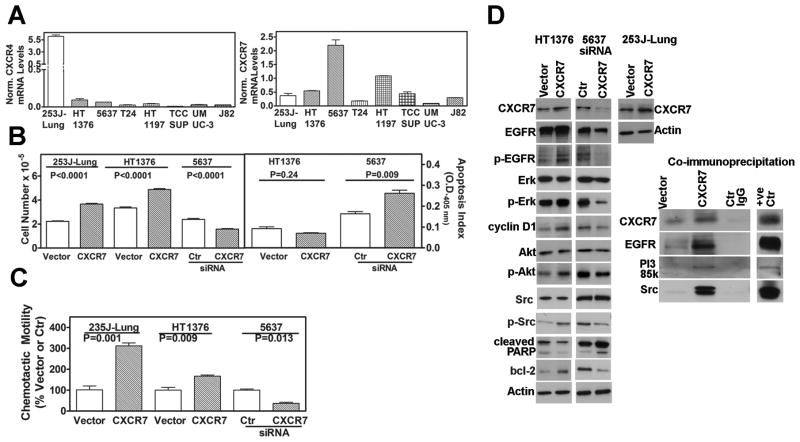
In all BCa cell lines, except for 253J-Lung, CXCR7 mRNA levels were 5–37-fold higher than CXCR4 levels (Figure 1A). Furthermore, the expression of CXCR7 in 5637 and HT1197 cell lines was 3–10-fold higher than in other cell lines.
Figure 1. CXCR7 expression and function in bladder cancer cells.
A: Analysis of CXCR4, CXCR7 mRNA levels in BCa cells, by Q-PCR. Data: Mean±sd. CXCR4 and CXCR7 values: 23J-Lung: 6.2±0.4, 0.4±0.1; HT1376: 0.1±0.02 0.6±0.02; 5637: 0.06±0.001, 2.2±0.3; T24: 0.03±0.006, 0.2±0.01; HT1197: 0.04±0.005, 1.1±0.1; TCCSUP: 0.01±0.007, 0.5±0.1; UMUC-3: 0.03±0.01, 0.1±0.007; J82: 0.03±0.002, 0.3±0.007 B: Effect of CXCR7 expression on growth and apoptosis. Left panel: Cell counting data at 72 hours following transfection either with vector, CXCR7 (253J-Lung and HT1376) or CXCR7 siRNA (5637). 253J-Lung: vector: 2.2±0.12, CXCR7: 3.7±0.17; HT1376: vector: 3.5±0.21, CXCR7: 4.9±0.22. 5637: control siRNA: 2.4±0.22, CXCR7 siRNA: 1.6±0.14. Right panel: Transfectants were assayed for apoptosis; Apoptosis index for each transfectant is presented. Data: Mean ± sd. HT136: vector: 0.09±0.02, CXCR7: 0.07±0.005; 5637: control siRNA: 0.16±0.02, CXCR7 siRNA: 0.26±0.03. C: Determination of the chemotactic motility of the CXCR7 overexpressing (HT1376, 253J-Lung) and CXCR7 siRNA (5637) transfectants. Data: Mean±sd. HT1376: vector: 100±33, CXCR7: 311±25; 253J-Lung: 100±22, CXCR7: 167±10; 5637: control siRNA: 100±7, CXCR7 siRNA: 37±7 D: Analysis of CXCR7 transfectants. HT1376 and 253J-Lung cells were transiently transfected with a CXCR7 plasmid. 5637 cells were transfected with CXCR7 siRNA. Cell lysates were analyzed by immunoblotting; loading control: actin. Right panel: Cell lysates of the 253J-Lung vector and CXCR7 transfectants were immunoprecipitated using an anti-CXCR7 antibody and the immunoprecipitates were immunoblotted for various antigens.
CXCR7 enhances proliferation and motility in BCa cells
To determine the functional significance of CXCR7, we either transiently overexpressed CXCR7 in 253J-Lung and HT1376 cells or downregulated CXCR7 expression in 5637 cells. CXCR7 overexpression or downregulation was confirmed by Q-PCR (data not shown). CXCR7 overexpression increased whereas CXCR7 downregulation decreased the growth of respective transfectants by 1.5-fold (P<0.0001; Figure 1B). Downregulation of CXCR7 also significantly decreased the growth of HT1197 cells (Cell number: control: 6.5±0.45×104; CXCR7 siRNA: 3.4±0.25; P<0.001).
CXCR7 expression regulates apoptosis (23,24). CXCR7 downregulation in 5637 cells induced apoptosis (Figure 1B right panel; apoptosis index: control: 0.16±0.02; CXCR7 siRNA: 0.26±0.03). The downregulation of CXCR7 in HT1197 also induced apoptosis (control: 0.1±0.015; CXCR7 siRNA: 0.21±0.025; P=0.0027). Low basal level of apoptosis was observed in HT1376 cells (0.09±0.02) and CXCR7 overexpression caused a small decrease (0.07±0.006) in this basal level of apoptosis (Figure 1B right panel).
