Introduction
Genomics research is making significant contributions to our understanding of the biology and treatment of several human diseases. With varying degrees of success, genomics has also contributed to the development of new diagnostic tools and prevention strategies in the public health settings (Brand et al. 2008). A high proportion of diseases with significant public health impact occur due to the interplay between genetic and environmental factors. In this regard, conducting genomics research on diseases with strong environmental determinants is useful not only to identify genetic causes but also to improve public health approaches that aim to modify environmental risk factors (Agurs-Collins et al. 2008; Burke et al. 2010). Genomics research may contribute to the latter by presenting evidence for stratifying targeted population by level of genetic risk. This risk stratification approach has the potential to refine disease prevention strategies by more effectively targeting individuals differentially affected by the disease (Khoury et al. 2005). Moreover, given that most common complex diseases have both genetic and environmental components, community health interventions that utilize information from both risk factors promise to have higher impact (Morabia and Costanza 2005).
Family health history (FHH) is the most commonly applied genomics/genetics tool in the stratification of disease risk at community level. FHH of a disease is a composite indicator of the effects of factors such as genetics, environment, culture, behavior, and the complex interplay between these factors in families. It is a “low tech” but powerful community health genomics tool for risk prediction and stratification, disease prevention and control, and health promotion (Yoon et al. 2002). For example, a study showed that 86 % of early strokes aggregated in 11 % of families, and 72 % of all early chronic heart disease cases clustered in only 14 % of families (Williams et al. 2001), demonstrating the efficiency of FHH in predicting disease risk (Scheuner 2003). Moreover, a randomized clinical trial showed effectiveness of family-oriented education and behavioral interventions. Individuals that received preventive messages tailored to an individual's familial risk had more compliance to the recommendations than those that received standard prevention messages (Ruffin et al. 2011). Despite existing knowledge gaps, the important role of the FHH tool for clinical and primary health practice was reflected in the National Institutes of Health's Consensus Development Program (http://consensus.nih.gov/2009/familyhistory.htm). Importantly, FHH offers unique values for disease prevention in low-income countries for several reasons including the fact that it is well proven, free, and is readily available to all persons (Guttmacher et al. 2004). However, there is paucity of practical evidence on applicability of FHH for informing disease prevention programs in low-income countries, particularly in Africa.
The aim of this article is to describe a practical lesson gained from epidemiologic and genetic studies in Ethiopia that informed community-based prevention approaches for targeting children at high risk for podoconiosis. First, we provide a brief description of podoconiosis and the resource challenges of prevention in endemic communities. Second, we review evidence for the role of genetic and environmental risk factors for podoconiosis, and the usefulness of FHH as a “genomics tool” capturing both risk factors for identification of high risk individuals and resource targeting. Third, we discuss an experience from a pedigree study and a community-based genomics research project that demonstrated the scientific basis and feasibility of FHH. Fourth, we highlight the practical application of this approach by the local community-oriented organization in developing a model podoconiosis prevention program that targets children at high risk. Finally, we point out a strategic direction that can be implemented at primary health care facilities in Ethiopia for systematizing and scaling up the implementation of FHH to speed up elimination of podoconiosis.
