Abstract
We conducted a deliberative engagement to assess attitudinal changes regarding biobank research, governance, and the return of results. We recruited African-Americans from two Southside Chicago health care facilities that serve communities of very different socioeconomic and educational backgrounds in order to examine similarities and differences within the African-American population. We used a mixed method, deliberative engagement process involving a convenience sample of parents recruited from a Federally Qualified Health Clinic (FQHC) [n = 23] and a university-based practice (UBP) [n = 22]. Four coding categories illustrate similarities and differences between participants from the two different practices: (1) reasons for and against participation; (2) trust and mistrust; (3) return of research results; and (4) religion. Overall, there was strong interest in receiving results, which was a main motivator for participation. While participants from both health care facilities expressed distrust of research, UBP participants also expressed trust in the research enterprise. FQHC participants more frequently mentioned religion. Studies about participation in biobanks often focus on participants’ race as the sole significant variable, while our work supports the importance of other demographic factors. Medical researchers must move beyond research analyses that consider the African-American population to be monolithic and value the diversity within it.
Keywords: Socioeconomic status, Religion, African-Americans, Biobanks, Genetic research, Return of results
Introduction
There is a growing literature on participants’ perspectives regarding biobanks and biobank-based genetic research. In general, the literature shows that the majority of Americans are willing to participate in biobanks (Lipworth et al. 2011; Murphy et al. 2008; Pulley et al. 2008; Kaphingst et al. 2006; McQuillan et al. 2003; McQuillan et al. 2006; McQuillan and Porter, 2011), and that virtually all support the return of research results (Lipworth et al. 2011; Murphy et al. 2008; Kaphingst et al. 2006; Streicher et al. 2011). One of the main hurdles for enrollment is trust—trust that the researchers will use donated samples to promote health and not to facilitate discrimination, and trust that the participants will have access to the diagnostic tests and therapies derived from such research (Lipworth et al. 2011; Heiney et al. 2010; James et al. 2008). Much of the research on participants’ attitudes makes comparisons and conclusions based on race/ethnicity (Heiney et al. 2010; James et al. 2008; Bussey-Jones et al. 2010; Zimmerman et al. 2006; Durant et al. 2011; Hipps et al. 2003; Streicher et al. 2011; Goldenberg et al. 2011). Many of the studies collect other demographic information including income and education, but even in large population studies, rarely do researchers perform analyses that might discover whether any of these other demographic characteristics might better correlate with attitudes and perspectives (Bussey-Jones et al. 2010; Hipps et al. 2003; Goldenberg et al. 2011).
We conducted a mixed method deliberative engagement to assess pre- and post-engagement attitudinal changes regarding biobank research, governance, and the return of results. A deliberative engagement involves educational programs followed by focus groups to examine the attitudes of an informed public. The deliberative engagement method was used to ensure an informed group of participants who would not simply give their raw opinions but rather would provide well-informed opinions that they could discuss with other community participants. In this manuscript, we compare the perspectives of informed African-American participants from two health care facilities that serve communities of different socioeconomic backgrounds. We focused on African-Americans because they are less likely to provide genetic samples for genetic-based biobank research (McQuillan et al. 2006), and we wanted to understand the underlying reasons for this hesitancy and whether this hesitancy might be explained by other demographic factors.
Methods
We conducted a mixed method, deliberative engagement project on the South Side of Chicago. A convenience sample of African-American parents was recruited from two pediatric health care facilities—a Federally Qualified Health Clinic (FQHC) and a university-based practice (UBP). The inclusion criteria were: (1) self-identification as African-American; (2) ≥18 years; and (3) knowledge and understanding of English.
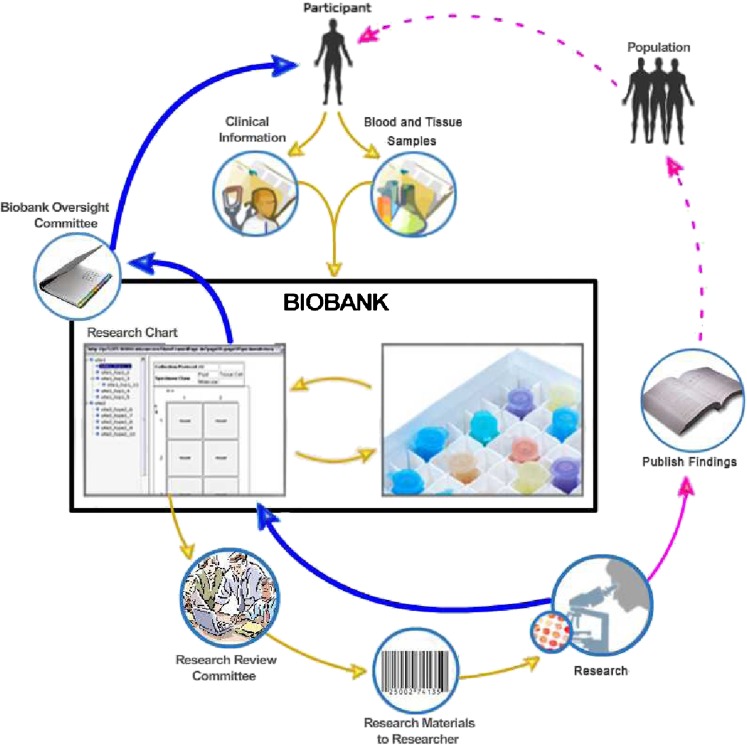
Participants attended four sets of engagements, which consisted of educational and focus group sessions, and took place on two consecutive Saturdays. On the first day, the educational program began with a brief overview of genetics and the ways in which medical conditions are inherited across generations. The focus then shifted to the purpose of a biobank and how it functioned. The content and process of informed consent for biobank-based research participation was described, as were the potential benefits and harms of participating in a biobank. The second day focused on the return of research results to both adult and child participants. On both days, participants were shown an image we developed to explain how data are collected, stored, and retrieved from a biobank for use by researchers, how and when results can be reported back, and the various junctures where governance is needed; that is where oversight of biobank processes can occur (see Fig. 1). Additional details of the educational program and the discussion guides have been reported elsewhere (Lemke et al. 2012; available from corresponding author).
