Abstract
Phosphate is one of the most abundant minerals in the body, and its serum levels are regulated by a complex set of processes occurring in the intestine, skeleton, and kidneys. The currently known main regulators of phosphate homeostasis include parathyroid hormone (PTH), calcitriol, and a number of peptides collectively known as the “phosphatonins” of which fibroblast growth factor-23 (FGF-23) has been best defined. Maintenance of extracellular and intracellular phosphate levels within a narrow range is important for many biological processes, including energy metabolism, cell signaling, regulation of protein synthesis, skeletal development, and bone integrity. The presence of adequate amounts of phosphate is critical for the process of apoptosis of mature chondrocytes in the growth plate. Without the presence of this mineral in high enough quantities, chondrocytes will not go into apoptosis, and the normal physiological chain of events that includes invasion of blood vessels and the generation of new bone will be blocked, resulting in rickets and delayed growth. In the rest of the skeleton, hypophosphatemia will result in osteomalacia due to an insufficient formation of hydroxyapatite. This review will address phosphate metabolism and its role in bone health.
Keywords: Phosphate, Vitamin D, FGF-23, Parathyroid hormone, Growth plate, Chondrocytes
Introduction
Maintaining physiological phosphate balance is of crucial biological importance for bone health. Body phosphate homeostasis is determined by modulation of intestinal uptake of dietary phosphate, renal phosphate reabsorption and excretion, and the exchange of phosphate between extracellular and bone storage pools [1]. From the physiology standpoint, recent knowledge has added phosphate transporters in the gut, bone, and kidney, as well as “phosphatonins” to the regulators of phosphate homeostasis [2–7]. From the pathophysiology angle, bench and clinical research has showed that phosphate is not a secondary player, but rather one of the major factors in the maintenance of bone health, and its deficiency results in bone pathology and clinical illness [1, 8, 9]. This review focuses on phosphate homeostasis and its role in bone health. A following review will address the pathophysiological and clinical implications of hypophosphatemia caused by renal tubular disorders.
Phosphate homeostasis
Phosphorus is an essential element and plays an important role in multiple biological processes [10]. Phosphorus-containing compounds have important roles in cell structure (maintenance of cell membrane integrity and nucleic acids), cellular metabolism (generation of ATP), regulation of subcellular processes (cell signaling through protein phosphorylation of key enzymes), maintenance of acid–base homeostasis (urinary buffering), and bone mineralization [8, 11]. Therefore, the maintenance of appropriate phosphorus homeostasis is critical for the well-being of the organism and for an optimal calcium–phosphate product for the mineralization of bone without its deposition in vascular and other soft tissues. In biological systems, phosphorus is present as phosphate, and these two terms are commonly used interchangeably.
Phosphate is the most abundant anion in the human body and comprises approximately 1 % of total body weight [12]. It is a predominantly intracellular anion where its concentration is 100-fold greater than that in the plasma. The majority of phosphate is present in bone and teeth (85 %), with the remainder distributed between other tissues (14 %) and extracellular fluid (1 %). In the skeleton, phosphate is primarily complexed with calcium in the form of hydroxyapatite crystals; the remaining phosphate appears as amorphous calcium phosphate [12]. In soft tissue and cell membranes, phosphorus exists mainly as phosphate esters and to a lesser extent as phosphoproteins and free phosphate ions. In the extracellular fluid, about one-tenth of the phosphorus content is bound to proteins, one-third is complexed to sodium, calcium, and magnesium, and the remainder is present as inorganic phosphate [11]. Serum phosphate concentration varies with age, with the highest concentration being in infants [normal range 4.5–8.3 mg/dL (1.50–2.65 mmol/L, conversion factor 0.322], who require more of the mineral for bone growth and soft tissue buildup, and concentrations declining towards adulthood [normal range 2.5–4.5 mg/dL (0.8–1.5 mmol/L)] [10–14]. Accordingly, in both the intestinal tract and the kidney, there is an age-related decline in phosphate absorption and reabsorption, respectively, that is correlated with decreased gene and protein expression of sodium–phosphate co-transporters [15].
In human adults, under steady state conditions, a regular Western diet provides between 1000 and 1,600 mg/day (approx. 20 mg/kg/day) of phosphorus [16]. Of this, approximately 16 mg/kg/day is absorbed in the proximal intestine, predominantly in the jejunum. Approximately 3 mg/kg/day is secreted into the intestine via pancreatic, bile, and intestinal secretions, giving a net phosphorus absorption of approximately 13 mg/kg/day, while 7 mg/kg/day appear in the feces. The absorbed phosphorus enters the extracellular fluid pool and moves in and out of bone as needed (approx. 3 mg/kg/day). The rate of bone remodeling is important in determining the concentration of plasma phosphorus, as disproportionate increased bone resorption will lead to a higher plasma phosphorus concentration whereas increased mineralization will lead to a lower one [10]. An example of the latter is the condition termed “hungry bones”, which follows parathyroidectomy in patients with secondary hyperparathyroidism. Unlike plasma calcium, which is partially bound to proteins and hence only filtered in part, plasma phosphorus is filtered almost completely and enters the tubular fluid in approximately the same concentrations as those present in plasma [10]. In the adult at zero metabolic balance, the amount of phosphorus excreted in the urine is equivalent to that absorbed in the intestine, namely about 13 mg/kg/day (approximating 900 mg). Thus, in states of metabolic phosphate equilibrium and normal renal function, the amount of phosphorus appearing in the urine can serve as a rough approximation of the amount absorbed in the intestine. In particular, in states of phosphate deprivation resulting from an inadequate intake of phosphorus or low absorption of phosphorus from the intestine, urinary phosphorus amounts are low and may serve as indicators of altered phosphorus regulation [10].
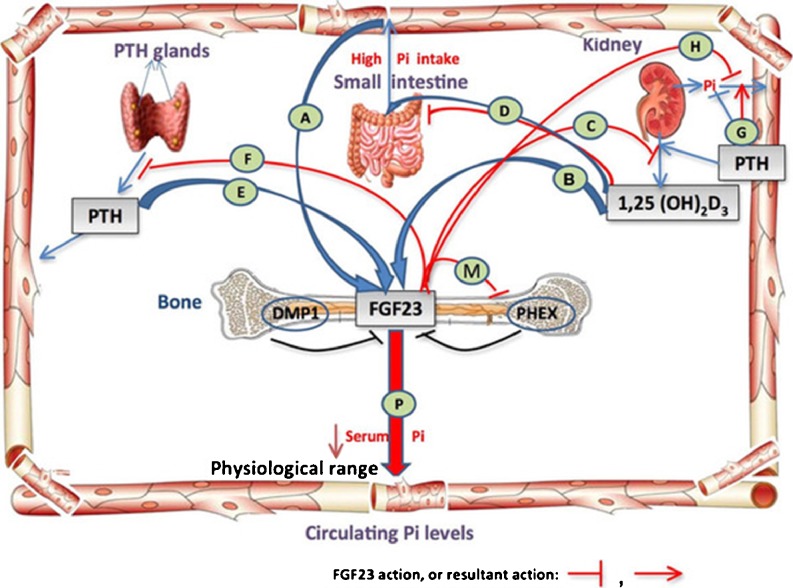
Until not too long ago, only three main regulators of phosphate metabolism had been identified: (1) dietary phosphate intake and absorption, (2) calcitriol, which increases phosphate absorption from the gut and bone, and (3) parathyroid hormone (PTH) which directly causes phosphate resorption from bone and decreases its reabsorption in the proximal tubule, and indirectly by stimulating the production of calcitriol. However, more recent findings have also demonstrated the physiological importance of bone and phosphatonins, such as fibroblast growth factor-23 (FGF-23) in phosphate regulation (see section Hormones involved in phosphate homeostasis) (Fig. 1) [10, 14, 17].