Podoconiosis—an environmentally preventable neglected tropical disease
Podoconiosis is a good example of a preventable disease caused by the interplay between genetic and environmental factors. Podoconiosis, also known as endemic non-filarial elephantiasis, is a non-infectious chronic disease resulting in bilateral swelling of the lower legs (Fig. 1). Available evidence indicates that mineral particles present in red clay soils are absorbed through the skin of the foot in barefooted individuals and are taken up into macrophages in the lower limb lymphatics where they induce an inflammatory response leading to fibrosis and obstruction of the vessel lumen (Price 1976a). The disease is caused by barefoot exposure to red clay soil derived from volcanic rock (Fig. 2) and mainly occurs in poor rural farming communities. Podoconiosis is an important, yet neglected, problem of public health importance in countries across tropical Africa, central and south America, and north India (Price 1990; Davey et al. 2007b). Recently, the World Health Organization included podoconiosis in its list of Neglected Tropical Diseases (http://www.who.int/neglected_diseases/diseases/ podoconiosis/en/). It is estimated that the total number of cases per country is highest in Ethiopia—up to one million people are affected. Estimates based on the distribution of the red clay soil showed that a further 11 million people in Ethiopia are at risk of developing podoconiosis. Of these, over five million are estimated to be children aged 1–18 years (Destas et al. 2003; CSA and ORC Macro 2007). Studies showed the average prevalence of podoconiosis in endemic areas of Ethiopia to be over 5 % (Destas et al. 2003; Davey et al. 2007b). Podoconiosis imposes huge economic burden that worsens the prevailing poverty, and results in high social stigmatization associated with the belief that the condition is familial and incurable (Tekola et al. 2006; Yakob et al. 2008). Prevention and treatment of podoconiosis in Ethiopia is mainly conducted by a local non-governmental organization—the Mossy Foot Treatment and Prevention Association (MFTPA). Despite considerable social, economic, and public health burden imposed by podoconiosis, there is minimal government involvement in prevention and treatment of the disease.
Fig. 1.
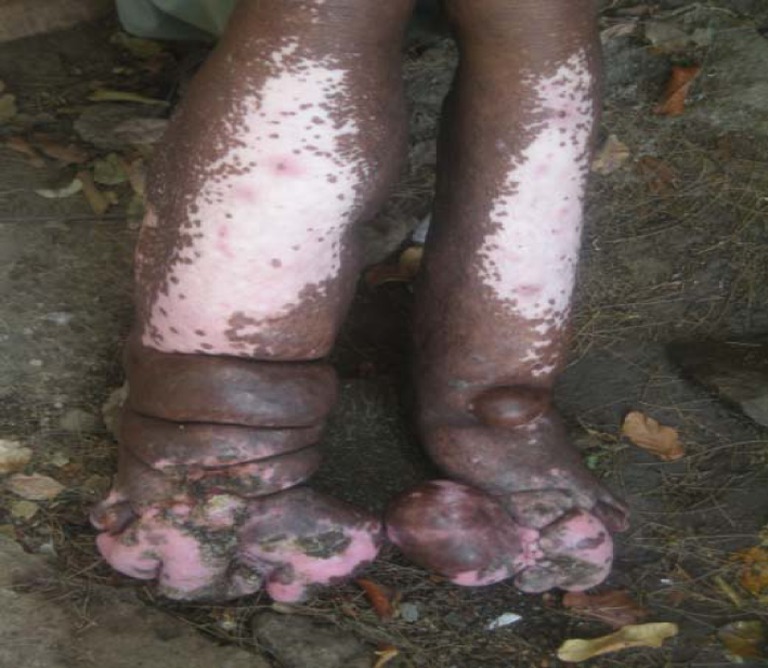
Advanced stage podoconiosis in a 42-year-old adult from southern Ethiopia
Fig. 2.

Barefoot farming in red clay soils of podoconiosis endemic areas, southern Ethiopia
Podoconiosis can be prevented and disease progression, especially at early stages, can be controlled by using measures that curb exposure of feet to the red clay soil and by maintaining proper foot hygiene including consistent washing with soap and water. Primary prevention (prevention of disease onset) that targets young individuals in endemic areas has been suggested as a cost-effective approach to minimize the burden imposed by podoconiosis (Tekola et al. 2006). Interventions targeting podoconiosis prevention in resource poor settings embrace health education and counseling on personal hygiene and shoe-wearing practice, provision of locally made and affordable shoes, and improving access to water and soap for washing feet.
Although consistent use of footwear helps to prevent podoconiosis, many rural people in endemic areas cannot afford shoes. A study showed that patients in southern Ethiopia went barefooted primarily because they were too poor to buy shoes (Yakob et al. 2008). Resource constraint is also a major challenge for the organizations involved in podoconiosis prevention and treatment activities. For example, the MFTPA, the only organization providing prevention and treatment in southern Ethiopia, has contact with and provides treatment for only one third of the patients in its operational area. The combined efforts of all the major non-governmental organizations involved in podoconiosis activities in Ethiopia have reached less than 4 % of the existing one million patients (Davey and Burridge 2009). This clearly shows the need to target individuals at “high risk” to optimize effectiveness of scarce resources on disease prevention programs.
