Abstract
The P4 subfamily of P-type ATPases includes phospholipid transporters. Moving such bulky amphipathic substrate molecules across the membrane poses unique mechanistic problems. Recently, three papers from three different laboratories have offered insights into some of these problems. One effect of these experiments will be to ignite a healthy debate about the path through the enzyme taken by the substrate. A second effect is to suggest a counterintuitive model for the critical substrate-binding site. By putting concrete hypotheses into play, these papers finally provide a foundation for investigations of mechanism for these proteins.
Keywords: P4 ATPase, Phospholipid transport, ATPase reaction cycle
Framing the problem
P-type ATPases are ATP-dependent ion transporters that derive their name from an aspartate that becomes transiently phosphorylated during the reaction cycle [17]. The structures of several members of the family have been determined at atomic resolution in the last decade, and they all have a large tripartite cytoplasmic domain and a small extracellular domain connected by six to ten transmembrane helices. The P4 subfamily of P-type ATPases was first defined in 1996, when the gene encoding an aminophospholipid translocase, ATP8A1, from bovine chromaffin granules [26] was found to be a relative of the previously identified yeast P-type ATPase, Drs2p [23]. P4 type ATPases are found in all eukaryotic organisms but not in prokaryotes. In vertebrates, there are 14 genes that encode P4 ATPases [9] while in yeast there are five; Drs2, Dnf1, Dnf2, Dnf3, and Neo1 [19]. Unlike the other subfamilies of P-type ATPases, which all transport simple ions, the only known substrates of the P4 ATPases are phospholipids.
The transport mechanism of the P-type ATPases depends on sequential partial reactions of ATP hydrolysis—phosphorylation and dephosphorylation of the critical aspartic acid residue in the cytoplasmic P domain—and linked sequential conformational changes; coordination between chemical and conformational steps drives substrate across the membrane against a concentration gradient. The conformational changes link a binding pocket for the transported ion to either the cytoplasm (the E1 conformation) or the extracytoplasmic, or lumenal space (the E2 conformation). In the classic Post–Albers model [1, 22], binding of ions in the E1 conformation enables phosphorylation to the E1P form; this form is conformationally unstable, and converts to the E2P conformation. Loss of ions into the luminal space from that conformation is the key to dephosphorylation to the E2 form. The latter is also conformationally unstable, and the enzyme thus converts back into the E1 conformation [1, 22].
There is a subtlety about this sequence of events which should be mentioned here, particularly as it applies to the E2P conformation. This latter designation must define two conformations; indeed, both have been crystallized in the case of the Ca-ATPase [15, 16, 27]. One is the form that releases the cytoplasmically bound ions into the lumen in reversible fashion; this form readily rebinds the ions and converts back to the E1P form. Excess ADP can react with the E1P form to regenerate ATP and E1, making this E2P conformation the “ADP-sensitive” form. Once the E2P form has bound lumenal substrate, it can no longer bind cytoplasmic substrate, and must change conformation to both prevent lumenal substrate dissociation and poise the enzyme for dephosphorylation; this form can no longer readily revert to the E1P conformation, and is thus operationally the “ADP-insensitive” form. This distinction emphasizes the central feature of the Post–Albers cycle for P-type ATPases: the enforced sequential order of these reactions and enzyme shape changes must be combined with occlusion of the substrate-binding pocket at the point of conformation change to prevent backflow of substrate [21]. Thus, the ion substrate does not travel through a simple pore; instead, the enzyme creates and destroys a binding site at different points in the cycle, and the site is accessible to opposite sides of the membrane at the points of site creation and destruction in the cycle [11].
Common mechanism of phosphorylation and dephosphorylation
Sequence comparisons show that P-Type ATPases share many conserved motifs. The treasury of atomic structures that have accumulated over the last decade locates most of these motifs in the cytoplasmic domains, where they play key roles in ATP hydrolysis and the transduction of this hydrolysis into changes in protein conformation. By necessity, the transmembrane region is the locus of substrate binding and release; since the P-type ATPases have diverged in their substrate specificity [2], it is not surprising that the transmembrane region is variable between different classes of P-type ATPase. Nevertheless, the similarity in overall structure between members of different subfamilies shows clearly that the basic mechanisms by which they operate are the same, with substrates that are transported out of the cytoplasm loaded in the E1 conformation, and released at the luminal side in the E2P conformation. Since phospholipids are pumped from the outer, lumenal leaflet to the inner leaflet, phospholipids should bind to the E2P form; that binding (and occlusion of the bound phospholipid) would then be the prerequisite for dephosphorylation of the enzyme. In fact, the addition of phospholipid has been shown to promote dephosphorylation of the E2P form of the P4 enzyme [6, 7, 10].
