Abstract
Background:
Locoregional recurrence is the major cause of treatment failure after surgery for oral squamous cell carcinoma. Molecular diagnostics have the potential to improve on clinicopathological parameters to predict this recurrence and plan adjuvant treatment. The test most frequently applied is based on detecting TP53 mutations, but alternative methodology is required for cases that harbour the wild-type gene.
Methods:
One hundred and two cases with tumour-adjacent margins, considered to be clear margins by microscopy, were examined using carefully optimised molecular diagnostics based on detection of the TP53 and Ly-6D markers. The markers were also combined to provide a dual approach.
Results:
The dual molecular diagnostic identified cases with a significant increase in the probablility of developing locoregional recurrence when tumour-adjacent positive and clear margins were compared (P=0.0001). These tests were most useful when the clearance at the resection margins was 5 mm or less. The TP53-based diagnostic was a better predictor of locoregional recurrence than established clinicopathological parameters.
Conclusion:
The optimised TP53-based diagnostic rapidly identifies an important subgroup of cases with close margins that will benefit from new treatment modalities to reduce the risk of recurrence.
Keywords: molecular diagnostics, head and neck cancer, residual cancer, recurrence, TP53 mutation, prognosis
Oral squamous cell carcinomas (OSCC) arise in genetically altered fields, of which only a small group can be identified clinically as a white or red patch. Many more are recognised microscopically as dysplasia. Locoregional recurrence is the major cause of treatment failure after surgery for OSCC. Recurrence may develop due to evolution of a new focus of carcinoma in a field that has not been excised. Alternatively, tumour spread beyond the resection margins that was not identified by histological examination may be the source of cells responsible for this recurrence. Application of ultrasensitive diagnostics based on finding carcinoma-associated mutations in the surrounding normal tissue may reveal the extent of spread of genetically altered keratinocytes, or residual carcinoma to identify cases at high risk of recurrence after surgery (Brennan et al, 1995; Partridge et al, 2000; van Houten et al, 2002; van Houten et al, 2004; Huang et al, 2007).
The TP53 gene is frequently chosen as a target for detection of precancerous change and minimal residual carcinoma as it is mutated in 50–60% of OSCC and a rate-limiting step in this process (Smeets et al, 2011). However, these mutations may occur anywhere in this gene, such that diagnostics for assessment of tumour-adjacent margins, based on detecting the same TP53 mutation in a carcinoma and the surrounding normal tissues, must be customised for each different mutation. Development of a method to achieve a sensitivity for TP53 mutation detection of at least one mutant transcript in 1000 wild-type, and ideally 1 in 10 000, requires multistep molecular techniques, as quantitative reverse-transcription PCR (QRT-PCR) cannot detect mutant TP53 against a large background of wild type. Preliminary studies applying new methods to detect TP53 mutations in tumour-adjacent normal tissues often appear promising but may be limited to analysis of selected cases for which a custom test is available (Shi et al, 2004; Poeta et al, 2009). Larger studies often reveal the need for further optimisation to achieve reproducible and robust assays with a high sensitivity for mutation detection. Once these goals are achieved, molecular diagnostics provide the clinician with a powerful new resource that can give information about the risk of recurrence within a short time frame.
There is also a need to develop ways to identify precancerous fields where progression is likely and tumour spread when TP53 is wild type. Until other highly recurrent carcinoma-specific mutations are identified, application of a surrogate marker for carcinoma, based on the detection of a squamous cell-specific target Ly-6D, recognised by the E48 antibody and often termed Ly-6D, in the deep tissues has been proposed (Nieuwenhuis et al, 2003; Graveland et al, 2009). This antigen has been shown to be expressed by normal, transitional and malignant squamous epithelia (Quak et al, 1990) and 80–90% of OSCC (Graveland et al, 2009). The importance of molecular screening of deep as well as mucosal margins was established by Huang et al (2007) and provides a rational basis to include the Ly-6D-based approach a surrogate marker so that all cases can be tested.