Fig. 1.
Regulatory mechanisms of phosphate homeostasis. A–H Intestine–bone–kidney–parathyroid axis. In the intestine, phosphate is actively absorbed through the cells or passively through the paracellular pathway. Its serum concentration is tightly regulated by parathyroid hormone (PTH), bone, and kidney. PTH stimulates phosphate excretion and calcitriol synthesis in the kidney; in turn, low phosphate and calcitriol directly inhibit PTH production. In the bone, PTH may stimulate fibroblast growth factor-23 (FGF-23) production and increase phosphate release following an increase in bone resorption. In the kidney, FGF-23 suppresses phosphate reabsorption and the synthesis of 1α, 25-dihydroxyvitamin D [1,25(OH)2D3], and in the parathyroid glands FGF23 suppresses the production and secretion of PTH. FGF23 is negatively regulated by bone signaling mechanisms (DMP1 and PHEX) and is positively regulated by systemic factors [serum inorganic phosphate (Pi), 1,25(OH)2D3, PTH (?)]. (Diagram is modified with permission from R. Sapir-Koren and G. Livshits: Bone mineralization and regulation of phosphate homeostasis. IBMS BoneKEy 8:286–300, 2011)
The gastrointestinal–bone–renal axis
Intestinal phosphate absorption
Phosphate is ubiquitous and present in all natural foods. All foods composed of animal or plant cells are rich in phosphate, with the major sources being protein-rich foods and cereal grains. Milk and its products are the richest sources of phosphate in the diet. Other good sources of phosphate are meat, fish, poultry eggs, and peanuts. Phosphate interacts with several dietary minerals, such as calcium, sodium, and magnesium. For example, an increase in dietary magnesium results in a decrease in phosphate absorption, whereas an adequate luminal concentration of sodium is essential to ensure phosphate absorption.
Dietary phosphate, 1α, 25-dihydroxyvitamin D (1,25(OH)2D3), and PTH are thought to be the most important physiological regulators of intestinal phosphate absorption, although epidermal growth factor (EGF) [18], glucocorticoids [19], estrogens [20], metabolic acidosis [21] and, as more recently shown, phosphatonins [10] and secreted frizzled related protein-4 (sFRP-4) also affect intestinal phosphate absorption [12].
Intestinal phosphorus absorption occurs through both cellular and paracellular pathways. Phosphate ingested through the diet is absorbed by the epithelium of the small intestine (duodenum and jejunum) via both a passive diffusional, load-dependent process and an active sodium-dependent process [8]. While the former depends on the amount of phosphorus in the gut, the latter is increased by 1,25(OH)2D3. In a feedback loop, 1,25(OH)2D3 is regulated by serum phosphate such that a decrease in the latter’s concentration leads to an increase in the synthesis of the hormone [10].
At the sub-cellular level, phosphate absorption is thought to depend on the function of sodium-dependent phosphate transporters that are members of the solute carrier family SLC34 [22], which include three type II co-transporters: NaPiIIa (SLC34A1), NaPiIIb (SLC34A2), and NaPiIIc (SLC34A3). NaPiIIa and NaPiIIc are primarily expressed in the apical brush border membrane of the renal proximal tubule and are central to the process of renal phosphate reabsorption (see below). The transporter primarily expressed in the small intestine is NaPiIIb (Fig. 2) and is regulated by 1,25(OH)2D3. NaPiIIb has a low affinity for phosphate, and humans with NaPiIIb-inactivating mutations do not have reduced serum phosphate levels [22]. Indeed, studies in NaPiIIb null mice have demonstrated that while the transporter has a central role in active intestinal phosphate absorption, its deletion results in a reciprocal increase in the expression of NaPiIIa in the renal proximal tubule and, consequently, the serum phosphate concentration remains normal. Changes in intestinal phosphate absorption may, therefore, affect renal phosphate handling, perhaps indirectly through alterations in serum phosphate concentration, or possibly through the production of intestinally derived circulating peptides or by FGF-23 or other phosphatonins (see below) [2].
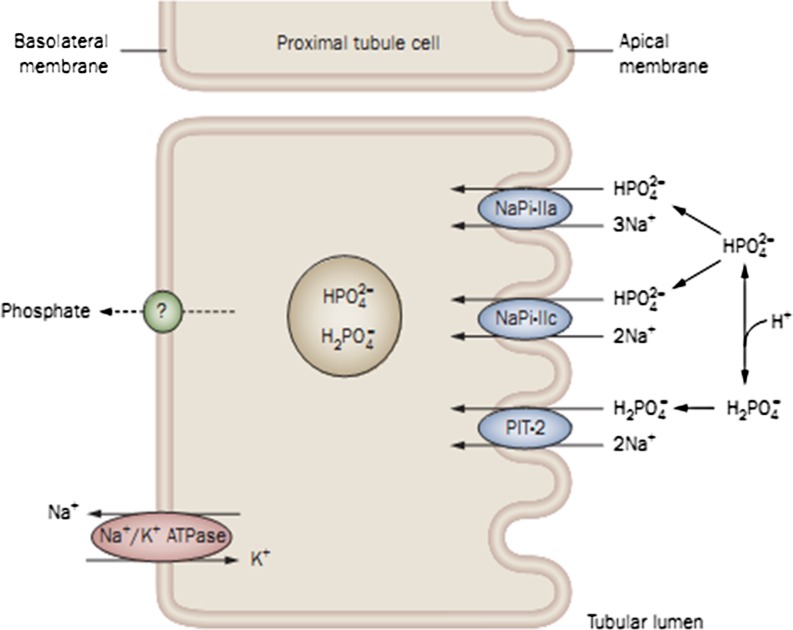
Fig. 2.
