Abstract
Background
Although clinicians have a number of measures to use to describe walking performance, few, if any, of the measures capture a person's perceived effort in walking. Perceived effort of walking may be a factor in what a person does versus what he or she is able to do.
Objective
The objective of this study was to examine the relationship of perceived effort of walking with gait, function, activity, fear of falling, and confidence in walking in older adults with mobility limitations.
Design
This investigation was a cross-sectional, descriptive, relational study.
Methods
The study took place at a clinical research training center. The participants were 50 older adults (mean age=76.8 years, SD=5.5) with mobility limitations. The measurements used were the Rating of Perceived Exertion (RPE) for walking; gait speed; the Modified Gait Abnormality Rating Scale; energy cost of walking; Late Life Function and Disability Instrument (LLFDI) for total, basic, and advanced lower-extremity function and for disability limitations; activity and restriction subscales of the Survey of Activities and Fear of Falling in the Elderly (SAFFE); activity counts; SAFFE fear subscale; and Gait Efficacy Scale (GES). The relationship of the RPE of walking with gait, function, activity, fear, and confidence was determined by using Spearman rank order coefficients and an analysis of variance (adjusted for age and sex) for mean differences between groups defined by no exertion during walking and some exertion during walking.
Results
The RPE was related to confidence in walking (GES, R=−.326, P=.021) and activity (activity counts, R=.295, P=.044). The RPE groups (no exertion versus some exertion) differed in LLFDI scores for total (57.9 versus 53.2), basic (68.6 versus 61.4), and advanced (49.1 versus 42.6) lower-extremity function; LLFDI scores for disability limitations (74.9 versus 67.5); SAFFE fear subscale scores (0.346 versus 0.643); and GES scores (80.1 versus 67.8) (all P<.05).
Limitations
The range of RPE scores for the participants studied was narrow. Thus, the real correlations between RPE and gait, physical function, and psychological aspects of walking may be greater than the relationships reported.
Conclusions
The perceived effort of walking was associated with physical activity and confidence in walking. Reducing the perceived effort of walking may be an important target of interventions to slow the decline in function of older adults with mobility limitations.
Fatigue is associated with functional limitations, disability, mortality, and other adverse outcomes in older adults.1–3 Fatigability is the feeling of fatigue related to a specific task, such as walking.4 Fatigability relates a change in fatigue to a change in the intensity, duration, or frequency of activity. Ferrucci5 conceptualized the idea of fatigability by categorizing total energy that can be expended for a day into component parts: resting metabolic rate, energy cost of daily activities (eg, activities of daily living [ADLs], walking, talking, and thinking), and functional reserve.6 A greater feeling of fatigue could result from an increase in resting metabolic rate or an increase in the energy cost of daily activities (or both) that reduce the functional reserve of energy. A reduction in reserve energy capacity means that an older adult expends a greater proportion of his or her maximum energy for life support and necessary daily activities.5–7 Many of the daily activities of older adults involve walking.8,9 Thus, compared with an older adult without gait abnormalities, an older adult with gait dysfunction expends greater effort on daily tasks10 and, therefore, is more likely to perceive greater fatigability.
According to Robertson and Noble,11 perceived exertion is defined as the subjective intensity of effort, strain, discomfort, and fatigue experienced during physical exercise. The Rating of Perceived Exertion (RPE)12 provides a global quantification of an individual's effort or fatigue, taking into account physiological, psychological, and performance factors.11 The RPE scale has been correlated (r=.80–.90) with several physiological measures of performance effort, including heart rate during activity.13 The RPE scale is currently being used in a validation study of the National Institutes of Health Patient-Reported Outcome Measurement Information System project to record perceived fatigue or effort before and after walking activity (eg, Six-Minute Walk Test) in people with congestive heart failure before and after a heart transplant.14
Although many measures of walking are available for clinicians to describe a patient's walking performance (eg, speed and gait characteristics, distance walked, need for assistance, and severity of abnormalities), most do not capture fatiguability, or the patient's perception of the effort of walking. In our clinical experience, people who walk at 1.0 m/s15,16 or who can walk 45 m (150 ft) (Functional Independence Measure17) or 330 m (capable of community ambulation18) sometimes achieve similar levels of walking ability through different means and with various degrees of effort in performance (unpublished data). Fatigability, or perceived effort of walking, may be an important modifier of what a person does versus what the person can do in daily life.19 The impact of interventions to improve gait on activity and participation may be influenced by a patient's perception of the ability to walk. Thus, if walking is perceived to be effortful, regardless of the ability to walk at an adequate speed and for an adequate distance in daily and community activities, then an older adult may limit (ie, do less) physical function in daily life and physical activity for recreation and promotion of good health.
The purpose of this study was to determine the relationship of perceived effort of walking with gait and mobility-related physical function and activity, fear of falling, and confidence in walking in older adults with mobility limitations. We expected greater perceived effort of walking to be associated with poorer gait and physical function, less physical activity, greater fear of falling, and less confidence in walking.