In this report, we present our findings utilising this dual approach in a prospective clinical trial powered to establish whether the molecular tests identify cases with a 20% increase in the risk of recurrence 2 years post surgery.
Materials and methods
One hundred and forty cases undergoing resection of OSCC were recruited to the UKCRNID7493 clinical trial. The Research Ethics Committees approved this prospective study at all collaborating centres. Tissue samples were collected as previously described (Huang et al, 2007). Briefly after tumour resection, the surgeon excised mucosal and deep tumour-adjacent margins from the tissues remaining at the edge of the defect for molecular examination. When the base of the resection was in continuity with the neck dissection and there were no true deep tumour-adjacent margins, the samples analysed were excised from the lateral aspects of the defect. An equal number of mucosal and deep margins were collected for each carcinoma. Each margin was divided into two halves. One part was snap frozen in isopentane for molecular analysis, the other part was fixed in formalin and processed into paraffin for examination of step-serial sections by light microscopy. A portion of each carcinoma was snap frozen and stored at −80 °C until nucleic acids were extracted. The minimum tumour clearance at the mucosal and deep margins was also reported by the pathologist. One hundred and two cases, with resection and tumour-adjacent margins assessed as being carcinoma-free by light microscopy, were forwarded for molecular analysis. All cases were followed for a minimum of 24 months to assess the impact of the molecular diagnostics on recurrence. Distant metastases were not analysed, as the study aim was to assess the effect of positive tumour-adjacent margins on the rate of recurrence.
Examination of mucosal and deep tumour-adjacent margins for cases where the carcinoma harboured mutant TP53
TP53 mutation status was established (Huang et al, 2007), and the presence or absence of the same TP53 mutations in the matched tumour-adjacent margins was also established using the LigAmp assay (Shi et al, 2004; Poeta et al, 2009) with the following important refinements. To develop the LigAmp assay for each TP53 mutation, the relevant exon was PCR amplified and purified for all carcinomas and the tumour-adjacent margins to be tested. Serial dilutions of each carcinoma-derived TP53 cDNA were diluted in the corresponding wild-type cDNA prepared from normal oral mucosa. Tumour-adjacent margins were processed undiluted. Optimal conditions for the ligations were 109 TP53 amplicons in 1 μl combined with 2.5 μl 10 × pfu ligase buffer, 0.5 μl 4 U μl−1 pfu ligase (Stratagene, Agilent Technologies, Stockport, UK), 0.25 μl 4 μℳ upstream ligation oligonucleotide (containing the nucleotide complementary to the TP53 mutation and a mismatched G or T, at the third base from the mutation site when feasible), 0.125 μl 4 μℳ downstream TP53-specific oligonucleotide and 20.125 μl nuclease-free water. The subsequent QRT-PCR reaction was performed with 50 nℳ of M13-specific primers, with each sample analysed in triplicate using an AB 7700 detection system (Applied Biosytems, Warrington, UK). Primer sequences that amplify TP53 mutations with a sensitivity greater than I mutant copy in 10 000 wild type are shown in Supplementary Table 1.
Molecular margins were scored as TP53 mutation-positive or -negative according to the 88–95–99.7% rule, using the formula Nct−3NSD=X (where Nct is the mean of the normal tissue cycle threshold values and NSD is the s.d. of the normal ct value). A margin was scored as TP53 mutation-positive when the ct was less than X (Flys et al, 2005). All cases were analysed using gDNA and cDNA, and positive results validated from new tissue sections. Cases with at least one positive tumour-adjacent mucosal or deep margin were considered to have a positive molecular test.