Phosphate transport in the intestine. The sodium-dependent phosphate transporters of the NaPi-IIb type are present at the luminal surface of the enterocyte (brush border membrane). NaPi-IIb transporters are electrogenic and have high affinity for inorganic phosphate (Pi). Energy for this transport process is provided by an inward downhill sodium gradient, maintained by transport of Na+ from the cell via a Na+/K+ ATPase cotransporter at the basolateral membrane. The phosphate incorporated into the enterocytes by this mechanism is transferred to the circulation by poorly understood mechanisms. Phosphate absorption also occurs via a sodium-independent process(es), such as diffusional movement across the intercellular spaces in the intestine. (Adapted from T.O. Carpentar: Chapter 10: Primary disorders of phosphate Mmtabolism, 2010, with permission from ENDOTEXT. Reprinted from www.endotext.org)
The current view is that, unlike the direct control by PTH of NaPiIIa in the kidney, PTH does not regulate NaPiIIb expression in the gut directly, but affects it indirectly by its stimulatory effect on 1,25(OH)2D3 synthesis, which enhances NaPiIIb protein expression [1]. However, the finding that upregulation of NaPiIIb in response to a low phosphate diet occurs in vitamin D receptor null mice, as well as in 1-OHase-deficient mice suggests that diet-related alterations in intestinal phosphate absorption can occur independently of changes in 1,25(OH)2D3 [23, 24]. As discussed below, this phenomenon may be explained, at least in part, by changes in circulating levels of FGF-23, which also affect phosphate transporters in the gut.
Bone
Crucial to the activity of osteoblasts and osteocytes in the process of matrix mineralization is the maintenance of adequate inorganic phosphorus levels. Inorganic phosphorus is one of the two main ionic components required for hydroxyapatite formation during the mineralization of the extracellular matrix [25]. The enzymatic activity of alkaline phosphatase is required to generate enough of the free mineral. This enzyme is located at the plasma membrane with its catalytic unit oriented outside. The enzyme cleaves phosphorus from beta-glycerol phosphate, and the free mineral enters the cell via a sodium-dependent phosphate transporter and is maintained intracellularly within a narrow range. In cases of hypophosphatemia, alkaline phosphatase activity rises in order to try to provide more phosphate to the bone cells. Once adequate amounts of phosphate are provided, the enzyme’s activity decreases; as such, it is a good indictor of phosphate homeostasis within the bone [9]. Monitoring the activity of bone-specific alkaline phosphatase may therefore provide more precise information about bone metabolism than can be obtained by measuring total alkaline phosphatase activity.
A function of FGF-23 is to protect the cells from too high levels of phosphorus, which negatively impact osteoblasts and practically result in cell death. Although numerous observations have shown the association between increased dietary phosphate load and concomitant high serum phosphate, due to either increased intake or decreased clearance (as in renal failure) and increased production of FGF-23, the precise mechanism of this association is yet unknown [2, 4, 7, 26].
Renal phosphate reabsorption
The kidney is the major organ involved in the regulation of minute-to-minute phosphate homeostasis. Renal handling of phosphate is regulated by a variety of hormonal and non-hormonal factors along the proximal convoluted and straight tubule of the kidney, including serum PTH, calcium, 1,25(OH)2D3 and bicarbonate concentrations, sodium reabsorption, hypercapnia or hypocapnia, dopamine, and serotonin [12]. In animals with intact parathyroid glands, phosphate reabsorption along the proximal convoluted tubule exceeds fluid reabsorption, such that the phosphate concentration in the proximal tubules is 70 % of the plasma level. There is little phosphate reabsorption in the proximal straight tubule in the presence of PTH. However, in the absence of PTH, phosphate is avidly reabsorbed along the proximal straight tubule, resulting in very low urinary excretion, and its concentration in this tubule is 30 % of that in plasma [12]. Changes in the urinary excretion of phosphate are almost invariably mirrored by changes in the apical expression of NaPiIIa and NaPiIIc in the proximal tubules [2, 12].
Phosphate transport across the renal proximal tubular cell is largely unidirectional and involves uptake across the brush border membrane, translocation across the cell, and efflux at the basolateral membrane. The overall rate-limiting step in the reabsorptive process is phosphate uptake at the apical membrane; consequently, this cell surface is a major site of phosphate regulation where type II transporters, NaPiIIa, and NaPiIIc, are expressed and collectively account for the reabsorption of 80 % of filtered phosphate. NaPiIIa has been shown to be localized throughout the proximal tubule (S1–S3 segments), with the highest protein levels found in the S1 segment (Fig. 3) [16]. PTH, FGF-23, and dietary phosphate are considered to be the major regulators of NaPiIIa protein levels. Border brush membrane expression of NaPiIIa is reduced within minutes in response to PTH [27] and within 2 h in response to altered dietary phosphate load [28]. This adaptation occurs through activation of molecular motifs within the protein that are responsible for its endocytosis or exocytosis, together with alterations in protein–protein interactions that stabilize the protein at the brush border membrane [29]. In mice, NaPiIIa is responsible for approximately 70 % of active phosphate transport.
Fig. 3.
Phosphate transcellular transport in the proximal tubule. Phosphate movement from the renal tubule fluid to the peritubular capillary blood indicates that phosphate reabsorption is principally a unidirectional process that proceeds by a transcellular mechanism. Phosphate enters the tubular cell across the luminal membrane via a saturable, active, and sodium-dependent transport system. The rate of phosphate transport is dependent on the abundance of transporters functioning in the membrane and the magnitude of the Na+ gradient maintained across the luminal membrane, with the latter depending on the Na+/ATPase or sodium pump on the basolateral membrane. The rate-limiting step in phosphate transcellular transport is likely the Na+-dependent entry of inorganic phosphate (Pi) across the luminal membrane. Several phosphate transport systems have been postulated to explain the still poorly understood transport of phosphate across the basolateral membrane, including Na+–Pi cotransport via type III Na –Pi cotransporters, passive diffusion, and anion exchange. (Figure is adapted from Naderi and Reilly [11] and reprinted with permission from Macmillan Publishers)
NaPiIIc protein is highly expressed in rodents during weaning, with levels diminishing with age, and this protein is only present in the S1 segment of the proximal tubule [30]. The expression of NaPiIIc protein in adult rodents is regulated by dietary phosphate, dietary magnesium, metabolic acidosis, and FGF-23 [1, 23]; however, there are conflicting reports on the role of PTH in NaPiIIc regulation [31]. Changes in NaPiIIc protein levels in response to dietary phosphate occur over a longer time course than those in NaPiIIa [28], and the cellular mechanisms of internalization of the transporter are different [32]. NaPiIIc may account for 30 % of renal phosphate reabsorption [1], and studies have shown that this protein plays a minor role in phosphate homeostasis in rodents [33] (Fig. 3). In humans, NaPiIIc might have a larger role in renal phosphate reabsorption, as inactivating mutations in the gene encoding NaPiIIc lead to hypophosphatemic syndromes (see below) [34].
Even in the absence of type II transporters, there is still residual renal phosphate reabsorption [33]. Thus, other phosphate transporters are able to maintain some phosphate reabsorption. PiT2 protein, a member of the SLC20 family and a type III transporter, has been localized in the kidney at the brush border membrane, and its abundance is regulated by dietary phosphate [28]. Under normal phosphate conditions the protein is restricted to the S1 segment, whereas dietary phosphate restriction induces expression of PiT2 protein in all segments of the proximal tubule. This type III transporter adapts to dietary phosphate composition more slowly than the type IIa transporters [28], and the involvement of PiT2 in phosphate uptake can vary with pH, making a modest contribution to renal phosphate reabsorption [35].