Fig. 1.
Lifecycle of a Biobank (modified from the original image which is in the public domain that can be found at: https://cabig.nci.nih.gov/tools/catissuesuite). This figure depicts the enrollment of a participant into a biobank through the reporting and return of results. Yellow arrows represent the movement of samples and data from the participant into the biobank and the movement of de-identified samples and data from the biobank to researchers. Pink arrows represent the movement of research results from the researchers to the public, while dashed lines represent the possible dissemination of benefits. Blue arrows represent the movement of research results back into the biobank and back to the participant. In the biobank figure, two governance junctures are portrayed: the research review committee (RRC) and the biobank oversight committee (BOC). The RRC is designed to evaluate whether a researcher’s proposal meets ethical and scientific standards and then permits the distribution of de-identified samples. The RRC may or may not be a formal institutional review board (IRB) since de-identified samples are non-human subjects and do not require IRB oversight. The BOC is designed to evaluate when and whether to return research results to individual participants. Some biobanks are designed not to permit the return of results, and in such biobanks this committee would not exist. Currently, there are no legal requirements as to the BOC’s membership or the criteria it would use to decide when to return results
Focus group sessions utilized open-ended questions and prompts relating directly to the educational material. Sessions were tape-recorded and then transcribed and coded by two investigators using Atlas.ti (version 6) after reaching standard intercoder reliability. Participants’ names were replaced with coded identifiers referencing health care facility (Q = FQHC, U = UBP), gender (F = female, M = male), and a unique number assigned to each participant.
Participants were also asked to complete pre- and post-engagement evaluations of self-assessed knowledge and attitudes about genetics and research. Evaluations of the deliberative engagement process were completed at the end of each Saturday. These results are reported elsewhere (Lemke et al. 2012).
This study was approved by both the Institutional Review Board (IRB) of the University of Chicago and Northwestern University with waiver of written informed consent. The IRB approved $50 compensation for each session a participant attended, with a $50 additional incentive for attending all four sessions (maximum $250).
Results
A total of 45 individuals participated in the engagement process. Ninety-five people were approached for recruitment at the FQHC and 13 (14 %) declined to participate, while 97 people were approached at the UBP, of whom 49 (42 %) declined to participate. Of the 82 persons from the FQHC who agreed to be re-contacted, 43 consented to participate over the phone (52 %) and 22 (51 % of those consented and 27 % of those initially recruited) attended the first session. Of the 48 persons from the UBP who agreed to be re-contacted, 31 consented to participate over the phone (65 %) and 23 (74 % of those consented and 48 % of those initially recruited) attended the first session. All but one person from each facility attended all four sessions.
The participants from the two practices were similar with regard to gender (~75 % female) and age (mean of 40 years at the UBP and 42 at the FQHC), although they differed significantly with regard to education (participants from the UBP having higher education, p < .05) and number of children (participants from the FQHC having more, p < .05) (Table 1). Although we did not collect economic information from individual participants, over 75 % of patients at the UBP have private insurance in contrast to less than 10 % at the FQHC (personal communication with clinic administrators, August 2011). Prior to the deliberative engagement, only three participants (7 %) self-described as highly knowledgeable about biobanks (see Table 1). Post-intervention, 18 of 35 (51 %) self-described as highly informed about biobanks.
Table 1.