Examination of deep tumour-adjacent margins for cases wherein the carcinoma harboured wild-type TP53
Deep margins for cases where the carcinoma was wild type for TP53 were analysed for expression of Ly-6D by QRT-PCR, as previously described (Nieuwenhuis et al, 2003; Graveland et al, 2009), using the two-standard curve method for data analysis. YHAWS was selected as the internal reference, as the expression of this gene is stable across a broad range of oral tissues (n>250). For primer sequences, see Supplementary Table 2. The threshold level of Ly-6D expression above which deep margins were scored as positive was defined as the mean ct and s.d. using 30 normal samples harvested from different tissue types, including muscle, minor salivary glands and oral submucosa.
The dual approach
The TP53 and Ly-6D markers were also combined in a dual approach in which a positive score with one marker provided a positive molecular test.
Statistical analysis
Data was analysed using Stata Release 11.1 (StataCorp, College Station, TX, USA). Significance was pre-determined at α=0.05. Monte Carlo simulations were used to obtain exact probabilities (Good, 2006). Single-ordered data was analysed using the Kruskal–Wallis test and ‘survival’ data was analysed by the Kaplan–Meier survival curves in conjunction with the log rank test. Proportional hazard models were used to determine the univariate prognostic significance of clinicopathological variables and molecular diagnostics with respect to local and locoregional recurrence. P-values for hazards ratios (HRs) were calculated using the likelihood-ratio test relative to a reference group. Multivariate analysis logistic regression was also applied with model parameter selection based on Bayesian information (Raftery, 1995).
Results
The clinical and pathological features of 102 cases with carcinoma-free resection margins are shown in Table 1. Forty-six cases received post-operative radiotherapy. The follow-up time for the study ranged from 24 to 146 months, with the median being 43 months. At the time of assessment, 16 out of 102 cases developed local and 27 out of 102 cases developed locoregional recurrence (Table 1). Fifty-one carcinomas harboured mutant TP53 and 51 were wild type. Three recurrent TP53 mutations were identified (175.2 G-A, 282.1 C-T and 248.2 G-A) with each detected for three cases.
Table 1. Clinicopathological details for 102 cases analysed with respect to tumour TP53 gene status.
| TP53 mutant cases (n=51) | Wild-typecases ( n =51) | All cases ( n =102) | |
|---|---|---|---|
| Age mean (range) | 62 (34–90) | 63 (36–82) | |
| Gender | |||
| Male | 32 | 32 | 64 |
| Female | 19 | 19 | 38 |
| UICC stage | |||
| I or II | 11 | 14 | 25 |
| III or IV | 40 | 37 | 87 |
| Tumour stage | |||
| T1 or T2 | 11 | 14 | 25 |
| T3 or T4 | 14 | 35 | 49 |
| Lymph nodes | |||
| N0 | 21 | 21 | 42 |
| N1 | 10 | 10 | 20 |
| N2 | 19 | 17 | 36 |
| N3 | 1 | 1 | |
| Resection margins clear at (mm) | |||
| 2 | 46 | 39 | 85 |
| 3 | 34 | 35 | 69 |
| 5 | 22 | 20 | 42 |
| Tumour differentiation | |||
| Well | 16 | 16 | 32 |
| Moderate | 27 | 27 | 54 |
| Poor | 8 | 8 | 16 |
| Vascular invasion | 11 | 9 | 20 |
| Neural invasion | 18 | 10 | 28 |
| Invasion pattern | |||
| Pushing | 16 | 19 | 35 |
| Infiltrative | 35 | 32 | 67 |
| Dysplasia at resection margin | 19 | 14 | 33 |
| Dysplasia at molecular margin | 8 | 5 | 13 |
| Post-operative radiotherapy | |||
| Yes | 26 | 20 | 46 |
| No | 25 | 31 | 56 |
| Recurrence | |||
| Local | 9 | 7 | 16 |
| Regional only | 6 | 0 | 6 |
| Local and regional | 10 | 11 | 21 |
| Alive at study end | 20 | 29 | 49 |
| DOC | 5 | 3 | 8 |
| DOD | 24 | 19 | 43 |
| Lost to follow-up | 2 | 0 | 2 |
Abbreviations: DOC=died as a result of other causes; DOD=died of disease.