One important aspect of phospholipid transport by the P4 ATPases cannot be illuminated by extrapolation from structural and sequence information about the better known ion transporters. The P4 ATPases operate as heterodimers in combination with protein subunits from the Cdc50 family of membrane proteins [24]. Members of this family all contain two transmembrane helices linked by a substantial lumenal domain [12]. These two proteins associate with each other in the ER [24, 25], and transport activity requires the presence of the subunit [4, 5, 13]. The role of the subunit in the reaction cycle of transport is not clear, but there is evidence that the subunit/transporter interactions are dynamic, and that the subunit is most strongly associated with the transporter at the E2P conformation [13], suggesting that it may play a role in phospholipid substrate binding or occlusion.
Giant substrate problem
Although the E1/E2 mechanism is a starting point for thinking about how P4 ATPases convert the energy of ATP hydrolysis into uphill transport of substrate across the membrane, the pumping mechanisms of the P4 ATPases must be unique because of the size of the amphipathic substrate that it transports. Indeed, the substrate specificity of the P4-Type ATPases is complex—transport is headgroup dependent, in some cases transporting aminophospholipids such as phosphatidylserine (PS) but not phosphatidylcholine (PC) [20, 28]. It is also stereospecific, but not for the headgroup (D vs L serine, for example) but for the C2 carbon of the glycerol backbone [8]. Transport is also backbone-specific, moving glycerophospholipids, but not sphingolipids [20]. In addition, the substrate of the P4 transporters imposes another requirement: once bound, ions do not have to move in the binding pocket as the P-type ATPase changes conformations, whereas phospholipid transport demands that the ligand physically reorient during the transport process. The binding site for the substrate must therefore not only be relatively large, but most also provide access to a pathway for this reorientation.
Models for the binding site
Where is the binding site and transport pathway in P4 ATPases? Knowing the pathway for loading and unloading the phospholipids from the transporter is not only essential for understanding substrate specificity, but also potentially informative about key features of the transport cycle. The binding sites for ions in the better studied P-type ATPases are not obviously helpful—the conserved ionic and polar residues making up these sites in the Ca and Na/K ATPases are not present in the P4 ATPases, largely replaced by non-polar residues [26]. However, the known structures do suggest two quite different models, and recent experiments speak to these models.
The proton transporter model
The first model is an extrapolation from the structure of the H+ (P3A) transporters [18]. These transporters also lack the anionic and polar residues that form the ion-binding sites in the Ca and Na/K transporters. Instead, the transported proton is bound to a conserved aspartate residue in the E1P stage of the cycle; when the enzyme conformation changes to the E2P form, a conserved arginine is brought close enough to form a salt bridge with this aspartate when the proton dissociates and is released into the lumen. The relevance of this protein for the P4 enzymes comes from the fact that the critical aspartate and arginine residues face a modest water-filled cavity in the core of the transmembrane domains, a cavity large enough to house a phospholipid headgroup. Recently, the relevance of this model for the P4 ATPases has received experimental support. In a collaboration between laboratories at Aarhus and Vancouver, Coleman et al. [6] showed that enzyme activity is strongly reduced by mutation of a highly conserved lysine (K873) near the center of the M5 transmembrane helix in the mammalian ATP8A2 enzyme. This reduction in enzyme activity is due, at least in part, to weaker PS binding, as measured by the ability of PS to stimulate dephosphorylation of E2P. The authors suggested that this positively charged side chain might complex directly with the phosphate of the phospholipid head group. This lysine aligns with a serine that is near to the ion-binding pocket in Ca-ATPases, but points away from the Ca ions. Notably, this residue is one turn of the helix (in the cytoplasmic direction) from the conserved arginine that faces the central hydrated cavity in the proton transporters. Although by no means definitive, these results are positive evidence that the phospholipid may follow a path through a space in the transporter which is homologous to the aqueous cavity that forms the path for the hydrogen ion in the proton transporters