Participant characteristics
| Federally qualified health center (FQHC) N = 22 | University-based practice (UBP) N = 23a | |
|---|---|---|
| n (%) | n (%) | |
| Race | N = 20a | |
| Only Black/African-American | 22 (100) | 20 (87) |
| Black and other races | 0 | 3 (13) |
| Gender | ||
| Female | 16 (73) | 18 (78) |
| Male | 6 (27) | 5 (22) |
| Education | ||
| ≤High school | 8 (36)* | 2 (9)* |
| >HS, <BA | 12 (55)* | 14 (61)* |
| ≥BA | 2 (9)* | 7 (30)* |
| Number of children | ||
| 1–3 | 14 (64)* | 22 (96)* |
| 4 or more | 8 (36)* | 1 (4)* |
| Age | Years | Years |
| Average age (± standard deviation) | 40 (±13) | 42 (±13) |
| Range | 20–63 | 22–62 |
| Self-rated knowledge | ||
| Prior knowledge about biobanks | n = 22 (%) | n = 22 (%) |
| Highly informed | 2 (9 %) | 1 (5 %) |
| Moderately to minimally informed | 14 (63 %) | 16 (73 %) |
| Not informed | 6 (27 %) | 5 (23 %) |
| Post knowledge about biobanks | n = 20 (%) | n = 15 (%) |
| Highly informed | 11 (55 %) | 7 (47 %) |
| Moderately to minimally informed | 8 (40 %) | 8 (53 %) |
| Not informed | 1 (5 %) | 0 |
| Clinic characteristics | % | % |
| Private insurance | <10 | >75 |
| Patients who self-identify as African-American | >90 | 75 |
This table is modified from Lemke et al. 2012. It is expanded to include clinic characteristics and self-rated knowledge scores
*p < .05
aN = 23 unless otherwise specified
We analyzed all transcripts for 36 themes which we used 2,463 times in the transcripts, 1,308 codes from the FQHC and 1,155 codes from the UBP. Some themes were coded infrequently (fewer than 20 times in the transcripts) such as access to health care, whereas others were used over 100 times (e.g., trust; reasons to want to know one’s research results). Three of the more commonly coded themes were: (1) reasons to and not to participate; (2) trust and mistrust; and (3) the return of research results. A fourth theme, less frequently coded (53 times), related to religion and the sacred. We examined similarities and differences in the themes between participants from these two health care facilities. For the first three themes, we also report-related quantitative data (Table 2). There were no statistically significant changes in attitudes between pre- and post-engagement for these three themes.
Table 2.
Attitudes towards genetic research and return of results
| Federally Qualified Health Center (FQHC) (N = 22) | University-based practice (UBP) (N = 23) | |||
|---|---|---|---|---|
| Pre-engagement n/d (%) agreea | Post-engagement n/d (%) agreea | Pre-engagement n/d (%) agreea | Post-engagement n/d (%) agreea | |
| How much do you trust genetic research? | 11/19 (57) | 15/20 (75) | 19/23 (83) | 14/15 (92) |
| Researchers should share overall, or group, results with participants. | 18/21 (86) | 17/19 (89) | 20/22 (91) | 13/15 (87) |
| Researchers should share individual research results, if a participant asks for them. | 20/22 (91) | 17/19 (89) | 23/23 (100) | 14/15 (93) |
| How concerned are you about the protection of privacy of genetic information that is collected in research? | 20/22 (91) | 18/20 (90) | 18/22 (82) | 14/15 (93) |
No changes were statistically significant
n/d numerator over denominator. Denominator varies due to nonresponders, who were excluded
aResponders were given a five-point scale = strongly agree, moderately agree, neutral, moderately disagree, and strongly disagree. Percentage of agree includes both those who strongly and moderately agree
Reasons to and not to participate
Most of our codes for this topic came from several specific questions that were asked to elucidate the reasons for and against participation. On the first session of the first day, participants were asked: “What are some reasons a person would participate in a biobank?” and “What are some reasons that a person would not participate in a biobank?” At the end of each session, participants were asked: “How do you feel about participating in a biobank?”
Most participants from both health care facilities expressed strong interest in enrolling in a biobank. Reasons participants gave to participate as well as not to participate were also similar. The most frequently mentioned motivation in both groups was altruism, although both groups also agreed that personal benefit was also a motivation. One FQHC participant stated, “I’m not thinking about myself; I’m thinking about [helping] the world” (QM04). Similarly, a UBP participant mentioned, “I think we’d be doing humankind a great service” (UM05).
Despite prompting by focus group moderators, the participants rarely gave reasons they would not enroll. One UBP participant stated she would not enroll unless she was paid. However, when prompted why members of the general public might not want to participate, participants offered numerous explanations. The most common reason was lack of understanding about what a biobank is and what functions it serves. A lack of trust in the institution recruiting for the biobank was another deterrent. Others mentioned that prospective donors might be afraid of receiving certain results. Finally, some participants mentioned their unease about phlebotomy, which would prevent them from giving a blood sample. “I don’t like getting poked” (QF05).