Analysis of mucosal and deep tumour-adjacent margins
Forty-two LigAmp TP53 assays were optimised for 51 cases. Thirty-three assays had a sensitivity for TP53 mutation detection greater than I mutant amplicon in 10 000 wild type, with the sensitivity for the remaining mutations being greater than 1 in 1000. Careful optimisation of the reaction conditions for the ligation and QRT-PCR steps for each mutation ensured that each customised test was robust, reproducible and achieved high sensitivity for TP53 mutation detection. Once optimised for a specific TP53 mutation, a test result could be available within 3 working days. Subsequently, 111 mucosal tumour-adjacent margins and 97 deep margins were analysed. For representative example, see Supplementary Figure 1. Thirty-four of the 51 cases (66.6%) showed TP53 mutations in one or more margins examined. Eight of the 51 cases had only mucosal margins that were scored as TP53 mutation-positive, 8 had only TP53 mutation-positive deep margins and 18 were positive at both sites. Eleven of the 26 (42%) positive had morphological evidence of dysplasia at the margins of the resected carcinoma, and 6 of 26 (15.3%) showed dysplasia at the tumour-adjacent mucosal margins.
Overall, there was excellent concordance of the LigAmp assay when gDNA and cDNA were analysed with three cases showing discordant results for one or more of the four margins tested, but the overall score for these cases was unchanged (Supplementary Table 3).
Fifty-one cases, wherein the tumour harboured wild-type TP53, with 94 deep tumour-adjacent margins, which could not be examined with the TP53 LigAmp, were analysed with the surrogate tumour marker Ly-6D, and 14 cases were scored as having positive margins.
A subgroup of cases wherein the tumour harboured mutant TP53 was analysed with both the TP53 LigAmp and the Ly-6D QRT-PCR assay (n=30). All available deep tumour-adjacent margins were tested. Concordant results were observed for 10 cases, with the margins scored as positive with both tests for 6 cases and negative for the other 4. A further 10 cases were TP53 mutation-positive but Ly-6D negative; the other cases were Ly-6D-positive but TP53-negative.
No immunostained cells with morphological features considered to be adequate to categorically diagnose minimal residual carcinoma were identified in the part of each margin forwarded for immunohistology, although normal squamous epithelia was reported in deep margins (n=4).
Relationship between molecular analysis of tumour-adjacent margins and locoregional recurrence
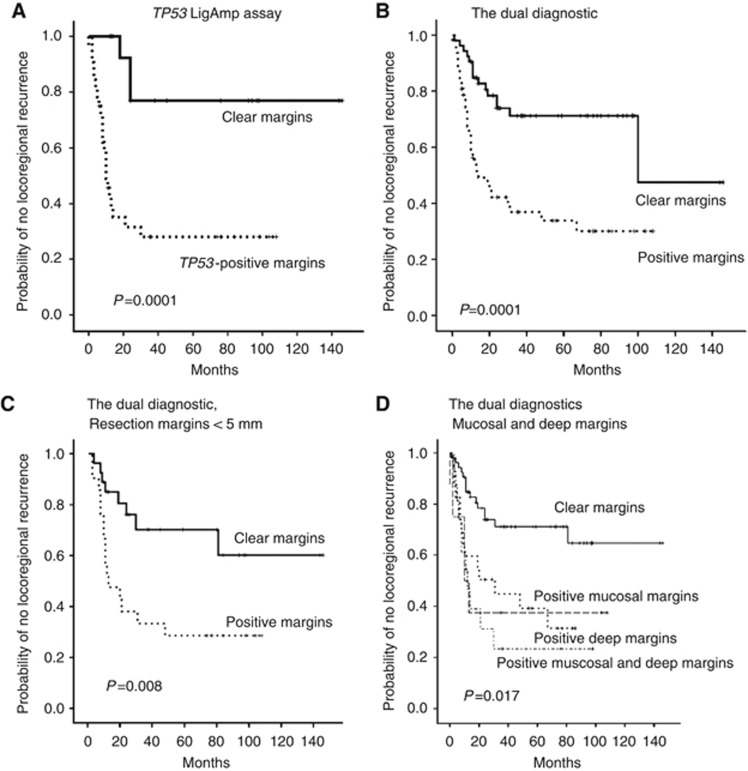
A high number of cases with TP53 mutation-positive tumour-adjacent margins developed locoregional recurrence, 16 out of 34 (47% Table 1). The effect of TP53 mutation-positive margins on locoregional recurrence was assessed by Kaplan–Meier estimates and log rank tests. This analysis revealed that the probability of developing locoregional recurrence was significantly higher for the group with TP53 mutation-positive margins when compared with the group with clear margins (P=0.0001; Figure 1A).
