Abstract
Background:
Although widely fragmented BMs have been associated with adverse outcome in several cancer types, comparatively little is known with respect to its effect on the prognosis of pancreatic cancer. The aim of the current study was therefore to determine the prognostic value of tumour basement membrane (BM) continuity in two anatomically closely related, however, prognostically different tumours, pancreatic head- and periampullary cancer.
Methods:
Tumour BM continuity was determined by immunohistochemical staining of its two major components, laminin and collagen type IV. Associations were made with recurrence free survival (RFS), cancer-specific survival (CSS), overall survival (OS) and conventional prognostic factors.
Results:
Fifty-nine and 61% of pancreatic head and periampullary tumours, respectively, showed limited BM laminin expression. Whereas 43% and 41% of pancreatic head and periampullary cancers, respectively, showed limited BM collagen type IV expression. Limited BM laminin was associated with poor outcome following curative resection of pancreatic head cancer (P=0.034, 0.013 and 0.017 for RFS, CSS and OS, respectively). Two and a half times as many patients with ⩾25% BM laminin were recurrence free and alive 5 years following resection compared with those with limited BM laminin. Although staining patterns of both BM components were weakly correlated with each other, BM collagen type IV expression was not significantly associated with outcome in either tumour type.
Conclusion:
Discontinuous BMs, determined by laminin expression, are associated with poor outcome following curative resection of pancreatic head cancer.
Keywords: pancreatic head cancer, periampullary cancer, basement membrane, laminin, collagen type IV, prognosis
Pancreatic cancer is one of the most lethal human cancers. Resection currently offers the only potential for cure. Owing to locally advanced disease or the presence of distant metastasis, only 20% of patients are amendable for resection and even following resection, recurrence remains a major problem (Li et al, 2004). In fact, almost half of the patients develop recurrent disease within the first year (Boeck et al, 2007).
Cancer progression and the formation of metastasis is a complex multi-step process coordinated by the dynamic interaction of tumour cells with their environment. Under normal circumstances epithelial cells are separated from the surrounding stroma by a highly crosslinked and insoluble sheet-like structure called the basement membrane (BM). Its two major components are laminin and collagen type IV, where laminin is the centre piece of the network and collagen type IV provides the scaffold. Apart from its barrier function, BMs provide structural support and regulate cell behaviour. Basement membranes are dynamic rather than static structures, being continuously remodelled by glycoprotein rupture and synthesis. Tumour BMs are significantly less crosslinked and therefore more susceptible to proteolysis, remodelling and turnover than BMs of normal tissue (Liotta et al, 1983; Martinez-Hernandez and Amenta, 1983; Kalluri, 2003).
Conceivably, BM continuity is the net effect of tumour matrix interaction and consequently a reflection of tumour behaviour. Widely fragmented BMs have been associated with poor outcome in bladder- (Conn et al, 1987; Daher et al, 1987; Schapers et al, 1990), colorectal- (Forster et al, 1984; Forster et al, 1986; Havenith et al, 1988), lung (ten Velde et al, 1991) and hepatocellular cancer (Grigioni et al, 1991). An irregular and discontinuous deposition of type IV collagen and laminin has also been observed for BMs of pancreatic cancer (Mollenhauer et al, 1987; Lee et al, 1994; Imamura et al, 1995; Shimoyama et al, 1995; Linder et al, 2001), however, except for the study by Linder et al, expression patterns were not studied for their association with outcome.
We therefore decided to study the distribution of BM laminin and collagen type IV in relation to conventional prognostic factors and clinical behaviour of two anatomically closely related, however, prognostically different pancreatic tumours, pancreatic head and periampullary cancer.
Patients and Methods
Patient population
Retrospectively, 231 patients treated for pancreatic ductal adenocarcinoma with curative intend at Erasmus Medical Center between 1987 and 2008, who had no microscopically residual tumour (R0), were identified. Tumours were classified by location, having their origin either in the pancreatic head or periampullary region, the latter group comprising of tumours originating in the Ampulla of Vater or the distal common bile duct. Tumour samples originating before the new 2002 UICC TNM classification were re-evaluated according to these new criteria.