Insulin enhances proximal tubule phosphate reabsorption by stimulating brush–border membrane Na–Pi cotransport and prevents the phosphaturic action of PTH. Growth hormone stimulates proximal tubule Na–Pi cotransport, an effect that is partially mediated by insulin-like growth factor 1 (IGF1). Epidermal growth factor stimulates phosphate reabsorption in perfused proximal tubules, but inhibits phosphate transport in opossum kidney cells. Thyroid hormone increases proximal tubule phosphate reabsorption by specifically enhancing brush–border membrane Na–Pi cotransport. Calcitonin and glucocorticoids inhibit proximal tubule brush–border reabsorption [11, 12]. Several phosphatonins, including FGF-23, FRP-4, and matrix extracellular phosphoglycoprotein (MEPE), which are associated with different pathophysiological states of renal phosphate handling also influence apical expression of NaPiIIa [27]. Reduced or increased phosphate intake results in the increased or decreased expression of NaPiIIa, respectively.
During periods of rapid growth and development, as in infancy and puberty, a positive phosphate balance is established. Thus, the ratio of phosphorus tubule maximum to glomerular filtration rate ()TP/GFR) is higher in infants than in older children and adults [1]. At least part of this phenomenon can be attributed to the effect of growth hormone on phosphate reabsorption.
Hormones involved in phosphate homeostasis
Parathyroid hormone
The primary function of PTH is to tightly regulate serum calcium concentration. In this regard, hypocalcemia stimulates the parathyroid glands to produce and release the hormone. PTH increases proximal tubular expression of 25(OH)D 1α-hydroxylase, resulting in increased production of 1,25(OH)2D3 and, consequently, increased calcium absorption in the gut. PTH also enhances calcium reabsorption in the distal convoluted tubule [12]. In the bone, PTH stimulates the release of calcium into the extracellular fluid by increasing osteoclastic bone resorption. In addition to its effects on calcium, PTH is one of the best characterized hormonal regulators of plasma phosphate concentration. In vitro or in vivo delivery of PTH results in reduced expression of NaPiIIa and NaPiIIc in the apical membrane of the proximal tubule [27]. This effect results from the relatively rapid internalization and subsequent degradation of NaPiIIa and NaPiIIc proteins, a process that in the short term occurs independently of the regulation of the expression of the co-transporters at the transcriptional level. The “bottom line” here is that the action of PTH on tubular phosphate handling is immediate (see below). The end result of the presence of long-standing high PTH concentration in the setting of normal kidney function is a decrease in serum phosphate, which in certain disease conditions may lead to rickets and osteomalacia.
Experiments have defined the FGF-23 signal transduction in the parathyroid gland as well as its effect on PTH secretion, showing that FGF-23 acts on the receptor complex in the parathyroid glands to decrease PTH gene expression and that PTH secretion levels out over the short term through activation of the MAPK pathway [36]. The ability of FGF-23 to reduce serum PTH levels may further enhance the activity of FGF-23 as a counter-regulatory hormone for 1,25(OH)2D3 and contribute to a long negative feedback loop involving bone, kidney, and parathyroid gland [37].
Calcitriol
Whereas PTH is one of the strongest stimulators of 1,25(OH)2D3 production, the latter acts to suppress PTH. Thus, 1,25(OH)2D3 not only largely regulates serum phosphate concentration directly by increasing its intestinal absorption but also indirectly by increasing its tubular reabsorption through its suppression of PTH. The opposing effects of PTH and vitamin D on the kidney and the intestine, respectively, balance phosphate levels while preserving calcium ion homeostasis. These two regulators are tightly intertwined with FGF-23 physiology, as discussed below.
Fibroblast growth factor-23
The phosphatonins are a group of proteins that have emerged as novel candidates in the regulation of phosphate homeostasis. The phosphatonins of current interest are FGF-23, FGF-7, sFRP-4, and MEPE [2, 10]. All have been shown to induce hypophosphatemia through a reduction in NaPiIIa protein expression, causing increased renal phosphate excretion, and they may have a role in the pathogenesis of some disorders of renal phosphate wasting [2]. These proteins inhibit renal phosphate reabsorption in vitro and in vivo; however, only FGF-23 and sFRP-4 are considered to play an important role in inhibiting the synthesis of 1,25(OH)2D3 [2] (Fig. 4).
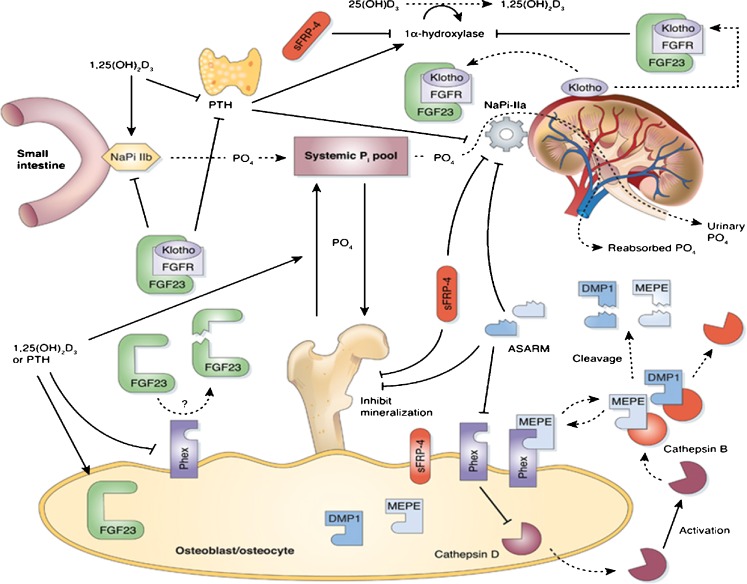
Fig. 4.