The external surface model
In addition to the question of the pathway for phospholipid headgroup movement during transport, there is the issue of the binding site for the phospholipid that is transported. A specific proposal for such a site was suggested by the crystal structure of SERCA in the E2 form (PDB 2AGV) [14]. In this structure, a phospholipid glycerol backbone is tightly bound in a crevasse between the M4 and M2 transmembrane helices. Several years ago, it was proposed that phospholipid binding at this site was an important part of the reaction cycle in P4 ATPases [12]. The original basis for this proposal was the observation that the binding site in this structure consists primarily of interactions with the glycerol backbone of the phospholipid, echoing the stereospecificity of transport for the glycerol backbone mentioned above. More positive evidence for the importance of this site has now come from a recent mutagenesis study of the yeast transporter Dnf1 [3]. In an exhaustive series of experiments, Baldridge and Graham investigated the basis of headgroup specificity by taking advantage of the fact that Dnf1p, but not Drs2p, transports PC. They found that Tyr616 in Dnf1 is a key residue determining headgroup specificity—substitution of Phe at this position (the amino acid in Drs2p) results in PS transport, while substitution of Tyr at this position in Drs2 abolishes PS transport. Y616 in Dnf1 is equivalent to P314 the Ca-ATPase, one of the residues contacting the bound phospholipid in the 2AGV structure, providing direct evidence that binding of phospholipid at this site plays an important role in the P4 ATPase reaction cycle.
Both the original proposal for the role of this site and the work by Baldridge and Graham included a suggestion that the pathway for phospholipid movement to this site might occur at the membrane-facing surface of the protein. This proposal for the pathway taken by the substrate is quite different from the internal path suggested by the result of Coleman et al. It is not clear how these two views will be reconciled, but until they are, it is valuable to consider some of the implications of the evidence for the existence of a specificity-determining site at this part of the protein, and how these implications conform to a variety of other observations.
Implications of the cytoplasmic-binding site for the reaction cycle
The key feature of the evidence for the role of Y616 in determining specificity is that this site is on the wrong side of the membrane—the cytoplasmic side (red circles, Fig. 1). Absent this evidence, active transport from the lumenal to the cytoplasmic side implies that the substrate concentration on the lumenal side should be low, leading to the expectation that substrate should bind to a high-affinity site on the lumenal side. By analogy with the reaction cycle of the Na/K ATPase, a simple model for the reaction cycle would have binding and occlusion of a phospholipid at this lumenal site in the E2P conformation, leading to dephosphorylation to the E2 form, with associated phospholipid movement to the cytoplasmic surface. The concentration of substrate in this leaflet is high, suggesting that the substrate must be weakly bound so that it can be readily released into the cytoplasmic leaflet of the membrane. Presumably, something very like this process normally occurs during the reaction cycle of the Ca-ATPase as the phospholipid bound to the E2 form is ejected upon transition to the E1 form of the enzyme. But if the phospholipid bound to this site on the cytoplasmic side determines specificity, it cannot be a low affinity site, and proper binding at this site must determine whether the reaction cycle proceeds. The simple model for the reaction cycle is not tenable, and several modifications to the simple model are required.
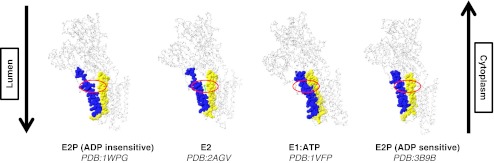
Fig. 1.