As shown in Figure 1C, CXCR7 overexpression also increased the chemotactic motility in HT1376 and 253J-Lung cells by 1.7- and 3.1-fold, respectively. Conversely, CXCR7 downregulation decreased the motility by 2.7-fold in HT1376 cells.
CXCR7 expression regulates EGF-receptor, Erk and Akt activation
Figure 1D shows CXCR7 overexpression and downregulation in various transfectants. CXCR7 expression has been shown to regulate cell cycle progression by modulating cyclin D1 levels (19). Consistent with the effect of CXCR7 on cell growth, CXCR7 overexpression increased (> 2-fold) and CXCR7 downregulation decreased (~ 2-fold) cyclin D1 levels in HT1376 and 5637 cells, respectively (Figure 1D). Consistent with the increase in apoptosis, CXCR7 downregulation induced PARP cleavage in 5637 cells (Figure 1D).
CXCR7 has been shown to form a functional complex with EGFR (19). As shown in Figure 1D, CXCR7 overexpression increased EGF-receptor (Tyr1173) phosphorylation by > 2-fold and CXCR7 downregulation, inhibited EGFR phosphorylation. CXCR7 overexpression or downregulation did not affect EGF-receptor mRNA levels (data not shown). In-cell Co-IP coupled with confocal microscopy showed that both EGF-receptor and CXCR7 co-localize on the plasma membrane of 253J-Lung cells transfected with a CXCR7 construct (Figure 2A).
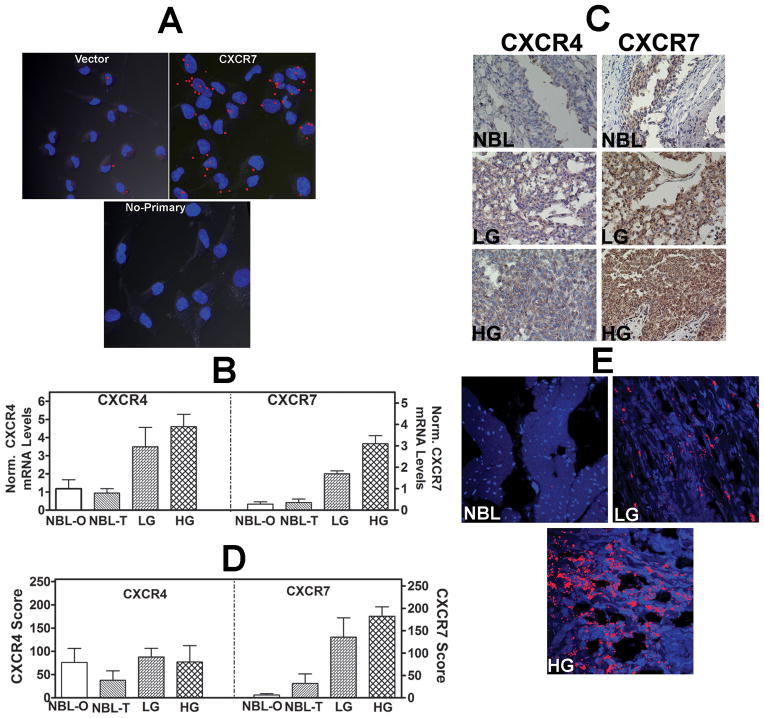
Figure 2. Analysis of CXCR7 transfectants and expression of CXCR4 and CXCR7 in bladder tissues.
A: In-cell co-IP of 253J-Lung vector and CXCR7 transfectants using the anti-CXCR7 and anti-EGFR antibodies; lower panel, no primary antibody control for the CXCR7 transfectant. Red dots indicate co-localization of CXCR7 and EGF-R. Magnification: 400×. B: CXCR4 and CXCR7 transcript levels in bladder tissues. NBL: normal bladder; NBL-O: NBL tissue obtained from organ donors; NBL-T: NBL tissue obtained from BCa patients at the time of cystectomy. LG: low-grade BCa; HG: high-grade BCa. The mean±sd levels are shown. C: CXCR4 and CXCR71 were localized in normal bladder, LG and HG BCa tissues by IHC. Representative specimens from each category are shown. D: Mean±sd staining scores of CXCR4 and CXCR7 in bladder specimens are shown. Due to poor fixation which resulted in the loss of tissues during staining, 22 high-grade, 7 low-grade tissues and 15 NBL tissues could be stained. E: Localization of CXCR7 in bladder endothelial cells. Bladder tissues were stained with anti-CD31 (endothelial cell marker) and CXCR7 using the in-cell co-IP technique. Red staining represents co-localization of CD31 and CXCR7, i.e., localization of CXCR7 in endothelial cells. Magnification: 400×.
Erk- and PI3-kinase/Akt signaling is downstream of EGF-receptor activation, and CXCR7 induces Erk and Akt activation (19,24,25). Overexpression of CXCR7 increased phospho-Erk, phospho-Akt levels ~ 2-fold (Figure 1D). Consistent with the cross-talk between EGFR and src (26,27), CXCR7 overexpression increased and its downregulation decreased phosphor-src levels by ≥ 2-fold (Figure 1D). Consistent with the modulation of Akt signaling, CXCR7 overexpression increased and its downregulation decreased bcl-2 levels.
