Abstract
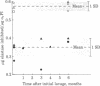
The elastase inhibitory capacity per mg of alpha 1-proteinase inhibitor (alpha 1 PI) was measured in the bronchoalveolar lavage (BAL) fluid from 26 healthy smokers and 24 nonsmokers. Activity was decreased by 40% in smokers' BAL fluid compared to nonsmokers. This effect was demonstrable by using human neutrophil elastase as well as porcine pancreatic elastase as test enzyme (elastase, EC 3.4.21.11) and was reproducible when selected individuals in each group underwent lavage on repeated occasions. In contrast, the functional activity of alpha 1-antichymotrypsin was not decreased in smokers' BAL fluid. Crossed antigen-antibody electrophoresis confirmed that inactivation of alpha 1 PI was responsible for the decrease in the elastase inhibitory capacity of smokers' BAL fluid. alpha 1 PI purified from smokers' BAL fluids contained methionine sulfoxide (4 mol/mol of inactive alpha 1 PI), whereas alpha 1 PI from nonsmokers' BAL fluid did not. Smokers' alpha 1 PI was indistinguishable from nonsmokers' alpha 1 PI on the basis of electrophoretic mobility, molecular weight, and immunoreactivity. Thus, oxidation of methionine residues in lung alpha 1 PI is associated with cigarette smoking. Because chemical oxidation of alpha 1 PI in vitro causes loss of its elastase inhibitory activity, the present observations suggest that methionine oxidation may also be the cause of decreased functional activity of lung alpha 1 PI in smokers. Oxidative inactivation of alpha 1 PI in the lungs of cigarette smokers may play a role in the development of pulmonary emphysema in this group.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams W. R., Eliraz A., Kimbel P., Weinbaum G. The effect of the oxidizing agents chloramine-T and cigarette smoke on dog serum proteinase inhibitor(s). Exp Lung Res. 1980 Aug;1(3):211–223. doi: 10.3109/01902148009065461. [DOI] [PubMed] [Google Scholar]
- Auerbach O., Hammond E. C., Garfinkel L., Benante C. Relation of smoking and age to emphysema. Whole-lung section study. N Engl J Med. 1972 Apr 20;286(16):853–857. doi: 10.1056/NEJM197204202861601. [DOI] [PubMed] [Google Scholar]
- Banda M. J., Clark E. J., Werb Z. Limited proteolysis by macrophage elastase inactivates human alpha 1-proteinase inhibitor. J Exp Med. 1980 Dec 1;152(6):1563–1570. doi: 10.1084/jem.152.6.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty K., Bieth J., Travis J. Kinetics of association of serine proteinases with native and oxidized alpha-1-proteinase inhibitor and alpha-1-antichymotrypsin. J Biol Chem. 1980 May 10;255(9):3931–3934. [PubMed] [Google Scholar]
- Bieth J., Spiess B., Wermuth C. G. The synthesis and analytical use of a highly sensitive and convenient substrate of elastase. Biochem Med. 1974 Dec;11(4):350–357. doi: 10.1016/0006-2944(74)90134-3. [DOI] [PubMed] [Google Scholar]
- Bignon J., Lenfant C., Scarpa G. L. Emphysema: past, present and future. Bull Eur Physiopathol Respir. 1980;16 (Suppl):423–428. doi: 10.1016/b978-0-08-027379-2.50045-4. [DOI] [PubMed] [Google Scholar]
- Bjerrum O. J., Bhakdi S., Bog-Hansen T. C., Knüfermann H., Wallach D. F. Quantitative immunoelectrophoresis of proteins in human erythrocyte membranes. Analysis of protein bands obtained by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biochim Biophys Acta. 1975 Nov 3;406(4):489–504. doi: 10.1016/0005-2736(75)90027-9. [DOI] [PubMed] [Google Scholar]