Conformations of the TM2 and TM4 transmembrane helices at several steps in the reaction cycle in SERCA. TM2 and TM4 are shown in spacefill: TM2 is rendered yellow while TM4 is rendered blue. The location corresponding to the cytoplasmic phospholipid-binding site in the E2 conformation is circled in red in each case. In the E2 conformation, the phospholipid head group observed in the 2AGV structure is shown in green. The crease between M2 and M4 opens during the E2 ADP-insensitive conformation and remains open during the E2 conformation, but closes during the transition between E2 and E1. The PDB code for each of the SERCA structures are given
The evidence that the specificity of transport is determined by the characteristics of a binding site on the cytoplasmic surface has several implications. First, if the phospholipid substrate is strongly bound at the cytoplasmic site in the E2 conformation, this interaction must be disrupted if the cycle is to proceed to the E1 conformation. Overcoming the energy barrier posed by this dissociation requirement should slow the E2 to E1 transition; in fact, Lenoir and colleagues have now shown that the E2 to E1 transition is the rate-limiting step in the reaction cycle [10]. Second, binding at the cytoplasmic site must be the key to dephosphorylation of the E2P form—if dephosphorylation was coincident with or preceded binding at the cytoplasmic site, the characteristics of that site could not determine the specificity of transport, because the cycle is not readily reversible following dephosphorylation. Third, these considerations do not eliminate the need for high affinity binding on the lumenal side, since that requirement is dictated by the low concentration of substrate in that leaflet. However, phospholipid bound to this lumenal site must readily exchange with the cytoplasmic site in the E2P (ADP-insensitive) form, since otherwise the enzyme would be incapable of dephosphorylation. In fact, Coleman et al observed just such facile phospholipid movement between the cytoplasmic and the lumenal side of the membrane when the ATPase is blocked by mutation at the E2P stage [6].
Ready movement of the phospholipid headgroup from one side of the membrane to the other implies the existence of a hydrophilic path between the two sides in the E2P form of the enzyme. In the E2 form, however, this pathway must be broken or the lumenal site eliminated in order to occlude the substrate at the cytoplasmic surface. This requirement may be the basis for the role of the subunit during the reaction cycle, either because it provides part of the transmembrane hydrophilic pathway, or because it provides part of the lumenal binding site. The dynamic association of the subunit with the transporter mentioned above, which is at its strongest at the E2P step of the cycle, and at its weakest at the E1 step [13], is consistent with such a functional role in the cycle.
The cycle
The key fact in these arguments is the existence of the substrate-binding pocket on the cytoplasmic side of the enzyme. Examining the crystal structures of SERCA during different phases of the transport cycle reveals that this pocket is only transiently present. As SERCA moves from E2 to E1, the pocket is closed, and the clustered residues that form the binding site are dispersed [12]. The pocket remains closed in the ADP-sensitive E2~P form (see Fig. 1, right). However, in the ADP-insensitive E2~P form, where the phospholipid should be bound on the lumenal side and sequestered from the outer leaflet, the triangular gap reappears, formed by the kink in TM4 and TM2. These observations are consistent with the idea that this is the point at which the phospholipid gains access to the cytoplasmic side of the membrane.
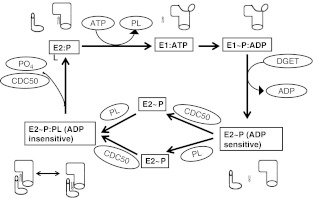
P4 ATPases are unstable in the E1 conformation [13]. However, a direct attempt failed to identify a counterion that might be loaded in the E1 conformation [6]. If there is no counterion, it is likely that formation of E1P, and rearrangement to the E2P conformation is very fast, implying that the enzyme spends the majority of the cycle waiting for phospholipid to bind at the ADP-sensitive E2P form. The overall result is a cycle that can be summarized as shown in Fig. 2.
Fig. 2.
Schematic diagram of the P4-ATPase reaction cycle. Enzyme conformations and complexes are shown in boxes while substrates and binding factors are represented in circles. DGET is the P4 ATPase sequence corresponding to the TGES motif in Ca and Na/K ATPases. Insertion of the DGET/TGES loop into the site vacated by ADP is the key step in the conversion of E1~P to E2~P. Phospholipid and CDC50 subunit both interact with the E2~P conformation, but the binding sequence is not known and so both scenarios are shown. Under physiological conditions, the ATP that donates the phosphate to the E1~P intermediate may be prebound at low affinity in the E2 conformation. In the cartoon figures, the cytoplasmic side is up, and the transmembrane region corresponds to the overlap between the stems of the transporter and subunit
Conclusion
The mechanisms by which P-type ATPases harness ATP hydrolysis to the uphill transport of ions have been greatly illuminated by the combination of reverse genetics and structural analysis. In the main, the resulting conclusions about ATP hydrolysis and enzyme conformation changes are very likely to be general across the entire family. The conclusions that are not general are those about the chemistry of the transported substrate, since these differ across the family. The relevant studies of the molecular mechanisms of phospholipid transport by P4 ATPases have for many years been handicapped by technical difficulties stemming from the physical characteristics of the substrate, the nonavailability of suitable enzyme preparations, and ignorance of important features of the enzyme. In the past year, experiments from a several different laboratories have shown that these roadblocks are beginning to crumble, and the results of the experiments have begun to reveal important idiosyncrasies in the reaction cycle of the P4 subfamily of P-type ATPases. The resulting details of the reaction cycle, diagrammed in Fig. 2, pose many interesting problems which are unresolved, but not unapproachable. We can hope for more such investigations, in anticipation of that day when the atomic details of the structure of this subfamily become revealed.