To determine how CXCR7 expression regulates phosphorylation of EGFR, Akt or src, we examined whether CXCR7 forms a complex with EGFR, src and PI3-kinase. As shown in Figure 1D, EGFR, p85 subunit of PI3-kinase and src were co-immunoprecipitated with CXCR7. Furthermore, overexpression of CXCR7 significantly increased the co-immunoprecipitation of these proteins together with CXCR7. Therefore, CXCR7 may promote BCa cell growth and motility by activating EGFR and downstream signaling. Contrary to previous reports (23,28), CXCR7 overexpression or its downregulation did not significantly alter VEGF and IL-8 transcript levels (data not shown).
CXCR7 expression is increased in BCa tissues
Transcript expression
Figure 2B shows that when compared to NBL (NBL-O: 1.2±1.4; NBL-T: 0.94±1.0) tissues, CXCR4 mRNA levels were 3–4-fold elevated in low-grade (3.5±2.8; P≤ 0.04) and high-grade (4.2±4.6; P≤0.03) BCa tissues. CXCR7 levels were 5–9-fold elevated in both low-grade (2.0±0.4; P≤0.009) and high-grade (3.1±2.3; P≤0.001) BCa tissues when compared to NBL tissues (NBL-O: 0.33±0.4; NBL-T: 0.4±0.8). No significant correlation was observed between tumor grade and CXCR4 (P= 0.39) or CXCR7 (P=0.13) levels. CXCR4 (P=0.32) and CXCR7 (P=0.25) levels also did not correlate with stage. Furthermore, CXCR4 or CXCR7 levels were not significantly different among patients with non-muscle invasive (stages Ta, T1) or muscle invasive (stages ≥ T2; P > 0.05) disease.
In this study 8 patients received neo-adjuvant chemotherapy. CXCR4 or CXCR7 transcript levels were not significantly different in these patients when compared to those who did not received neo-adjuvant chemotherapy (P> 0.5). Six patients received radiation therapy following cystectomy due to advanced disease. Both CXCR4 (P=0.093) and CXCR7 (P=0.055) levels were not significantly different among patients who received radiation versus those who did not.
Protein expression
Expression of CXCR4 and CXCR7 proteins was evaluated by IHC in the same set of BCa tissues. Figure 2C shows that in both NBL and BCa tissues, CXCR4 and CXCR7 were expressed only in urothelial cells. CXCR4 levels were similar in both NBL (NBL-O: 76±95; NBL-T: 38±45) and BCa (LG: 88±86; HG: 77±93) tissues. However, CXCR7 expression was elevated in BCa specimens (LG: 136±114; HG: 183±99), when compared to NBL tissues (NBL-O: 6.3±9.2; NBL-T: 32±47; Figure 2C). CXCR4 expression was not significantly different between NBL and BCa tissues, regardless of the tumor grade (P = 0.72; Figure 2D). However, CXCR7 staining was significantly higher in both low- and high-grade tissues when compared to NBL tissues (P 0.002; Figure 2D). The staining inferences for CXCR4 (P=0.57) and CXCR7 (P=0.22) did not significantly correlate with tumor grade (CXCR4, P=0.57; CXCR7: P=0.22) or stage (CXCR4, P= 0.0114; CXCR7 (P=0.78).
Association of CXCR4 and CXCR7 expression with metastasis
In this study, the majority of the patients had high-grade (n=37) and muscle invasive (n=33) BCa. In univariate analysis stage, lymph node, CXCR7 transcript levels and CXCR7 staining score significantly associated with metastasis (Table 2A). In the multivariate model, stage and CXCR7 mRNA levels associated with metastasis (Table 2B). For disease specific mortality, only gender and CXCR7 staining scores were significant predictors (Table 2B).
Table 2. Determination of the association between metastasis and pre- and postoperative parameters and CXCR4 and CXCR7 expression.
The pre- and post-operative parameters included age, gender, tumor-grade, stage, lymph node status and concomitant presence of CIS. Only significant parameters (P<0.05) are shown in both A and B. A: Univariate analysis: ND: Not determined for parameters that did not reach significance. B: Multivariate analysis: Cox proportional hazards analysis was performed by including pre- and post-operative parameters and the transcript levels/staining scores of CXCR4 and CXCR7.