In our pre- and post-engagement surveys, >80 % of participants from both health care facilities expressed moderate to strong concern regarding the protection of privacy of genetic information collected in research (Table 2), and privacy and confidentiality risks were addressed in three of four educational sessions. However, privacy concerns were not a probe in our discussion guide and were rarely raised in the focus group sessions as a reason not to participate. Rather, participants from both health care facilities expressed the belief that in an era of WikiLeaks, Facebook, and Twitter, traditional conceptions of privacy were outmoded. The inability to guarantee absolute privacy to biobank participants was accepted as a fact of life and was not perceived as a reason not to participate: “Everyone’s so upset over this whole privacy thing, and it’s like, just get over it” (QM01).
Return of results
Most of our codes for this theme occurred in discussions on the second day in which the focus was on the return of results. In the morning, participants were asked: “If you participated in biobank genetic research, would you want to get group (summary) research results back?” and “If you participated in biobank genetic research, would you want to get individual research results back?” Probes were also asked about return of specific results: “What kinds of individual research results would you want to know, if any?” and “Are there certain kinds of individual research results that you would not want to know?” These same questions were asked in the afternoon regarding their children.
Participants were asked about the receipt of individual and aggregate research results. Over 85 % of participants from both health care facilities expressed interest in receiving such results in the pre- and post-engagement surveys (Table 2). In fact, several participants from each health care facility stated that they would not donate to a biobank that did not plan to return individual results. Especially representative comments were: “I wouldn’t participate in something that is not gonna give any feedback back” (QF01) and “I wouldn’t do it because I’m still not getting …the results of yea or nay” (UF11). Most participants from both health care facilities stated that they would like all of their individual results returned without exception. “I don’t care what it is,” said one woman, “I want to know everything” (QF06). In fact, “everything” was the most frequent response when participants from the FQHC were asked what types of results they would like back if they were to enroll in a biobank.
Despite overall strong interest in receiving individual results, some participants expressed fear: “Some people may be afraid of what the results might be. They might find [they] have a trait or a gene or some type of disease, and some people don’t want to deal with that. They would rather just not know” (QF13). “A lot of people are afraid to even learn what might be going on with them” (UF17). Similarly, several comments from both groups referred to disinterest in the return of results due to participants’ belief that learning this information would cause them to become paranoid or hypochondriacal. “I think some people overreact [to health information] sometimes” (UF10). “When you find out something, people acting crazy” (QF05).
When probed about specific types of research results that could be discovered, a more nuanced perspective emerged. With respect to non-actionable results, such as results about gene changes with unknown implications and results about untreatable conditions, the majority of comments expressed by FQHC participants opposed the receipt of individual results. In contrast, UBP participants were divided and actually made more comments expressing interest in such results than expressing disinterest.
Neurological and psychological conditions were mentioned by participants from both health care facilities. Individual research results about Alzheimer disease and dementia were desired by participants in both the UBP and FQHC. “I wanna know when I start losing my mind what it is. I wanna make sure everybody else is not making me seem crazy. I already know what it is: I’m getting Alzheimer’s” (UF13). Results about all other mental disorders were explicitly unwanted in both health care facilities. “You’ll go crazy thinking you’re about to go crazy” (QM01), cautions one FQHC participant.
Although there was no prompt for the return of results about “fatal conditions” in the discussion guides, participants from the FQHC—but not the UBP—raised this issue. The vast majority of comments supported receiving these results. “[If] I have six months to live, that’s something I would want to know,” said one woman (QF07). Some individuals, however, explicitly did not want such results. “They shouldn’t tell people that. ‘You only got one year to live.’ Why you even tell people something like that?” (QF05).
Trust and mistrust
Our questions about trust were focused on whom participants would and would not trust involved in biobank oversight. “What individuals or groups should decide if research findings should be returned to participants?” “What individuals or groups would you not want involved in deciding if research findings should be returned to participants?”
Participants from both health care facilities acknowledged the valuable role of the government in funding biobanks, though they simultaneously describe the government as an untrustworthy institution to be involved in oversight. “I don’t want the government having access to my DNA…It’s the one person that has the access that we don’t want, and there’s no way around that!” (UF13). Participants from the FQHC—but not the UBP—also mentioned mistrust of the police. One participant called the police untrustworthy because “they’re corrupt” (QF07). Another participant said that if the police were to get a hold of one’s biobank information, “the police gon’ track ‘em down” (QM02). Some even related anecdotes of falsified DNA tests being used to frame innocent civilians. One individual acted out a hypothetical fraud by a police officer and his victim: “‘This guy right here is a bad element. We got to get him out of there!’ Then there’s a big to-do, and a couple months down the line, ‘Oh, we found something in the DNA!’ … ‘No, they have set me up!’” (QM01). Another FQHC participant stated that the use of genetics in forensics is always troubled by the inherent malleability of genetic evidence: “They can taint information; they can twist information in any way they want, to meet their specific analysis” (QM05).
The FQHC participants were unanimously mistrustful of the role of commercial entities in biobank-based research. The mere mention of sharing research data with commercial entities was deemed “exploitation” (QM04) by one participant. Another individual said flatly of biobank-based research, “I wouldn’t want anybody to make money off of this” (QM02). “I feel like [if] money …has a lot to do with [biobanking],” one man cautioned, “it’s gonna cripple what people are trying to do here” (QM04).