Figure 1.
Kaplan–Meier estimates for (A) The TP53-based diagnostic, no locoregional recurrence with respect to the presence or absence of TP53 mutations in the tumour-adjacent margins examined. (B) No locoregional recurrence with the dual TP53- and Ly-6D-based diagnostic. (C) No locoregional recurrence with the dual diagnostic for cases wherein the tumour-adjacent margins examined were from cases in which the clearance at the resection margins was 5 mm or less. (D) No local recurrence with the dual diagnostic with respect to clear tumour-adjacent margins, TP53 mutation-positive tumour-adjacent mucosal margins, positive deep margins or positivity at both sites.
Fourteen of 51 (27%) cases wherein the carcinoma was wild type for TP53 had deep margins scored as Ly-6D-positive. Two of these cases developed local and 7seven developed locoregional recurrence, but the Kaplan–Meier estimates for the comparisons between cases with Ly-6D-positive and -negative margins were not significant.
The results obtained with both the TP53 and Ly-6D-based diagnostics were also combined, so that all 102 cases were analysed with a molecular test and the probability of developing locoregional recurrence was significant (P=0.0001; Figure 1B). Detailed analysis of the 102 cases examined with the combined diagnostics revealed that when the clearance at the resection margins was 5 mm or less, the difference in the recurrence rate with a positive tumour-adjacent margin was significant for locoregional recurrence (P=0.008; Figure 1C). In contrast, this difference was not significant when the clearance at the resection margins was greater than 5 mm (P=0.628).
When the value of testing tumour-adjacent mucosal or deep margins was compared with the dual diagnostic, the difference in the locoregional recurrence rate was significant when cases with clear and positive deep margins were compared. The difference in the recurrence rate was also significant when cases with clear and positive mucosal and deep margins were compared (P=0.017; Figure 1D).
Concordance between TP53 mutation-positive and LY-6D-positive tumour-adjacent margins, development of recurrence and the effect of radiotherapy
The finding of TP53 mutation-positive tumour-adjacent margins correctly identified 22 of 25 cases developing locoregional recurrence. Fifteen of 17 cases with TP53 mutation-negative margins and 17 of 34 cases with TP53 mutation-positive tumour-adjacent margins did not develop recurrence, Table 2. These relationships are also shown for the Ly-6D-based diagnostic and the combined approach, together with the sensitivity, specificity, positive and negative predictive values for recurrence.
Table 2. The sensitivity, specificity, positive and negative predictive values for the TP53 LigAmp and Ly-6D QRT-PCR assays for locoregional recurrence.