| Metastasis | Disease Specific Mortality | |||||
|---|---|---|---|---|---|---|
| Parameter | Chi- square | P-value | Odds Ratio | Chi- square | P-value | Odds Ratio |
| A: Univariate analysis | ||||||
| Stage | 8.36 | 0.004* | 5.0; 2.1 – 20 | 8.6 | 0.0034* | 3.74; 1.8 – 10.8 |
| Grade | 0.01 | 0.94 | ND | 4.01 | 0.045* | 6.0; 1.6 – 90.9 |
| Lymph node | 4.43 | 0.035* | 3.7; 1.3 – 16.1 | 3.5 | 0.06 | ND |
| CXCR7 | 8.05 | 0.0046* | 3.0; 1.4 – 6.8 | 0.09 | 0.77 | ND |
| CXCR4 | 3.68 | 0.055 | 1.21; 1.01 – 1.54 | 0.18 | 0.67 | ND |
| CXCR7 IHC | 3.9 | 0.049* | 3.24; 1.2 – 14.9 | 0.3 | 0.59 | ND |
| CXCR4 IHC | 2.6 | 0.11 | ND | 3.8 | 0.05 | 1.01; 1.0 – 1.03 |
| B: Multi-variate analysis | ||||||
| Parameter | Chi- square | P-value | Risk ratio | Chi- square | P-value | Risk ratio |
| Stage | 7.6 | 0.006* | 3.3; 1.4 – 10.5 | |||
| Gender | 4.8 | 0.029* | 3.5; 1.1 – 14.1 | 7.5 | 0.006 | 5640; Not defined |
| CXCR7 | 5.5 | 0.019* | 4.0; 1.2 – 20 | |||
| CXCR7 Staining | 4.53 | 0.033* | 4.3; 1.12 – 32.2 | |||
Although, the number of specimens was limited, CXCR7 transcript levels (cut-off 2.4) had 81.2% sensitivity and 70% specificity to associate with metastasis, respectively. For both CXCR7 mRNA levels and CXCR7 staining scores, the sensitivity (61%, 100%) and specificity (55%, 39%) were low for predicting disease-specific mortality.
In hepatocellular carcinoma and meningioma, CXCR7 is expressed in tumor endothelial cells (29, 30). As shown in Figure 2E, visualization of CXCR7 expression in endothelial cells by “In-cell co-IP” technique showed no CXCR7 expression in endothelial cells in NBL tissue and weak expression in low-grade BCa tissue. However, there was high CXCR7 expression in endothelial cells in the high-grade BCa tissue.
CXCR7 mRNA expression is increased in exfoliated urothelial cells from BCa patients
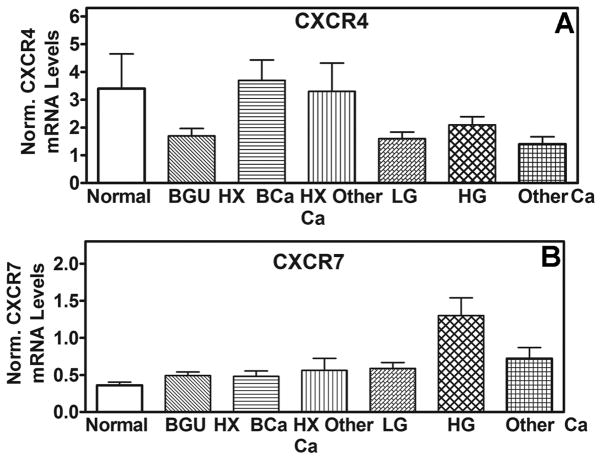
We used the Q-PCR assay to measure the transcript levels of CXCR4 and CXCR7 exfoliated urothelial cells present in urine specimens. As shown in Fig. 3A, normalized CXCR4 mRNA levels were not significantly different among BCa patients and the other categories of individuals (i.e., Normal, BGU, HxBCa, HX other Ca, other Ca) and ranged between 1.4 and 3.7. Contrarily, CXCR7 levels were significantly elevated in patients with high-grade BCa (Fig 3B, Table 3A). CXCR7 levels in various categories were as follows: normal: 0.4±0.2; BGU: 0.5±0.4; HXBCa: 0.5±0.4; HX other Ca: 0.4±0.1; LG: 0.6±0.3; HG: 1.3±1.5; other Ca: 0.7±0.5. The differences in CXCR7 levels between patients with high-grade BCa and normal (P=0.0002), BGU (P=0.0002) or history of BCa or other cancers (0.002) categories were significant. The differences in CXCR7 levels among patients with low-grade BCa and the control categories were not significant (P>0.05).
Figure 3. CXCR4 and CXCR7 levels in bladder tissues and exfoliated urothelial cells. A and B.
The mean±sd mRNA levels of CXCR4 (B) and CXCR7 (C) among different categories are shown. Mean levels of CXCR4 and CXCR7 among Group 1 (2.6±1.8; 0.38±0.26) and Group 2 (4.9±10.6; 0.33±0.12; P>0.05) of normal individuals, explained in Table 2, were not significantly different.
Table 3.
Determination of the association between presence of BCa and CXCR4 orCXCR7 transcript levels in exfoliated urothelial cells. A: Univariate analysis. The cut-off limit generated by the ROC curve was used to determine the sensitivity and specificity and accuracy of CXCR7 to detect BCa; in each column mean±sd and 95% CI values obtained by cross-validation (boot-strap modeling; specific sampling rate = 0.5; re-sampling = 104) are shown. B: Analysis of sensitivity by tumor grade and of specificity by non-BCa conditions. BGU category also included 8 patients with HX of other urologic cancers.
