Abstract
Background:
Pharmacological inhibitors of vascular endothelial growth factor (VEGF) receptors, like vatalanib, have been tested in randomised trials (CONFIRM (Colorectal Oral Novel therapy For the Inhibition of Angiogenesis and Retarding of Metastases) 1 and 2) in colorectal cancer showing activity in a subgroup of patients with high serum LDH expression. In the current study, we assessed the predictive role of vascular density (VD) in patients treated in the above trials.
Methods:
Paraffin-embedded materials from 141 patients were analysed with immunohistochemistry for the expression of the CD31 (pan-endothelial cell marker) and of phosphorylated pVEGFR2/KDR on endothelial cells. The VD was correlated with response to therapy and with progression-free (PFS) and overall survival (OS).
Results:
A significant association of pVEGFR2/KDR+ VD with poor response in the placebo group was noted (response rates (RRs) 15% (3/20) when high VD vs 52% (26/50) when low VD; P=0.006). The RR increased from 15 (3/20) to 50% (11/22) in tumours with high VD when vatalanib was added to chemotherapy (P=0.02). A significantly improved PFS was noted in patients with high pVEGFR2/KDR+ VD when treated with vatalanib (P=0.002). A similar effect was also noted in patients with high CD31+ VD (P=0.07). Overall survival was marginally improved (P=0.07).
Conclusion:
Assessment of the activated vessel density may allow the stratification of patients recruited in randomised trials with VEGFR-targeting anti-angiogenic agents, unmasking their therapeutic potential and enabling their introduction in the clinical practice for the benefit of specific patient subgroups, at the same time reducing the cost of therapy.
Keywords: vatalanib, colorectal cancer, CONFIRM, CD31, VEGFR2, KDR
Vascular endothelial growth factor (VEGF), one of the most potent angiogenic factors in tumours (Ferrara and Davis-Smyth, 1997), is a major target for the development of anti-angiogenic therapies. Vascular endothelial growth factor acts on specific tyrosine kinase receptors, VEGFR-1 (flt-1), VEGFR-2 (KDR/flk-1) and VEGFR-3 (flt-4). The addition of the anti-VEGF monoclonal antibody ‘bevacizumab’ to standard chemotherapy improved the efficacy and outcome of patients with a variety of malignancies, including colon cancer (Hurwitz et al, 2005).
The development of pharmacological inhibitors of VEGF receptor activation seems, therefore, a promising therapeutic approach (Zhong and Bowen, 2007). The CONFIRM (Colorectal Oral Novel therapy For the Inhibition of Angiogenesis and Retarding of Metastases) I and II trials are among the largest randomised trials, investigating the efficacy of vatalanib (PTK787/ZK222584), a small-molecule tyrosine kinase receptor inhibitor, in metastatic colorectal cancer (Hecht et al, 2011; Van Cutsem et al, 2011). Although vatalanib did not offer an overall survival (OS) advantage, analysis according to the prospectively stratified serum LDH levels showed a 40% reduction in time of tumour progression in patients with high LDH serum levels (Hecht et al, 2011), a subgroup of colorectal cancer patients with poor prognosis. This finding was further analysed using immunohistochemistry in a subset of patients providing further evidence that cancer cell LDH-A overexpression defines poor prognosis, an event that is partially averted by the administration of vatalanib (Koukourakis et al, 2011).
In the current study, we assessed the vascular density (VD) in paraffin-embedded material from the primary tumours of patients recruited in the CONFIRM randomised trials. This was performed by immunohistochemistry using an antibody to the pan-endothelial cell marker CD31 and also an antibody recognising the phosphorylated (active) form of the VEGFR2/KDR receptor (pVEGFR2/KDR) on endothelial cells. The role of these parameters in the response of colorectal tumours to chemotherapy with or without vatalanib, and also in the progression and OS of patients was analysed. As serum LDH and tissue LDHA levels have been previously reported by us to be of predictive value in vatalanib trials (Koukourakis et al, 2011), these were analysed in parallel with VD.
Materials and methods
In the phase III multinational CONFIRM trials 1 and 2, the therapeutic role of vatalanib in combination with chemotherapy in patients with metastatic colorectal cancer was assessed (Hecht et al, 2011; Van Cutsem et al, 2011). CONFIRM 1 and 2 phase III trials included patients with confirmed metastatic adenocarcinoma of the colon or rectum. CONFIRM 1 recruited patients for first-line chemotherapy while CONFIRM 2 comprised patients with previously treated metastatic colorectal adenocarcinoma whose disease had recurred or progressed during or within 6 months of treatment with irinotecan in combination with a fluoropyrimidine. The primary end points of the CONFIRM 1 and 2 trials was the progression-free (PFS) and the OS. The secondary end points in CONFIRM 2 were the overall and the PFS in patients with high serum LDH levels. Patients received PTK/ZK plus FOLFOX4 or placebo plus FOLFOX4 (day 1: oxaliplatin 85 mg m−2 i.v. infusion and LV 200 mg m−2 racemate i.v. followed by FU 400 mg m−2 IV bolus and FU 600 mg m−2 IV as a 22-h continuous infusion; day 2: LV 200 mg m−2 racemate (or 100 mg m−2 ℒ-LV) i.v. over 120 min followed by FU 400 mg m−2 i.v. bolus and FU 600 mg m−2 i.v. as a 22-h continuous infusion)(Ferrara and Davis-Smyth, 1997) was administered every 2 weeks in combination with oral PTK/ZK (1250 mg) or placebo on a once-daily continuous schedule.