Representative tumour areas were encircled on original haematoxylin/eosin slides by a GI pathologist (KB) with special expertise in pancreatic pathology and staining was performed on corresponding formalin-fixed, paraffin-embedded tissue.
During the above-mentioned period two randomised control trials were ongoing in our centre. Between September 1987 and April 1995, 17 patients were randomised to the treatment arm of the EORTC 40891 trial, receiving two courses of 5-FU as a continuous infusion (max 1500 mg per day) followed by radiotherapy (20 Gy). From June 2000 up to its closure in March 2007, 32 patients were randomised to the treatment arm of a trial combining intra-arterial chemotherapy and radiotherapy. Patients received six cycles of intra-arterial mitoxantrone (10 mg m−2), folinic acid (170 mg m−2 per day), 5-FU (600 mg m−2 per day) and cisplatinum (60 mg m−2), the first cycle followed by radiotherapy (54 Gy). These trials and the results have been described in detail elsewhere (Smeenk et al, 2007; Morak et al, 2008).
At the time of the present report, the median follow-up duration was 19 months (range 0–192 months). Recurrence-free survival (RFS) was defined as the time from date of surgery to the date of first proof of disease recurrence (locally, distant or both) or to death without relapse. Overall survival (OS) was computed as the number of months from resection to death of any cause as registered by the social security death index, whereas for cancer-specific survival (CSS) only the pancreatic cancer-related deaths were counted. Patients who died in hospital following procedure-related complications were excluded from analysis with respect to survival, as their death was considered unrelated to tumour biology. This was verified by an evaluation of in hospital death in relation to the tumour variable BM.
Expression of BM components by immunohistochemistry
Immunohistochemistry was performed according to the protocol used in clinical practice at our institution and was optimised for laminin and type IV collagen.
Briefly, 4 μℳ sections were deparaffinised in xylene and rehydrated through decreasing ethanol series ending in distilled water. In case of collagen type IV staining, antigen retrieval was performed by microwave heating (20 min preheating followed by 20 min of cooking) in Tris-EDTA buffer pH 9.0, whereas for laminin staining, proteinase K was applied for 10 min. Endogenous peroxidase activity was quenched using 0.3% hydrogen peroxide in PBS for 20 min. Sections were incubated overnight at 4 °C with a monoclonal mouse antibody to collagen type IV (CIV 22, M0785, Dako Netherlands BV, Heverlee, Belgium) or laminin (4C7, reacts with laminin alpha5; M0638, Dako) at dilutions of 40 × and 20 × , respectively, in Dako REAL antibody diluent (S2022, Dako), which reduces background staining without the need for additional blocking steps. Following incubation with the secondary antibody (Dako REAL Envision HRP Rabbit/Mouse) for 30 min at room temperature, immunostaining was developed by immersion in diaminobenzidine. Slides were washed extensively between each of the above steps. Nuclei were counterstained with Harris Haematoxylin. Next, slides were dehydrated, fixated and finally covered using Leica multistainer and robotic cover slipper (ST5020 and CV 5030, Leica Microsystems BV, Rijswijk, The Netherlands). Positive and negative controls were included in each run.
Tissue evaluation
Slides were examined by light microscopy and scored separately by three observers (JAvdZ; BMD and TLMtH) blinded to both clinical and pathological data. As previously described by Havenith et al (1988) and ten Velde et al (1991), the expression of BM components was quantified using a visual grading system based on the percentage of epithelial cell lining. The epithelial cell lining was either <25% (i.e., limited), 25–75% or >75%.
With respect to laminin expression inter-observer agreement was moderate (the weighted kappa ranged from 0.48 to 0.56), whereas inter-observer agreement for collagen type IV was less good (weighted kappa ranged from 0.34 to 0.48). Discrepant scores were resolved by consensus.