| Table 3 A
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Biomarker | Chi- square | P-value | Odds Ratio; 95% CI | AUC | Cut-off | Sensitivity | Specificity | Accuracy |
|
| ||||||||
| CXCR4 | 1.77 | 0.18 | ND | 0.53 | ND | ND | ND | ND |
|
| ||||||||
| CXCR7 | 16.053 | 0.0001 | 6.4; 2.7 – 16.8 | 0.787 | 0.52 | 80.7% (46/57) 79.5 ± 5.0; 78.5 – 80.5 |
75% (90/120) 75.5 ± 5.0 74.5 – 76.5 |
76.8% 77.5 – 3.9; 76.7 – 78.3 |
|
| ||||||||
| Table 3B
| ||||||||
| CXCR7 | Sensitivity | Specificity | Other Cancer (% above the cut-off limit) | |||||
| LG | HG | Normal | HXBCa | BGU | ||||
|
| ||||||||
| 58.8% (10/17) | 90% 36/40) | 92.6% (25/27) | 76.7% (23/30) | 66.7% (42/63) | 66.7% (6/9) | |||
Efficacy of CXCR7 to detect BCa
Based on the cut-off values generated by ROC curves, CXCR7 transcript levels had 80.7% sensitivity to detect BCa. This reasonably high sensitivity was due to the high sensitivity of CXCR7 to detect high-grade BCa (Table 3B). The specificity of CXCR7 levels among normal individuals was high (Table 3B). However, the specificity of CXCR7 levels among patients with BGU conditions was low. This low specificity was because 10 out of 13 patients with nephrolithiasis had CXCR7 transcript levels above the cut-off limit (i.e., 0.52). Among the 30 patients with HxBCa, six recurred within 6 months. While 4 out of these 6 patients had CXCR7 levels above the cut-off limit CXCR7 marker, only 2 out of the 24 HxBCa patients who did not have recurrence had levels above the cut-off limit (chi-square: 10.2; P = 0.0014; relative risk = 8.0, 95% CI: 1.9 – 33.9).
DISCUSSION
This is the first study that establishes the functional and clinical significance of CXCR7 expression in BCa. The key findings of our study are: 1. Most BCa cell lines express higher levels of CXCR7 when compared to CXCR4. 2. CXCR7 expression promotes BCa cell growth, survival and motility; 3. CXCR7 forms a complex with EGFR, src, and PI3-kinase. Such a signaling complex may be responsible for the observed increase in the phosphorylation of EGF-receptor, Akt and src. 4. CXCR7 expression (both mRNA and protein) is elevated in bladder tumor tissues and associates with prognosis; 5. In exfoliated urothelial cells, CXCR7 mRNA expression is up-regulated in high-grade BCa patients.
Treatment of T24 and J82 BCa-cells with SDF-1 has been shown to increase cell proliferation and motility (11) and it is partially blocked by co-incubation with an anti-CXCR4 antibody (11). Our data show that in T24 and J82 cells, CXCR7 expression is 6–10-fold higher than CXCR4. Since CXCR7 has higher affinity for SDF-1, it can explain why anti-CXCR4 antibody only partially blocked the biological activity of SDF-1. In our study, CXCR7-stimulated cell proliferation, survival and chemotactic motility was observed in the absence of any externally added SDF-1. Furthermore, fetal bovine serum contains little SDF-1 (19). BCa cell lines also expressed low levels of SDF-1 and the expression did not correlate with either CXCR7 or CXCR4 expression (Unpublished results). Therefore, due to its high affinity for SDF-1, CXCR7-mediated signaling may occur at very low concentrations of SDF-1 or CXCR7 may function in a ligand-independent manner (19).
The co-localization and co-immunoprecipitation experiments show that CXCR7 and EGF-receptor form a complex that recruits downstream signaling molecules such as, PI3-kinase and src. This signaling complex also explains, why unlike CXCR4, although CXCR7 does not induce Gβγ activation (2–4), it still activates Akt (phosphorylation at Ser473) and Erk. The connection between CXCR7 and mitotic signaling is further strengthened by the observation that CXCR7 expression modulates cyclin D1 levels. The effect of CXCR7 expression on apoptosis is very likely due to Akt signaling, since CXCR7 expression also regulates bcl-2 levels. These results suggest that CXCR7 expression regulates proliferation, survival and motility in BCa cells through signaling events such as EGF-receptor activation and Akt signaling.
In our study CXCR7 expression did not alter IL-8 or VEGF expression. However, in hepatocellular carcinoma and meningioma, CXCR7 has been shown to localize in tumor endothelial cells (29,30). Our results show that CXCR7 expression in bladder endothelial cells increases with tumor grade; high-grade bladder tumors show high expression of CXCR7 in endothelial cells.
In most BCa cell lines, the ratio of CXCR7:CXCR4 expression was between 5 and 37, i.e., CXCR7 expression was significantly higher than CXCR4 expression in BCa cell lines. Contrarily in NBL tissues, CXCR7 expression was 2.5–3-fold lower than CXCR4 expression (CXCR7:CXCR4 ratio: NBL-O: 0.35; NBL-T: 0.36). The functional significance of this switch with respect to the CXCR7:CXCR4 ratio in BCa cell lines when compared to normal bladder is currently unknown. It is also noteworthy that the expression of both CXCR4 and CXCR7 was lower in BCa cell lines than in BCa tissues. It is possible that in BCa tissues, both tumor cells and endothelial cells contribute to the expression of these chemokine receptors. Nevertheless, in exfoliated bladder tumor cells, CXCR7 mRNA expression was higher (1.3±1.5) in patients with high-grade bladder tumors than in healthy individuals, patients with benign conditions or in patients with a history of BCa (~ 0.5±0.3). Therefore the differences in BCa cell lines and tumor cells in BCa tissues, with respect to the chemokine receptor expression, may be due to cell culture conditions, and established cell lines versus the actual tissue microenvironment.