Genetics of podoconiosis
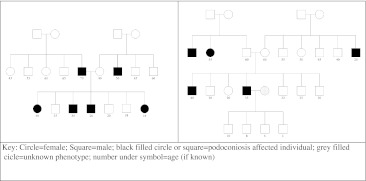
Initial support for the role of genetics in the development of podoconiosis came from pedigree studies conducted in the early 1970s on 80 families with more than one affected person. The study reported significant familial aggregation and the possibility of a genetic factor(s) with an estimated risk genotype frequency of 15–40 % (Price 1972; Price 1976b). Two recent genetic studies conducted by our group and other researchers showed that podoconiosis occurs when genetically susceptible individuals are exposed to the irritant red clay soil in endemic areas. First, we conducted a pedigree study based on 59 multi-generational families with multiple affected members from southern Ethiopia. Estimated heritability of podoconiosis with proband ascertainment was 0.629 (SE = 0.069, p = 1 × 10−7) suggesting that about 63 % of the variance in podoconiosis is due to inherited genetics; the sibling recurrence risk ratio was 5.07 indicating that siblings of an affected person are at five times increased risk of developing podoconiosis when compared to a randomly selected individual in the general population; and the most parsimonious model was an autosomal co-dominant major gene with age and footwear as significant environmental covariates (Davey et al. 2007a). Two sample pedigrees are shown in Fig. 3. Recently, using the genome-wide association study and family-based association approaches, to identified the specific genetic variants that confer susceptibility to podoconiosis (Tekola Ayele et al. 2012).
Fig. 3.
Two family pedigrees from southern Ethiopia
Usefulness of familial factors to identify individuals at “high risk” for podoconiosis
The non-genetic risk factors for podoconiosis (i.e., geographic, social, economic, and behavioral) are commonly shared by the majority of people that live in endemic areas (Table 1). For example, in our case–control designed genomics study on podoconiosis, the controls were individuals that had long-term exposure to the red clay soil (a minimum age of 50 years, no consistent use of shoes, and lived in the endemic area for a minimum of 25 years) yet did not develop the disease. An electron microscopy microanalysis of the lymphatic tissues of the lower limb of barefooted people identified no significant differences in the content of particles found in red clay soils between podoconiosis affected and unaffected individuals (Price and Henderson 1978). These observations indicate that the non-genetic risk factors, while important in the development of podoconiosis, lack the specificity needed to identify individuals at “high risk” within endemic areas.
Table 1.
Risk factors for podoconiosis in endemic areas
| Factor | Description | Reference |
|---|---|---|
| Geography | Altitude: high altitude >1,000 m above sea level | (Davey et al. 2007b; Price 1990) |
| Rainfall pattern and volume: seasonal rainfall, >1,000 mm per year | ||
| Soil: irritant red clay soil derived from volcanic rock | ||
| Socio-economic status | Occupation: the majority are farmers | (Price 1990; Destas et al. 2003; Mengistu et al. 1987; Kloos et al. 1992) |
| Literacy: the majority are not educated | ||
| Household economy: the majority are poor | ||
| Gender: males and females are equally affected | ||
| Shoe-wearing behavior (lifestyle) | The majority of patients are barefooted | (Davey et al. 2007b; Price 1990) |
| Familial factor/genetics | Familial clustering, high heritability (63 %), high sibling recurrence risk ratio (5.1), the most parsimonious genetic model being autosomal co-dominant major gene with age and footwear as significant environmental covariates. Genome-wide association study revealed significant susceptibility loci on chromosome 6 | (Price 1972; Davey et al. 2007a; Tekola Ayele et al. 2012) |
