Abstract
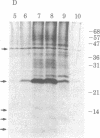
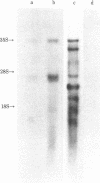
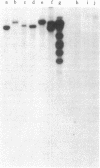
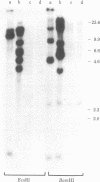
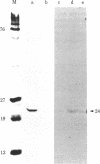
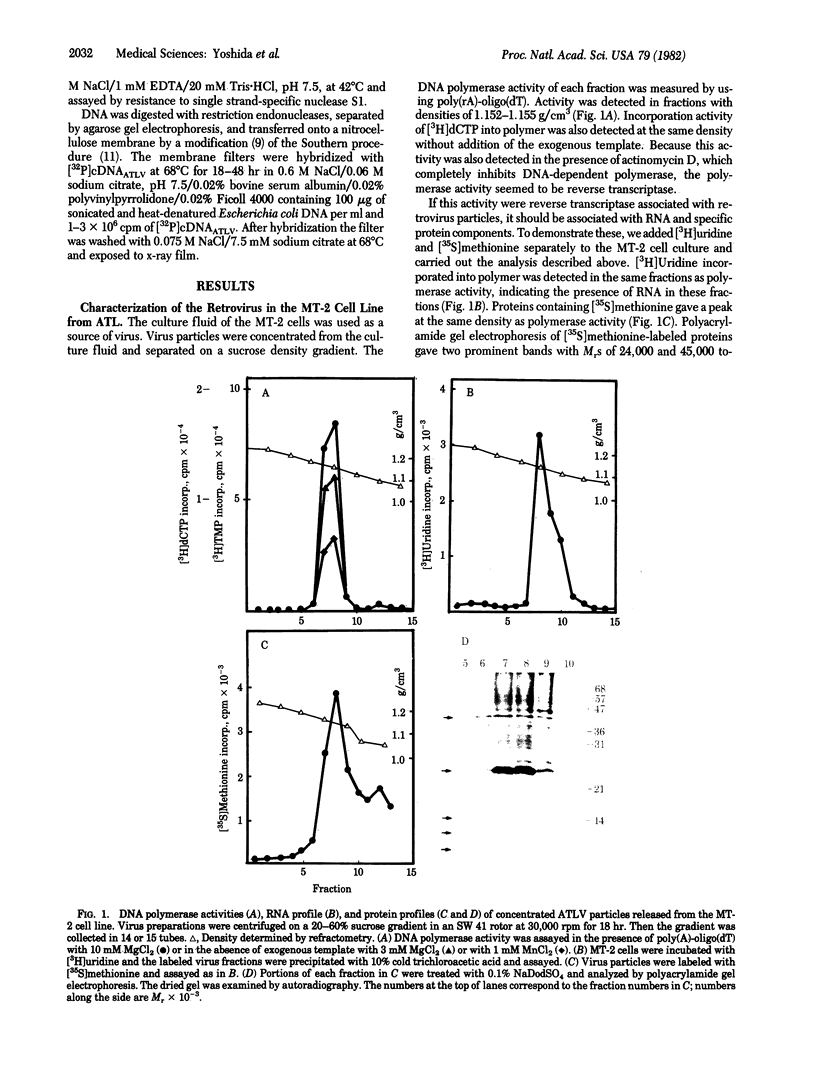
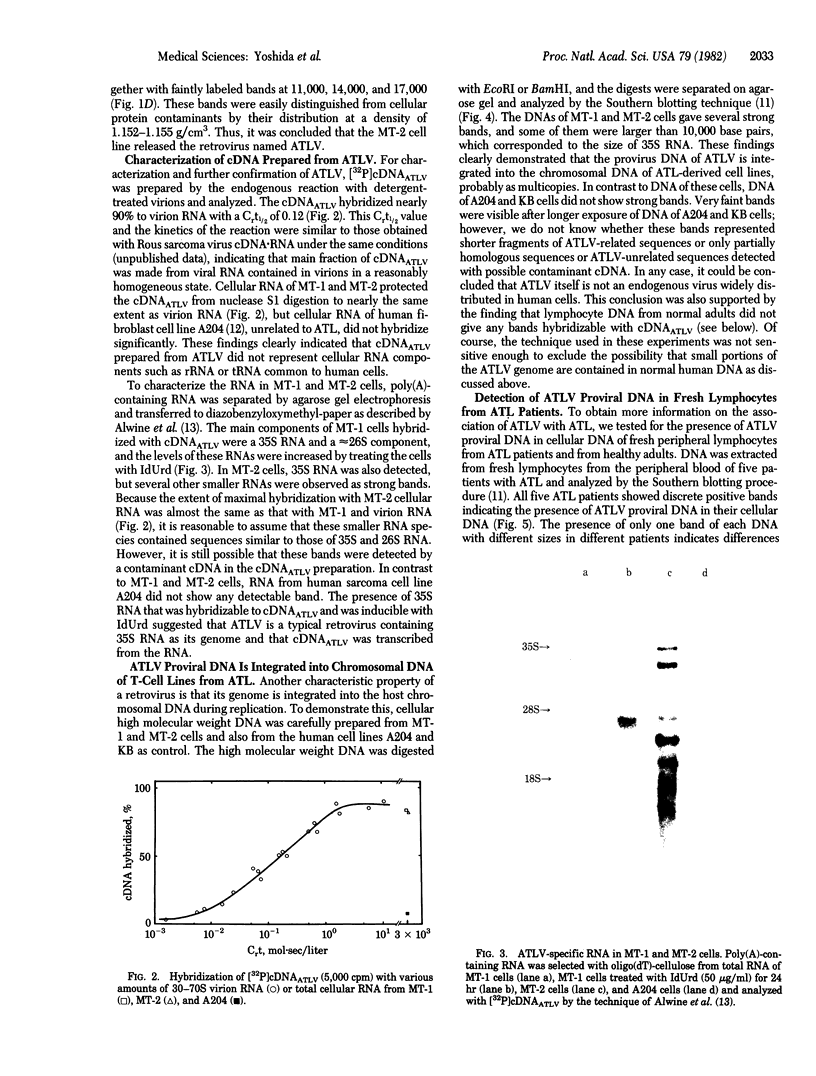
A retrovirus (ATLV) was unequivocally demonstrated in human adult T-cell leukemia (ATL) cell lines by density (1.152-1.155 g/cm3) in a sucrose gradient, reverse transcriptase activity insensitive to actinomycin D, RNA labeled with [3H]uridine, and specific proteins with molecular weights of 11,000, 14,000, 17,000, 24,000, and 45,000. Furthermore, cDNA prepared by endogenous reaction with detergent-treated virions hybridized to 35S RNA containing poly(A), which was inducible by IdUrd treatment of a T-cell line derived from leukemic cells of the ATL, and the integrated form of ATLV proviral DNA was detected in T-cell lines derived from ATL. The ATLV proviral DNA was also detected in fresh peripheral lymphocytes from all five patients with ATL tested so far but not in those from healthy adults. On the other hand, ATLV protein of Mr 42,000 was found to be at least one of the ATL-associated antigen(s) that were previously detected in ATL-leukemic cells by all sera from patients with ATL. These findings on the close association of ATLV protein and proviral DNA with ATL are direct evidence for the possible involvement of the retrovirus ATLV in leukemogenesis of human ATL.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Robinson H. L., Robinson W. S., Huebner R. J., Turner H. C. Proteins of Rous sarcoma virus. Virology. 1968 Sep;36(1):73–86. doi: 10.1016/0042-6822(68)90118-9. [DOI] [PubMed] [Google Scholar]