Method
This cross-sectional study of the relationship of perceived effort of walking with measures of gait, physical function and activity, and psychological factors of walking performance involved the baseline data recorded for a randomized controlled trial of 2 therapeutic activities to improve walking in older adults with slow and variable gait.20
Participants
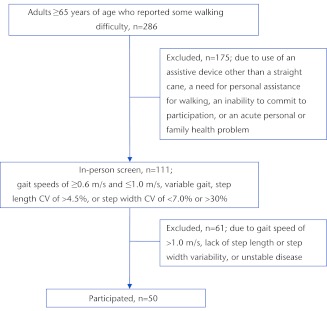
The study participants (Fig. 1) were 65 years of age or older and were able to walk independently with or without the use of a straight cane. Participants gave informed consent and provided written evidence of medical clearance from their physicians to participate in the low- to moderate-intensity exercise interventions used in the study. The Mini-Mental State Examination (MMSE) was used as a brief screening measure of cognitive function necessary to provide informed consent for participation. An MMSE score of >23 was required to be eligible to participate in the study.21 The older adults who were eligible to participate demonstrated slow (gait speeds of ≤1.0 m/s but ≥0.6 m/s22) and variable gait. Abnormal gait variability was defined as either a step length coefficient of variation of less than 4.5%23 or a step width coefficient of variation of less than 7% or greater than 30%24; these values were based on gait recorded over an instrumented walkway (see below). Demographic data on age, sex, highest educational level completed, and comorbidities were collected by participant report. A brief medical history was recorded using the Comorbidity Index. Participants reported whether a physician had ever told them they had any of 18 common conditions expected to have an impact on physical function using the Comorbidity Index.25
Figure 1.
Study flow chart. CV=coefficient of variation.
Measures
The perceived effort of walking was measured as the RPE. Participants rated the perceived effort of walking using the Borg RPE scale12 after a short walking task (total walk distance, ∼15 m [∼50 ft]) at a self-selected, comfortable pace. The RPE scale values ranged from 6 (“no exertion at all”) to 20 (“very, very hard exertion”).12 The RPE scale has been shown to have reproducibility for describing perceived exertion status and changes in perceived effort of performance in adults, including older adults in good health, and in people with various clinical disorders. The RPE scale has been validated by comparison with several measures of physiological performance effort13 as well as measures of standing balance and a stair test.26,27
Gait.
Gait was measured in various ways: gait speed and variability, Modified Gait Abnormality Rating Scale (GARSM), and energy cost of walking.
Gait speed was recorded on a 4-m instrumented walkway, the GaitMatII (E.Q. Inc, Chalfont, Pennsylvania),28 with 2-m noninstrumented walkway segments at each end to allow for acceleration and deceleration. After 2 practice trials, data were collected during 2 trials on the walkway. The gait speed recorded was the average of the speeds in the 2 trials.29 The coefficients of variation for step length and step width variability were derived from the standard deviation of all right and left steps recorded during the 2 walks and were calculated as follows: (mean standard deviation/mean step length or step width) × 100%.30,31 Step length and step width variability was used to determine eligibility for the study and not as an outcome measure.
Gait speed determined over the instrumented walkway was shown to have test-retest reliability (intraclass correlation coefficient [ICC]=.78) and concurrent validity by comparison with mean gait characteristics (eg, step length, step width, and stance time)32 and predictive validity for physical disability, institutionalization, and death.8,33–35 A meaningful change in gait speed has been determined through several corroborating methods (substantial change=0.1 m/s; small change=0.05 m/s).36
The GARSM, a 7-item, observational rating of gait abnormalities, was used as a measure of gait dysfunction related to a risk of falling in community-dwelling older adults.37,38 Gait abnormalities, variability, guardedness, staggering, foot contact, hip range of motion, shoulder extension, and arm–heel-strike synchrony were determined from a videotape recording of a participant walking for a short distance. A physical therapist (1 of the authors) who was experienced with using the GARSM rated the abnormalities using the criterion-based scale (0–3 points) for each item. Total scores ranged from 0 to 21, with higher scores indicating poorer gait performance. When performed by experienced assessors, the GARSM has been shown to have excellent reliability (ICC=.95–.99).37 Concurrent validity for the GARSM has been demonstrated with gait characteristics.37 The GARSM score can be used to distinguish between older adults with and without a history of recurrent fall risk with a sensitivity of 62.3% and a specificity of 87.1% at a cutoff value of 9 (determined from a receiver operating characteristic curve).38
The energy cost of walking was used as a physiological measure of the energy necessary for an older adult to walk. The energy cost of walking (mL/kg·m) was derived from the rate of oxygen consumption (mL/kg·min)—determined by open-circuit spirometry and analysis of expired gases with a VO2000 portable metabolic measurement system (Medgraphics, Minneapolis, Minnesota)—divided by gait speed (m/min). The rates of oxygen consumption during 3 minutes of treadmill walking at a self-selected pace and a physiological steady state were averaged.39–41 Participants were allowed 1 or 2 trial sessions to become accustomed to the treadmill before oxygen consumption measurements were taken. Trial sessions to establish familiarity with the treadmill were completed in 1 or 2 visits in less than 1 week.
The lower the energy cost of walking, the more efficient the gait.40,42 We used standardized methods for deriving the energy cost of walking from the oxygen consumption during a physiological steady state, as established in previous studies.41,43–47 When derived with standardized methods, the energy cost of walking (mL/kg·m)42,48 is a reproducible measure of the metabolic cost of gait.39,49,50 The measure of the energy cost of walking is influenced little, if at all, by changes in oxygen consumption related to improved fitness secondary to aerobic exercise49,50 and can be compared at different time points and across different people, regardless of changes in gait speed.41,49,50
Physical function.