Interestingly, observations in Rwanda and Burundi (Price 1976b) and the genetic studies described above show that among individuals in a community or a family that have common environmental exposure (i.e., barefoot, subsistence farming community with similar socio-environmental risk factors), only a subset with genetic susceptibility develop podoconiosis. The interplay between genetic and environmental factors in podoconiosis means that this information can be used to refine primary prevention by prioritizing interventions to individuals at “high risk” in terms of a positive FHH. Several characteristics of podoconiosis make it feasible to use FHH to identify individuals at “high risk” of developing podoconiosis: (1) there is evidence for a strong genetic component in podoconiosis as described above; (2) there is a need to prioritize use of limited resources in podoconiosis endemic areas towards individuals with the highest need; (3) most people in endemic communities can accurately report family history of podoconiosis because of the distinctive features of the disease (Fig. 1) (Desta et al. 2007) and the social stigma suffered by affected families; (4) FHH of podoconiosis captures most of the risk factors for the disease including social, geographical, economic, behavioral (being uneducated, employment history of subsistence farming, and not wearing shoes which exposes them to the irritant soil), and genetics (Table 1); and (5) the application of FHH for predicting risk of developing podoconiosis meets most recommended public health criteria including affordability of the tool, public health relevance, and availability of effective prevention methods (Wilson and Jungner 1968; Yoon et al. 2002) (Table 2).
Table 2.
Family health history criteria for predicting risk of podoconiosis in endemic areas
| Criteria (Wilson and Jungner 1968; Yoon et al. 2002) | Podoconiosis | Reference |
|---|---|---|
| Accuracy with which the disease can be recalled | Progressive swelling and disfigurement of the lower legs; the pattern of settlement and the strong family relationship in rural Ethiopia keeps families closely connected minimizing recall bias | (Price 1972; Davey et al. 2007b) |
| Prevalence of the disease in the population | Has high prevalence (average 5 %); prevalence higher than that of HIV/AIDS, malaria, and tuberculosis in endemic areas | (Price 1972; Destas et al. 2003; Mengistu et al. 1987; Kloos et al. 1992; Alemu et al. 2011; Geshere Oli et al. 2012) |
| Risk associated with family history | Significant familial aggregation with an estimated risk genotype frequency of 15–40 % | (Price 1972; Davey et al. 2007a) |
| High heritability (63 %), high sibling recurrence risk ratio (5.1) | ||
| Availability of effective early detection and prevention measures | Footwear and personal hygiene effective in prevention | (Price 1990; Kloos et al. 1992) |
| No stigma associated with being at above average risk | There is prevailing belief that podoconiosis has familial component. The resulting stigma is immense. Currently, the level of stigma is declining in southern Ethiopia because of community-based education programs and unaffected people are now appreciating the fact that podoconiosis is preventable with regular wearing of shoes and proper foot hygiene | (Yakob et al. 2008; Tekola et al. 2009) |
| Cost of the tool | Family health history can be obtained during routine household visits by existing fieldworkers/health extension workers or at clinic visits by patients | (Davey and Burridge 2009; Alemu et al. 2011; Datiko and Lindtjorn 2009) |
Genomics research informed a “high risk” approach to prevent podoconiosis
The genomics research project (pedigree and genome-wide association studies) to understand the genetic basis of podoconiosis in southern Ethiopia included a community outreach component that explored the best public health approaches to tailor prevention efforts towards “high risk” children in a resource poor environment. The community outreach component of the genomics research project involved two major areas: training fieldworkers and providing health education to community members with special emphasis on the role of regular wearing of shoes and consistent washing of feet to prevent podoconiosis. The MFTPA fieldworkers were trained on the use of a checklist to identify affected families, and a series of sensitization and education sessions were conducted with community members about the interacting roles of familial and environmental factors in the etiology of podoconiosis. The pedigree study and the community outreach activities in the genomics research provided opportunities for MFTPA to openly discuss the familial nature of the disease during routine health education and awareness raising campaigns targeting the community and health professionals contributed to reduction of social stigma associated with the familial nature of the disease and the erroneous prevailing belief that podoconiosis cannot be prevented or controlled (Tekola et al. 2009; Dunlop and Barlow-Stewart 2010).