Basement membranes can only properly be identified by electron microscopy; with their 40–60 nm thickness, they are beyond the resolving power of the light microscope. Although BM zone might therefore have been more appropriate to describe the observed epithelial cell lining by its major components laminin and collagen type IV (Kefalides et al, 1979); because of the wide use of the term BM in other studies, this was also used throughout the current paper.
Statistical analysis
Statistical analysis was performed using SPSS version 18.0 for Windows (IBM, Amsterdam, The Netherlands).
Differences in distribution of categorical clinico-pathological parameters between groups were compared with χ2 or Fisher’s exact tests when appropriate.
The distributions of RFS, CSS and OS were estimated using Kaplan–Meier methodology. For interpretation purposes analyses were stratified by tumour origin, that is, pancreatic head or periampullary. Univariate associations were tested using log-rank test. Cox proportional hazards regression model was used to test whether outcome measures were independent of other established prognostic factors (T status, nodal involvement and tumour differentiation). Besides these established prognostic factors, numbers were also corrected for adjuvant therapy to prove that results were independent of this potentially prognostic factor.
By the use of interaction terms it was investigated whether the prognostic effect of BM Laminin and collagen IV expression differed between pancreatic head and periampullary cancers.
All P-values reported are two sided and values ⩽0.05 were considered statistically significant.
Results
Patient population
Of 231 tumours, 209 were adequately stained for analysis of BM laminin expression and 214 for analysis of BM collagen type IV expression. Both patient cohorts consisted of slightly more males than females, 119 vs 90 and 123 vs 91 in the laminin and collagen type IV cohorts, respectively. The median age of the patients in both cohorts was 65 years (range 36–87). Of 47% of tumours the origin was the pancreatic head, the other 53% were periampullary cancers. In both cohorts and for both tumour origins fewer patients had T1/2 tumours than T3/4 tumours, this was more pronounced in pancreatic head cancers (15% and 36% T1/2 tumours for pancreatic head and periampullary cancers, respectively). The amount of patients with lymph node involvement was approximately the same as those without lymph node involvement in both cohorts and for both tumour origins. Furthermore, in both patient cohorts and for both tumour types, the majority of tumours were moderately differentiated, with an equal amount of well and poorly differentiated tumours (on average 16%). Ten patients died during their postoperative stay and were thus excluded from survival analyses.
Basement membrane laminin and collagen type IV expression
Fifty-nine per cent of pancreatic head cancers showed <25% BM laminin, thirty per cent 25–75% and eleven per cent >75% BM laminin expression.
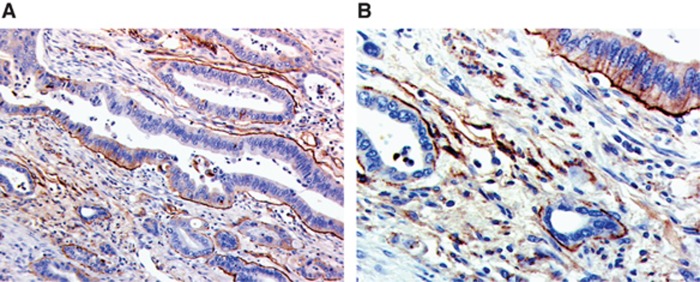
Sixty-one per cent of periampullary tumours showed <25% BM laminin, thirty-three per cent 25–75% and six per cent >75% BM laminin expression. An example of BM laminin staining is given in Figure 1. Interestingly, positive intracellular staining of tumour cells was also observed.
Figure 1.
Pancreatic cancer staining with anti-laminin showing both basement membrane and tumour cell staining. (A) × 10 magnification. (B) × 20 magnification.
In contrast, forty-three per cent of pancreatic head cancers showed <25% BM collagen type IV, forty-six per cent 25–75% and eleven per cent >75% BM collagen type IV expression. For periampullary cancer, expression rates were 41.4%, 47.4% and 11.2% for limited, 25–75% and >75% BM collagen type IV expression, respectively. Figure 2 is an example of BM collagen type IV staining. Apart from the BM, positive tumour stroma expression was also observed.
Figure 2.