- Giard D. J., Aaronson S. A., Todaro G. J., Arnstein P., Kersey J. H., Dosik H., Parks W. P. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973 Nov;51(5):1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Nagata K., Hanaoka M., Nakai M., Matsumoto T., Kinoshita K. I., Shirakawa S., Miyoshi I. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miyoshi I., Kubonishi I., Sumida M., Hiraki S., Tsubota T., Kimura I., Miyamoto K., Sato J. A novel T-cell line derived from adult T-cell leukemia. Gan. 1980 Feb;71(1):155–156. [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Uchiyama T., Yodoi J., Sagawa K., Takatsuki K., Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977 Sep;50(3):481–492. [PubMed] [Google Scholar]
- Weiss S. R., Varmus H. E., Bishop J. M. The size and genetic composition of virus-specific RNAs in the cytoplasm of cells producing avian sarcoma-leukosis viruses. Cell. 1977 Dec;12(4):983–992. doi: 10.1016/0092-8674(77)90163-5. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Kawai S., Toyoshima K. Genome structure of avian sarcoma virus Y73 and unique sequence coding for polyprotein p90. J Virol. 1981 May;38(2):430–437. doi: 10.1128/jvi.38.2.430-437.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Kawai S., Toyoshima K. Unifected avian cells contain structurally unrelated progenitors of viral sarcoma genes. Nature. 1980 Oct 16;287(5783):653–654. doi: 10.1038/287653a0. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Yamashita M., Nomoto A. Transformation-defective mutants of Rous sarcoma virus with longer sizes of genome RNA and their highly frequent occurrences. J Virol. 1979 May;30(2):453–461. doi: 10.1128/jvi.30.2.453-461.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]