The implemented educational program helped the community and the MFTPA fieldworkers understand that individuals at high risk for podoconiosis due to familial factors should have priority for access to water and shoes. During the research fieldwork, community members were given education on the universal recommendation to wear shoes in order to effectively prevent podoconiosis in endemic areas; the fieldworkers were also trained to give priority to children with positive FHH in resource-limited settings. This facilitated familial risk-communication between MFTPA and the community.
Implementation of the “high risk” approach by the MFTPA
Following the evidence presented by the pedigree study, the MFTPA introduced a special podoconiosis prevention program that targets children at “high risk” for the disease in Wolaita zone, southern Ethiopia where the prevalence of podoconiosis is 5.5 % (Destas et al. 2003). FHH was used to stratify children into high and low risk based on the average (general population) risk of developing podoconiosis. Children at “high risk” were operationally defined as those aged <15 years, living in podoconiosis endemic areas, and having a positive family history of podoconiosis in first-degree relatives. Tailored FHH checklists were completed by trained fieldworkers during patients' clinic visits or during fieldworkers' home visits. All adult patients were asked about the presence of children aged <15 years in the family, the number of affected children if any, and the relationship between the patient and the child. Identified children were then included in the special podoconiosis prevention program. Components of the program included (1) education and counseling of household members about the relevance of understanding FHH to prevent podoconiosis in children, (2) education of parents to encourage children to consistently wear shoes and wash their feet, (3) advising children to wear shoes during all waking hours including in non-cemented in-door environments, and (4) provision of shoes to children at regular time intervals, a strategy that would be impractical at a population-wide level. Over two years, the MFTPA distributed over 41,000 pairs of donated shoes to children. The shoes provided were similar to other locally used shoes so that children in the program would not become targets for stigmatization marked by the type of shoes they wear. Children were included in the program following consent by their parents or legal guardians.
The MFTPA integrated the newly introduced genetic (FHH)-based podoconiosis prevention strategy with the existing population-wide prevention program that covered individuals and families living in endemic areas. The population-wide prevention program entailed educational messages relayed at community gatherings such as churches, mosques, and schools to encourage use of footwear by all individuals living in endemic areas. The education stressed that podoconiosis is preventable, which helped minimize stigma directed at affected families and children recruited for the special prevention program. The population-wide program strengthened the special prevention program by facilitating identification of affected families. This model is a practical illustration that the approach of targeting certain groups (e.g., high-risk children) through genomics may have an added value on existing population-wide public health approaches (ten Kate 2005; Khoury et al. 2005). Furthermore, providing shoes to children has the added advantage of aiding sustainability of prevention efforts. This is so because early intervention may result in sustained behavioral change through instilling the habit of wearing shoes and washing feet on regular basis at the formative years of children in the endemic areas. Notably, provision of familial risk information reinforces these efforts. In addition, prevention of podoconiosis by providing footwear may enhance children's school attendance and performance, which would otherwise be challenged by the stigma and morbidity caused by the disease (Yakob et al. 2008; Tekola et al. 2009). In the long term, it is likely that well-educated and economically empowered children will be able to afford and use footwear as adult.
Policy recommendations—genomics tools in “elimination” of podoconiosis
Despite the well-established knowledge that podoconiosis is a non-communicable disease that can potentially be eliminated (Davey 2008), no systematic approach has been developed to guide large-scale elimination efforts. In the short term, elimination programs should focus on prevention of disease onset (measured by reduction in incidence; Dowdle 1998). Although no formal cost-effectiveness evaluation was conducted, FHH was used by the MFTPA to target children at “high risk” and to optimize the scarce resources available for prevention and elimination. Sustainability of prevention programs through increased community and government involvement, and large-scale application of FHH will empower individuals and enhance public health efforts to eliminate podoconiosis.