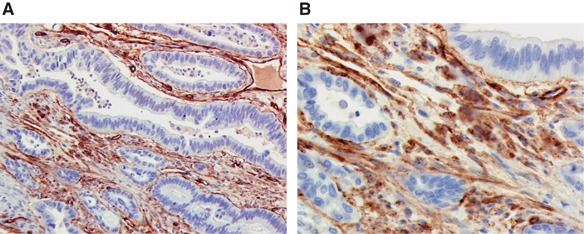
Pancreatic cancer staining with anti-collagen type IV showing both basement membrane staining and staining of tumour stroma. (A) × 10 magnification. (B) × 20 magnification.
Furthermore, the graded levels of BM laminin and collagen type IV expression correlated weakly with each other (Spearman r=0.43 and 0.33 for pancreatic head and periampullary cancers, respectively, P<0.001). In tumours with more discontinuous BM laminin staining, generally less BM collagen type IV staining was observed.
Pathologic correlations
Basement membrane laminin expression was not associated with any of the conventional prognostic factors (T or N status or grade of differentiation) in either tumour (i.e., pancreatic head or periampullary cancer). In contrast BM collagen type IV expression was associated with grade of differentiation in pancreatic head cancers (P=0.037). A more fragmented BM collagen type IV expression pattern was observed in less differentiated tumours.
Clinical correlations
The pancreatic tumour types described, pancreatic head and periampullar cancer, showed significant different survival behaviour. Approximately two times as many patients were recurrence free and alive 5 years following complete resection of periampullary cancer as compared with pancreatic head cancer (29% vs 16% P<0.001 for all outcome measures).
To test whether tumour epithelial BM continuity, as determined by expression of its two major components laminin and collagen type IV, affects prognosis of tumours differently, depending on the tumour origin as specified above, we tested for effect modification (interaction) by tumour origin in the Cox models. This interaction test showed that the prognostic effect of BM laminin did not significantly differ between pancreatic head- and periampullary cancer. This was true for all outcome measures (P=0.42, 0.19 and 0.35 for RFS, CSS and OS, respectively). Statistically, it would have been justified to take both tumours (i.e., pancreatic head and periampullary cancer) together for survival analysis. However, because of the significantly different survival of pancreatic head and periampullary cancer, both tumours were analysed separately for interpretation purposes.
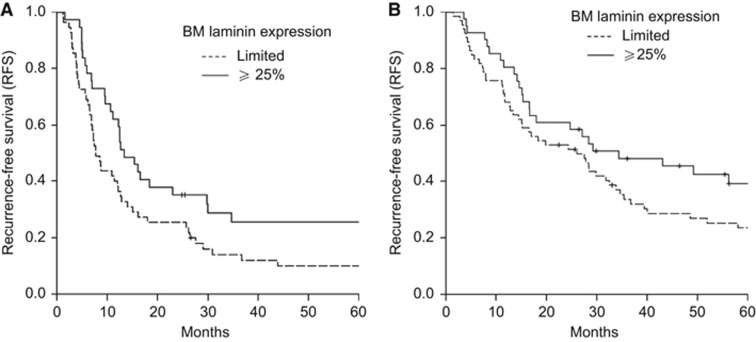
Because of the relatively small amount of tumours classified as having >75% BM laminin (9 out of 92 and 7 out of 107 of, respectively, pancreatic head and periampullary cancer), both categories (25–75% and >75% BM laminin) were taken together as ⩾25%. Survival analysis showed that BM laminin expression was significantly associated with outcome following curative resection of pancreatic head cancer (P=0.034, 0.013 and 0.017 for RFS, CSS and OS, respectively). Ten per cent of patients treated for pancreatic head cancer showing <25% (i.e., limited) epithelial BM laminin were recurrence free and alive 5 years following curative resection of the tumour, whereas more than twice as many patients (26%) were recurrence free and alive if their tumours showed ⩾25% BM laminin. A similar trend was observed for patients treated for periampullary cancer (24% and 39% for limited and ⩾25% BM, respectively), however, this difference in survival behaviour was not significant (P=0.16; 0.27 and 0.15 for RFS, CSS and OS, respectively). (Figure 3) By multivariate analysis it was shown that limited BM laminin expression is an independent predictor of poor OS and CSS following curative resection of pancreatic head cancer. This was not the case for RFS. When corrected for other prognostic factors such as tumour extent (T status), nodal involvement, grade of differentiation, BM laminin expression did however show a trend for an independent association with RFS (P=0.09) (Table 1). Even when corrected for the adjuvant treatment given to some patients, BM laminin expression remained a significant prognostic factor for CSS and showed a trend for a significant association with RFS and OS (P=0.11 and 0.058, respectively).