Physical function was measured with the Late Life Function and Disability Instrument (LLFDI). The LLFDI questionnaire was used to record self-reported physical function and disability.9,51 The total function component (LLFDI function component) included 32 questions about a person's ability to perform motor activities typical of daily life tasks. Two subscales of the function component were also used: the basic lower-extremity function subscale for activities that involve standing, stooping, and basic walking tasks; and the advanced lower-extremity function subscale for activities that require greater physical ability and endurance. Item scores ranged from 5 (“no difficulty in task performance”) to 1 (“unable to perform the task”). Raw scores were transformed to a 100-point scale, with higher scores indicating better function. The LLFDI function component has been shown to be reliable (ICC=.91–.98).9
The LLFDI for disability limitations (LLFDI disability component) was used to define the extent of difficulty that the older adults experienced in carrying out a variety of daily life tasks.51 Item scores ranged from 5 (“not limited at all”) to 1 (“completely limited”). Raw scores were transformed to a 100-point scale, with higher scores indicating better capability. The reproducibility of the LLFDI disability component has been reported to be greater than .80.51 The validity of the LLFDI function and disability components has been demonstrated by comparison with the activity and participation domains of the International Classification of Functioning, Disability and Health.52 In an exploratory factor analysis, the LLFDI physical function items loaded on 3 factors; 2 in the activities domain, mobility activities and daily activities, explained 24.4% and 24.3% of the variance, respectively, and 1 in the participation domain, social/participation, explained 12.4% of the variance. Thus, 61.1% of the total variance was explained.53 Meaningful change in LLFDI components has not been defined.
Physical activity.
Physical activity was measured in various ways: activity and restriction subscales of the Survey of Activities and Fear of Falling in the Elderly (SAFFE) and activity counts over 7 days.
The SAFFE questionnaire (administered in interviews) was used to determine how many of 11 usual daily activities the older adults performed (SAFFE activity subscale) and how many of the 11 activities they performed less than 5 years prior (SAFFE restriction subscale) because of a fear of falling. Internal consistency (Cronbach alpha=.91) and validity for fear of falling during usual daily activities were previously reported.54
Physical activity during daily activities was recorded with a CSA/MTI Actigraph accelerometer (Actigraph, Pensacola, Florida) worn at waist level55,56 during all waking hours for 7 consecutive days. Activity counts were recorded in counts per minute, which represented the mean activity counts per day, divided by the mean minutes worn per day, averaged over days worn. Accelerometers have been shown to have reliability for movement activity, providing more precise measures of walking than pedometers.57 The Actigraph accelerometer has been validated in a comparison with metabolic measures (r=.66–.89)58 and by the detection of differences in activity for walking on a treadmill or a track at a self-selected pace, including sensitivity to changes in gait speed.59
Fear and confidence.
Fear and confidence were measured with the SAFFE fear subscale and the Gait Efficacy Scale (GES).
The SAFFE fear subscale was used to record a participant's rating of fear of falling during each of 11 daily activities. The SAFFE fear subscale ratings, from 0 (“no fear at all”) to 3 (“very worried”), for the 11 activities performed were averaged to yield the SAFFE fear subscale score. Higher SAFFE fear subscale scores represented greater fear of falling.54
The GES was used to record confidence in walking under several challenging circumstances (eg, walking over grass, stepping onto and off of a curb, and going up and down stairs with and without a railing). Each of 10 items was scored from 1 (“no confidence”) to 10 (“complete confidence in the ability to perform the walking task”), for a possible score range of 10 to 100.60 The test-retest reliability (ICC) of the GES has been reported to be .93, and the construct validity of the GES has been demonstrated for gait performance (r=.49–.71), physical function (R=.59–.82), and mobility- or balance-related confidence and fear (r=.49–.87).61
Data Analysis
All statistical analyses were performed with PASW Statistics, version 18 (SPSS Inc, Chicago, Illinois). Means and standard deviations for baseline characteristics of all participants were determined. The relationship of the RPE of walking with gait, function, activity, fear, and confidence was defined using Spearman rank order coefficients. Older adults were then classified into 1 of 2 RPE groups: those who reported no exertion during walking (RPE of 6; 13 participants) and those who reported some exertion during walking (RPE of ≥7; 37 participants). An analysis of covariance model with age and sex as covariates was used to determine mean differences between RPE groups for each variable.
Role of the Funding Source
This study was supported by the Pittsburgh Older Americans Independence Center (NIA P30 AG024827), a Beeson Career Development Award (NIA K23 AG02676), and a Pittsburgh Clinical Research Training Grant in Geriatrics and Gerontology (T32 AG021885).
Results
Baseline Characteristics of Participants
The mean age of the participants was 76.8 years (SD=5.5). Approximately one third of the participants were men, and nearly three quarters of the participants had education beyond high school. The older adults studied had some comorbidities and mobility limitations, including slow gait and step width or step length variability, consistent with the eligibility criteria (Table).
Table.