- Carp H., Janoff A. In vitro suppression of serum elastase-inhibitory capacity by reactive oxygen species generated by phagocytosing polymorphonuclear leukocytes. J Clin Invest. 1979 Apr;63(4):793–797. doi: 10.1172/JCI109364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp H., Janoff A. Inactivation of bronchial mucous proteinase inhibitor by cigarette smoke and phagocyte-derived oxidants. Exp Lung Res. 1980 Aug;1(3):225–237. doi: 10.3109/01902148009065462. [DOI] [PubMed] [Google Scholar]
- Carp H., Janoff A. Possible mechanisms of emphysema in smokers. In vitro suppression of serum elastase-inhibitory capacity by fresh cigarette smoke and its prevention by antioxidants. Am Rev Respir Dis. 1978 Sep;118(3):617–621. doi: 10.1164/arrd.1978.118.3.617. [DOI] [PubMed] [Google Scholar]
- Carp H., Janoff A. Potential mediator of inflammation. Phagocyte-derived oxidants suppress the elastase-inhibitory capacity of alpha 1-proteinase inhibitor in vitro. J Clin Invest. 1980 Nov;66(5):987–995. doi: 10.1172/JCI109968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Stone P. J., El Hag A., Calore J. D., Franzblau C. Myeloperoxidase-catalyzed inactivation of alpha 1-protease inhibitor by human neutrophils. J Biol Chem. 1981 Apr 10;256(7):3348–3353. [PubMed] [Google Scholar]
- Clarke H. G., Freeman T. Quantitative immunoelectrophoresis of human serum proteins. Clin Sci. 1968 Oct;35(2):403–413. [PubMed] [Google Scholar]
- Cohen A. B. The effects in vivo and in vitro of oxidative damage to purified alpha1-antitrypsin and to the enzyme-inhibiting activity of plasma. Am Rev Respir Dis. 1979 Jun;119(6):953–960. doi: 10.1164/arrd.1979.119.6.953. [DOI] [PubMed] [Google Scholar]
- Damiano V. V., Sandler A., Abrams W. R., Meranze D. R., Cohen A. B., Kimbel P., Weinbaum G. Electron and light microscopic studies of the lungs of chloramine-T treated dogs. Bull Eur Physiopathol Respir. 1980;16 (Suppl):141–156. doi: 10.1016/b978-0-08-027379-2.50015-6. [DOI] [PubMed] [Google Scholar]
- Dunnill M. S. Aetiology of emphysema. Bull Eur Physiopathol Respir. 1979 Sep-Oct;15(5):1015–1029. [PubMed] [Google Scholar]
- Farmer D. A., Hageman J. H. Use of N-benzoyl-L-tyrosine thiobenzyl ester as a protease substrate. Hydrolysis by alpha-chymotrypsin and subtilisin BPN. J Biol Chem. 1975 Sep 25;250(18):7366–7371. [PubMed] [Google Scholar]
- Feinstein G., Janoff A. A rapid method of purification of human granulocyte cationic neutral proteases: purification and further characterization of human granulocyte elastase. Biochim Biophys Acta. 1975 Oct 22;403(2):493–505. doi: 10.1016/0005-2744(75)90077-7. [DOI] [PubMed] [Google Scholar]
- Gadek J. E., Fells G. A., Crystal R. G. Cigarette smoking induces functional antiprotease deficiency in the lower respiratory tract of humans. Science. 1979 Dec 14;206(4424):1315–1316. doi: 10.1126/science.316188. [DOI] [PubMed] [Google Scholar]
- Hoidal J. R., Fox R. B., LeMarbe P. A., Perri R., Repine J. E. Altered oxidative metabolic responses in vitro of alveolar macrophages from asymptomatic cigarette smokers. Am Rev Respir Dis. 1981 Jan;123(1):85–89. doi: 10.1164/arrd.1981.123.1.85. [DOI] [PubMed] [Google Scholar]