The UBP participants discussed their mistrust of two different commercial entities: insurance and pharmaceutical companies. Regarding the insurance industry, one respondent stated, “They [might] find out that African-Americans have a particular gene or something [then] they’ll be able to raise our rates higher than anybody else” (UF15). Regarding pharmaceutical companies, another respondent stated: “Within the last ten years, there have been so many instances where their profit margin and their vested interest were very closely tied as opposed to them wanting to affect the masses,” (UF03). However, two UBP participants also expressed positive feelings towards pharmaceutical companies: “I’ve taken a lot of pills in my day …that have alleviated a lot of pain, and I’ve got those pharmaceutical companies to thank for it” (UM03).
Many of the comments about trust, however, did not arise in response to our questions. Rather, they arose organically in response to other concerns—about whether to participate, about the consent process, and about the way in which participants could be affected by the research results. The UBP focus groups talked as frequently about trust as they did about mistrust. In the FQHC, however, there were three times more comments about mistrust than about trust.
Trust in science, medicine, and research differed between health care facilities. The UBP participants expressed strong support for the scientific enterprise, while the FQHC participants expressed mixed attitudes. For example, several comments from UBP participants reflected a willingness to trust that researchers would practice ethical discretion and hold participants’ best interest in mind. When asked whether limits should be placed on the range of research permitted from biobank samples, one UBP participant responded that he did not see the need for any: “If [the oversight committee] says this is a worthwhile project, have my spit” (UM03). In contrast, the FQHC participants never mentioned trusting the researchers themselves. One FQHC participant believed that, despite regulations to prevent ethical misconduct in research, researchers were still abusing participants’ rights: “No matter what is being said in this [focus] group, I feel it’s being done anyway” (QM01).
In our quantitative data, the majority of participants expressed trust in genetic research—with a trend, albeit not statistically significant, toward slightly greater trust in the UBP (75 %) compared to the FQHC (57 %). There was also a trend, albeit not statistically significant, towards increased expression of trust from pre- to post-engagement survey responses (Table 2).
Religion and the sacred
Religion was neither a topic in the educational sessions nor was it a question or prompt in the discussion guides. It did, however, appear as a minor theme in our coding. Religion-themed comments were coded nearly three times more frequently in the FQHC than in the UBP focus groups.
Only participants from the FQHC described their donated genetic samples as something of religious significance. One woman said, “That’s your sacred…You’re not supposed to pass it out [and] let them play with it” (QF08). Another participants from a different FQHC session said of donating a genetic sample, “A person is giving you one of the most sacred things that they have in life” (QM04).
Prayer was only mentioned in the FQHC focus groups. “It’s easy to pray…I learned that prayer changed stuff. I used to cry, [but] I learned to give myself 50 feet and 50 seconds and pray” (QF01). Another woman said that “[the doctors] said I couldn’t have a baby,” but then “I prayed about it and I asked God,” (QF08) and she believed that God listened to her prayer, allowing her to bear a child. FQHC participants also more often expressed beliefs that God does and should take an active role in our health. “I think what you guys [scientists] are doing here is good, but at the end of the day, God decides everything…If God don’t want you to know, you not gonna, and that’s that” (QM04). “If it’s intended [by God],” says one woman, “it’s gon’ happen” (QF13).
The FQHC participants mentioned that they would trust certain people with religious affiliations. One woman (QF04) said she would trust people from the church to protect the privacy of their biobank data. Another woman mentioned that she thought a preacher should be allowed to serve on a biobank oversight committee as long as he was “able to put aside his religion for medical reasons to save somebody” (QF02). However, participants from both health care facilities also expressed some discomfort with the role of clergy in biobanks. When asked whom they would not trust to protect the privacy of their genetic data, some FQHC participants mentioned ministers. One FQHC participant stated she would not trust ministers for this position “because they have ideas on certain things you shouldn’t do, and you shouldn’t bring religion [into biobank policy]” (QF08). A similar attitude was expressed by one UBP participant, who noted that “sometimes [ministers] may have knowledge of what they teach, but …they don’t necessarily have a grace in terms of how to present something” (UM02).
Discussion
Our research design was based on the principles of deliberative democracy (Fishkin 2009) but is more accurately described as a deliberative engagement, because it does not seek population representation. Rather our recruitment was intended to hear the diversity of opinions of African-Americans from different socioeconomic and educational backgrounds. The two clinics were not associated with each other and were chosen because they serve different populations on the South Side of Chicago. For instance, fewer than 10 % of the clientele at the FQHC were privately insured, while more than 75 % at the UBP were. While participants from the health care facilities were of similar age and gender, they differed in educational background (Table 1).
Recruitment uptake was quite different between the two health care facilities. Individuals from the UBP were less likely to agree to participate and often cited weekend work hours and family commitments as well as general disinclination to participate in research. However, those from the UBP who agreed to be re-contacted proved more likely to consent as well as to attend the first session. Individuals from the FQHC more frequently agreed to be re-contacted but were less likely to consent and to attend the sessions.