Although the CONFIRM 1 and 2 trials are not fully comparable, as they deal with two distinct populations (chemotherapy naïve and irinotecan-resistant patients), the collected samples obtained from patients recruited in these two trials allow a safe overall analysis, as the patients receiving or not receiving vatalanib are well balanced in numbers within the CONFIRM 1 and 2 tissue samples (31 vs 39 and 40 vs 31, respectively). Thus, analysis was performed in combined CONFIRM 1 and 2 data sets, and no separate analysis was attempted owing to the relatively low number of tissues collected.
In an attempt to investigate the association of PTZK/ZK therapeutic activity with tumour angiogenesis and anaerobic metabolic pathways, paraffin-embedded material from 164 patients with metastatic colorectal adenocarcinoma were recruited in the CONFIRM 1 and 2 trials. This series of patients has been previously analysed, to assess the predictive role of serum and tissue lactate dehydrogenase content (Koukourakis et al, 2011). Indeed, high serum LDH levels, an ominous prognostic marker in human cancer, was one of the strongest predictors of better response to vatalanib (Hecht et al, 2011).
In the current study, the CD31 pan-endothelial marker was used to highlight vessels and assess the tumour microvessel density (Parums et al, 1990). Similarly, the pVEGFR2/KDR (phosphorylated VEGF receptor 2/KDR) expression in tumour endothelial cells was assessed as a marker of activated VD (Stewart et al, 2003). Following blind assessment of the slides on the conference microscope, 23 samples were excluded from analysis as the tissue material or the quality of staining was considered poor for a reliable assessment of VD or they lacked tissue areas of the invading tumour edge. In this way, a total of 141 samples were included in the current analysis.
Tissue slides were collected by Bayer–Schering from centres participating in the trials and sent to the department of Pathology, Democritus University of Thrace, Alexandroupolis, Greece. Samples were achieved formalin-fixed paraffin-embedded material from the primary tumour obtained at the day of first surgery and these were surgical (not biopsy) samples. We did not analyse material from metastatic sites. Although tissues from primary tumours may exhibit different biological features, this was a risk we accepted before starting the whole project. In practice, it was impossible to collect an adequate number of tissues from metastatic sites to allow a reliable analysis. The pathologists that assessed the marker expression were blinded to the outcome of patients and to all other clinical and laboratory details. The local Ethics and Scientific committees approved the study.
The characteristics of patients analysed in the current study and response rates (RRs) according to treatment arm are shown in Table 1. Outcomes and response to treatment data were given by the independent data monitoring board. The follow-up of patients ranges from 1 to 1418 days (median 610 days). All patients were dead with progression of their disease at the time of analysis.
Table 1. Patient characteristics.
| Placebo | Vatalanib | |
|---|---|---|
| No. | 70 | 71 |
| CONFIRM 1/2a | 39/31 | 31/40 |
| PS 0/1, 2 | 43/27 | 34/37 |
| Sex M/F | 49/21 | 48/23 |
| Age (median, range) | 62 (38–82) | 64 (36–79) |
| CR+PR | 29/70 (41.4%) | 29/69 (42.0%)b |
| Median PFS (days) | 175 | 175 |
| Median OS (days) | 628 | 610 |
| Number of PFS events | 70 | 71 |
| Number of deaths | 70 | 71 |
Abbreviations: CR=complete response; OS=overall survival; PFS=progression-free survival; PR=partial response; PS=performance status.
Tissue samples collected from patients recruited in the CONFIRM 1 or 2 studies.
Two patients had unknown response status.
Immunohistochemistry
For CD31 (JC70 monoclonal antibody, DAKO, Glostrup, Denmark) assessment, an immunohistochemical alakaline phsophatase anti-alkaline phosphatase technique was used as previously reported (Koukourakis et al, 2005). A modified streptavidin technique was used for pVEGFR2/KDR immunohistochemistry, using the monoclonal antibody 34a (Stewart et al, 2003) raised against the Y1214 tyrosine residue of the KDR protein, as previously reported (Koukourakis et al, 2006).
In previous studies, including colorectal cancer (Giatromanolaki et al, 2000, 2007; Koukourakis et al, 2005), we have shown that active angiogenesis occurs mainly in the tumour invading edge and that inner tumour areas often share poor vascularity, which stress the necessity to assess angiogenesis in the invading front of the growing tumour. Thus, sections from primary tumours were scanned at low power ( × 40 and × 100). Areas of the highest vascularisation within the tumour invading front (adjacent to the normal colon) were chosen and microvessel counting followed on three chosen × 200 fields of the highest density. The final VD was the mean of the vessel counts obtained in these fields. As there are no established cutoff points for the grouping tumours according to the VD, the cutoff value were set to the maximum difference between the groups, which were different for the two VDs assessed. Thus, the median value (50%) and the 66th percentile of the VD recorded were used as a cutoff points to define two groups of tumours with high and low VD (for each one of the cutoff points). In this way, we could evaluate two different cutoff points that identify a group of higher than the median VD and a group of very high VD (upper one-third), in relation to the activity of vatalanib.
All staining scoring was performed separately by two independent observers. Any discrepancies were resolved on the conference microscope.