The detection of CXCR4 by a peptide antagonist has been suggested as a diagnostic tool to detect invasive BCa cells in urine (13). However, in our study, CXCR4 mRNA levels were similar in BCa patients and the individuals in various control categories. Contrarily, CXCR7 mRNA levels were significantly elevated in the exfoliated urothelial cells from high-grade BCa patients; 90% sensitivity. Based on a recent study which showed that bind of bacterial lipopolysaccarhide to the toll-like receptor 4 (TLR4) increased CXCR7 expression in a colon carcinoma cell line (31), one would have expected a consistent increase in CXCR7 expression among patients with urinary tract infection. However, CXCR7 mRNA levels were consistently increased among patients with nephrolithiasis but not among patients with other inflammatory conditions such as cystitis or urinary tract infections.
In this study, while CXCR7 mRNA levels independently correlated with metastasis, CXCR7 staining associated with disease specific mortality. This variability among mRNA levels and staining scores could be due to small cohort size and/or insufficient follow-up. Some patients with high-grade BCa didn’t metastasize and/or experience mortality as the endpoint within the available follow-up. These reasons also bring to light certain limitations of this study. First to validate the findings of the study, a multi-institutional study will be needed to address the inherent limitation of a single center study. The plausibility of tumor heterogeneity resulting in higher levels of CXCR7 mRNA as a limitation may be related to sampling during tissue analysis. This limitation however is self-limiting since stage ultimately correlated to metastasis in multivariate analysis.
Taken together, CXCR7 has multiple functions in BCa cells, including promoting proliferation, motility and survival. This study also suggests that a re-examination of the functions attributed to the binding of SDF-1 to CXCR4 in BCa cell lines may be required because we found higher expression of CXCR7 than CXCR4 in those very BCa cell lines. Furthermore in BCa, CXCR7 expression associates with invasion and metastasis. Based on these results a multi-center study to prospectively evaluate the prognostic potential of CXCR7 is warranted.
Acknowledgments
Grant Support: Women’s Cancer Association of University of Miami pilot award; Florida Department of Health – James and Esther King Biomedical Research Program (10KT-01; VBL – University of Miami site; Team Science Project Principal Investigator: Dr. Charles Rosser, M.D. Anderson Cancer Center Orlando); 2R01CA072821–11 (VBL). Judith Knapp was a fellow of the International Academy of Life Sciences, Biomedical Science Exchange Program.
We thank Mr. Gabriel Gaidosh for his help with the confocal microscopy. We are grateful to Dr. Mark Soloway, Chairman Emeritus, Department of Urology, University of Miami - Miller School of Medicine for critically reviewing the manuscript.
Abbreviations used
- BCa
bladder cancer
- BGU
benign genitourinary
- EGF
Epidermal growth factor
- HX
History of
- NBL
normal bladder tissue
- HG
high-grade
- LG
low-grade
- SDF
stroma-derived factor
Contributor Information
Travis J. Yates, Email: tyates@med.miami.edu, Sheila and David Fuente Graduate Program in Cancer Biology, Sylvester Comprehensive Cancer Center, University of Miami School of Medicine, P.O. Box 016960, Miami, Florida. Phone: (305) 243-6321; Fax: (305) 243-6893.
Judith Knapp, Email: judith.knapp@yahoo.de, Department of Urology, University of Miami School of Medicine, P.O. Box 016960, Miami, Florida. Phone: (305) 243-6321; Fax: (305) 243-6893.