Characteristics of 50 Participants and Relationship of Rating of Perceived Exertion (RPE) of Walking With Gait, Physical Function, Physical Activity, Fear of Falling, and Confidence in Walkinga
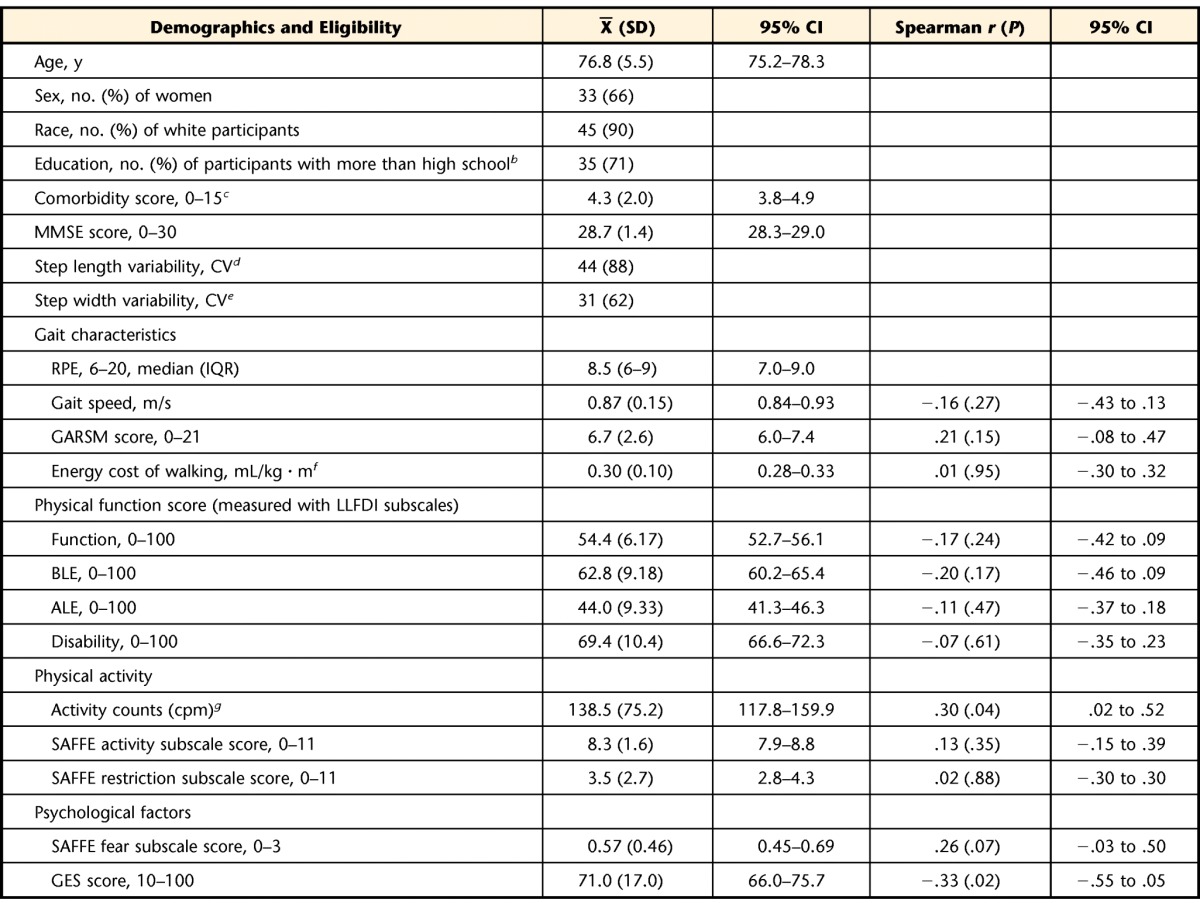
CI=confidence interval; MMSE=Mini-Mental State Examination21; CV=coefficient of variation, reported as a percentage [(SD/mean)×100]; IQR=interquartile range; GARSM=Modified Gait Abnormality Rating Scale; LLFDI=Late Life Function and Disability Instrument; cpm=counts per minute; SAFFE=Survey of Activities and Fear of Falling in the Elderly; GES=Gait Efficacy Scale.
Education data were available for 49 participants.
Comorbidity25 data were available for 46 participants.
Number (percentage) of participants with a step length CV of greater than 4.5%.
Number (percentage) of participants with a step width CV of less than 7% or greater than 30%.
Data on the energy cost of walking were available for 48 participants.
Activity counts were available for 47 participants.
The mean gait abnormalities determined with the GARSM were double the mean gait abnormalities characteristic of community-dwelling older adults without gait dysfunction.28 The mean energy cost of walking was 0.30 mL/kg·m, nearly twice the energy cost of normal walking.42 The mean SAFFE fear subscale score for the participants was at a level associated with fear of falling but not usually restricting activities because of fear54 (Table).
Relationship of Perceived Effort of Walking With Gait, Function, Activity, Fear, and Confidence
A greater perceived effort of walking (RPE) was associated with greater physical activity (activity counts, r=.30, P=.04) and less confidence in walking (GES, r=−.33, P=.02) and was marginally related to fear of falling (SAFFE fear subscale, r=.26, P=.07). The RPE was not correlated with gait characteristics or physical function, but the nonsignificant association was in the expected direction for the relationship, such that the greater the reported effort of walking, the poorer the performance or the report of physical function (Table).
RPE Group Differences (No Exertion Versus Some Exertion)
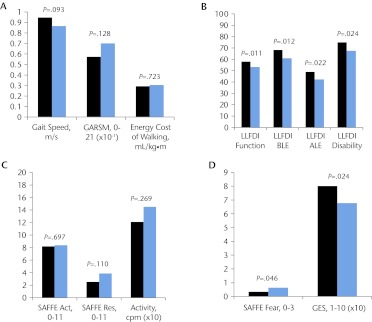
Although there were no differences in gait performance, there were differences in physical function and psychological factors between older adults who reported no exertion during walking and older adults who reported some exertion during walking (Fig. 2). Participants who reported no exertion during walking displayed greater physical function (as indicated by all components of the LLFDI), less fear of falling, and greater confidence in walking; P values for all adjusted mean differences were less than .05 (Figs. 2B and 2D).
Figure 2.
Between-group differences for the group with a Rating of Perceived Exertion (RPE) indicating no exertion during walking (an RPE value of 6; 13 participants; black bars) and the group with an RPE indicating some exertion during walking (an RPE value of ≥7; 37 participants; blue bars). The median RPE was 9 (range=7–15). (A) Gait characteristics. (B) Physical function. (C) Physical activity. (D) Fear of falling and confidence in walking. Activity=physical activity, recorded in counts per minute (cpm); GARSM=Modified Gait Abnormality Rating Scale; GES=Gait Efficacy Scale; LLFDI=Late Life Function and Disability Instrument for total (Function), basic (BLE), and advanced (ALE) lower-extremity function and for disability limitations (Disability); SAFFE=Survey of Activities and Fear of Falling in the Elderly (activity [Act], restriction [Res], and fear [Fear] subscales).