Overall picture of the physiology of FGF-23 and its role in phosphate homeostasis. FGF-23 inhibits renal Pi reabsorption by reducing the apical expression and activity of NaPi-IIa in the proximal tubule epithelium. FGF-23 also reduces intestinal absorption of dietary Pi through a vitamin D receptor (VDR)-dependent decrease in NaPi-IIb activity. The latter phenomenon is most likely secondary to FGF-23-mediated reduction of circulating 1,25(OH)2D3 synthesis through suppression of 1α(OH)ase expression in the kidney. The proteins required for mineralization [osteocalcin, bone sialoprotein, vitronectin, and dentin matrix protein 1 (DMP-1)] are in dynamic balance with the ASARM peptide, an inhibitor of mineralization. In vitro, data indicates that PHEX-sequestered matrix extracellular phosphoglycoprotein (MEPE) is protected from matrix proteases. The PHEX sequestration is likely reversible, and free unbound-MEPE is vulnerable to cleavage by extracellular matrix proteases/cathepsin B. Cleavage of MEPE would result in the release of the protease-resistant stable ASARM peptide (aspartic acid-rich motif). Free ASARM peptide may also sterically inhibit the NPT2 co-transporter by direct binding. PHEX is expressed in the parathyroid glands and may have a role in the normal regulation of PTH. The full-length uncleaved FGF-23 molecule may indirectly/directly downregulate the proteins required for mineralization. Thus, FGF-23 and/or other cytokines are likely directly/indirectly responsible for the changes in expression of these proteins. FGF-23 indirectly or directly suppresses expression of the NPT2 phosphate co-transporter, suppresses expression of 1α-hydroxylase, and increases expression of 24-hydroxylase [decrease in 1,25(OH)2D3]. FGF-23 and Klotho might function in a common single transduction pathway. (Modified from R. Pawel et al.: Recent advances in the renal–skeletal–gut axis that controls phosphate homeostasis. Lab Invest 89:7–14, 2009. Reprinted by permission from Macmillian Publishers)
FGF-23 is predominantly expressed in bone, but it is also expressed in a variety of human and mouse tissues, including brain, thymus, small intestine, heart, lung, liver, kidney, thyroid/parathyroid, lymph node, skeletal muscle, spleen, skin, stomach, and testis [6, 34, 38, 39]. Studies have shown that FGF-23 can also cause effects in these different tissues [40]. In bone, FGF-23 is predominantly generated by osteocytes, and to a lesser extent by various cells of the osteoblast lineage. In this context, the osteocyte lacuno–canalicular system should be viewed as an endocrine organ regulating phosphate metabolism [17]. By downregulating the sodium phosphate co-transporters in the proximal tubule, FGF-23 causes a decrease in phosphate reabsorption, thus causing phosphaturia and hypophosphatemia. By inhibiting the activity of renal 1α-hydroxylase and stimulating that of 24-hydroxylase, it diminishes the production of calcitriol and increases its catabolism, respectively, resulting in low serum levels of the active vitamin D metabolite [8]. In addition, FGF-23 inhibits the secretion of PTH [14]. However, it is important to remember that the main regulator of PTH secretion continues to be serumionized calcium.
Receptors to FGF-23 are present in many tissues, and its actions have recently been well described by Gattineni and Baum [7]. However, FGF-23 downstream signaling has been detected in only a very restricted number of tissues, including the kidney (the distal convoluted tubules), parathyroid glands, and the brain (the epithelium of the choroid plexus) [6]. The reason for strict localization of signaling is that in order to exert its effect on its receptor, FGF-23 has to also have its essential cofactor Klotho present. This cofactor is a transmembrane protein that is highly expressed in the kidney and, to a lesser extent, in the parathyroid glands and brain, and it serves as an obligate co-receptor, enabling FGF-23 to interact with its receptor, dictating which tissues will respond to FGF-23 [14, 37]. Studies in animals have shown that in the case of abnormal Klotho, the animal behaves as if it is deficient in FGF-23, exhibiting high serum phosphate and calcitriol levels [41]. Furthermore, animals will not manifest the effects of FGF-23—even in the face of high serum levels of the hormone—if at the same time the animal is deficient in Klotho [42].
FGF-23 is a 251-amino acid glycoprotein. It fulfils the criteria to be defined as a hormone and under normal physiological conditions it is in charge of maintaining phosphate balance, mainly by adjusting the body response to dietary phosphate load. Its N-terminal peptide binds to its receptor in the tissues and its C-terminal binds to Klotho; thus, both the N and C terminals are participants in the hormone’s activity [14].
As previously noted, the main effect of FGF-23 is on phosphate metabolism and the maintenance of phosphate homeostasis. Increased oral phosphate intake results in increased secretion and higher serum levels of FGF-23, causing increased phosphaturia and decreased calcitriol production. With higher blood levels of FGF-23, there will also be decreased absorption of phosphate from the gut. Alternatively, dietary phosphate restriction will result in lower blood levels of FGF-23 [26]. The mechanism by which dietary phosphate affects FGF-23 levels is as yet unknown. Studies in both research animals and humans show that both changes in dietary phosphate load and in serum phosphate induced by dietary intake have a direct stimulatory effect on FGF-23 secretion, as do high levels of calcitriol [7]. The latter creates a negative feedback mechanism by which calcitriol stimulates FGF-23, which in turn suppresses calcitriol production. In short, FGF-23 will increase phosphate renal losses, whereas calcitriol increases its absorption from the gut and bone.
In terms of phosphate homeostasis, the interactions between FGF-23, calcitriol, and PTH are quite complex. FGF-23 suppresses calcitriol and PTH production, calcitriol stimulates FGF-23 and suppresses PTH, and PTH stimulates calcitriol production. It is not fully clear whether PTH directly stimulates FGF-23 production or does it indirectly by the increased production of calcitriol. While studies have shown that the latter mechanism is the most likely [43], some bench research provides support for the former possibility [6, 36].
The effect of FGF-23 on proximal tubular phosphate reabsorption is independent of PTH and calcitriol [43]. Both PTH and FGF-23 stimulate phosphaturia; however, it appears that the effect of PTH is immediate, whereas that of FGF-23 requires more time, likely several hours. Whereas PTH promotes the production of calcitriol, FGF-23 suppresses it. The ability of FGF-23 to suppress PTH production may further contribute to decreased calcitriol synthesis.
Studies of FGF-23 deficiency in animal models and cell culture suggest that FGF-23, and the proteins that regulate FGF-23, have a direct effect on bone [44]. In these models, FGF-23 appears to directly regulate osteoblast differentiation [44], while a complete lack of the FGF-23 protein impairs skeletal mineralization despite adequate (even excessive) circulating levels of phosphorus and vitamin D. Although the mechanisms by which these effects on bone are mediated are unknown, they may involve a number of proteins that have been shown to regulate both FGF-23 levels and skeletal mineralization [17].
It is thus evident that FGF-23, calcitriol, and PTH are all involved in mineral metabolism in the gut, bone, kidney, and parathyroid gland, with the ultimate goal of maintaining calcium and phosphate homeostasis [43]. Conceptually, whereas most of the hormonal system associated with calcium metabolism is directed at raising its serum concentration, hormones involved in phosphate homeostasis are mostly aimed at keeping its serum level low. As such, both PTH and FGF-23 exert their effect at lowering serum phosphate, but whereas the response of the renal tubule to PTH is instantaneous, the one to FGF-23 requires a longer time, measured in hours and days.