| Locoregional recurrence | No locoregional recurrence | ||
|---|---|---|---|
| TA margins TP53 mutant | 22 | 12 | Positive predictive value 64.7% (95% CI, 46.5–80.3%) |
| TA margins TP53 wild type | 2 | 15 | Negative predictive value 88.2% (95% CI, 63.6–98.5%) |
| Sensitivity 91.7% | Specificity 55.6% | ||
| TA margins Ly-6D-positive | 8 | 6 | Positive predictive value 57.1% (95% CI, 28.9–82.3%) |
| TA margins Ly-6D-negative | 11 | 26 | Negative predictive value 70.3% (95% CI, 53–84.1%) |
| Sensitivity 42.2% | Specificity 81.3% | ||
| TA margins positive with the dual diagnostic | 30 | 18 | Positive predictive value 62.5% (95% CI, 47.4–76%) |
| TA margins negative with the dual diagnostic | 13 | 41 | Negative predictive value 75.9% (95% CI, 62.4–86.5%) |
| Sensitivity 69.8% | Specificity 69.5% |
Abbreviations: CI=confidence interval; QRT-PCR=quantitative reverse-transcription PCR; TA=tumour-adjacent.
It is to be expected that some cases with TP53 mutation-positive or Ly-6D-positive margins will not develop recurrence due to the effect of radiotherapy. Twenty-six of 51 (51%) cases wherein the carcinoma harboured mutant TP53 received radiotherapy and 11 (42%) developed locoregional recurrence. The recurrence rate for cases where the tumour was wild type for TP53 was slightly higher in that 21 of 51 cases received radiotherapy and 11 of 21 (52%) developed recurrences. However, overall, there was no difference in the rate of recurrence for TP53 mutant and wild-type cases.
Relationship between clinicopathological features of molecular examination of tumour-adjacent margins and development of recurrence
A combination of factors is associated with treatment failure, so we used univariate and HR analyses to look for relationships between the molecular test and clinicopathological features (TNM stage, T stage, N stage, tumour differentiation, perivascular or perineural spread, infiltrative or pushing margins and dysplasia at the resection margins) and development of recurrence.
Univariate analysis of the subset of TP53 mutant cases revealed that a positive tumour-adjacent margin was the only significant factor for locoregional recurrence (P=0.002), whereas positive tumour-adjacent margins (P=0.002) and TNM stage (P=0.035) were significant for the full data set. In contrast, only perineural spread was significant for locoregional recurrence (P=0.032) for the TP53 wild-type cases.
Application of HRs in survival analysis confirmed that a positive molecular test was significant for locoregional recurrence for the TP53 mutant cases, HR 4.8 (95% CI 1.66–14.2, P=0.004; Supplementary Table 4). The HR for the dual diagnostic was also significant, HR 3.1 (95% CI 1.7–5.7, P<0.001). HR analysis confirmed that perineural spread was a significant factor for locoregional recurrence for the wild-type cases HR 2.28 (95% CI 8.59–6.08, P=0.097).
In multivariate analysis, considering all clinicopathological factors together with the results from the molecular tests, for the cases where the carcinoma harboured mutant TP53, a positive molecular test was the best independent predictor of locoregional recurrence, followed by the combination of a positive molecular test and perineural spread. When the TP53 wild-type cases were considered, perineural spread remained the best predictor for locoregional recurrence.
Discussion
At present, the clinician relies on clinical staging and the histopathology report to gauge a patient’s prognosis and plan treatment. The challenge is to develop molecular markers that can provide more accurate predictions about the risk of recurrence and to establish which cases are most likely to benefit from the additional information provided by new diagnostics. In the present study, we successfully applied a dual molecular diagnostic to identify cases with clear resection margins at high risk of locoregional recurrence. We demonstrate for the first time that the LigAmp TP53-based diagnostic outperforms all established clinicopathological parameters for predicting the risk of locoregional recurrence. The result of this molecular test can now be available within 3 working days, facilitating translation of this test from a research to a routine diagnostic laboratory setting and incorporation into future clinical trials of new adjuvants.