The aim of the overall project was to assess pre- and post-engagement attitudinal changes regarding biobank-based research, biobank governance, and the return of results. Our main finding was that there were no attitudinal changes. This may be due to the neutrality of the educational material presented, materials that went through many iterative processes to achieve non-biased content (Lemke et al. 2012). Another possible explanation is that individuals who agreed to participate had strongly held knowledgeable views prior to participation, although our survey data (see Table 1) show otherwise. A final explanation is that attitudes do not directly correlate with knowledge but are based, at least in part, on other values and beliefs, including trust (Corbie-Smith et al. 1999).
Overall, our findings about the role of trust in biobank enrollment, the reasons to participate or not to participate, and the strong interest in the return of individual results were very similar to those of other qualitative studies on biobanks (Lipworth et al. 2011; Murphy et al. 2008; Kaphingst et al. 2006). The academic literature often focuses on the differences between groups based on race and ethnicity, despite consensus that these concepts are not biological but social constructs (American Anthropological Association Statement on “race” 1998; Condit 2007). Researchers often focus on race as the significant variable (Bussey-Jones et al. 2010; Zimmerman et al. 2006; Durant et al. 2011; Hipps et al. 2003; Streicher et al. 2011; Goldenberg et al. 2011; Sterling et al. 2006) without fully evaluating the importance of other demographic factors that they collect, such as income and education. In contrast, we deliberately sought to compare attitudes of African-Americans from different socioeconomic and educational backgrounds.
We found great commonality in reasons for and against enrollment in biobanks across socioeconomic and educational lines. Participants from both health care facilities expressed reasons to participate that are well documented in the biobank literature: altruism and personal benefit (Ormond et al. 2009; Kettis-Lindblad et al. 2006). Reasons not to enroll included lack of knowledge of biobanking and policies not to return results, reasons that are well documented in the literature (Kaufman et al. 2008; Godard et al. 2007). Like Kaufman and colleagues, we found concerns about privacy (Kaufman et al. 2009), but this did not seem to influence enrollment decision.
Consistent with the literature, our survey data showed that most participants were interested in having research results returned (Murphy et al. 2008; Fong et al. 2006; Shalowitz and Miller 2008). This holds true for research results of clinical significance and to a lesser extent for ambiguous (Wendler and Emanuel 2002), untreatable (Murphy et al. 2008), and mental health (Hipps et al. 2003; Trippitelli et al. 1998) genetic research results. More nuanced perspectives were expressed in our focus group sessions, with participants expressing some reservations regarding the return of ambiguous results and mental health findings.
We also found some instructive differences between the participants from the two health care facilities. Consistent with other studies, we found lack of trust as a key factor hindering recruitment and as a reason not to enroll (Heiney et al. 2010; James et al. 2008). Although both groups expressed distrust of the commercialization of biobank-based research and the role of the government in biobank funding, participants from the FQHC also expressed distrust of the police, whereas participants from the UBP expressed distrust of insurance companies and pharmaceutical companies. In addition, participants from the UBP were three times more likely to discuss issues of trust—trust in the value of both science and the research enterprise (Fisher and Wallace 2000).
A second difference between the two health care facilities revolved around discussions of religion. Comments about religion came up three times more often in the FQHC focus groups, and prayer was mentioned only by FQHC participants. The broader discourse of religion among FQHC participants is consistent with other studies that find an inverse correlation between education and the invocation of religion as a resource for addressing health challenges (Johnson 1997; Weathers et al. 2009).
There are several limitations to our project. First was the small sample size that hampers most qualitative research. Nevertheless, our strategy was deliberately to recruit African-Americans with diverse socioeconomic and educational attainment status. Our decision to hold separate deliberative engagement sessions with participants from the two health care facilities allowed us to assess socioeconomic and educational differences in attitudes, which may better correlate with support for research and willingness to participate. However, whether our results are generalizable to African-American communities outside of the South Side of Chicago needs to be further studied.
A second limitation was that our sample was a convenience sample, and those who agreed to participate were more likely to be supportive of this type of project than would be found in a random sample. We had to approach 192 individuals to get 45 participants (23 %) to attend the sessions. Reasons for non-participation varied but still suggest room for bias.
A third limitation was that our questions and probes about trust focused on trust with regard to biobank governance or oversight and less about trust in genetic research or in research more generally. Thus our discussion is narrower than can be found in more general discussions (see, for example, Corbie-Smith et al. 1999).
A fourth limitation is that we did not directly inquire about religion and the church in our deliberative engagement. Churches play an important health role in many African-American communities (Watson et al. 2003; Giger et al. 2008; Beeghley et al. 1981), and our failure to address religion and the Church and their possible roles in biobank governance and in participants’ support for genetic research was an omission that should be corrected in future research.
