Abstract
Introduction
Islet autotransplantation (IAT) is performed at the time of total pancreatectomy (TP) to prevent or minimize post-surgical diabetes. Corticosteroids induce insulin resistance and present a risk to islet autografts, through glucotoxicity and increased metabolic demand on a marginal islet mass.
Case Reports
We present four IAT recipients treated with oral or injected corticosteroids after transplant for medical conditions unrelated to chronic pancreatitis or TPIAT. Hyperglycemia or insulin resistance were evident in all four cases, including reversion to long-term insulin therapy in two cases. One patient receiving corticosteroid injections had a transient increase in hemoglobin A1c (+0.6% above baseline), and one patient given a one time dose of oral dexamethasone exhibited hyperglycemia despite high insulin (>200 mU/L) and C-peptide (15.3 ng/mL) production on an oral glucose tolerance test.
Conclusions
IAT recipients have insufficient islet mass to compensate for the insulin resistance induced by corticosteroids. Caution should be given to using these agents in IAT recipients. When corticosteroids are medically necessary, insulin therapy should be administered temporarily to compensate for the increased metabolic demand and minimize long-term risks on the islet graft.
Introduction
Total pancreatectomy and intraportal islet autotransplantation (TP-IAT) may be performed for treatment of severe chronic pancreatitis. The goal of the IAT is to prevent or minimize the otherwise inevitable surgical diabetes (1). Because patients undergoing this procedure have a chronically inflamed and scarred pancreatic parenchyme, the number of islets available for transplantation is limited and highly variable between individuals (2,3). In addition, islets may be lost during the difficult isolation procedure. Thus, all recipients receive fewer islets transplanted than are present in an average adult pancreas. With this limited islet mass, approximately one-third of patients achieve insulin independence, while a significant number of insulin dependent patients retain enough endogenous insulin secretion to maintain near normal blood sugars on low dose insulin therapy (1,4).
Attrition in islet function does occur in some insulin independent patients (5). Although the reasons for this decline are unclear, possible factors include exhaustion of a marginal beta cell mass or environmental toxicity, such as hyperglycemia (6). In order to preserve insulin independence as long as possible, we are particularly careful to avoid chronic or severe acute hyperglycemia, to minimize the risk of glucotoxicity on the beta cells.
Corticosteroids induce skeletal muscle and hepatic insulin resistance and may cause hyperglycemia in susceptible individuals (7). Because most healthy non-diabetic individuals tolerate such medications without any notable sequelae, physicians may not recognize the potential of these agents to induce immediate hyperglycemia and even long-term declines in beta cell function in “non-diabetic” IAT recipients. Such individuals have a marginal islet mass that cannot adequately compensate for the degree of insulin resistance induced. Hyperglycemia, when extreme or sustained, may cause irreversible damage to the transplanted islets.
Although we have seen such cases, there are few publications available in the existing medical literature to alert primary care providers to the importance of exercising caution in prescribing these agents. To illustrate the potential risks of corticosteroids in IAT recipients, herein we report four insulin-independent patients status post TP-IAT who received brief treatments with glucocorticoids, resulting in transient hyperglycemia and/or failure of the islet autotransplant.
Case Reports
Case #1 is a 30y.o female with idiopathic chronic pancreatitis who underwent TP-IAT at age 23 years of age with a transplanted islet mass of 3,062 IE/kg. She was maintained on daily glargine therapy during the first year post-transplant, with hemoglobin A1c (HbA1c) levels of 6.8% and 6.2% at 5 and 9 months post-transplant. Glycemic control subsequently improved, and she was weaned off all insulin therapy at approximately 1 year post-transplant and remained off insulin until 4.8 years post-transplant, with HbA1c 5.5–5.8%. At 4.7 years post-transplant, the patient presented to the emergency department for a severe allergic reaction manifesting as generalized hives. She received methylprednisolone 125mg IV and an antihistamine and was discharged on prednisone (20mg PO twice daily for 3 days, followed by 20 mg PO daily for 3 days). She was subsequently seen by her primary care provider, who prescribed additional prednisone 20 mg tablets for an unclear duration due to unresolved hives. The patient was not monitoring blood sugars during this interval. At 3 weeks after initiation of high dose corticosteroids, the patient presented with complaints of excessive thirst, muscle spasms, loss of appetite, and weight loss, with onset of symptoms after starting the prednisone. A random blood sugar at this time was 438 mg/dL and HbA1C level was 10%, a significant increase from 5.6% four months prior. The patient resumed insulin therapy with a basal-bolus regimen of glargine and aspart, and was later transitioned to an insulin pump. Her most recent HbAIc was 5.8% on a continuous subcutaneous insulin infusion by ambulatory insulin pump.