- Hoidal J. R., Fox R. B., Repine J. E. Defective oxidative metabolic responses in vitro of alveolar macrophages in chronic granulomatous disease. Am Rev Respir Dis. 1979 Sep;120(3):613–618. doi: 10.1164/arrd.1979.120.3.613. [DOI] [PubMed] [Google Scholar]
- Janoff A., Carp H., Lee D. K., Drew R. T. Cigarette smoke inhalation decreases alpha 1-antitrypsin activity in rat lung. Science. 1979 Dec 14;206(4424):1313–1314. doi: 10.1126/science.316187. [DOI] [PubMed] [Google Scholar]
- Janoff A., Carp H. Possible mechanisms of emphysema in smokers: cigarette smoke condensate suppresses protease inhibition in vitro. Am Rev Respir Dis. 1977 Jul;116(1):65–72. doi: 10.1164/arrd.1977.116.1.65. [DOI] [PubMed] [Google Scholar]
- Johnson D., Travis J. Inactivation of human alpha 1-proteinase inhibitor by thiol proteinases. Biochem J. 1977 Jun 1;163(3):639–641. doi: 10.1042/bj1630639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D., Travis J. Structural evidence for methionine at the reactive site of human alpha-1-proteinase inhibitor. J Biol Chem. 1978 Oct 25;253(20):7142–7144. [PubMed] [Google Scholar]
- Johnson D., Travis J. The oxidative inactivation of human alpha-1-proteinase inhibitor. Further evidence for methionine at the reactive center. J Biol Chem. 1979 May 25;254(10):4022–4026. [PubMed] [Google Scholar]
- Karlinsky J. B., Snider G. L. Animal models of emphysema. Am Rev Respir Dis. 1978 Jun;117(6):1109–1133. doi: 10.1164/arrd.1978.117.6.1109. [DOI] [PubMed] [Google Scholar]
- Livingston D. M. Immunoaffinity chromatography of proteins. Methods Enzymol. 1974;34:723–731. doi: 10.1016/s0076-6879(74)34094-3. [DOI] [PubMed] [Google Scholar]
- Matheson N. R., Wong P. S., Schuyler M., Travis J. Interaction of human alpha-1-proteinase inhibitor with neutrophil myeloperoxidase. Biochemistry. 1981 Jan 20;20(2):331–336. doi: 10.1021/bi00505a016. [DOI] [PubMed] [Google Scholar]
- Matheson N. R., Wong P. S., Travis J. Enzymatic inactivation of human alpha-1-proteinase inhibitor by neutrophil myeloperoxidase. Biochem Biophys Res Commun. 1979 May 28;88(2):402–409. doi: 10.1016/0006-291x(79)92062-x. [DOI] [PubMed] [Google Scholar]
- Ohlsson K., Fryksmark U., Tegner H. The effect of cigarette smoke condensate on alpha 1-antitrypsin, antileukoprotease and granulocyte elastase. Eur J Clin Invest. 1980 Oct;10(5):373–379. doi: 10.1111/j.1365-2362.1980.tb00048.x. [DOI] [PubMed] [Google Scholar]
- Shechter Y., Burstein Y., Patchornik A. Selective oxidation of methionine residues in proteins. Biochemistry. 1975 Oct 7;14(20):4497–4503. doi: 10.1021/bi00691a025. [DOI] [PubMed] [Google Scholar]
- Stoklosa J. T., Latz H. W. Molecular weight determinations of proteins by polyacrylamide gel electrophoresis with sodium dodecyl sulfate in just the sample solution. Biochem Biophys Res Commun. 1974 May 7;58(1):74–79. doi: 10.1016/0006-291x(74)90892-4. [DOI] [PubMed] [Google Scholar]
- Travis J., Garner D., Bowen J. Human alpha-1-antichymotrypsin: purification and properties. Biochemistry. 1978 Dec 26;17(26):5647–5651. doi: 10.1021/bi00619a010. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wong P. S., Travis J. Isolation and properties of oxidized alpha-1-proteinase inhibitor from human rheumatoid synovial fluid. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1449–1454. doi: 10.1016/0006-291x(80)90113-8. [DOI] [PubMed] [Google Scholar]