References
- 1.Albers RW. Biochemical aspects of active transport. Annu Rev Biochem. 1967;36:727–756. doi: 10.1146/annurev.bi.36.070167.003455. [DOI] [PubMed] [Google Scholar]
- 2.Axelsen KB, Palmgren MG. Evolution of substrate specificities in the P-type ATPase superfamily. J Mol Evol. 1998;46(1):84–101. doi: 10.1007/PL00006286. [DOI] [PubMed] [Google Scholar]
- 3.Baldridge RD, Graham TR. Identification of residues defining phospholipid flippase substrate specificity of type IV P-type ATPases. Proc Natl Acad Sci U S A. 2012;109(6):E290–E298. doi: 10.1073/pnas.1115725109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryde S, Hennrich H, Verhulst PM, Devaux PF, Lenoir G, Holthuis JC. CDC50 proteins are critical components of the human class-1 P4-ATPase transport machinery. J Biol Chem. 2010;285(52):40562–40572. doi: 10.1074/jbc.M110.139543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coleman JA, Molday RS. Critical role of the {beta}-subunit CDC50A in the stable expression, assembly, subcellular localization and lipid transport activity of the P4-ATPase ATP8A2. J Biol Chem. 2011;286:17205–17216. doi: 10.1074/jbc.M111.229419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coleman JA, Vestergaard AL, Molday RS, Vilsen B, Peter Andersen J. Critical role of a transmembrane lysine in aminophospholipid transport by mammalian photoreceptor P4-ATPase ATP8A2. Proc Natl Acad Sci U S A. 2012;109(5):1449–1454. doi: 10.1073/pnas.1108862109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding J, Wu Z, Crider BP, Ma Y, Li X, Slaughter C, Gong L, Xie XS. Identification and functional expression of four isoforms of ATPase II, the putative aminophospholipid translocase. Effect of isoform variation on the ATPase activity and phospholipid specificity. J Biol Chem. 2000;275(30):23378–23386. doi: 10.1074/jbc.M910319199. [DOI] [PubMed] [Google Scholar]
- 8.Hall MP, Huestis WH. Phosphatidylserine headgroup diastereomers translocate equivalently across human erythrocyte membranes. Biochim Biophys Acta Biomembr. 1994;1190(2):243–247. doi: 10.1016/0005-2736(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 9.Halleck MS, Pradhan D, Blackman C, Berkes C, Williamson P, Schlegel RA. Multiple members of a third subfamily of P-type ATPases identified by genomic sequences and ESTs. Genome Res. 1998;8(4):354–361. doi: 10.1101/gr.8.4.354. [DOI] [PubMed] [Google Scholar]
- 10.Jacquot A, Montigny C, Hennrich H, Barry R, Maire M, Jaxel C, Holthuis J, Champeil P, Lenoir G. Phosphatidylserine stimulation of Drs2p.Cdc50p lipid translocase dephosphorylation is controlled by phosphatidylinositol-4-phosphate. J Biol Chem. 2012;287(16):13249–13261. doi: 10.1074/jbc.M111.313916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jardetzky O. Simple allosteric model for membrane pumps. Nature. 1966;211(5052):969–970. doi: 10.1038/211969a0. [DOI] [PubMed] [Google Scholar]
- 12.Lenoir G, Williamson P, Holthuis JC. On the origin of lipid asymmetry: the flip side of ion transport. Curr Opin Chem Biol. 2007;11:1–8. doi: 10.1016/j.cbpa.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Lenoir G, Williamson P, Puts CF, Holthuis JC. Cdc50p plays a vital role in the ATPase reaction cycle of the putative aminophospholipid transporter drs2p. J Biol Chem. 2009;284(27):17956–17967. doi: 10.1074/jbc.M109.013722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obara K, Miyashita N, Xu C, Toyoshima I, Sugita Y, Inesi G, Toyoshima C. Structural role of countertransport revealed in Ca(2+) pump crystal structure in the absence of Ca(2+) Proc Natl Acad Sci U S A. 2005;102(41):14489–14496. doi: 10.1073/pnas.0506222102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olesen C, Picard M, Winther AM, Gyrup C, Morth JP, Oxvig C, Moller JV, Nissen P. The structural basis of calcium transport by the calcium pump. Nature. 2007;450(7172):1036–1042. doi: 10.1038/nature06418. [DOI] [PubMed] [Google Scholar]