Combination with LDHA analysis
As the hypoxic response generates many metabolic survival pathways that are essential for tumour growth, those tumours with already highly induced pathways may be more susceptible to the additional effect of vatalanib. We therefore analysed the association of VD with serum LDH and tissue LDHA (LDH5 isoform formed by four LDHA subunits) expression in multivariate models of progression and OS, using previously published LDH5 stratification (57/141 had high tissue LDH5 expression) (Koukourakis et al, 2011). Patients with serum LDH above normal were all grouped in the high serum LDH level category (55/141 patients).
Statistical analysis
Statistical analysis was performed using the GraphPad Prism 5.0 and the Instat 3.1 package (GraphPad Software Inc., San Diego, CA, USA). A Fisher’s exact test was used for testing relationships between categorical variables (contingency tables). Linear regression analysis was used to assess correlation with continuous variables. Progression free and overall (OS) survival were estimated using Kaplan–Meier curves. Curves were compared using the log-rank test (Mantel–Haenszel). The Gehan–Breslow–Wilcoxon method that gives more weight to events (death or relapse) at early time points was also used. This feature may be important when analysing progression events following therapy or death events expected to occur at a high rate early in the course of follow-up. A Cox proportional hazard model including all pre-outcome variables was used to further test the independent significance of variables proved of significance at univariate analysis. A two-tailed P-value of <0.05 was used for significance.
Results
Vascular density
The VD as assessed by the anti-CD31 antibody ranged from 5 to 35 vessels per × 200 optical field (median 15, and 66th percentile 17). Using the median and the 66th percentile values, 67/141 and 45/141 cases had high VD; Figure 1A.
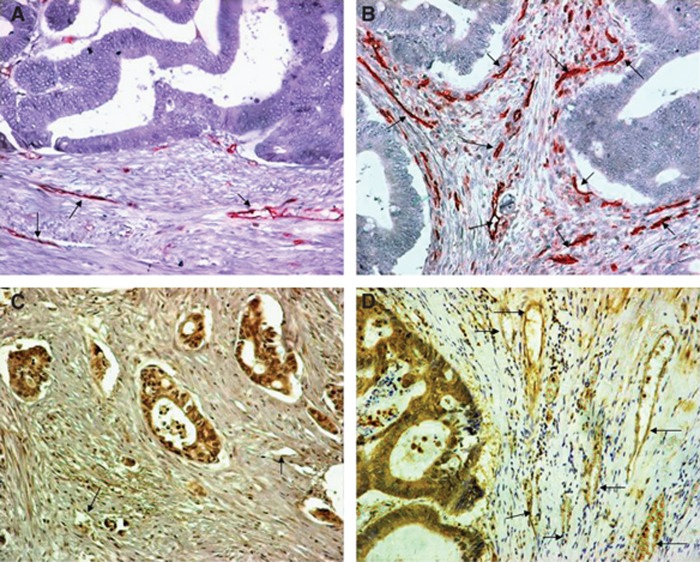
Figure 1.
Immunohistochemical figures of colon carcinomas with low (A) and high (B) CD31+ VD, and low (C) and high (D) pVEGFR2/KDR+ VD (magnification × 200; arrows show vessels).
The activated VD as assessed by the pVEGFR2/KDR antibody ranged from 1–10 vessels per optical field (median 7, 66th percentile 10). Using the median and the 66th percentile values, 64/141 and 42/141 cases had high VD Figure 1B.
Linear regression analysis between CD31 and pVEGFR2/KDR-positive VD revealed a significant association (P<0.0001; r=0.39). Figure 1C.
The ratio of VD assessed by pVEGFR2/KDR to that scored by CD31 was also calculated to derive the percentage of VEGF-activated tumour vessels (activation ratio). This ranged from 0.06 to 1.00 (median 0.50, 66th percentile 0.66). Using the median and the 66th percentile values, 69/141 and 51/141 cases had high activation ratio.
Association of immunohistochemistry with patient and disease variables
Vascular density, whether assessed with CD31 or pVEGFR2/KDR immunostaining, did not show any correlation with the age, sex or the performance status of patients (data not shown). The tumour burden was calculated as the sum of maximum assessable tumour dimensions in cm (range 1–40 cm, median 8.3 cm). There was no association of VD of the primary tumour with the tumour burden of the metastatic disease assessed before recruitment in the study. Similarly, the activation ratio of vessels was not related to any of the above variables.