Figure 3.
Recurrence-free survival (RFS) of patients treated for, respectively, pancreatic head (A) and periampullary cancer (B) shows shorter RFS in patients with tumours with limited basement membrane (BM) laminin expression compared to those with tumours with more continuous BM laminin expression. This different survival behaviour, however, was only significant for pancreatic head cancers (P=0.034 and 0.16 for pancreatic head and periampullary cancer, respectively).
Table 1. 5-Year survival and multivariate analysis of conventional prognostic factors and tumour BM laminin expression on outcome following curative resection of pancreatic head cancer.
|
RFS
|
CSS
|
OS
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor | N | % 5 Year | HR | 95% CI | P- value | % 5 Year | HR | 95% CI | P- value | % 5 Year | HR | 95% CI | P- value |
| Tumour extension | 0.30 | 0.18 | 0.39 | ||||||||||
| T1/2a | 14 | 29 | 31 | 29 | |||||||||
| T3/4 | 76 | 13 | 1.41 | 0.74–2.69 | 17 | 1.67 | 0.78–3.56 | 14 | 1.33 | 0.69–2.55 | |||
| Nodal involvement | 0.024 | 0.006 | 0.006 | ||||||||||
| Noa | 43 | 30 | 35 | 32 | |||||||||
| Yes | 49 | 3 | 1.75 | 1.08–2.86 | 3 | 2.10 | 1.24–3.54 | 2 | 1.98 | 1.22–3.22 | |||
| Differentiation | 0.09 | 0.08 | 0.10 | ||||||||||
| Wella | 15 | 33 | 38 | 33 | |||||||||
| Moderately | 60 | 12 | 1.99 | 1.04–3.80 | 0.037 | 15 | 1.84 | 0.89–3.83 | 0.10 | 13 | 1.97 | 1.03–3.76 | 0.040 |
| Poorly | 16 | 13 | 2.12 | 0.98–4.59 | 0.056 | 13 | 2.69 | 1.14–6.36 | 0.024 | 13 | 2.02 | 0.93–4.41 | 0.08 |
| BM Laminin expression | 0.09 | 0.043 | 0.050 | ||||||||||
| Limiteda | 55 | 10 | 11 | 10 | |||||||||
| ⩾25% | 37 | 26 | 0.67 | 0.42–1.07 | 31 | 0.59 | 0.35–0.98 | 27 | 0.63 | 0.39–1.00 | |||
Abbreviations: BM=basement membrane; 95% CI=95% confidence interval; CSS=cancer-specific survival; HR=hazard ratio; OS=overall survival; RFS=recurrence-free survival; % 5 year=% 5 year survival by univariate analysis.
Reference category.
In contrast to BM laminin, BM collagen type IV expression was not associated with outcome in either type of pancreatic cancer (P=0.35, 0.19 and 0.35; and 0.94, 0.89 and 0.85 for RFS, CSS and OS of pancreatic head- and periampullary cancer, respectively) (data not shown).
Discussion
This is the largest study to date studying the expression of BM components in pancreatic cancer specimens and investigating its potential relation with prognosis following curative resection.
In the current study, approximately one-tenth of pancreatic head and periampullary tumours showed ⩾75% tumour epithelial cell lining by BM major components laminin or collagen type IV. This could be either due to increased turnover by proteolytic enzymes or decreased synthesis. In the 1980s, Liotta et al already showed that the rate of spontaneous metastases correlated with collagen type IV degradation activity in cells. Since then several matrix metalloproteinase′s have been identified. In the current study some tumour cells showed immunoreactivity for laminin, whereas immunostaining for collagen type IV was also observed of tumour stroma. Accumulation of collagen type IV in the interstitium was also described by Kalluri (2003) in association with fibrosis.