Role of phosphate in the growth plate: physiology and pathophysiology
The epiphyseal growth plate has a crucial role in bone growth. Growth plate thickness is determined by two opposing processes: chondrocyte proliferation and hypertrophy on one hand and chondrocyte apoptosis and vascular invasion of the growth plate followed by conversion into primary bone spongiosa on the other [45]. Vascular invasion requires mineralization of the growth plate cartilage and is delayed or prevented by calcium or phosphorus deficiencies [45]. The growth plate is bounded by the epiphysis on one side and the metaphysis on the other. There are three zones in the cartilaginous component: reserve or germinal, proliferative, and hypertrophic. Only the reserve layer has a vascular supply. Cell division and enlargement along with matrix production occur in the proliferative zone, which is characterized by linear columns of chondrocytes. This area is metabolically active, and there is a complex regulatory loop for the conversion of proliferative cells to hypertrophic cells. The cells enlarge by five- to tenfold in the hypertrophic zone [45]. The growth plate hypertrophic layer is divided into upper (early) and lower (late) hypertrophic zones, and the terminal cell of the lower calcified hypertrophic zone undergoes apoptosis [9].
Terminal differentiation of hypertrophic chondrocytes is characterized by the expression of signaling molecules that promote vascular invasion, apoptosis, and replacement of hypertrophic chondrocytes by osteoblasts that lay down the primary spongiosa of bone. Aberrant regulation of this developmental process results in growth plate disorders [46]. Under the latter circumstances, growth plate cartilage continues to accumulate and the growth plate thickens. The chondrocytes of the growth plate become disorganized, losing their regular straight-columned orientation. In the bone below the growth plate (metaphysis), the mineralization defect leads to the accumulation of osteoid. This growth plate abnormality observed in growing animals and humans is called rickets. Although calcium has been shown to play an important role in regulating chondrocyte maturation [46], apoptosis of terminally differentiated hypertrophic chondrocytes is dependent upon normal levels of circulating phosphate [47]. Studies in both humans and mice have demonstrated that the preservation of normal mineral ion homeostasis can compensate for the absence of 1,25(OH)2D3 or functional vitamin D receptors, resulting in a normal growth plate phenotype. These studies raised the question as to whether normalization of serum calcium, phosphate, or both is required to prevent the rachitic phenotype [48, 49].
The apoptosis of epiphyseal chondrocytes is multifactorial and both provides a mechanism for the removal of terminally differentiated cells from cartilage columns and promotes invasion of the vascular elements of bone marrow, for the generation of new bone [9]. This apoptosis process is defective in rickets, and the availability of phosphorus is essential for proper apoptosis of the terminal hypertrophic cell. The observation that inhibition of phosphate transport prevents phosphate-mediated apoptosis in hypertrophic chondrocytes [25] further reinforces the notion that circulating phosphate, rather than the presence of local mineralized matrix, is a key determinant of hypertrophic chondrocyte apoptosis [50].
The prevention of rickets in patients with VDR mutations and in VDR knock-out mice administered high amounts of dietary calcium demonstrates that neither vitamin D nor the VDR is required for normal growth plate maturation and that rickets develops due to impaired mineral ion homeostasis [9]. Furthermore, apoptosis in VDR null mice was rescued by restoring phosphorus serum levels without any correction of serum calcium levels [9]. Similar studies on Hyp mice, a model of X-linked hypophosphatemia, and on mice that were weaned onto a phosphorus-restricted/high-calcium diet revealed the same findings observed in VDR null mice—namely, healing of the rickets with phosphate supplementation alone. Prior to treatment, hypophosphatemia was associated with a decrease in the number of apoptotic hypertrophic chondrocytes and expansion of the growth plate, indicating that the histological findings observed are not unique to VDR null mice, but rather a general phenomenon coincident with hypophosphatemia [47].
It is important to remember that in addition to the growth plate, the rest of the skeleton is also affected by hypophosphatemia, resulting in osteomalacia, i.e., undermineralized soft bones. In the child, both rickets and osteomalacia will be manifested, whereas the adult will have only osteomalacia. Thus, craniotabes in the infant, bowed legs in the older child, and bone pain and fractures in the adult are all clinical manifestations of osteomalacia.
Conclusions
Whereas for many generations physicians were taught to think of rickets as a calcium disorder, evidence in recent years points to phosphate as the main culprit. The presence of phosphate is crucial for bone growth and mineralization and in its absence in sufficient amounts, rickets and osteomalacia will develop. The maintenance of optimal phosphate balance is managed by complex interactions between the gut, kidney, and bone, involving multiple regulators. However, the overall picture is still not fully clear due to, among other things, the fact that up to the present no “phosphate sensing receptor” has been found. Future studies will hopefully provide additional insight into phosphate homeostasis.
Questions (answers are provided following the reference list)
Phosphate transport in the intestine is accomplished by:
NaPi-IIa (SLC34A1)
NaPi-IIc (SLC34A3)
NaPi-IIb (SLC34A2)
All of them
- The role of phosphate in growth plate physiology involves:
- Chondrocyte proliferation
- Hypertrophic chondrocyte apoptosis
- Accumulation of osteoid
- Vascular maturation
The more important regulator of phosphorus transporter in the gut is:
PTH
Dietary phosphate
1,25(OH)2D3
Phosphatonins
The main P transporters in the kidney are:
NaPiIIa and NaPiIIc
NaPiIIa and NaPiIIb
NaPiIIb and NaPiIIc
FGF-23
What is not true regarding FGF-23:
It needs the cofactor Klotho to exert its effect
It diminishes the production of calcitriol
It increases secretion of PTH
Increased oral phosphate intake increases serum levels of FGF-23
Footnotes
Answers:
A, 3; B, 2; C, 3; D, 1; E, 3.