- 16.Olesen C, Sorensen TL, Nielsen RC, Moller JV, Nissen P. Dephosphorylation of the calcium pump coupled to counterion occlusion. Science. 2004;306(5705):2251–2255. doi: 10.1126/science.1106289. [DOI] [PubMed] [Google Scholar]
- 17.Palmgren MG, Nissen P. P-type ATPases. Annu Rev Biophys. 2011;40:243–266. doi: 10.1146/annurev.biophys.093008.131331. [DOI] [PubMed] [Google Scholar]
- 18.Pedersen BP, Buch-Pedersen MJ, Morth JP, Palmgren MG, Nissen P. Crystal structure of the plasma membrane proton pump. Nature. 2007;450(7172):1111–1114. doi: 10.1038/nature06417. [DOI] [PubMed] [Google Scholar]
- 19.Pomorski T, Holthuis JC, Herrmann A, Meer G. Tracking down lipid flippases and their biological functions. J Cell Sci. 2004;117(Pt 6):805–813. doi: 10.1242/jcs.01055. [DOI] [PubMed] [Google Scholar]
- 20.Pomorski T, Lombardi R, Riezman H, Devaux PF, Meer G, Holthuis JC. Drs2p-related P-type ATPases Dnf1p and Dnf2p are required for phospholipid translocation across the yeast plasma membrane and serve a role in endocytosis. Mol Biol Cell. 2003;14(3):1240–1254. doi: 10.1091/mbc.E02-08-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Post RL, Hegyvary C, Kume S. Activation by adenosine triphosphate in the phosphorylation kinetics of sodium and potassium ion transport adenosine triphosphatase. J Biol Chem. 1972;247(20):6530–6540. [PubMed] [Google Scholar]
- 22.Post RL, Kume S, Tobin T, Orcutt B, Sen AK. Flexibility of an active center in sodium-plus-potassium adenosine triphosphatase. J Gen Physiol. 1969;54(1):306–326. doi: 10.1085/jgp.54.1.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ripmaster TL, Vaughn GP, Woolford JL., Jr DRS1 to DRS7, novel genes required for ribosome assembly and function in Saccharomyces cerevisiae. Mol Cell Biol. 1993;13(12):7901–7912. doi: 10.1128/mcb.13.12.7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saito K, Fujimura-Kamada K, Furuta N, Kato U, Umeda M, Tanaka K. Cdc50p, a protein required for polarized growth, associates with the Drs2p P-type ATPase implicated in phospholipid translocation in Saccharomyces cerevisiae. Mol Biol Cell. 2004;15(7):3418–3432. doi: 10.1091/mbc.E03-11-0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takatsu H, Baba K, Shima T, Umino H, Kato U, Umeda M, Nakayama K, Shin HW. ATP9B, a P4-ATPase (a putative aminophospholipid translocase), localizes to the trans-Golgi network in a CDC50-independent manner. J Biol Chem. 2011;286:38159–38167. doi: 10.1074/jbc.M111.281006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang X, Halleck MS, Schlegel RA, Williamson P. A subfamily of P-type ATPases with aminophospholipid transporting activity. Science. 1996;272(5267):1495–1497. doi: 10.1126/science.272.5267.1495. [DOI] [PubMed] [Google Scholar]
- 27.Toyoshima C, Nomura H, Tsuda T. Lumenal gating mechanism revealed in calcium pump crystal structures with phosphate analogues. Nature. 2004;432(7015):361–368. doi: 10.1038/nature02981. [DOI] [PubMed] [Google Scholar]
- 28.Zachowski A, Henry JP, Devaux PF. Control of transmembrane lipid asymmetry in chromaffin granules by an ATP-dependent protein. Nature. 1989;340(6228):75–76. doi: 10.1038/340075a0. [DOI] [PubMed] [Google Scholar]