We recommend that as part of a program to eliminate podoconiosis, the FHH approach should be expanded to other endemic areas in Ethiopia through integration with the rural health system. The health extension program of the government of Ethiopia offers an untapped opportunity to achieve this goal within a shorter time frame and at minimal incremental cost. This government program is led by the health extension workers (i.e., trained community health workers) who visit rural households on regular basis mainly to provide health education. Research showed that involving health extension workers improved tuberculosis case detection and treatment success rate (Datiko and Lindtjorn 2009). We have demonstrated that health extension workers can successfully implement community-based podoconiosis surveys in west Ethiopia (Alemu et al. 2011), and can recruit patients into a start-up podoconiosis clinic program in north Ethiopia. Therefore, this economically efficient health worker system is well placed to identify podoconiosis affected families and to support disease prevention programs involving high risk children.
Conclusions
There are two key lessons from the experience presented in this paper. First, we observe that large-scale genomics research projects when coupled with community-based activities can positively influence the way prevention programs are practiced. Second, the experience of the “high risk” children prevention program showed that FHH is a helpful tool for the identification and stratification of individuals most at risk for interventions especially in resource-challenged settings. Despite the current paucity of genomics data from populations of low-income countries such as those in Africa, there are promising initiatives (e.g., the Human Heredity and Health in Africa Project -H3 Africa, http://h3africa.org) that can potentially generate useful data. However, “translation” of genomics into clinical and public health practice for the major diseases in low-income countries may take years (Knoppers et al. 2010). In the mean time, FHH can be used as a low-cost, simple, and readily available risk-stratifying tool for optimizing resources to prevent disease. It is however important to develop a systematic approach to evaluate the clinical utility and validity of the FHH tool in different settings. Furthermore, because of the genetic and socio-cultural diversity of the African population (Campbell and Tishkoff 2008), it will be important to validate our experience in different population groups. Overall, we posit that by using genetic strategies such as FHH, public health practitioners can enhance community-based intervention programs especially in resource poor settings.
Acknowledgments
We thank Meskele Ashine for allowing us to use data from the Mossy Foot Treatment and Prevention Association of Ethiopia. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official view of the National Institutes of Health (NIH). The podoconiosis project is supported by the Wellcome Trust. The authors are supported by the Intramural Research Program of the National Human Genome Research Institute, NIH, in the Center for Research on Genomics and Global Health (CRGGH). The CRGGH is support by the National Institute of Diabetes and Digestive and Kidney Diseases at NIH.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Agurs-Collins T, Khoury MJ, Simon-Morton D, Olster DH, Harris JR, Milner JA. Public health genomics: translating obesity genomics research into population health benefits. Obesity (Silver Spring) 2008;16(Suppl 3):S85–S94. doi: 10.1038/oby.2008.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemu G, Tekola Ayele F, Daniel T, Ahrens C, Davey G. Burden of podoconiosis in poor rural communities in Gulliso woreda, West Ethiopia. PLoS Negl Trop Dis. 2011;5:e1184. doi: 10.1371/journal.pntd.0001184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A, Brand H, Schulte in den Baumen T. The impact of genetics and genomics on public health. Eur J Hum Genet. 2008;16(1):5–13. doi: 10.1038/sj.ejhg.5201942. [DOI] [PubMed] [Google Scholar]