Conclusion
Across socioeconomic and educational backgrounds, participants held many similar opinions, although the differences are instructive. Distrust was a major theme for all participants, although those from the health care facility that served mainly people with private health insurance expressed greater trust. Religion was more frequently invoked as a means to address health challenges by participants from the health care facility that served mainly those on public aid. Our findings suggest that medical researchers must move beyond analyses that consider the African-American population to be monolithic and value the diversity within it.
Acknowledgments
We thank Amy Lemke for her role in study design, training, and coding; Connie Robinson and Melanie Brown for serving as focus group facilitators; and Ellen Wright Clayton and Elizabeth Heitman for serving as expert consultants. The project was funded by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1RR024999.
References
- American Anthropological Association Statement on “race.” (May 17, 1998) On the web at: http://www.aaanet.org/stmts/racepp.htm. Last accessed 7 Feb 2012
- Beeghley L, Velsor E, Bock EW. The correlates of religiosity among Black and White Americans. Sociol Q. 1981;22:403–412. doi: 10.1111/j.1533-8525.1981.tb00670.x. [DOI] [Google Scholar]
- Bussey-Jones J, Garrett J, Henderson G, Moloney M, Blumenthal C, Corbie-Smith G. The role of race and trust in tissue/blood donation for genetic research. Genet Med. 2010;12:116–121. doi: 10.1097/GIM.0b013e3181cd6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condit C. How culture and science make race “genetic”: motives and strategies for discrete categorization of the continuous and heterogeneous. Lit Med. 2007;26:240–268. doi: 10.1353/lm.2008.0000. [DOI] [PubMed] [Google Scholar]
- Corbie-Smith G, Thomas SB, Williams MV, Moody-Ayers S. Attitudes and beliefs of African Americans toward participation in medical research. J Gen Int Med. 1999;14:537–546. doi: 10.1046/j.1525-1497.1999.07048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durant RW, Legedza AT, Marcantonio ER, Freeman MB, Landon BE. Willingness to participate in clinical trials among African Americans and Whites previously exposed to clinical research. J Cult Divers. 2011;18:8–19. [PMC free article] [PubMed] [Google Scholar]
- Fisher CB, Wallace SA. Through the community looking glass: reevaluating the ethical and policy implications of research on adolescent risk and sociopathology. Ethics Behav. 2000;10:99–118. doi: 10.1207/S15327019EB1002_01. [DOI] [PubMed] [Google Scholar]
- Fishkin JS. When the people speak: Deliberative democracy and public consultation. Oxford: Oxford University Press; 2009. [Google Scholar]
- Fong M, Braun KL, Chang M-L. Native Hawaiian preferences for informed consent and disclosure of results from genetic research. J Cancer Educ. 2006;21(Suppl):S47–S52. doi: 10.1207/s15430154jce2101s_10. [DOI] [PubMed] [Google Scholar]
- Giger JN, Appel SJ, Davidhizar R, Davis C. Church and spirituality in the lives of the African American community. J Transcult Nurs. 2008;19:375–383. doi: 10.1177/1043659608322502. [DOI] [PubMed] [Google Scholar]
- Godard B, Marshall J, Laberge C. Community engagement in genetic research: results of the first public consultation for the Quebec CARTaGENE project. Community Genet. 2007;10:147–158. doi: 10.1159/000101756. [DOI] [PubMed] [Google Scholar]
- Goldenberg AJ, Hull SC, Wilfond BS, Sharp RR. Patient perspectives on group benefits and harms in genetic research. Public Health Genom. 2011;14:135–142. doi: 10.1159/000317497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiney SP, Adams SA, Wells LM, Johnson H. Evaluation of conceptual framework for recruitment of African American patients with breast cancer. Oncol Nurs Forum. 2010;37:E160–E167. doi: 10.1188/10.ONF.E160-E167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipps Y, Roberts JS, Farrer LA, Green RC. Differences between African Americans and Whites in their attitudes toward genetic testing for Alzheimer’s disease. Genet Test. 2003;7:39–44. doi: 10.1089/109065703321560921. [DOI] [PubMed] [Google Scholar]
- James RD, Yu J-H, Henrikson NB, Bowen DJ, Fullerton SM. Strategies and stakeholders: minority recruitment in cancer genetics research. Community Genet. 2008;11:241–249. doi: 10.1159/000116878. [DOI] [PubMed] [Google Scholar]
- Johnson DC. Formal education vs. religious belief: soliciting new evidence with multinomial logic modeling. J Sci Study Relig. 1997;36:231–246. doi: 10.2307/1387555. [DOI] [Google Scholar]
- Kaphingst KA, Janoff JM, Harris LN, Emmons KM. Views of female breast cancer patients who donated biologic samples regarding storage and use of samples for genetic research. Clin Genet. 2006;69:393–398. doi: 10.1111/j.1399-0004.2006.00614.x. [DOI] [PubMed] [Google Scholar]