Discussion
The intent of the present study was to explore the relationship of perceived effort of walking with gait performance, physical function and activity, fear of falling, and confidence in walking in older adults. A measure of an individual's perceived effort of walking may provide a clinician with some insights into the experience of older adults with walking problems, including what they might do and not just what they can do with their walking ability. The association of greater perceived effort of walking with less confidence in walking and greater fear of falling during ADLs is consistent with expectations based on previously determined relationships of self-efficacy, fear of falling, and physical function.62–64 Self-efficacy (ie, reported confidence in one's own physical abilities) has been shown to be associated with reported physical activity, physical function, and functional difficulties64 and to partially mediate the impact of physical activity on functional limitations.65 The previously determined associations of self-efficacy, fear of falling, and physical function and the relationship of RPE and confidence in walking demonstrated in the present study highlight the potential importance of designing intervention approaches to improve walking and to address these personal factors as well. The marginal relationship between perceived effort of walking and activity-related fear of falling cannot predict whether a person's perception of effort of walking will lead to fear in the performance of daily activities, but perceived energy deficits have been shown to influence a person's decision making and risk taking.66
We did not expect the positive relationship between perceived effort of walking and physical activity. We can only speculate about the reasons for the relationship between greater perceived effort of walking and greater physical activity in daily life. It is possible that older adults who are more active have greater exposure to walking activity and therefore are better able to “judge or rate” their perceived effort. Perceived effort of walking may be more representative of physiological effort in people who have more experience with walking than in those who walk little. A similar phenomenon of perception of performance accuracy was described for self-reported physical function in community-dwelling older adults. Older adults who walked more reported physical functional limitations more consistent with their demonstrated performance on batteries of walking; those who walked less tended to underreport their physical functional limitations.67–69
We expected RPE to be related to the energy cost of walking partly because of the conceptual model of fatigability of Ferrucci5 and Schrack et al6—specifically, an increase in the energy cost walking means that the energy expended for daily activities (most of which are based on walking) represents a larger proportion of the total energy that can be expended; thus, the functional reserve of energy is reduced. Finding no relationship between perceived fatigue and the energy cost of walking, we considered other explanations based on the model of fatigability and potential implications for interventions to improve walking and reduce disability. One way to preserve the functional reserve of energy despite a greater energy cost of walking would be to have a greater total energy capacity. Some of the older adults studied may have had sufficient aerobic capacity such that even if their energy cost of walking meant that energy expended for daily activities represented a larger proportion of the available total energy expenditure, the functional reserve of energy was maintained and, thus, RPE values were lower than expected. Greater aerobic capacity allowed these older adults to better tolerate a greater energy cost of walking without perceived fatigue.6,7 Alternatively, older adults with low aerobic capacity (ie, total energy expenditure) may have reported a greater perceived effort of walking even with minimal gait abnormalities contributing to a mildly elevated energy cost of walking because the slight elevation in the energy expended for daily activities represented a substantial proportion of the available total daily energy expenditure. For clinicians working with older adults who have walking problems and a goal of reducing or preventing disability, 2 intervention approaches appear to be supported: (1) improve aerobic capacity to enable older adults to tolerate an elevated energy cost of walking and (2) reduce gait abnormalities and restore timing and coordination in gait to maintain or decrease the energy cost of walking.
Older adults who reported some exertion during walking demonstrated poorer physical function, specifically with ADLs, than older adults who reported no exertion during walking (Fig. 2). These results support Ferrucci's conceptualization of fatigue; greater effort for ADLs results in greater fatigue.5,6 Although we might have expected older adults with gait dysfunction to have poor advanced lower-extremity function, the marked difference in basic lower-extremity function between the RPE groups is alarming. The LLFDI basic lower-extremity function subscale reflects the activities necessary for ADLs. Thus, older adults who have a greater perceived effort of walking have greater difficulty with basic ADLs. Greater difficulty with basic ADLs may result in a loss of independence, activity restriction, and poorer health-related quality of life.4 The difference between the RPE groups in physical function but not in gait characteristics illustrates the additional information that clinicians may gain from a patient's report of exertion during walking. The older adults' reports of exertion during walking may have been influenced by their daily experience of exertion during all walking activities and therefore may reflect physical function but not gait characteristics alone. Clinicians may gain useful insight about physical function in daily life simply from a patient's RPE during walking.
Although gait performance did not differ between the RPE group showing no exertion and the RPE group showing some exertion, the differences in gait speed and abnormalities between the groups were clinically meaningful. Older adults in the group showing no exertion walked, on average, 0.08 m/s faster than those in the group showing some exertion. A mean difference in gait speed of 0.08 m/s was greater than a small meaningful difference but less than a substantial difference.36 Similarly, although mean gait abnormalities did not differ between the RPE groups, the mean between-group difference in the GARSM of 1.03 was greater than an estimated small difference (0.2 × GARSM; SD=0.52) but less than a substantial difference (0.5 × GARSM; SD=1.30) in mean gait abnormalities.
Some of the older adults with the slowest gait speeds and several gait abnormalities reported the lowest RPE for walking. Perceived exertion is relative to an individual's usual daily experience. Our interest in exploring the use of the RPE for walking was to capture the walking experience of older adults with mobility limitations. It is possible that the older adults recalibrated their perception of “usual effort” for walking through the experience of walking with substantial gait abnormalities for a sustained period. Because gait speed decline is often progressive, we believe that many of the older adults with the slowest gait speeds had experienced walking difficulties for a substantial length of time. Because of their experience with gait dysfunction, they might have become accustomed to the level of effort of walking70 and, therefore, might have reported the current level of effort to be little or no exertion during walking. Interestingly, the older adults with gait speeds below the median for the sample (≤0.89 m/s) had the most gait abnormalities and the greatest energy cost while reporting that walking required no effort (RPE=6).