References
- 1.Marks J, Edward S, Debnam ES, Unwi RJ. Phosphate homeostasis and the renal-gastrointestinal axis. Am J Physiol Renal Physiol. 2010;299:F285–F296. doi: 10.1152/ajprenal.00508.2009. [DOI] [PubMed] [Google Scholar]
- 2.Berndt T, Thomas LF, Craig TA, Sommer S, Li X, Bergstralh EJ, Kumar R. Evidence for a signaling axis by which intestinal phosphate rapidly modulates renal phosphate reabsorption. Proc Natl Acad Sci USA. 2007;104:11085–11090. doi: 10.1073/pnas.0704446104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaikh A, Berndt T, Kumar R. Regulation of phosphate homeostasis by the phosphatonins and other novel mediators. Pediatr Nephrol. 2008;23:1203–1210. doi: 10.1007/s00467-008-0751-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giral H, Caldas Y, Sutherland E, Wilson P, Breusegem SY, Barry N, Blaine J, Jiang T, Wang XX, Levi M. Regulation of the rat intestinal Na-dependent phosphate transporters by dietary phosphate. Am J Physiol Renal Physiol. 2009;297:F1466–F1475. doi: 10.1152/ajprenal.00279.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reining SC, Liesegang A, Betz H, Biber J, Murer H, Hernando N. Expression of renal and intestinal Na/Pi cotransporters in the absence of GABARAP. Pflügers Arch. 2010;460:201–217. doi: 10.1007/s00424-010-0832-2. [DOI] [PubMed] [Google Scholar]
- 6.Ramon I, Kleynen P, Jean-Jacques Body J, Karmali R. Fibroblast growth factor 23 and its role in phosphate homeostasis. Eur J Endocrinol. 2010;162:1–10. doi: 10.1530/EJE-09-0597. [DOI] [PubMed] [Google Scholar]
- 7.Gattineni J, Baum M. Regulation of phosphate transport by fibroblast growth factor 23 (FGF-23): implications for disorders of phosphate metabolism. Pediatr Nephrol. 2010;25:591–601. doi: 10.1007/s00467-009-1273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amanzadeh J, Reilly RF., Jr Hypophosphatemia: an evidence-based approach to its clinical consequences and management. Nat Clin Pract Nephrol. 2006;2:136–148. doi: 10.1038/ncpneph0124. [DOI] [PubMed] [Google Scholar]
- 9.Tiosano D, Hochberg Z. Hypophosphatemia: the common denominator of all rickets. J Bone Miner Metab. 2009;27:392–401. doi: 10.1007/s00774-009-0079-1. [DOI] [PubMed] [Google Scholar]
- 10.Berndt TJ, Schiavi S, Kumar R. “Phosphatonins” and the regulation of phosphorus homeostasis. Am J Physiol Renal Physiol. 2005;289:F1170–F1182. doi: 10.1152/ajprenal.00072.2005. [DOI] [PubMed] [Google Scholar]
- 11.Alizadeh Naderi AS, Reilly RF. Hereditary disorders of renal phosphate wasting. Nat Rev Nephrol. 2010;6:657–665. doi: 10.1038/nrneph.2010.121. [DOI] [PubMed] [Google Scholar]
- 12.Farrow EG, White KE. Recent advances in renal phosphate handling. Nat Rev Nephrol. 2010;6:207–217. doi: 10.1038/nrneph.2010.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergwitz C, Huppner H. Regulation of phosphate homeostasis by PTH, vitamin D and FGF-23. Annu Rev Med. 2010;61:91–104. doi: 10.1146/annurev.med.051308.111339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alon US. Clinical practice: Fibroblast growth factor (FGF)23: a new hormone. Eur J Pediatr. 2011;170:545–554. doi: 10.1007/s00431-010-1382-5. [DOI] [PubMed] [Google Scholar]
- 15.Xu H, Bai L, Collins JF, Ghishan FK. Age-dependent regulation of rat intestinal type IIb sodium-phosphate cotransporter by 1,25-(OH)2 vitamin D3. Am J Physiol Cell Physiol. 2002;282:C487–C493. doi: 10.1152/ajpcell.00412.2001. [DOI] [PubMed] [Google Scholar]
- 16.Tenenhouse HS. Regulation of phosphorus homeostasis by the type IIa Na/phosphate cotransporter. Annu Rev Nutr. 2005;25:197–214. doi: 10.1146/annurev.nutr.25.050304.092642. [DOI] [PubMed] [Google Scholar]
- 17.Wesseling-Perry K. FGF-23 in bone biology. Pediatr Nephrol. 2010;25:603–608. doi: 10.1007/s00467-009-1384-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu H, Collins JF, Bai L, Kiela PR, Ghishan FK. Regulation of the human sodium-phosphate cotransporter NaPi-IIb gene promoter by epidermal growth factor. Am J Physiol Cell Physiol. 2001;280:C628–C636. doi: 10.1152/ajpcell.2001.280.3.C628. [DOI] [PubMed] [Google Scholar]
- 19.Arima K, Hines ER, Kiela PR, Drees JB, Collins JF, Ghishan FK. Glucocorticoid regulation and glycosylation of mouse intestinal type IIb Na-Pi cotransporter during ontogeny. Am J Physiol Gastrointest Liver Physiol. 2002;283:G426–G434. doi: 10.1152/ajpgi.00319.2001. [DOI] [PubMed] [Google Scholar]
- 20.Xu H, Uno JK, Inouye M, Xu L, Drees JB, Collins JF, Ghishan FK. Regulation of intestinal NaPi-IIb cotransporter gene expression by estrogen. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1317–G1324. doi: 10.1152/ajpgi.00172.2003. [DOI] [PubMed] [Google Scholar]
- 21.Stauber A, Radanovic T, Stange G, Murer H, Wagner CA, Biber J. Regulation of intestinal phosphate transport. II. Metabolic acidosis stimulates Na+−dependent phosphate absorption and expression of the Na+−Pi cotransporter NaPi-IIb in small intestine. Am J Physiol Gastrointest Liver Physiol. 2005;288:G501–G506. doi: 10.1152/ajpgi.00168.2004. [DOI] [PubMed] [Google Scholar]
- 22.Murer H, Forster I, Biber J. The sodium phosphate cotransporter family SLC34. Pflügers Arch. 2004;447:763–767. doi: 10.1007/s00424-003-1072-5. [DOI] [PubMed] [Google Scholar]
- 23.Segawa H, Kaneko I, Yamanaka S, Ito M, Kuwahata M, Inoue Y, Kato S, Miyamoto K. Intestinal Na-P(i) cotransporter adaptation to dietary P(i) content in vitamin D receptor null mice. Am J Physiol Renal Physiol. 2004;287:F39–F47. doi: 10.1152/ajprenal.00375.2003. [DOI] [PubMed] [Google Scholar]
- 24.Capuano P, Radanovic T, Wagner CA, Bacic D, Kato S, Uchiyama Y, St Arnoud R, Murer H, Biber J. Intestinal and renal adaptation to a low-Pi diet of type II NaPi cotransporters in vitamin D receptor- and 1 OHase-deficient mice. Am J Physiol Cell Physiol. 2005;288:C429–C434. doi: 10.1152/ajpcell.00331.2004. [DOI] [PubMed] [Google Scholar]
- 25.Magne D, Bluteau G, Faucheux C, Palmer G, Vignes-Colombeix C, Pilet P, Rouillon T, Caverzasio J, Weiss P, Daculsi G, Guicheux J. Phosphate is a specific signal for ATDC5 chondrocyte maturation and apoptosis-associated mineralization: possible implication of apoptosis in the regulation of endochondral ossification. J Bone Miner Res. 2003;18:1430–1442. doi: 10.1359/jbmr.2003.18.8.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antoniucci DM, Yamashita T, Portale AA. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab. 2006;91:3144–3149. doi: 10.1210/jc.2006-0021. [DOI] [PubMed] [Google Scholar]