In line with the concept that synthesis and modulation of BM components have a major role in morphogenesis (Martinez-Hernandez and Amenta, 1983), BM collagen type IV expression was associated with tumour differentiation of pancreatic head cancers in our patient cohort. The more patchy BM collagen type IV staining, the least differentiated the tumour. In contrast, tumour differentiation was not associated with BM laminin expression. Both poorly differentiated tumours and well-differentiated tumours showed limited BM laminin expression, the same was true for tumour extent, an observation that suggests that disruption of BM laminin is an early process in tumour progression. The correlation of BM continuity with differentiation is in line with observations in bladder-, colorectal-, hepatocellular-, breast-, endometrial cancer and an earlier report on pancreatic cancer (Albrechtsen et al, 1981; Forster et al, 1984, 1986; Stenback et al 1985; Mollenhauer et al, 1987; Schapers et al, 1990; Grigioni et al, 1991; Lazaris et al, 2003; Souza et al, 2007). BM continuity has also been associated with stage in some tumours (Havenith et al, 1988; Schapers et al, 1990) and metastasis in others (Forster et al, 1984; Forster et al, 1986; Mielcarek-Kuchta et al, 2008).
As could have been expected by its highly crosslinked structure (Kalluri, 2003), BM laminin expression was associated with BM collagen type IV expression. Generally, tumours with limited BM laminin deposits also showed scarce collagen type IV expression.
Although widely fragmented BMs have been observed in pancreatic cancer before (Lee et al, 1994; Imamura et al, 1995; Shimoyama et al, 1995), there is only one small study of 16 patients analysing several extracellular matrix proteins and integrins stating that staining patterns were comparable irrespective of patient survival (Linder et al, 2001). The lack of an association between the BM patterns and outcome observed by Linder and co-workers could be due to the small patient sample. In contrast, in our study more than twice as many patients were recurrence free and alive 5 years following resection if their tumours had ⩾25% BM laminin expression compared with patients with tumours with <25% BM laminin. In fact, limited BM laminin expression proved to be an independent predictor of poor survival following curative resection of pancreatic head cancer. Although BM laminin and BM collagen IV expression patterns were weakly correlated with each other, only BM laminin expression was associated with outcome. BM continuity by collagen type IV expression was not associated with outcome. BM by laminin was also associated with outcome in colorectal- (Forster et al, 1984; Forster et al, 1986; Lazaris et al, 2003), bladder- (Schapers et al, 1990) and hepatocellular cancer (Grigioni et al, 1991). In contrast to our findings a relation with BM by collagen IV was also observed in several tumours (Daher et al, 1987; Havenith et al, 1988; Schapers et al, 1990; Grigioni et al, 1991; ten Velde et al, 1991; Lazaris et al, 2003).
There are two mechanisms, by which laminin is thought to be involved in the formation of metastases. First, laminin has been reported to be involved in the formation of hemidesmosomes, biological structures that enable static cell adhesion. Consequently, decreased expression could cause disassembly or a reduction in the number of hemidesmosomes, with failure of cell anchoring.
Second, the cleaved form of laminin has been observed to stimulate motility of epithelial cell types. Therefore, an increased expression of MMPs that cleave laminin could stimulate cell motility (Giannelli and Antonaci, 2000). A process in which activated pancreatic stellate cells might have an essential role (Schneiderhan et al, 2007).
The lack of a relation of collagen type IV expression with outcome in our study could theoretically have been caused by the fair-to-moderate agreement in scoring between the three observers. Apparently scoring BM collagen type IV expression is rather complicated. To draw definite conclusions with respect to the prognostic value of BM collagen type IV expression, other scoring systems need to be explored.