Limitations of the Study
One limitation of this research is the narrow range of RPE values reported. Although it is true that the majority of RPE values reported fell between 6 and 9 (range=6–15), we believe that the ratings were representative of the sample, which included older adults with mild to moderate difficulties with gait. The correlations of RPE with gait, physical function, and psychological aspects of walking might have been negatively affected by the restricted range of RPE scores for the majority of the sample, meaning that the real relationships might have been more significant than the reported ones but were not likely to be less significant. A further limitation is that we do not know the aerobic capacity, total energy expenditure, or duration of walking difficulties for the older adults studied; thus, we are unable to substantiate our interpretation of the potential impact of each of these factors on the relationship found for RPE.
The method used with the RPE scale in the present study was the estimation paradigm, which involves the quantification of perceived effort at a self-selected walking speed. Future research with the RPE scale might include the use of the production paradigm method, which involves people performing a walking task at designated RPE levels,71 and examination of gait characteristics and gait abnormalities in people at different RPE levels.
Conclusion
The perceived effort of walking was associated with physical activity and confidence in walking. Older adults who reported some exertion during walking had poorer physical function, more disability, greater fear of falling, and less confidence in walking than those who reported no exertion during walking. Perceived effort of walking raises some important person-centered considerations for the design and outcomes of interventions intended to improve walking and physical function in older adults with mobility limitations.
The Bottom Line
What do we already know about this topic?
Rating of perceived exertion (RPE) has been used as a comprehensive measure of a person's self-reported effort or fatigue experienced during exercise, and it is related to physiological measures of performance.
What new information does this study offer?
This study found the RPE to be a useful measure in capturing perception of effort in walking, which is not represented by other clinical measures of walking performance (eg, speed and gait characteristics, distance walked, assistance, severity of abnormalities, confidence).
If you're a patient, what might these findings mean to you?
In older adults with some walking problems, perceived effort of walking is related to confidence in walking and physical activity and may be an influence on physical function in daily life.
Footnotes
Dr Julius and Dr VanSwearingen provided concept/idea/research design and data analysis. All authors provided writing. Dr Brach and Dr VanSwearingen provided data collection and fund procurement. Dr VanSwearingen provided project management. Dr Brach and Dr Wert provided consultation (including review of manuscript before submission).
This study was approved by the Institutional Review Board for Human Subject Research of the University of Pittsburgh.
This research was presented as a poster presentation at the 2012 Annual Scientific Meeting of the American Geriatric Society; May 3–5, 2011; Seattle, Washington.
This study was supported by the Pittsburgh Older Americans Independence Center (NIA P30 AG024827), a Beeson Career Development Award (NIA K23 AG02676), and a Pittsburgh Clinical Research Training Grant in Geriatrics and Gerontology (T32 AG021885).
References
- 1. Avlund K. Fatigue in older adults: an indicator of the aging process? Aging Clin Exp Res. 2010;22:100–115 [DOI] [PubMed] [Google Scholar]
- 2. Avlund K, Schultz-Larsen K, Davidsen M. Tiredness in daily activities at age 70 as a predictor of mortality during the next 10 years. J Clin Epidemiol. 1998;51:323–333 [DOI] [PubMed] [Google Scholar]
- 3. Avlund K, Vass M, Hendriksen C. Onset of mobility disability among community dwelling older men and women: the role of tiredness in daily activities. Age Ageing. 2003;32:579–584 [DOI] [PubMed] [Google Scholar]
- 4. Alexander NB, Taffet GE, Horne FM, et al. Bedside-to-bench conference: research agenda for idiopathic fatigue and aging. J Am Geriatr Soc. 2010;58:967–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferrucci L. Effects of gender, weight, diet, and physical activity on energetic predictors of fatigue. In: Proceedings of Idiopathic Fatigue and Aging: the NIA Bedside-to-Bench Conference; September 3–5, 2008; Bethesda, Maryland [Google Scholar]
- 6. Schrack JA, Simonsick EM, Ferrucci L. The energetic pathway to mobility loss: an emerging new framework for longitudinal studies on aging. J Am Geriatr Soc. 2010;58:S329–S336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fiser WM, Hays NP, Rogers SC, et al. Energetics of walking in elderly people: factors related to gait speed. J Gerontol A Biol Sci Med Sci. 2010;65A:1332–1337 [DOI] [PubMed] [Google Scholar]
- 8. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94 [DOI] [PubMed] [Google Scholar]
- 9. Haley SM, Jette AM, Coster WJ, et al. Late Life Function and Disability Instrument, II: development and evaluation of the function component. J Gerontol A Biol Sci Med Sci. 2002;57:M217–M222 [DOI] [PubMed] [Google Scholar]
- 10. Wert DM, Brach JS, Perera S, et al. Gait biomechanics, spatial and temporal characteristics, and the energy cost of walking in older adults with impaired mobility. Phys Ther. 2010;90:977–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Robertson RJ, Noble BJ. Perception of physical exertion: methods, mediators, and applications. Exerc Sport Sci Rev. 1997;25:407–452 [PubMed] [Google Scholar]