- 27.Bacic D, Lehir M, Biber J, Kaissling B, Murer H, Wagner CA. The renal Na+/phosphate cotransporter NaPi-IIa is internalized via the receptor-mediated endocytic route in response to parathyroid hormone. Kidney Int. 2006;69:495–503. doi: 10.1038/sj.ki.5000148. [DOI] [PubMed] [Google Scholar]
- 28.Villa-Bellosta R, Ravera S, Sorribas V, Stange G, Levi M, Murer H, Biber J, Forster IC. The Na+−Pi cotransporter PiT-2 (SLC20A2) is expressed in the apical membrane of rat renal proximal tubules and regulated by dietary Pi. Am J Physiol Renal Physiol. 2009;296:F691–F699. doi: 10.1152/ajprenal.90623.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biber J, Hernando N, Forster I, Murer H. Regulation of phosphate transport in proximal tubules. Pflügers Arch. 2009;458:39–52. doi: 10.1007/s00424-008-0580-8. [DOI] [PubMed] [Google Scholar]
- 30.Segawa H, Kaneko I, Takahashi A, Kuwahata M, Ito M, Ohkido I, Tatsumi S, Miyamoto K. Growth-related renal type II Na/P i cotransporter. J Biol Chem. 2002;277:19665–19672. doi: 10.1074/jbc.M200943200. [DOI] [PubMed] [Google Scholar]
- 31.Prie D, Urena TP, Friedlander G. Latest findings in phosphate homeostasis. Kidney Int. 2009;75:882–889. doi: 10.1038/ki.2008.643. [DOI] [PubMed] [Google Scholar]
- 32.Segawa H, Yamanaka S, Ito M, Kuwahata M, Shono M, Yamamoto T, Miyamoto K. Internalization of renal type IIc Na-Pi cotransporter in response to a high-phosphate diet. Am J Physiol Renal Physiol. 2005;288:F587–F596. doi: 10.1152/ajprenal.00097.2004. [DOI] [PubMed] [Google Scholar]
- 33.Segawa H, Onitsuka A, Kuwahata M, Hanabusa E, Furutani J, Kaneko I, Tomoe Y, Aranami F, Matsumoto N, Ito M, Matsumoto M, Li M, Amizuka N, Miyamoto K. Type IIc sodium-dependent phosphate transporter regulates calcium metabolism. J Am Soc Nephrol. 2009;20:104–113. doi: 10.1681/ASN.2008020177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bergwitz C, Roslin NM, Tieder M, Loredo-Osti JC, Bastepe M, Abu-Zahra H, Frappier D, Burkett K, Carpenter TO, Anderson D, Garabedian M, Sermet I, Fujiwara TM, Morgan K, Tenenhouse HS, Juppner H. SLC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am J Hum Genet. 2006;78:179–192. doi: 10.1086/499409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Villa-Bellosta R, Sorribas V. Compensatory regulation of the sodium/phosphate cotransporters NaPi-IIc (SCL34A3) and Pit-2 (SLC20A2) during Pi deprivation and acidosis. Pflügers Arch. 2010;459:499–508. doi: 10.1007/s00424-009-0746-z. [DOI] [PubMed] [Google Scholar]
- 36.Silver J, Naveh-Many T. FGF-23 and the parathyroid glands. Pediatr Nephrol. 2010;25:2241–2245. doi: 10.1007/s00467-010-1565-3. [DOI] [PubMed] [Google Scholar]
- 37.Kuro-o M. Overview of the FGF-23 -Klotho axis. Pediatr Nephrol. 2010;25:583–590. doi: 10.1007/s00467-009-1260-4. [DOI] [PubMed] [Google Scholar]
- 38.Liu S, Zhou J, Tang W, Jiang X, Rowe DW, Quarles LD. Pathogenic role of FGF-23 in Hyp mice. Am J Physiol Endocrinol Metab. 2006;291:38–49. doi: 10.1152/ajpendo.00008.2006. [DOI] [PubMed] [Google Scholar]
- 39.Kolek OI, Hines ER, Jones MD, LeSueur LK, Lipko MA, Kiela PR, Collins JF, Haussler MR, Ghishan FK. 1 α,25-Dihydroxyvitamin D3 upregulates FGF-23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1036–G1042. doi: 10.1152/ajpgi.00243.2005. [DOI] [PubMed] [Google Scholar]
- 40.Yoshiko Y, Wang H, Minamizaki T, Ijuin C, Yamamoto R, Suemune S, Kozai K, Tanne K, Aubin JE, Maeda N. Mineralized tissue cells are a principal source of FGF-23. Bone. 2007;40:1565–1573. doi: 10.1016/j.bone.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 41.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T. Targeted ablation of FGF-23 demonstrates an essential physiological role of FGF-23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113:561–568. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF-23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 43.Shimada T, Yamazaki Y, Takahashi M, Hasegawa H, Urakawa I, Oshima T, Ono K, Kakitani M, Tomizuka K, Fujita T, Fukumoto S, Yamashita T. Vitamin D receptor-independent FGF-23 actions in regulation phosphate and vitamin D metabolism. Am J Physiol Renal Physiol. 2005;289:F1088–F1095. doi: 10.1152/ajprenal.00474.2004. [DOI] [PubMed] [Google Scholar]
- 44.Wang H, Yoshiko Y, Yamamoto R, Minamizaki T, Kozai K, Tanne K, Aubin JE, Maeda N. Overexpression of fibroblast growth factor 23 suppresses osteoblast differentiation and matrix mineralization in vitro. J Bone Miner Res. 2008;23:939–948. doi: 10.1359/jbmr.080220. [DOI] [PubMed] [Google Scholar]
- 45.Hunter WL, Arsenault AL, Hodsman AB. Rearrangement of the metaphyseal vasculature of the rat growth plate in rickets and rachitic reversal: a model of vascular arrest and angiogenesis renewed. Anat Rec. 1991;229:453–461. doi: 10.1002/ar.1092290404. [DOI] [PubMed] [Google Scholar]
- 46.Chang W, Tu C, Pratt S, Chen TH, Shoback D. Extracellular Ca(2+)-sensing receptors modulate matrix production and mineralization in chondrogenic RCJ3.1C5.18 cells. Endocrinology. 2002;143:1467–1474. doi: 10.1210/en.143.4.1467. [DOI] [PubMed] [Google Scholar]
- 47.Sabbagh Y, Carpenter TO, Demay MB. Hypophosphatemia leads to rickets by impairing caspase-mediated apoptosis of hypertrophic chondrocytes. Proc Natl Acad Sci USA. 2005;102:9637–9642. doi: 10.1073/pnas.0502249102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li YC, Amling M, Pirro AE, Priemel M, Meuse J, Baron R, Delling G, Demay MB. Normalization of mineral ion homeostasis by dietary means prevents hyperparathyroidism, rickets, and osteomalacia, but not alopecia in vitamin D receptor-ablated mice. Endocrinology. 1998;139:4391–4396. doi: 10.1210/en.139.10.4391. [DOI] [PubMed] [Google Scholar]
- 49.Dardenne O, Prud’homme J, Glorieux FH, St-Arnaud R. Rescue of the phenotype of CYP27B1 (1alpha-hydroxylase)-deficient mice. J Steroid Biochem Mol Biol. 2007;89–90:327–330. doi: 10.1016/j.jsbmb.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 50.Miedlich SU, Zalutskaya A, Zhu ED, Demay MB. Phosphate-induced apoptosis of hypertrophic chondrocytes is associated with a decrease in mitochondrial membrane potential and is dependent upon Erk1/2 phosphorylation. J Biol Chem. 2010;285:18270–18275. doi: 10.1074/jbc.M109.098616. [DOI] [PMC free article] [PubMed] [Google Scholar]