- Burke W, Burton H, Hall AE, Karmali M, Khoury MJ, Knoppers B, Meslin EM, Stanley F, Wright CF, Zimmern RL. Extending the reach of public health genomics: what should be the agenda for public health in an era of genome-based and "personalized" medicine? Genet Med. 2010;12(12):785–791. doi: 10.1097/GIM.0b013e3182011222. [DOI] [PubMed] [Google Scholar]
- Campbell MC, Tishkoff SA. African genetic diversity: implications for human demographic history, modern human origins, and complex disease mapping. Annu Rev Genomics Hum Genet. 2008;9:403–433. doi: 10.1146/annurev.genom.9.081307.164258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The 2007 Population and Housing Census of Ethiopia. Addis Ababa: CSA; 2007. [Google Scholar]
- Datiko DG, Lindtjorn B. Health extension workers improve tuberculosis case detection and treatment success in southern Ethiopia: a community randomized trial. PLoS One. 2009;4(5):e5443. doi: 10.1371/journal.pone.0005443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey G. Podoconiosis: let Ethiopia lead the way. Ethiop J Health Dev. 2008;22(1):1–2. [Google Scholar]
- Davey G, Burridge E. Community-based control of a neglected tropical disease: the mossy foot treatment and prevention association. PLoS Negl Trop Dis. 2009;3(5):e424. doi: 10.1371/journal.pntd.0000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey G, Gebrehanna E, Adeyemo A, Rotimi C, Newport M, Desta K. Podoconiosis: a tropical model for gene-environment interactions? Trans R Soc Trop Med Hyg. 2007;101(1):91–96. doi: 10.1016/j.trstmh.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Davey G, Tekola F, Newport MJ. Podoconiosis: non-infectious geochemical elephantiasis. Trans R Soc Trop Med Hyg. 2007;101(12):1175–1180. doi: 10.1016/j.trstmh.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Desta K, Ashine M, Davey G. Predictive value of clinical assessment of patients with podoconiosis in an endemic community setting. Trans R Soc Trop Med Hyg. 2007;101(6):621–623. doi: 10.1016/j.trstmh.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Destas K, Ashine M, Davey G. Prevalence of podoconiosis (endemic non-filarial elephantiasis) in Wolaitta, Southern Ethiopia. Trop Doct. 2003;33(4):217–220. doi: 10.1177/004947550303300410. [DOI] [PubMed] [Google Scholar]
- Dowdle WR. The principles of disease elimination and eradication. Bull World Health Organ. 1998;76(Suppl 2):23–25. [PMC free article] [PubMed] [Google Scholar]
- Dunlop K, Barlow-Stewart K. 'Start the conversation': the New South Wales (Australia) family health history campaign. Public Health Genomics. 2010;13(5):301–309. doi: 10.1159/000253121. [DOI] [PubMed] [Google Scholar]
- Geshere Oli G, Tekola Ayele F, Petros B (2012) Parasitological, serological, and clinical evidence for high prevalence of podoconiosis (non-filarial elephantiasis) in Midakegn district, central Ethiopia. Trop Med Int Health (in press) [DOI] [PMC free article] [PubMed]
- Guttmacher AE, Collins FS, Carmona RH. The family history-more important than ever. N Engl J Med. 2004;351(22):2333–2336. doi: 10.1056/NEJMsb042979. [DOI] [PubMed] [Google Scholar]
- Khoury MJ, Davis R, Gwinn M, Lindegren ML, Yoon P. Do we need genomic research for the prevention of common diseases with environmental causes? Am J Epidemiol. 2005;161(9):799–805. doi: 10.1093/aje/kwi113. [DOI] [PubMed] [Google Scholar]
- Kloos H, Bedri Kello A, Addus A. Podoconiosis (endemic non-filarial elephantiasis) in two resettlement schemes in western Ethiopia. Trop Doct. 1992;22(3):109–112. doi: 10.1177/004947559202200306. [DOI] [PubMed] [Google Scholar]