In conclusion, the current study is the first study that identifies an independent relationship between tumour BM laminin expression and prognosis of pancreatic head cancer. Routine tumour BM laminin staining could potentially differentiate between different prognostic subgroups of pancreatic head cancer and consequently aid in therapeutic decision making.
Acknowledgments
We thank H van Dekken for his previous work on the studies pathology specimen and the Department of Clinical Pathology for using their equipment.
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
The authors declare no conflict of interest.
09/10/2012
This paper has been modified since advance online publication, an acknowledgement has been added
References
- Albrechtsen R, Nielsen M, Wewer U, Engvall E, Ruoslahti E (1981) Basement membrane changes in breast cancer detected by immunohistochemical staining for laminin. Cancer Res 41: 5076–5081 [PubMed] [Google Scholar]
- Boeck S, Ankerst DP, Heinemann V (2007) The role of adjuvant chemotherapy for patients with resected pancreatic cancer: systematic review of randomized controlled trials and meta-analysis. Oncology 72: 314–321 [DOI] [PubMed] [Google Scholar]
- Conn IG, Crocker J, Wallace DM, Hughes MA, Hilton CJ (1987) Basement membranes in urothelial carcinoma. Br J Urol 60: 536–542 [DOI] [PubMed] [Google Scholar]
- Daher N, Abourachid H, Bove N, Petit J, Burtin P (1987) Collagen IV staining pattern in bladder carcinomas: relationship to prognosis. Br J Cancer 55: 665–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster SJ, Talbot IC, Clayton DG, Critchley DR (1986) Tumour basement membrane laminin in adenocarcinoma of rectum: an immunohistochemical study of biological and clinical significance. Int J Cancer 37: 813–817 [DOI] [PubMed] [Google Scholar]
- Forster SJ, Talbot IC, Critchley DR (1984) Laminin and fibronectin in rectal adenocarcinoma: relationship to tumour grade, stage and metastasis. Br J Cancer 50: 51–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannelli G, Antonaci S (2000) Biological and clinical relevance of laminin-5 in cancer. Clin Exp Metastasis 18: 439–443 [DOI] [PubMed] [Google Scholar]
- Grigioni WF, Garbisa S, D′Errico A, Baccarini P, Stetler-Stevenson WG, Liotta LA, Mancini AM (1991) Evaluation of hepatocellular carcinoma aggressiveness by a panel of extracellular matrix antigens. Am J Pathol 138: 647–654 [PMC free article] [PubMed] [Google Scholar]
- Havenith MG, Arends JW, Simon R, Volovics A, Wiggers T, Bosman FT (1988) Type IV collagen immunoreactivity in colorectal cancer. Prognostic value of basement membrane deposition. Cancer 62: 2207–2211 [DOI] [PubMed] [Google Scholar]
- Imamura T, Manabe T, Ohshio G, Wang ZH, Yamaki K, Yoshimura T, Suwa H, Imamura M (1995) Immunohistochemical staining for type IV collagen and laminin in the stroma of human pancreatic cancer. Int J Pancreatol 18: 95–99 [DOI] [PubMed] [Google Scholar]
- Kalluri R (2003) Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer 3: 422–433 [DOI] [PubMed] [Google Scholar]
- Kefalides NA, Alper R, Clark CC (1979) Biochemistry and metabolism of basement membranes. Int Rev Cytol 61: 167–228 [DOI] [PubMed] [Google Scholar]
- Lazaris A, Tzoumani AN, Thimara I, Theodoropoulos GE, Thomopoulou G, Dicoglou C, Panoussopoulos D, Davaris P (2003) Immunohistochemical assessment of basement membrane components in colorectal cancer: prognostic implications. J Exp Clin Cancer Res 22: 599–606 [PubMed] [Google Scholar]