Analysis of RRs
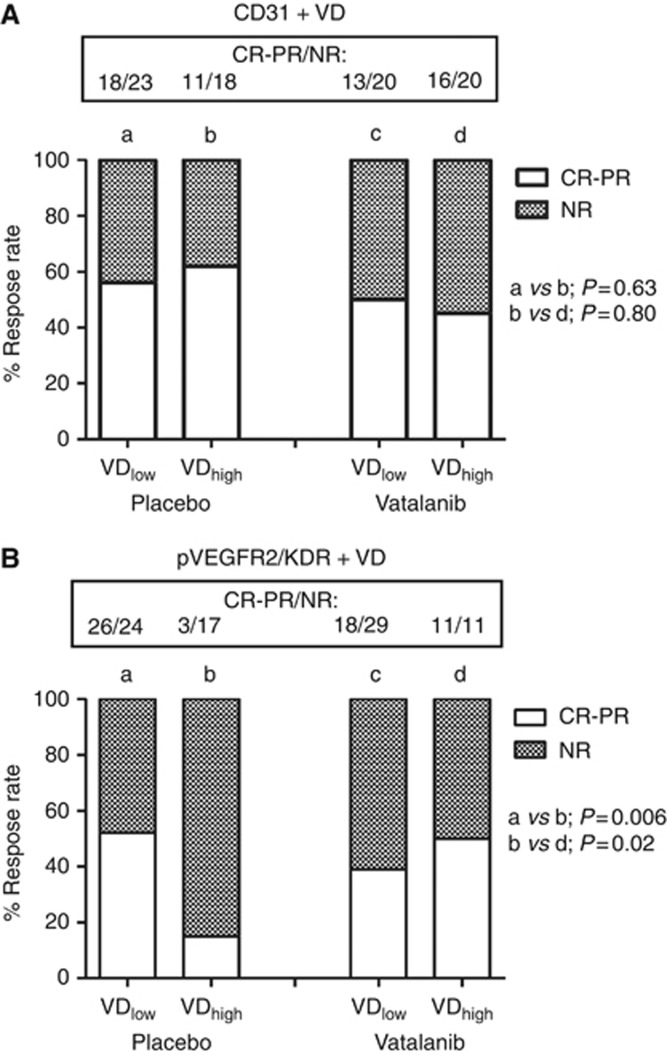
In two patients the RR was unknown, so analysis was performed in 139 cases. Group analysis (contingency tables) of the association of the CD31+ VD with RRs (progressive and stable disease vs partial and complete response) in patients receiving or not receiving vatalanib showed no significant associations whether the median or the 66th percentile was used for grouping (P>0.60). Figure 2A shows the analysis using the median CD31+ VD.
Figure 2.
Response rates (complete and partial vs non-response) according to the administration of vatalanib and the CD31+ (A) and pVEGFR2/KDR+ (B) VD.
However, a significant association of pVEGFR2/KDR+ VD with poor response in the placebo group was noted. Using the 66th percentile as a cutoff point, the RRs was as low as 15% (3/20) in patients with high VD vs 52% (26/50) in patients with low VD (P=0.006); Figure 2B. A significant, still weaker association was noted when the median value was used as a cutoff point (P=0.04). The adverse effect of pVEGFR2/KDR+ VD noted in patients not receiving vatalanib was no longer evident in the group receiving the anti-angiogenic drug (P=0.43). Analysis within the group of patients with high VD showed that the RR increased from 15 (3/20) to 50% (11/22) when vatalanib was added to chemotherapy (P=0.02). No association of the activated ratio with the RR was noted.
Survival analysis
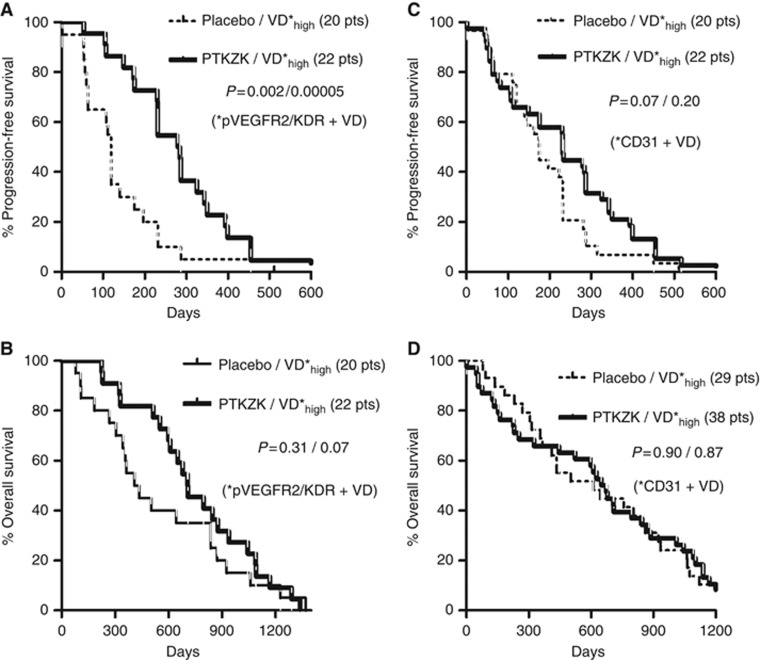
Analysis of the PFS, according to the VD parameters, showed a significant improved survival of patients with high pVEGFR2/KDR+ VD (using as a cutoff point the 66th percentile), when they were treated with vatalanib (Figure 3A; P=0.002). A similar effect was also noted in patients with high CD31+ VD (using the median VD as a cutoff point), but the difference was of marginal significance (Figure 3C; P=0.07). No association of the activation ratio with PFS was noted.
Figure 3.
Progression-free and OS stratified for vatalanib administration in patients with high pVEGFR2/KDR+ (A and B) and high CD31+ (C and D) VD. P-values refer to Mantel–Haenszel and Gehan–Breslow–Wilcoxon methods, respectively. pts, patients.
Analysis of the disease-specific OS showed a marginal correlation of vatalanib administration in cases with high pVEGFR2/KDR VD (cutoff the 66th percentile; P=0.07), but there was no association in cases with high CD31+ VD (Figures 3B and D).