We also show that molecular assessment of tissues beyond the resection margins identifies cases at high risk of local or locoregional recurrence, and that the molecular diagnostics are most useful when the clearance at the surgical margins is less than 5 mm. The difference in the local recurrence rate was most significant when cases with clear and tumour-positive deep margins were compared, confirming the importance of applying molecular tests to mucosal as well as deep margins. Our observations also confirm the importance of perineural invasion as a prognostic marker (Soo et al, 1986; Fagan et al, 1998; Rahima et al, 2004). The concordant results when cDNA and gDNA were used indicates that false-positive tests due to DNA leaking from tumour cells is less of a concern than previously suggested (van Houten et al, 2002), although we now routinely use cDNA as the starting material for molecular diagnostics.
The TP53 LigAmp cannot be used to quantify the burden of residual carcinoma for an individual case, only to score a tumour-adjacent margin as positive or negative in relation to the threshold, as the percentage of malignant cells in each carcinoma is different. To address this point, Poeta et al (2009) used plasmids containing mutant TP53 as well as carcinoma-derived DNA to quantify the LigAmp assay, but their results were not significant. These authors subsequently applied recursive partitioning trees based on information about whether recurrence developed or not, to set the cut point for deciding whether a test should be scored as positive or negative. The Kaplan–Meier ‘survival’ analysis in this earlier report must thus be interpreted with caution. However, the present larger study based on an optimised methodology confirms the clinical utility of this TP53-based diagnostic to provide a test with a high sensitivity for mutation detection.
In contrast, the relatively quantitative Ly-6D-based test for molecular analysis of deep margins lacks the discriminatory sensitivity of the TP53-based diagnostics in terms of identifying cases at risk of recurrence. Nevertheless, the high specificity of the Ly-6D-based approach for predicting no risk of recurrence means that a negative test provides useful information. Thus, combining both molecular diagnostics allows consecutive cases to be screened for evidence of minimal residual carcinoma, with the caveat that only deep margins are examined with the Ly-6D QRT-PCR.
The high rate of false-positive tests obtained with the Ly-6D QRT-PCR suggests that there are probably normal squamous epithelia in the deep margins forwarded for analysis, and this was confirmed by light microscopy. Contamination from normal cells as the margins are harvested seems most likely, but as the molecular and histological examination was performed on different parts of each margin, no firm conclusions can be made. Thus, although the Ly-6D-based diagnostic can provide useful prognostic information, there are drawbacks associated with this approach. This highlights the need to identify a highly recurrent mutation, a fusion gene or an intragenic fusion, similar to the markers that have been so successful in developing effective strategies to monitor for the presence of residual leukaemia, to develop an alternative robust molecular diagnostic for OSCC.
Acknowledgments
This research was supported by grants from the Coco Marcus Trust, the Rosetrees Trust and the Association for International Cancer Research.
Supplementary Information accompanies the paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
09/10/2012
This paper has been modified since advance online publication, an acknowledgement has been added
Supplementary Material
References
- Brennan JA, Mao L, Hruban RH, Boyle JO, Eby YJ, Koch KM, Goodman SN, Sidransky D (1995) Molecular assessment of histopathological staging in squamous-cell carcinoma of the head and neck. N Engl J Med 332: 429–435 [DOI] [PubMed] [Google Scholar]