- Lee CS, Montebello J, Georgiou T, Rode J (1994) Distribution of type IV collagen in pancreatic adenocarcinoma and chronic pancreatitis. Int J Exp Pathol 75: 79–83 [PMC free article] [PubMed] [Google Scholar]
- Li D, Xie K, Wolff R, Abbruzzese JL (2004) Pancreatic cancer. Lancet 363: 1049–1057 [DOI] [PubMed] [Google Scholar]
- Linder S, Castanos-Velez E, von Rosen A, Biberfeld P (2001) Immunohistochemical expression of extracellular matrix proteins and adhesion molecules in pancreatic carcinoma. Hepatogastroenterology 48: 1321–1327 [PubMed] [Google Scholar]
- Liotta LA, Rao CN, Barsky SH (1983) Tumor invasion and the extracellular matrix. Lab Invest 49: 636–649 [PubMed] [Google Scholar]
- Liotta LA, Tryggvason K, Garbisa S, Hart I, Foltz CM, Shafie S (1980) Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature 284: 67–68 [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A, Amenta PS (1983) The basement membrane in pathology. Lab Invest 48: 656–677 [PubMed] [Google Scholar]
- Mielcarek-Kuchta D, Olofsson J, Golusinski W (2008) Laminin expression in advanced laryngeal squamous cell carcinoma does not correlate to neck metastases. Eur Arch Otorhinolaryngol 265: 1257–1261 [DOI] [PubMed] [Google Scholar]
- Mollenhauer J, Roether I, Kern HF (1987) Distribution of extracellular matrix proteins in pancreatic ductal adenocarcinoma and its influence on tumor cell proliferation in vitro. Pancreas 2: 14–24 [DOI] [PubMed] [Google Scholar]
- Morak MJ, van der Gaast A, Incrocci L, van Dekken H, Hermans JJ, Jeekel J, Hop WC, Kazemier G, van Eijck CH (2008) Adjuvant intra-arterial chemotherapy and radiotherapy versus surgery alone in resectable pancreatic and periampullary cancer: a prospective randomized controlled trial. Ann Surg 248: 1031–1041 [DOI] [PubMed] [Google Scholar]
- Schapers RF, Pauwels RP, Havenith MG, Smeets AW, van den Brandt PA, Bosman FT (1990) Prognostic significance of type IV collagen and laminin immunoreactivity in urothelial carcinomas of the bladder. Cancer 66: 2583–2588 [DOI] [PubMed] [Google Scholar]
- Schneiderhan W, Diaz F, Fundel M, Zhou S, Siech M, Hasel C, Moller P, Gschwend JE, Seufferlein T, Gress T, Adler G, Bachem MG (2007) Pancreatic stellate cells are an important source of MMP-2 in human pancreatic cancer and accelerate tumor progression in a murine xenograft model and CAM assay. J Cell Sci 120: 512–519 [DOI] [PubMed] [Google Scholar]
- Shimoyama S, Gansauge F, Gansauge S, Oohara T, Beger HG (1995) Altered expression of extracellular matrix molecules and their receptors in chronic pancreatitis and pancreatic adenocarcinoma in comparison with normal pancreas. Int J Pancreatol 18: 227–234 [DOI] [PubMed] [Google Scholar]
- Smeenk HG, van Eijck CH, Hop WC, Erdmann J, Tran KC, Debois M, van Cutsem E, van Dekken H, Klinkenbijl JH, Jeekel J (2007) Long-term survival and metastatic pattern of pancreatic and periampullary cancer after adjuvant chemoradiation or observation: long-term results of EORTC trial 40891. Ann Surg 246: 734–740 [DOI] [PubMed] [Google Scholar]
- Souza LF, Souza VF, Silva LD, Santos JN, Reis SR (2007) Expression of basement membrane laminin in oral squamous cell carcinomas. Braz J Otorhinolaryngol 73: 768–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenback F, Risteli J, Risteli L, Wasenius VM (1985) Basement membrane laminin and type IV collagen in endometrial adenocarcinoma: relation to differentiation and treatment. Oncology 42: 370–376 [DOI] [PubMed] [Google Scholar]
- ten Velde GP, Havenith MG, Volovics A, Bosman FT (1991) Prognostic significance of basement membrane deposition in operable squamous cell carcinomas of the lung. Cancer 67: 3001–3005 [DOI] [PubMed] [Google Scholar]