Combination with LDHA analysis
There was a strong association between LDH5 expression and VD. Out of 57 cases with high LDH5 expression, 27 (47.3) and 39 (68%) had high pVEGFR2/KDR+ and CD31+ VD, respectively, vs 15/84 (17.8) and 28/84 (33.3%) cases with low LDH5 expression (P=0.0003 and P<0.0001, respectively). The 66th percentile and the median VD, for pVEGFR2/KDR and CD31, were used as cutoff points for the above analysis. There was no significant association of serum LDH levels with VD parameters.
Table 2 shows the univariate and multivariate analysis of PFS in the placebo and the vatalanib groups. pVEGFR2/KDR+ VD was the only variable of independent significance for PFS, defining a significant poor prognosis in the placebo group (P=0.008, hazard ratio 2.1, 95% CI 1.1–3.8) and a better prognosis in the vatalanib group (P=0.03, hazard ratio 0.5, 95% CI 0.3–0.9). There was no independent significant association with OS for any of the three variables assessed.
Table 2. Univariate and multivariate analysis of PFS.
|
PFS
|
||||
|---|---|---|---|---|
|
Univariate
|
Multivariate
|
|||
| Variable | HR (95% CI) | P -value | HR (95% CI) | P -value |
| Placebo group | ||||
| VD (CD31) | 1.4 (0.8–2.4) | 0.19/0.43 | 0.92 (0.4–1.4) | 0.92 |
| VD (pVEGFR2/KDR) | 2.7 (1.3–5.3) | 0.003/0.001 | 2.10 (1.1–3.8) | 0.008 |
| LDH5 score | 1.4 (0.7–2.5) | 0.25/0.05 | 1.20 (0.6–2.1) | 0.51 |
| LDH serum | 1.2 (0.6–2.3) | 0.58/0.52 | 1.43 (0.7–2.6) | 0.26 |
| Vatalanib group | ||||
| VD (CD31) | 0.6 (0.3–1.0) | 0.08/0.09 | 0.81 (0.4–1.4) | 0.46 |
| VD (pVEGFR2/KDR) | 0.5 (0.3–0.9) | 0.02/0.0004 | 0.57 (0.3–0.9) | 0.03 |
| LDH5 score | 0.8 (0.5–1.3) | 0.41/0.34 | 1.01 (0.6–1.6) | 0.94 |
| LDH serum | 1.2 (0.7–1.9) | 0.45/0.17 | 1.22 (0.7–1.9) | 0.39 |
Abbreviations: CI=confidence interval; HR=hazard ratio; LDH=lactate dehydrogenase; PFS=progression-free survival; VD=vascular density.
P-values in univariate analysis refer to Mantel–Haenszel and Gehan–Breslow–Wilcoxon methods, respectively. Bold values show statistical significance.
Discussion
In the present study, we examined the prognostic role of standard and activated VD, using anti-CD31 and anti-pVEGFR2/KDR immunostaining, in a series of colorectal carcinomas recruited in two randomised trials investigating the VEGF-receptor tyrosine kinase inhibitor vatalanib (Wood et al, 2000). Vatalanib is expected to have a direct effect on tumour vessels overexpressing VEGF receptors. Indeed, hypoxia has also been shown to upregulate the expression of the VEGFR2/KDR gene (Takagi et al, 1996).
Vatalanib is expected to have an effect on tumours with high angiogenic activity. Vascular endothelial growth factor is certainly a major angiogenic pathway and its inhibition has been shown to induce regression of the immature vasculature, resulting in an effect known as ‘vascular normalisation’ that restores a better blood flow, thus a better intratumoural distribution of the injected chemotherapy (Willett et al, 2006). Thus increased response to chemotherapy is expected to improve the efficacy of the FOLFOX regimen widely used in the treatment of colorectal cancer, as also applied in the CONFIRM trials. However, this effect is not shown in all experimental systems (Kerbel, 2009).
In the current study, we had 141 tissue samples from the primary tumours of patients treated in the CONFIRM trials and they matched well the overall composition of the study (Koukourakis et al, 2011). Using the CD31 pan-endothelial cell marker we only observed a marginal association of the administration of vatalanib with improved PFS when tumours had a high VD. Thus, this method is unlikely to be of use for patient selection, and shows the need for more specific identification of endothelial biology related to response.
Analysis of VD, as assessed after staining with an antibody recognising the phosphorylated/active form of the VEGFR2/KDR receptor, revealed important aspects of the clinical role of activated VEGF angiogenic pathway and also of the interaction of vatalanib with the effect of chemotherapy. Tumours with high pVEGFR2/KDR+ VD had a significantly poorer response to FOLFOX chemotherapy compared with those with low VD. Addition of vatalanib significantly reduced the resistance of these tumours to chemotherapy. Although the marker was assessed on the primary tumours and response on their metastasis, the results obtained were significant suggesting that in a large part of metastatic tumours the primary angiogenic phenotype persists. A hypothesis that may explain the synergy noted is the phenomenon of vascular normalisation induced by anti-angiogenic therapy (Jain, 2005), as the drug flow and availability could increase in cases receiving vatalanib. This hypothesis is in direct contrast, however, with a recent study showing that anti-VEGF monoclonal antibodies reduced the perfusion and influx of radiolabelled docetaxel for 4 days in non-small-cell lung cancer patients (Van der Veldt et al, 2012). Another suggestion may be a direct effect of vatalanib on cancer cells as VEGFR2/KDR receptors are also extensively expressed by cancer cells (Stewart et al, 2003; Koukourakis et al, 2006). Further analysis of PFS and OS showed that prognosis of patients with activated VEGF-receptor angiogenic pathway experienced, at least in the current series, substantial benefit when they received vatalanib in addition to chemotherapy. This beneficial effect was not substantiated in the group of patients with high VD as assessed with the pan-endothelial marker CD31. This probably reflects different angiogenic pathways that may be active in different tumours, so that vatalanib, being a VEGFR2/KDR inhibitor, exerts its anti-angiogenic activity only when VEGF/receptor pathway is active on tumour endothelium.