- 12. Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2:92–98 [PubMed] [Google Scholar]
- 13. Borg GAV. Perceived exertion: a note of “history” and methods. Med Sci Sports Exerc. 2010;5:90–93 [PubMed] [Google Scholar]
- 14. National Institutes of Health. Validity Studies: Validating PROMIS Instruments in Congestive Heart Failure Patients Receiving a Heart Transplant. PROMIS website. Available at: http://www.nihpromis.org/documents/Protocol_Summaries_CHF.pdf. Accessed March 7, 2011
- 15. Ferrucci L, Baninelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625 [DOI] [PubMed] [Google Scholar]
- 16. Guralnik JM, Ferrucci L, Balfour JL, et al. Progressive versus catastrophic loss of the ability to walk: implications for the prevention of mobility loss. J Am Geriatr Soc. 2001;49:1463–1470 [DOI] [PubMed] [Google Scholar]
- 17. Granger CV, Hamilton BB, Linacre JM, et al. Performance profiles of the Functional Independence Measure. Am J Phys Med Rehabil. 1993;72:84–90 [DOI] [PubMed] [Google Scholar]
- 18. Lerner-Frankiel MB, Vargas S, Brown M, et al. Functional community ambulation: what are your criteria? Clinical Management. 1986;6:12–15 [Google Scholar]
- 19. Simonsick EM, Guralnik JM, Fried LP. Who walks? Factors associated with walking behavior in disabled older women with and without self-reported walking difficulty. J Am Geriatr Soc. 1999;47:672–680 [DOI] [PubMed] [Google Scholar]
- 20. VanSwearingen JM, Perera S, Brach JS, et al. A randomized trial of two forms of therapeutic activity to improve walking: effect on the energy cost of walking. J Gerontol A Biol Sci Med Sci. 2009;64:1190–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 22. Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322 [DOI] [PubMed] [Google Scholar]
- 23. Maki BE. Gait changes in older adults: predictors of falls or indicators of fear? J Am Geriatr Soc. 1997;45:313–320 [DOI] [PubMed] [Google Scholar]
- 24. Brach JS, Berlin JE, VanSwearingen JM, et al. Too much or too little step width variability is associated with a fall history in older persons who walk at or near normal gait speed. J Neuroeng Rehabil. 2005;2:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rigler SK, Studenski S, Wallace D, et al. Comorbidity adjustments for functional outcomes in community-dwelling older adults. Clin Rehabil. 2002;16:428. [DOI] [PubMed] [Google Scholar]
- 26. Faulkner J, Eston RG. Perceived exertion research in the 21st century: developments, reflections and questions for the future. J Exerc Sci Fit. 2008;6:1–14 [Google Scholar]
- 27. Fry D, Pfalzer LA. Reliability of four functional tests and rating of perceived exertion in persons with multiple sclerosis. Physiother Can. 2006;58:212–220 [Google Scholar]
- 28. Walsh JP. Foot fall measurement technology. In: Craik RL, Oatis CA. Gait Analysis: Theory and Application. St Louis, MO: Mosby-Year Book Inc; 1995:125–142 [Google Scholar]
- 29. Brach JS, Perera S, Studenski S, et al. Reliability and validity of measures of gait variability in community-dwelling older adults. Arch Phys Med Rehabil. 2008;89:2293–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brach JS, Berthold R, Craik RL, et al. Gait variability in community-dwelling older adults. J Am Geriatr Soc. 2001;49:1646–1650 [DOI] [PubMed] [Google Scholar]
- 31. Brach JS, Studenski S, Perera S, et al. Stance time and step width variability have unique contributing impairments in older persons. Gait Posture. 2008;27:431–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Imms FJ, Edholm OG. Studies of gait and mobility in the elderly. Age Ageing. 1981;10:147–156 [DOI] [PubMed] [Google Scholar]
- 33. Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guralnik JM, Ferrucci L, Pieper C, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the Short Physical Performance Battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231 [DOI] [PubMed] [Google Scholar]
- 35. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Perera S, Mody SH, Woodman RC, et al. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749 [DOI] [PubMed] [Google Scholar]
- 37. VanSwearingen JM, Paschal KA, Bonino P, Yang J-F. The Modified Gait Abnormality Rating Scale for recognizing the risk of recurrent falls in community-dwelling elderly adults. Phys Ther. 1996;76:994–1002 [DOI] [PubMed] [Google Scholar]
- 38. VanSwearingen JM, Paschal KA, Bonino P, Chen TW. Assessing recurrent fall risk of community-dwelling, frail older veterans using specific tests of mobility and the Physical Performance Test of function. J Gerontol A Biol Sci Med Sci. 1998;53:M457–M464 [DOI] [PubMed] [Google Scholar]
- 39. Boyd R, Fatone S, Rodda J, et al. High- or low-technology measurements of energy expenditure in clinical gait analysis? Dev Med Child Neurol. 1999;41:676–682 [DOI] [PubMed] [Google Scholar]
- 40. McArdle WD, Katch FI, Katch VL. Exercise Physiology: Energy, Nutrition, and Human Performance. 5th ed. Baltimore, MD: Lippincott Williams & Williams; 2001 [Google Scholar]
- 41. Waters RL, Lunsford BR. Energy cost of paraplegic ambulation. J Bone Joint Surg. 1985;67:1245–1250 [PubMed] [Google Scholar]
- 42. Waters RL. Energy expenditure. In: Perry J. Gait Analysis: Normal and Pathologic Function. Thorofare, NJ: Slack Inc; 2004:443–489 [Google Scholar]
- 43. Bernardi M, Macaluso A, Sproviero E, et al. Cost of walking and locomotor impairment. J Electromyogr Kinesiol. 1999;9:149–157 [DOI] [PubMed] [Google Scholar]
- 44. Gersten J, Orr W. External work of walking in hemiparetic patients. Scand J Rehabil Med. 1971;3:85–88 [PubMed] [Google Scholar]
