Abstract
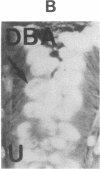
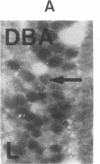
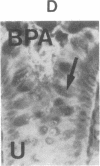
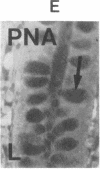
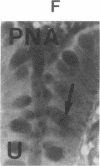
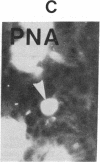
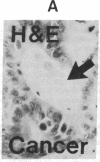
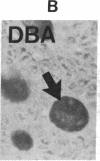
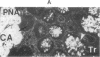
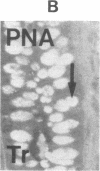
The binding of fluorescein isothiocyanate (FITC)-conjugated lectins to mucin in the human colon was studied by using fluorescence microscopy. In normal mucosa, lectins that preferentially bind to exposed N-acetyl-galactosamine residues (Dolichos biflorus agglutinin and soybean agglutinin) bound selectively to the goblet cell mucin of well-differentiated cells in the upper colonic crypt. By contrast, lectins that require exposed non-reducing galactose residues for binding (Ricinus communis agglutinin1 and Bauhinia purpurea agglutinin) preferentially labeled the mucin of less-differentiated goblet cells located in the lower portion of the colonic crypt. The lectin derived from Arachis hypogaea (peanut agglutinin) has a high affinity for a carbohydrate structure not normally exposed in human tissues. This lectin did not label the goblet cell mucin in the normal colon. However, the mucin was labeled in all 21 colon cancer specimens examined. Additionally, the nonmalignant epithelium immediately adjacent to colon cancer (termed "transitional mucosa") also contained goblet cell mucin that was labeled by FITC-peanut agglutinin. Three conclusions may be drawn from the selective binding characteristics of FITC-lectins to colonic mucins. First, an alteration in the exposed, nonreducing carbohydrate residues occurs in human colonic mucin during the process of goblet cell differentiation. Second, an exposed carbohydrate structure that is not normally present in human tissues is expressed in the mucin produced by malignant colonic epithelium. Third, the presence of the cancer-associated carbohydrate structure in the mucin of transitional mucosa suggests that this tissue may be in the process of early malignant transformation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A. Structure of gastrointestinal mucus glycoproteins and the viscous and gel-forming properties of mucus. Br Med Bull. 1978 Jan;34(1):28–33. [PubMed] [Google Scholar]
- Artzt K., Dubois P., Bennett D., Condamine H., Babinet C., Jacob F. Surface antigens common to mouse cleavage embryos and primitive teratocarcinoma cells in culture. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2988–2992. doi: 10.1073/pnas.70.10.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhours J. F., Glickman R. M. Rat intestinal glycolipids. II. Distribution and biosynthesis of glycolipids and ceramide in villus and crypt cells. Biochim Biophys Acta. 1976 Jul 20;441(1):123–133. doi: 10.1016/0005-2760(76)90287-3. [DOI] [PubMed] [Google Scholar]
- Carlson D. M. Structures and immunochemical properties of oligosaccharides isolated from pig submaxillary mucins. J Biol Chem. 1968 Feb 10;243(3):616–626. [PubMed] [Google Scholar]
- Dawson P. A., Filipe M. I. An ultrastructural and histochemical study of the mucous membrane adjacent to and remote from carcinoma of the colon. Cancer. 1976 May;37(5):2388–2398. doi: 10.1002/1097-0142(197605)37:5<2388::aid-cncr2820370531>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Etzler M. E., Branstrator M. L. Differential localization of cell surface and secretory components in rat intestinal epithelium by use of lectins. J Cell Biol. 1974 Aug;62(2):329–343. doi: 10.1083/jcb.62.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipe M. I., Branfoot A. C. Abnormal patterns of mucus secretion in apparently normal mucosa of large intestine with carcinoma. Cancer. 1974 Aug;34(2):282–290. doi: 10.1002/1097-0142(197408)34:2<282::aid-cncr2820340211>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Fischer K., Poschmann A., Stegner H. E. Nachweis von Mammakarzinom-Zellen mit der Immunfluoreszenztechnik. Dtsch Med Wochenschr. 1977 Aug 26;102(34):1227–1227. doi: 10.1055/s-0028-1105484. [DOI] [PubMed] [Google Scholar]