These data strongly support that small-molecule VEGF tyrosine kinase receptor inhibitors are indeed active in tumours bearing the target, that is, endothelium with activated VEGFR2/KDR receptors. This is the first study suggesting that immunohistochemical assessment of VEGF-activated vasculature may prove a potent predictor of the efficacy of angiogenesis inhibitors. In a study by Jubb et al (2006), a subset of tumour samples from colorectal patients treated in a randomised trial of irinotecan/5-fluorouracil with or without bevacizumab (anti-VEGF monoclonal antibody), the assessment of CD34+ VD was not revealed as a prognostic indicator of bevacizumab activity. Although, as in the current study, assessment of VD using a pan-endothelial cell antigen like CD31 or CD34 may be a weak marker to predict the likelihood to respond to anti-VEGF therapy, it is stressed that the usage of multi-tissue arrays (MTAs) for the vessel counting (like the method applied in the study by Jubb et al) should not be considered as equivalent to whole tissue sample evaluation. The areas of intense vascularisation are more often located in the invading tumour edge (Giatromanolaki et al, 2000; Koukourakis et al, 2005) and are not homogeneously distributed, so that small tissue cores included in the MTAs are rather unreliable for vessel counting.
Zhao et al (2012), in lung cancer patients treated with chemotherapy combined with bevacizumab, found a positive correlation of tumour shrinkage with undifferentiated VD (exhibiting double CD31 and CD34 positivity), but not CD34 alone. However, only 16 patients were studied, this was not randomised, and the combination with chemotherapy may well have targeted proliferating vessels included in the total score in addition to the anti-VEGF effects, so it is not possible to separate biomarkers for VEGF alone in that study. It is equally plausible that CD31+CD34+ is a marker for chemotherapy sensitivity.
On the other hand, as confirmed in the current study, assessment of the activated VD expressing phosphorylated VEGF receptors, or even expressing VEGF/VEGF-receptor complex, as previously reported using specific antibodies (Brekken et al, 1998; Koukourakis et al, 2000), may be a more reliable method to predict a benefit from anti-VEGF anti-angiogenic therapies. Assessment of high and activated VD in primary tumours was significantly linked with poor response to chemotherapy of metastatic disease, a feature that was blocked by the addition of vatalanib. The angiogenic ability differences between primary and metastatic tumours are poorly studied in the literature, although in a previous study of ours comparing the VD in primary and metastatic to the lymph nodes breast cancer showed that cancer cells migrating to the nodes have rather similar angiogenic abilities to the parental cells of the primary tumour (Arapandoni-Dadioti et al, 1999). In any case, the current data suggest that although metastatic disease may change its angiogenic phenotype compared with the primary, highly angiogenic primary tumours seem to exhibit highly angiogenic metastasis, so that assessment of the angiogenic status in primary tissues sustains a strong predictive relevance for vatalanib activity.
Regarding the LDHA-combined analysis, both pVEGFR2+ and CD31+ were significantly linked with high LDHA cancer cell expression, suggesting a close link of hypoxia pathways with VEGF-activated angiogenic pathways in colorectal cancer. Indeed, in a previous analysis, hypoxia-inducible factors HIF1a and HIF2a were significantly linked with VEGF and LDHA expression in the same material (Koukourakis et al, 2007). In multivariate analysis, only pVEGFR2/KDR+ VD was revealed as an independent factor of poor PFS in the placebo group and of better PFS in the vatalanib group. LDH5 that had been previously shown to be of independent prognostic relevance (Koukourakis et al, 2011) did not sustain its statistical value in models that included the direct target of vatalanib, namely the activated VEGFR2/KDR pathway on vessels. This finding further supports the strong relevance of the assessment of this activated VD as a predictor of activity of VEGF tyrosine kinase inhibitors. The important issue raised is that these agents target a subgroup of patients with particularly poor prognosis so that stratification of patients according to VEGF-activated vasculature should easily provide statistical differences, even with low numbers of stratified patients. Incorporating such markers in prospective randomised trials could facilitate the selection of patients for VEGF-targeting agents and appropriate use in clinical practice.
It is stressed that the current study has several limitations as this is a retrospective attempt to evaluate a role of tumour angiogenic activity in response to anti-angiogenic therapy combined with chemotherapy. Retrospective collection of tissues is difficult and the random collection of tissue blocks from <10% of the total number of patients recruited in the CONFIRM trials is not deprived of bias. Moreover, the two CONFIRM trials were not addressed to the same patient population. The relatively low number of samples and the multiple variable analysis also have a risk of bias from multiple comparisons.