- 45. Macko RF, DeSouza CA, Tretter LD, et al. Treadmill aerobic exercise training reduces the energy expenditure and cardiovascular demands of hemiparetic gait in chronic stroke patients: a preliminary report. Stroke. 1997;28:326–330 [DOI] [PubMed] [Google Scholar]
- 46. Macko RF, Smith GV, Dobrovolny NA, et al. Treadmill training improves fitness reserve in chronic stroke patients. Arch Phys Med Rehabil. 2001;82:879–884 [DOI] [PubMed] [Google Scholar]
- 47. Waters RL, Barnes G, Husserl T, et al. Comparable energy expenditure following arthrodesis of the hip and ankle. J Bone Joint Surg Am. 1988;70:1032–1037 [PubMed] [Google Scholar]
- 48. Ijzerman KJ, Baardman G, van't Hof MA, et al. Validity and reproducibility of crutch force and heart rate measurements to assess energy expenditure of paraplegic gait. Arch Phys Med Rehabil. 1999;80:1017–1023 [DOI] [PubMed] [Google Scholar]
- 49. Hood VL, Granat MH, Maxwell DJ, Hasler JP. A new method of using heart rate to represent energy expenditure: the Total Heart Beat Index. Arch Phys Med Rehabil. 2002;83:1266–1273 [DOI] [PubMed] [Google Scholar]
- 50. MacGregor J. The objective measurement of physical performance with long term ambulatory physiological surveillance equipment (LAPSE). In: Stott FD, Raftery EB, Goulding L. ISAM 1979: Proceedings of the Third International Symposium on Ambulatory Monitoring. London, United Kingdom: Academic Press; 1980:29–39 [Google Scholar]
- 51. Jette AM, Haley SM, Coster WJ, et al. Late Life Function and Disability Instrument, I: development and evaluation of the disability component. J Gerontol A Biol Sci Med Sci. 2002;57:M209–M216 [DOI] [PubMed] [Google Scholar]
- 52. International Classification of Functioning, Disability and Health: ICF. Geneva, Switzerland: World Health Organization; 2001 [Google Scholar]
- 53. Jette AM, Haley SM, Kooyoomijian JT. Are the ICF activity and participation dimensions distinct? J Rehabil Med. 2003;35:145–149 [DOI] [PubMed] [Google Scholar]
- 54. Lachman ME, Howland J, Tennstedt S, et al. Fear of falling and activity restriction: the Survey of Activities and Fear of Falling in the Elderly (SAFE). J Gerontol B Psychol Sci Soc Sci. 1998;53:P43–P50 [DOI] [PubMed] [Google Scholar]
- 55. Schwartz AM, Strath SJ, Bassett DR. Estimation of energy expenditure using CSA accelerometers at hip and wrist sites. Med Sci Sports Exerc. 2000;32:S450–S456 [DOI] [PubMed] [Google Scholar]
- 56. Welk GJ, Schaben JA, Morrow JR. Reliability of accelerometry-based activity monitors: a generalizability study. Med Sci Sports Exerc. 2004;36:1637–1645 [PubMed] [Google Scholar]
- 57. Bassett DR, Ainsworth BE, Leggett SR, et al. Accuracy of five electronic pedometers for measuring distance walked. Med Sci Sports Exerc. 1996;28:1071–1077 [DOI] [PubMed] [Google Scholar]
- 58. Melanson EL, Jr, Freedson PS. Validity of the Computer Science and Applications, Inc. (CSA) activity monitor. Med Sci Sports Exerc. 1995;27:934–940 [PubMed] [Google Scholar]
- 59. Nichols JF, Morgan CG, Chabot LE, et al. Assessment of physical activity with the Computer Science and Applications, Inc., accelerometer: laboratory versus field validation. Res Q Exerc Sport. 2000;71:36–43 [DOI] [PubMed] [Google Scholar]
- 60. Hess RJ, Brach JS, Piva SR, et al. Walking skill can be assessed in older adults: validity of Figure-of-8 Walk Test. Phys Ther. 2010;90:89–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Newell A, VanSwearingen JM, Hile ES, et al. The Modified Gait Efficacy Scale: establishing the psychometric properties in older adults. Phys Ther. 2012;92:318–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cumming RG, Salkeld G, Thomas M, et al. Prospective study of the impact of fear of falling on activities of daily living, SF-36 scores, and nursing home admission. J Gerontol A Biol Sci Med Sci. 2000;55:299–305 [DOI] [PubMed] [Google Scholar]
- 63. Friedman SM, Munoz B, West SK, et al. Falls and fear of falling: which comes first? A longitudinal prediction model suggests strategies for primary and secondary prevention. J Am Geriatr Soc. 2002;50:1329–1335 [DOI] [PubMed] [Google Scholar]
- 64. McAuley E, Konopack JF, Morris KS, et al. Physical activity and functional limitations in older women: influence of self-efficacy. J Gerontol B Psychol Sci Soc Sci. 2006;61:P270–P277 [DOI] [PubMed] [Google Scholar]
- 65. McAuley E, Morris KS, Doerksen SE, et al. Effects of change in physical activity on physical function limitations in older women: mediating roles of physical function performance and self-efficacy. J Am Geriatr Soc. 2007;55:1967–1973 [DOI] [PubMed] [Google Scholar]
- 66. Symmonds M, Emmanuel JJ, Drew ME, et al. Metabolic state alters economic decision making under risk in humans. PLoS ONE. 2010;5:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Brach JS, VanSwearingen JM, Newman AB, et al. Identifying early decline of physical function in community-dwelling older adults: performance-based and self-report measures. Phys Ther. 2002;82:320–328 [PubMed] [Google Scholar]
- 68. Elam JT, Graney MJ, Beaver T, et al. Comparison of subjective ratings of function with observed functional ability of frail older persons. Am J Public Health. 1991;81:1127–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kelly-Hayes M, Jette AM, Wolf PA, et al. Functional limitations and disability among elders in the Framingham Study. Am J Public Health. 1992;82:841–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Marcora S. Counterpoint: afferent feedback from fatigued locomotor muscles is not an important determinant of endurance exercise performance. J Appl Physiol. 2010;108:454–456 [DOI] [PubMed] [Google Scholar]
- 71. Groslambert A, Mahon AD. Perceived exertion: influence of age and cognitive development. Sports Med. 2006;36:911–928 [DOI] [PubMed] [Google Scholar]