- Knoppers BM, Leroux T, Doucet H, Godard B, Laberge C, Stanton-Jean M, Fortin S, Cousineau J, Monardes C, Girard N, Levesque L, Durand C, Farmer Y, Dion-Labrie M, Bouthillier ME, Avard D. Framing genomics, public health research and policy: points to consider. Public Health Genomics. 2010;13(4):224–234. doi: 10.1159/000279624. [DOI] [PubMed] [Google Scholar]
- Mengistu G, Humber DP, Ersumo M, Mamo T. High prevalence of elephantiasis and cutaneous leishmaniasis in Ocholo, south-west Ethiopia. Ethiop Med J. 1987;25(4):203–207. [PubMed] [Google Scholar]
- Morabia A, Costanza MC. Re: "do we need genomic research for the prevention of common diseases with environmental causes?". Am J Epidemiol. 2005;162(8):815. doi: 10.1093/aje/kwi282. [DOI] [PubMed] [Google Scholar]
- Price EW. A possible genetic factor in non-filarial elephantiasis of the lower legs. Ethiop Med J. 1972;10(3):87–93. [PubMed] [Google Scholar]
- Price EW. The association of endemic elephantiasis of the lower legs in East Africa with soil derived from volcanic rocks. Trans R Soc Trop Med Hyg. 1976;70(4):288–295. doi: 10.1016/0035-9203(76)90078-X. [DOI] [PubMed] [Google Scholar]
- Price EW. Endemic elephantiasis of the lower legs in Rwanda and Burundi. Trop Geogr Med. 1976;28(4):283–290. [PubMed] [Google Scholar]
- Price E. Podoconiosis: non-filarial elephantiasis. Oxford: Oxford Medical; 1990. [Google Scholar]
- Price EW, Henderson WJ. The elemental content of lymphatic tissues of barefooted people in Ethiopia, with reference to endemic elephantiasis of the lower legs. Trans R Soc Trop Med Hyg. 1978;72(2):132–136. doi: 10.1016/0035-9203(78)90048-2. [DOI] [PubMed] [Google Scholar]
- Ruffin MT, Nease DE, Sen A, Pace WD, Wang C, Acheson LS, Rubinstein WS, O'Neill S, Gramling R. Effect of preventive messages tailored to family history on health behaviors: The Family Healthware Impact Trial. Ann Fam Med. 2011;9(1):3–11. doi: 10.1370/afm.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuner MT. Genetic evaluation for coronary artery disease. Genet Med. 2003;5(4):269–285. doi: 10.1097/01.GIM.0000079364.98247.26. [DOI] [PubMed] [Google Scholar]
- Tekola Ayele F, Adeyemo A, Finan C, Hailu E, Sinnott P, Diaz Burlinson N, Aseffa A, Rotimi C, Newport MJ, Davey G (2012) HLA class II locus and susceptibility to podoconiosis. N Engl J Med (in press) [DOI] [PMC free article] [PubMed]
- Tekola F, Mariam DH, Davey G. Economic costs of endemic non-filarial elephantiasis in Wolaita Zone, Ethiopia. Trop Med Int Health. 2006;11(7):1136–1144. doi: 10.1111/j.1365-3156.2006.01658.x. [DOI] [PubMed] [Google Scholar]
- Tekola F, Bull S, Farsides B, Newport MJ, Adeyemo A, Rotimi CN, Davey G. Impact of social stigma on the process of obtaining informed consent for genetic research on podoconiosis: a qualitative study. BMC Med Ethics. 2009;10:13. doi: 10.1186/1472-6939-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kate LP. Community genetics: a bridge between clinical genetics and public health. Community Genet. 2005;8(1):7–11. doi: 10.1159/000083330. [DOI] [PubMed] [Google Scholar]
- Williams RR, Hunt SC, Heiss G, Province MA, Bensen JT, Higgins M, Chamberlain RM, Ware J, Hopkins PN. Usefulness of cardiovascular family history data for population-based preventive medicine and medical research (the Health Family Tree Study and the NHLBI Family Heart Study) Am J Cardiol. 2001;87(2):129–135. doi: 10.1016/S0002-9149(00)01303-5. [DOI] [PubMed] [Google Scholar]
- Wilson J, Jungner G. Principles and practice of screening for disease. Geneva: World Health Organization; 1968. [Google Scholar]
- Yakob B, Deribe K, Davey G. High levels of misconceptions and stigma in a community highly endemic for podoconiosis in southern Ethiopia. Trans R Soc Trop Med Hyg. 2008;102(5):439–444. doi: 10.1016/j.trstmh.2008.01.023. [DOI] [PubMed] [Google Scholar]
- Yoon PW, Scheuner MT, Peterson-Oehlke KL, Gwinn M, Faucett A, Khoury MJ. Can family history be used as a tool for public health and preventive medicine? Genet Med. 2002;4(4):304–310. doi: 10.1097/00125817-200207000-00009. [DOI] [PubMed] [Google Scholar]