- Kaufman D, Murphy J, Scott J, Hudson K. Subjects matter: a survey of public opinions about a large genetic cohort study. Genet Med. 2008;10:831–839. doi: 10.1097/GIM.0b013e31818bb3ab. [DOI] [PubMed] [Google Scholar]
- Kaufman DJ, Murphy-Bollinger J, Scott J, Hudson KL. Public opinion about the importance of privacy in biobank research. Am J Hum Genet. 2009;85:643–654. doi: 10.1016/j.ajhg.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettis-Lindblad Å, Ring L, Viberth E, Hansson M. Genetic research and donation of tissue samples to biobanks. What do potential sample donors in the Swedish general public think? Eur J Public Health. 2006;16:433–440. doi: 10.1093/eurpub/cki198. [DOI] [PubMed] [Google Scholar]
- Lemke AA, Halverson CME, Ross LF (2012) Biobank participation and returning research results: perspectives from a deliberative engagement in Southside Chicago. Am J Med Genet. doi:10.1002/ajmg.a.34414 [DOI] [PMC free article] [PubMed]
- Lipworth W, Forsyth R, Kerridge I. Tissue donation to biobanks: a review of sociological studies. Sociol Health Illn. 2011;33:792–811. doi: 10.1111/j.1467-9566.2011.01342.x. [DOI] [PubMed] [Google Scholar]
- McQuillan GM, Porter KS. Consent for future genetic research: the NHANES experience in 2007-2008. IRB. 2011;33:9–14. [PubMed] [Google Scholar]
- McQuillan GM, Porter KS, Agelli M, Kington R. Consent for genetic research in a general population: the NHANES experience. Genet Med. 2003;5:35–42. doi: 10.1097/00125817-200301000-00006. [DOI] [PubMed] [Google Scholar]
- McQuillan GM, Pan Q, Porter KS. Consent for genetic research in a general population: an update on the National Health and Nutrition Examination Survey experience. Genet Med. 2006;8:354–360. doi: 10.1097/01.gim.0000223552.70393.08. [DOI] [PubMed] [Google Scholar]
- Murphy J, Scott J, Kaufman D, Geller G, LeRoy L, Hudson K. Public expectations for return of results from large-cohort genetic research. Am J Bioeth. 2008;8:36–43. doi: 10.1080/15265160802513093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormond KE, Cirino AL, Helenowski IB, Chisholm RL, Wolf WA. Assessing the understanding of biobank participants. Am J Med Genet A. 2009;149A:188–198. doi: 10.1002/ajmg.a.32635. [DOI] [PubMed] [Google Scholar]
- Pulley JM, Brace MM, Bernard GR, Masys DR. Attitudes and perceptions of patients towards methods of establishing a DNA biobank. Cell Tissue Bank. 2008;9:55–65. doi: 10.1007/s10561-007-9051-2. [DOI] [PubMed] [Google Scholar]
- Shalowitz D, Miller F. Communicating the results of clinical research to participants: attitudes, practices, and future directions. PLoS Med. 2008;5:0714–0720. doi: 10.1371/journal.pmed.0050091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling R, Henderson GE, Corbie-Smith G. Public willingness to participate in and public opinions about genetic variation research: a review of the literature. Am J Public Health. 2006;96:1971–1978. doi: 10.2105/AJPH.2005.069286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streicher SA, Sanderson SC, Jabs EW, Diefenbach M, Smirnoff M, Peter I, Horowitz CR, Brenner B, Richardson LD. Reasons for participating and genetic information needs among racially and ethnically diverse biobank participants: a focus group study. J Community Genet. 2011;2:153–163. doi: 10.1007/s12687-011-0052-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trippitelli CL, Jamison KR, Folstein MF, Bartko JJ, DePaulo JR. Pilot study on patients’ and spouses’ attitudes toward potential genetic testing for bipolar disorder. Am J Psychiatry. 1998;155:899–904. doi: 10.1176/ajp.155.7.899. [DOI] [PubMed] [Google Scholar]
- Watson D, Bisesi L, Tanamly S, Sim T, Branch C, William E. The role of small and medium-sized African American churches in promoting health lifestyle. J Relig Health. 2003;42:191–200. doi: 10.1023/A:1024835500987. [DOI] [Google Scholar]
- Weathers B, Kessler L, Collier A, Stopfer J, Domchek S, Halbert CH. Utilization of religious coping strategies among African American women at increased risk for hereditary breast and ovarian cancer. Fam Community Health. 2009;32:218–227. doi: 10.1097/FCH.0b013e3181ab3b53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendler D, Emanuel E. The debate over research on stored biological samples: what do sources think? Arch Int Med. 2002;162:1457–1462. doi: 10.1001/archinte.162.13.1457. [DOI] [PubMed] [Google Scholar]
- Zimmerman RK, Tabbarah M, Nowalk MP, Raymund M, Jewell IK, Wilson SA, Ricci EM. Racial differences in beliefs about genetic screening among patients at inner-city neighborhood health centers. J Natl Med Assoc. 2006;98:370–377. [PMC free article] [PubMed] [Google Scholar]