Miguel Gosalbez, Email: mg672@hotmail.com, Department of Urology, University of Miami School of Medicine, P.O. Box 016960, Miami, Florida. Phone: (305) 243-6321; Fax: (305) 243-6893
Soum D. Lokeshwar, Email: soumlokeshwar@yahoo.com, Sylvester Comprehensive Cancer Center, University of Miami School of Medicine, P.O. Box 016960, Miami, Florida. Phone: (305) 243-6321; Fax: (305) 243-6893
Christopher S. Gomez, Email: cgomez7@med.miami.edu, Department of Urology, University of Miami School of Medicine, P.O. Box 016960, Miami, Florida. Phone: (305) 243-6591; Fax: (305) 243-6893
Anaid Benitez, Email: abenitez@med.miami.edu, Department of Urology, University of Miami School of Medicine, P.O. Box 016960, Miami, Florida. Phone: (305) 243-6321; Fax: (305) 243-6893
Obi O. Ekwenna, Email: oekwenna@med.miami.edu, Department of Urology, University of Miami School of Medicine, P.O. Box 016960, Miami, Florida. Phone: (305) 243-3670; Fax: (305) 243-6893
Ezekiel E. Young, Email: eyoung@med.miami.edu, Department of Urology, University of Miami School of Medicine, P.O. Box 016960, Miami, Florida. Phone: (305) 243-3670; Fax: (305) 243-6893
Murugesan Manoharan, Email: mmanoharan@.med.miami.edu, Department of Urology, University of Miami School of Medicine, P.O. Box 016960, Miami, Florida. Phone: (305) 243-6591; Fax: (305) 243-6893
Vinata B. Lokeshwar, Email: vlokeshw@med.miami.edu, Departments of Urology, Cell Biology and Anatomy, Sylvester Comprehensive Cancer Center, University of Miami School of Medicine, P.O. Box 016960, Miami, Florida. Phone: (305) 243-6321; Fax: (305) 243-6893
References
- 1.Kaufman DS, Shipley WU, Feldman AS. Bladder Cancer. Lancet. 2009;374:239–249. doi: 10.1016/S0140-6736(09)60491-8. [DOI] [PubMed] [Google Scholar]
- 2.Vandercappellen J, Van Damme J, Struyf S. The role of CXC chemokines and their receptors in cancer. Cancer Lett. 2008;267:226–44. doi: 10.1016/j.canlet.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 3.Maksym RB, Tarnowski M, Grymula K, Tarnowska J, Wysoczynski M, Liu R, et al. The role of stromal-derived factor-1--CXCR7 axis in development and cancer. Eur J Pharmacol. 2009;625:31–40. doi: 10.1016/j.ejphar.2009.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun X, Cheng G, Hao M, Zheng J, Zhou X, Zhang J, et al. CXCL12/CXCR4/CXCR7 chemokine axis and cancer progression. Cancer Metastasis Rev. 2010;29:709–22. doi: 10.1007/s10555-010-9256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 2010;16:2927–31. doi: 10.1158/1078-0432.CCR-09-2329. [DOI] [PubMed] [Google Scholar]
- 6.Altenburg JD, Broxmeyer HE, Jin Q, Cooper S, Basu S, Alkhatib G. A naturally occurring splice variant of CXCL12/stromal cell-derived factor 1 is a potent human immunodeficiency virus type 1 inhibitor with weak chemotaxis and cell survival activities. J Virol. 2007;81:8140–8. doi: 10.1128/JVI.00268-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Ji R, Li J, Gu Q, Zhao X, Sun T, et al. Correlation effect of EGFR and CXCR4 and CCR7 chemokine receptors in predicting breast cancer metastasis and prognosis. J Exp Clin Cancer Res. 2010;29:16–25. doi: 10.1186/1756-9966-29-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andre F, Xia W, Conforti R, Wei Y, Boulet T, Tomasic G, et al. CXCR4 expression in early breast cancer and risk of distant recurrence. Oncologist. 2009;14:1182–1188. doi: 10.1634/theoncologist.2009-0161. [DOI] [PubMed] [Google Scholar]
- 9.Saigusa S, Toiyama Y, Tanaka K, Yokoe T, Okugawa Y, Kawamoto A, et al. Stromal CXCR4 and CXCL12 expression is associated with distant recurrence and poor prognosis in rectal cancer after chemoradiotherapy. Ann Surg Oncol. 2010;17:2051–2058. doi: 10.1245/s10434-010-0970-y. [DOI] [PubMed] [Google Scholar]
- 10.Retz MM, Sidhu SS, Blaveri E, Kerr SC, Dolganov GM, Lehmann J, et al. CXCR4 expression reflects tumor progression and regulates motility of bladder cancer cells. Int J Cancer. 2005;114:182–189. doi: 10.1002/ijc.20729. [DOI] [PubMed] [Google Scholar]
- 11.Eisenhardt A, Frey U, Tack M, Rosskopf D, Lümmen G, Rübben H, et al. Expression analysis and potential functional role of the CXCR4 chemokine receptor in bladder cancer. Eur Urol. 2005;47:111–117. doi: 10.1016/j.eururo.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Pignot G, Bieche I, Vacher S, Güet C, Vieillefond A, Debré B, et al. Large-scale real-time reverse transcription-PCR approach of angiogenic pathways in human transitional cell carcinoma of the bladder: identification of VEGFA as a major independent prognostic marker. Eur Urol. 2009;56:678–88. doi: 10.1016/j.eururo.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 13.Nishizawa K, Nishiyama H, Oishi S, Tanahara N, Kotani H, Mikami Y, et al. Fluorescent imaging of high-grade bladder cancer using a specific antagonist for chemokine receptor CXCR4. Int J Cancer. 2010;127:1180–1187. doi: 10.1002/ijc.25145. [DOI] [PubMed] [Google Scholar]