- Fagan JJ, Collins B, Barnes L, D’Amico F, Myers EN, Johnson JT (1998) Perineural invasion in squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg 124: 637–640 [DOI] [PubMed] [Google Scholar]
- Flys T, Nissley DV, Claasen CW, Jones D, Shi C, Guay LA, Musoke P, Mmiro F, Strathern JN, Jackson JB, Eshleman JR, Eshleman SH (2005) Sensitive drug-resistance assays reveal long-term persistence of HIV-1 variants with the K103N nevirapine (NVP) resistance mutation in some women and infants after the administration of single-dose NVP: HIVNET 012. J Infect Dis 192: 24–29 [DOI] [PubMed] [Google Scholar]
- Good PI (2006) Resampling Methods: A Practical Guide to Data Analysis. 3rd edn. Birkhäuser: Boston [Google Scholar]
- Graveland AP, de Maaker M, Braakhuis BJM, de Bree R, Eerenstein SEJ, Bloemena E, Leemans CR, Brakenhoff RH (2009) Molecular detection of minimal residual cancer in surgical margins of head and neck cancer patients. Cellular Oncol 31: 317–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Pateromichelakis S, Hills A, Sherriff M, Lyons A, Odell E, Morgan P, Harrison J, Partridge M (2007) TP53 mutations in deep tissues are more strongly associated with recurrence than mutation-positive mucosal margins. Clin Cancer Res 13: 6099–06 [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis EJ, Leemans CR, Kummer JA, Denkers F, Snow GB, Brakenhoff RH (2003) Assessment and clinical significance of micrometastases in lymph nodes of head and neck cancer patients detected by LY-6D (Ly-6D) quantitative reverse transcription-polymerase chain reaction. Lab Invest 83: 1233–1240 [DOI] [PubMed] [Google Scholar]
- Partridge M, Li SR, Pateromichelakis S, Francis R, Phillips E, Huang XH, Tesfa-Selase F, Langdon JD (2000) Detection of minimal residual cancer to investigate why oral tumours recur despite seemingly adequate treatment. Clin Cancer Res 6: 2718–2725 [PubMed] [Google Scholar]
- Poeta ML, Manola J, Goldenberg D, Forastiere A, Califano JA, Ridge JA, Goodwin J, Kenady D, Saunders J, Westra W, Sidransky D, Koch WM (2009) The LigAmp TP53 assay for detection of minimal residual disease in head and neck squamous cell carcinoma surgical margins. Clin Cancer Res 15: 7658–7665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quak JJ, Balm AJ, van Dongen GA, Brakkee JG, Scheper RJ, Snow GB, Meijer CJ (1990) A 22-kD surface antigen detected by monoclonal antibody E 48 is exclusively expressed in stratified squamous and transitional epithelia. Am J Pathol 136: 191–197 [PMC free article] [PubMed] [Google Scholar]
- Raftery A (1995) Bayesian Model Selection in Social Research. Sociol Methodol 25: 111–163 [Google Scholar]
- Rahima B, Shingaki S, Nagata M, Saito C (2004) Prognostic significance of perineural invasion in oral and oropharyngeal carcinoma. Oral Surg Oral Med Oral Path 97: 423–431 [DOI] [PubMed] [Google Scholar]
- Shi C, Eshleman SH, Jones D, Fukishima N, Hua L, Parker AR, Yeo CJ, Hruban RH, Goggins MG, Eshleman JR (2004) LigAmp for sensitive detection of single-nucleotide differences. Nat Methods 1: 141–147 [DOI] [PubMed] [Google Scholar]
- Smeets SJ, van der Plas M, Schaaij-Visser TB, van Veen EA, van Meerloo J, Braakhuis BJ, Steenbergen RD, Brakenhoff RH (2011) Immortalisation of oral keratinocytes by functional inactivation of the TP53 and pRb pathways. Int J Cancer 28: 1596–05 [DOI] [PubMed] [Google Scholar]
- Soo K-C, Carter RL, O’Brien C, Barr L, Bliss JM, Shaw HJ (1986) Prognostic implications of perineural spread in squamous carcinoma of the head and neck. Laryngoscope 96: 1145–1148 [DOI] [PubMed] [Google Scholar]
- Van Houten VM, Leemand CR, Kummer JA, Kuik DJ, van den Brekel MWM, Snow GB, Brakenhoff RH (2004) Molecular diagnosis of surgical margins and local recurrence in head and neck cancer patients a prospective study. Clin Cancer Res 10: 3614–3620 [DOI] [PubMed] [Google Scholar]
- Van Houten VMM, Tabor M, Van Den Brekel MWM, Kummer JA, Denkers F, Dijkstra J, Leemans CR, Van Der Waal I, Snow GB, Brakenhoff RH (2002) Mutated TP53 as a marker for the diagnosis of head and neck cancer. J Pathol 198: 476–486 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.