Despite the loss of commercial interest on the vatalanib molecule, the current study provides several insights that may be useful in the future for the clinical development of VEGF-targeting anti-angiogenic therapies. Assessment of the activated vessel density in whole tissue samples from the primary tumours is feasible using specific antibodies (e.g., the 34a or the 11B5 Abs). The stratification of patients according to the actual target of the compound, in our case the endothelial VEGF receptors, may be important for unmasking the therapeutic potential of VEGF-receptor-targeting tyrosine kinase inhibitors, enabling the introduction of such molecules in the clinical practice for the benefit of specific patient subgroups, at the same time reducing the financial burden from such novel therapies. Nevertheless, the current study should be considered as a pilot study with several limitations and confirmation of the eventual usage of pVEGFR2/KDR as a marker of activated vasculature responsive to anti-VEGFR2/KDR drugs should be confirmed in prospective trials. Such trials should consider assessment of activated VD is as many as possible tissue areas adjacent to normal colon (areas of the highest angiogenic activity), so that special care should be taken during the collection of the tissue material for analysis. As there are no established cutoff points for the grouping tumours according to the VD, the current study suggests that if the CD31+ VD is to be applied the median value gives better correlation with post-chemotherapy survival parameters, while best statistical correlations are obtained when using the 66th percentile for pVEGFR2/KDR VD. Such cutoff points are recommended for future trials.
Acknowledgments
The study was financially supported by the Tumour and Angiogenesis Research Group and the Bayer-Schering Pharma. Additional support was from the Oxford NIHR Comprehensive Biomedical Research Centre (ALH).
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Author TT – Consultant or Advisory Role; Schering. Author GF – Honoraria; Novartis. Authors MMS and DL – Employment or Leadership Position; Novartis. Author TJ – Employment or Leadership Position; Bayer Oy. Author DL – Employment or Leadership Position; Bayer Pharma AG. Author GM – Employment or Leadership Position; Bayer Pharmaceuticals. The remaining authors declare no conflict of interest.
09/10/2012
This paper has been modified since advance online publication, an acknowledgement has been added
References
- Arapandoni-Dadioti P, Giatromanolaki A, Trihia H, Harris AL, Koukourakis MI (1999) Angiogenesis in ductal breast carcinoma. Comparison of microvessel density between primary tumour and lymph node metastasis. Cancer Lett 137: 145–150 [DOI] [PubMed] [Google Scholar]
- Brekken RA, Huang X, King SW, Thorpe PE (1998) Vascular endothelial growth factor as a marker of tumor endothelium. Cancer Res 58: 1952–1959 [PubMed] [Google Scholar]
- Ferrara N, Davis-Smyth T (1997) The biology of vascular endothelial growth factor. Endocr Rev 18: 4–25 [DOI] [PubMed] [Google Scholar]
- Giatromanolaki A, Koukourakis MI, Sivridis E, Chlouverakis G, Vourvouhaki E, Turley H, Harris AL, Gatter KC (2007) Activated VEGFR2/KDR pathway in tumour cells and tumour associated vessels of colorectal cancer. Eur J Clin Invest 37: 878–886 [DOI] [PubMed] [Google Scholar]
- Giatromanolaki A, Koukourakis MI, Sivridis E, O’Byrne K, Gatter KC, Harris AL (2000) ‘Invading edge vs. inner’ (edvin) patterns of vascularization: an interplay between angiogenic and vascular survival factors defines the clinical behaviour of non-small cell lung cancer. J Pathol 192: 140–149 [DOI] [PubMed] [Google Scholar]
- Hecht JR, Trarbach T, Hainsworth JD, Major P, Jäger E, Wolff RA, Lloyd-Salvant K, Bodoky G, Pendergrass K, Berg W, Chen BL, Jalava T, Meinhardt G, Laurent D, Lebwohl D, Kerr D (2011) Randomized, placebo-controlled, phase III study of first-line oxaliplatin-based chemotherapy plus PTK787/ZK 222584, an oral vascular endothelial growth factor receptor inhibitor, in patients with metastatic colorectal adenocarcinoma. J Clin Oncol 29: 1997–2003 [DOI] [PubMed] [Google Scholar]
- Hurwitz HI, Fehrenbacher L, Hainsworth JD, Heim W, Berlin J, Holmgren E, Hambleton J, Novotny WF, Kabbinavar F (2005) Bevacizumab in combination with fluorouracil and leucovorin: an active regimen for first-line metastatic colorectal cancer. J Clin Oncol 23: 3502–3508 [DOI] [PubMed] [Google Scholar]
- Jain RK (2005) Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307(5706): 58–62 [DOI] [PubMed] [Google Scholar]
- Jubb AM, Hurwitz HI, Bai W, Holmgren EB, Tobin P, Guerrero AS, Kabbinavar F, Holden SN, Novotny WF, Frantz GD, Hillan KJ, Koeppen H (2006) Impact of vascular endothelial growth factor-A expression, thrombospondin-2 expression, and microvessel density on the treatment effect of bevacizumab in metastatic colorectal cancer. J Clin Oncol 24: 217–227 [DOI] [PubMed] [Google Scholar]