- 14.D’Alterio C, Consales C, Polimeno M, Franco R, Cindolo L, Portella L, et al. Concomitant CXCR4 and CXCR7 Expression Predicts Poor Prognosis in Renal Cancer. Curr Cancer Drug Targets. 2010;10:772–781. doi: 10.2174/156800910793605839. [DOI] [PubMed] [Google Scholar]
- 15.Iwakiri S, Mino N, Takahashi T, Sonobe M, Nagai S, Okubo K, et al. Higher expression of chemokine receptor CXCR7 is linked to early and metastatic recurrence in pathological stage I nonsmall cell lung cancer. Cancer. 2009;115:2580–2593. doi: 10.1002/cncr.24281. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Shiozawa Y, Wang J, Wang Y, Jung Y, Pienta KJ, et al. The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer. J Biol Chem. 2008;283:4283–4294. doi: 10.1074/jbc.M707465200. [DOI] [PubMed] [Google Scholar]
- 17.Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miao Z, Luker KE, Summers BC, Berahovich R, Bhojani MS, Rehemtulla A, et al. CXCR7 (RDC1) promotes breast and lung tumor growth in vivo and is expressed on tumor-associated vasculature. Proc Natl Acad Sci U S A. 2007;104:15735–15740. doi: 10.1073/pnas.0610444104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh RK, Lokeshwar BL. The IL-8-regulated chemokine receptor CXCR7 stimulates EGFR signaling to promote prostate cancer growth. Cancer Res. 2011;71:3268–3277. doi: 10.1158/0008-5472.CAN-10-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masters JR, Hepburn PJ, Walker L, Highman WJ, Trejdosiewicz LK, Povey S, Parkar M, Hill BT, Riddle PR, Franks LM. Tissue culture model of transitional cell carcinoma: characterization of twenty-two human urothelial cell lines. Cancer Res. 1986 Jul;46(7):3630–6. [PubMed] [Google Scholar]
- 21.Lokeshwar VB, Lopez LE, Munoz D, et al. Antitumor activity of hyaluronic acid synthesis inhibitor 4-methylumbelliferone in prostate cancer cells. Cancer Res. 2010;70:2613–2623. doi: 10.1158/0008-5472.CAN-09-3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kramer MW, Escudero DO, Lokeshwar SD, et al. Association of hyaluronic acid family members (HAS1, HAS2, and HYAL-1) with bladder cancer diagnosis and prognosis. Cancer. 2010;117:1197–1209. doi: 10.1002/cncr.25565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kollmar O, Rupertus K, Scheuer C, Nickels RM, Haberl GC, Tilton B, et al. CXCR4 and CXCR7 regulate angiogenesis and CT26. WT tumor growth independent from SDF-1. Int J Cancer. 2010;126:1302–1315. doi: 10.1002/ijc.24956. [DOI] [PubMed] [Google Scholar]
- 24.Hattermann K, Held-Feindt J, Lucius R, Müerköster SS, Penfold ME, Schall TJ, et al. The chemokine receptor CXCR7 is highly expressed in human glioma cells and mediates antiapoptotic effects. Cancer Res. 2010;70:3299–3308. doi: 10.1158/0008-5472.CAN-09-3642. [DOI] [PubMed] [Google Scholar]
- 25.Odemis V, Boosmann K, Heinen A, Küry P, Engele J. CXCR7 is an active component of SDF-1 signalling in astrocytes and Schwann cells. J Cell Sci. 2010;123:1081–1088. doi: 10.1242/jcs.062810. [DOI] [PubMed] [Google Scholar]
- 26.Schulze WX, Deng L, Mann M. Phosphotyrosine interactome of the ErbB-receptor kinase family. Mol Syst Biol. 2005;1:1–13. doi: 10.1038/msb4100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato K, Nagao T, Iwasaki T, Nishihira Y, Fukami Y. Src-dependent phosphorylation of the EGF receptor Tyr-845 mediates Stat-p21waf1 pathway in A431 cells. Genes Cells. 2003;8:995–1003. doi: 10.1046/j.1356-9597.2003.00691.x. [DOI] [PubMed] [Google Scholar]
- 28.Zheng K, Li HY, Su XL, Wang XY, Tian T, Li F, et al. Chemokine receptor CXCR7 regulates the invasion, angiogenesis and tumor growth of human hepatocellular carcinoma cells. J Exp Clin Cancer Res. 2010;29:31–45. doi: 10.1186/1756-9966-29-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monnier J, Boissan M, L’Helgoualc’h A, Lacombe ML, Turlin B, Zucman-Rossi J, Théret N, Piquet-Pellorce C, Samson M. CXCR7 is up-regulated in human and murine hepatocellular carcinoma and is specifically expressed by endothelial cells. Eur J Cancer. 2012;48:138–148. doi: 10.1016/j.ejca.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 30.Würth R, Barbieri F, Bajetto A, Pattarozzi A, Gatti M, Porcile C, Zona G, Ravetti JL, Spaziante R, Florio T. Expression of CXCR7 chemokine receptor in human meningioma cells and in intratumoral microvasculature. J Neuroimmunol. 2011;234:115–123. doi: 10.1016/j.jneuroim.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 31.Xu H, Wu Q, Dang S, Jin M, Xu J, Cheng Y, Pan M, Wu Y, Zhang C, Zhang Y. Alteration of CXCR7 expression mediated by TLR4 promotes tumor cell proliferation and migration in human colorectal carcinoma. PLoS One. 2011;6(12):e27399. doi: 10.1371/journal.pone.0027399. [DOI] [PMC free article] [PubMed] [Google Scholar]