- Freeman H. J., Etzler M. E., Garrido A. B., Kim Y. S. Alterations in cell surface membrane components of adapting rat small intestinal epithelium. Studies with lectins after massive proximal jejunoileal resection and jejunoileal transposition. Gastroenterology. 1978 Dec;75(6):1066–1072. [PubMed] [Google Scholar]
- Freeman H. J., Lotan R., Kim Y. S. Application of lectins for detection of goblet cell glycoconjugate differences in proximal and distal colon of the rat. Lab Invest. 1980 Apr;42(4):405–412. [PubMed] [Google Scholar]
- Glickman R. M., Bouhours J. F. Characterization, distribution and biosynthesis of the major ganglioside of rat intestinal mucosa. Biochim Biophys Acta. 1976 Jan 22;424(1):17–25. doi: 10.1016/0005-2760(76)90045-x. [DOI] [PubMed] [Google Scholar]
- Gold D., Miller F. Chemical and immunological differences between normal and tumoral colonic mucoprotein antigen. Nature. 1975 May 1;255(5503):85–87. doi: 10.1038/255085a0. [DOI] [PubMed] [Google Scholar]
- Howard D. R., Batsakis J. G. Cytostructural localization of a tumor-associated antigen. Science. 1980 Oct 10;210(4466):201–203. doi: 10.1126/science.6997995. [DOI] [PubMed] [Google Scholar]
- Kim Y. S., Isaacs R., Perdomo J. M. Alterations of membrane glycopeptides in human colonic adenocarcinoma. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4869–4873. doi: 10.1073/pnas.71.12.4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin S., Russell E. C., Blanchard D., McWilliams N. B., Maurer H. M., Mohanakumar T. Receptors for peanut agglutinin (Arachus hypogea) in childhood acute lymphoblastic leukemia: possible clinical significance. Blood. 1980 Jan;55(1):37–39. [PubMed] [Google Scholar]
- Limas C., Lange P. Altered reactivity for A, B, H antigens in transitional cell carcinomas of the urinary bladder. A study of the mechanisms involved. Cancer. 1980 Sep 15;46(6):1366–1373. doi: 10.1002/1097-0142(19800915)46:6<1366::aid-cncr2820460613>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Lipkin M. Phase 1 and phase 2 proliferative lesions of colonic epithelial cells in diseases leading to colonic cancer. Cancer. 1974 Sep;34(3):suppl–suppl:888. doi: 10.1002/1097-0142(197409)34:3+<878::aid-cncr2820340715>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Lotan R., Skutelsky E., Danon D., Sharon N. The purification, composition, and specificity of the anti-T lectin from peanut (Arachis hypogaea). J Biol Chem. 1975 Nov 10;250(21):8518–8523. [PubMed] [Google Scholar]
- Nairn R. C., Fothergill J. E., McEntegart M. G., Richmond H. G. Loss of Gastro-intestinal-specific Antigen in Neoplasia. Br Med J. 1962 Jun 30;1(5295):1791–1793. doi: 10.1136/bmj.1.5295.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman R. A., Klein P. J., Rudland P. S. Binding of peanut lectin to breast epithelium, human carcinomas, and a cultured rat mammary stem cell: use of the lectin as a marker of mammary differentiation. J Natl Cancer Inst. 1979 Dec;63(6):1339–1346. [PubMed] [Google Scholar]
- Reisner Y., Gachelin G., Dubois P., Nicolas J. F., Sharon N., Jacob F. Interaction of peanut agglutinin, a lectin specific for nonreducing terminal D-galactosyl residues, with embryonal carcinoma cells. Dev Biol. 1977 Nov;61(1):20–27. doi: 10.1016/0012-1606(77)90338-4. [DOI] [PubMed] [Google Scholar]
- Reisner Y., Sharon N., Haran-Ghera N. Expression of peanut agglutinin receptors on virus-induced preleukemic cells in mice. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2244–2246. doi: 10.1073/pnas.77.4.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell R. H., Levin B. Ultrastructure of the "transitional" mucosa adjacent to large bowel carcinoma. Cancer. 1977 Nov;40(5 Suppl):2509–2522. doi: 10.1002/1097-0142(197711)40:5+<2509::aid-cncr2820400918>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Springer G. F., Desai P. R., Banatwala I. Blood group MN antigens and precursors in normal and malignant human breast glandular tissue. J Natl Cancer Inst. 1975 Feb;54(2):335–339. [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem. 1973 Apr 10;248(7):2536–2541. [PubMed] [Google Scholar]