- Kerbel RS (2009) Issues regarding improving the impact of antiangiogenic drugs for the treatment of breast cancer. Breast 18: S41–S47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koukourakis MI, Giatromanolaki A, Sivridis E, Gatter KC, Harris AL (2006) Lactate dehydrogenase 5 expression in operable colorectal cancer: strong association with survival and activated vascular endothelial growth factor pathway--a report of the Tumour Angiogenesis Research Group. J Clin Oncol 24: 4301–4308 [DOI] [PubMed] [Google Scholar]
- Koukourakis MI, Giatromanolaki A, Sivridis E, Gatter KC, Harris AL, Tumour and Angiogenesis Research Group (2005) Inclusion of vasculature-related variables in the Dukes staging system of colon cancer. Clin Cancer Res 11: 8653–8660 [DOI] [PubMed] [Google Scholar]
- Koukourakis MI, Giatromanolaki A, Sivridis E, Gatter KC, Trarbach T, Folprecht G, Shi MM, Lebwohl D, Jalava T, Laurent D, Meinhardt G, Harris AL (2011) Prognostic and predictive role of lactate dehydrogenase 5 expression in colorectal cancer patients treated with PTK787/ZK 222584 (vatalanib) antiangiogenic therapy. Clin Cancer Res 17: 4892–4900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koukourakis MI, Giatromanolaki A, Sivridis E, Gatter KC, Harris AL, Trarbach T, Folprecht G, Shi MM, Meinhardt G (2007) Intratumoral lactate dehydrogenase 5 (LDH5) protein is associated with the expression of angiogenesis markers and hypoxia in patients with colorectal cancer. J Clin Oncol 2007 ASCO Annual Meeting Proceedings Part I. 25(18S (20 June Supplement)): 4107 [Google Scholar]
- Koukourakis MI, Giatromanolaki A, Thorpe PE, Brekken RA, Sivridis E, Kakolyris S, Georgoulias V, Gatter KC, Harris AL (2000) Vascular endothelial growth factor/KDR activated microvessel density versus CD31 standard microvessel density in non-small cell lung cancer. Cancer Res 60: 3088–3095 [PubMed] [Google Scholar]
- Parums DV, Cordell JL, Micklem K, Heryet AR, Gatter KC, Mason DY (1990) JC70: a new monoclonal antibody that detects vascular endothelium associated antigen on routinely processed tissue sections. J Clin Pathol 43: 752–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart M, Turley H, Cook N, Pezzella F, Pillai G, Ogilvie D, Cartlidge S, Paterson D, Copley C, Kendrew J, Barnes C, Harris AL, Gatter KC (2003) The angiogenic receptor KDR is widely distributed in human tissues and tumours and relocates intracellularly on phosphorylation. An immunohistochemical study. Histopathology 43: 33–39 [DOI] [PubMed] [Google Scholar]
- Takagi H, King GL, Ferrara N, Aiello LP (1996) Hypoxia regulates vascular endothelial growth factor receptor KDR/Flk gene expression through adenosine A2 receptors in retinal capillary endothelial cells. Invest Ophthalmol Vis Sci 37: 1311–1321 [PubMed] [Google Scholar]
- Van Cutsem E, Bajetta E, Valle J, Köhne CH, Hecht JR, Moore M, Germond C, Berg W, Chen BL, Jalava T, Lebwohl D, Meinhardt G, Laurent D, Lin E (2011) Randomized, placebo-controlled, phase III study of oxaliplatin, fluorouracil, and leucovorin with or without PTK787/ZK 222584 in patients with previously treated metastatic colorectal adenocarcinoma. J Clin Oncol 29: 2004–2010 [DOI] [PubMed] [Google Scholar]
- Van der Veldt AA, Lubberink M, Bahce I, Walraven M, de Boer MP, Greuter HN, Hendrikse NH, Eriksson J, Windhorst AD, Postmus PE, Verheul HM, Serné EH, Lammertsma AA, Smit EF (2012) Rapid decrease in delivery of chemotherapy to tumors after anti-VEGF therapy: implications for scheduling of anti-angiogenic drugs. Cancer Cell 21: 82–91 [DOI] [PubMed] [Google Scholar]
- Willett CG, Kozin SV, Duda DG, di Tomaso E, Kozak KR, Boucher Y, Jain RK (2006) Combined vascular endothelial growth factor-targeted therapy and radiotherapy for rectal cancer: theory and clinical practice. Semin Oncol 33: S35–S40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JM, Bold G, Buchdunger E, Cozens R, Ferrari S, Frei J, Hofmann F, Mestan J, Mett H, O’Reilly T, Persohn E, Rösel J, Schnell C, Stover D, Theuer A, Towbin H, Wenger F, Woods-Cook K, Menrad A, Siemeister G, Schirner M, Thierauch KH, Schneider MR, Drevs J, Martiny-Baron G, Totzke F (2000) PTK787/ZK 222584, a novel and potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, impairs vascular endothelial growth factor-induced responses and tumor growth after oral administration. Cancer Res 60: 2178–2189 [PubMed] [Google Scholar]
- Zhao YY, Xue C, Jiang W, Zhao HY, Huang Y, Feenstra K, Resau JH, Qian CN, Zhang L (2012) Predictive value of intratumoral microvascular density in patients with advanced non-small cell lung cancer receiving chemotherapy plus bevacizumab. J Thorac Oncol 7: 71–75 [DOI] [PubMed] [Google Scholar]
- Zhong H, Bowen JP (2007) Molecular design and clinical development of VEGFR kinase inhibitors. Curr Top Med Chem 7: 1379–1393 [DOI] [PubMed] [Google Scholar]