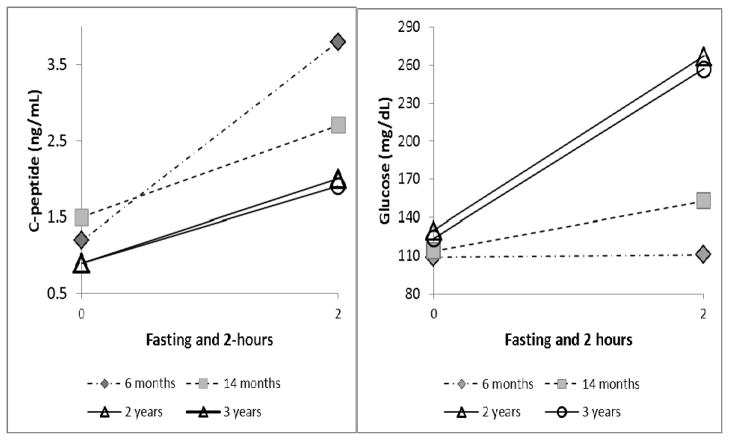
Case #2 is a 68 year old woman who underwent TPIAT for idiopathic chronic pancreatitis at age 65 years. She received 3,248 IE/kg transplanted intraportally. She subsequently maintained borderline islet function, off insulin, with HbA1c of 6.7% at 3 and 6 months post-transplant, and 6.9% at 14 months posttransplant. C-peptide levels from a mixed meal tolerance tests (Boost HP, 360mL) are displayed in figure 1. At 17 months post-transplant, HbA1c was increased to 7.5%, and the patient initiated correction scale aspart but no other insulin. She was started on gliperimide by her primary care physician per the patient’s desire to try to remain off insulin. At 22 months posttransplant, due to leg cramps, the patient was prescribed high dose prednisone (50 mg daily for two days, subsequently tapered down by 10 mg every 2 days). The patient noted an immediate increase in self-monitored blood sugars to greater than 400 mg/dL, and prednisone was stopped after less than 1 week. Islet function was subsequently retested at 2 years post-transplant (off prednisone), and revealed HbA1c 8.7%, decreased C-peptide response to MMTT, and elevated glucoses on MMTT (figure 1). She was restarted on insulin therapy, and most recently requires 10 units glargine daily with correction scale aspart. HbA1c was improved (6.9%) and C-peptide stable on mixed meal testing at 3 year follow up.
Figure 1.
C-peptide and glucose values fasting and 2 hours after a mixed meal stimulus (Boost HP, 360 mL) for case #2 at various time points. Corticosteroids were administered between the 14 month and 2 year time points. Fasting and 2 hour stimulated C-peptide levels were lower and glucoses higher at the 2 and 3 year follow up, compared to earlier assessments. However, the function appeared similar between 2 and 3 years.
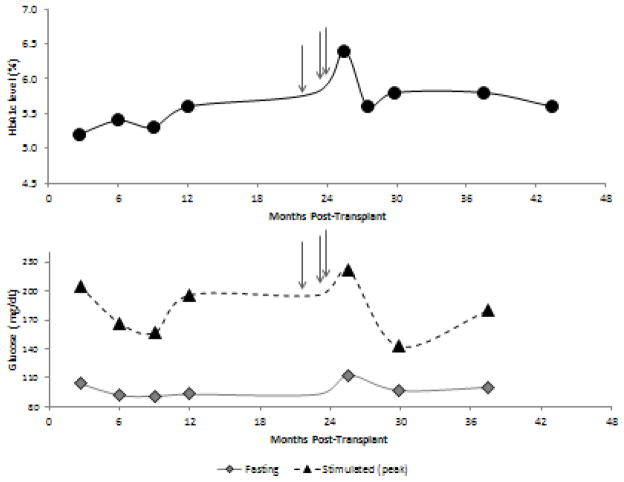
Case #3 of age. She received 3,758 IE/kg intraportally, and discontinued all insulin therapy at 2 months post-transplant. She maintained HbA1c levels of 5.2–5.6% at 3–12 months post-transplant. She developed shoulder and neck pain, and was treated with steroid injections at 21 months (one injection of unknown dose) and 24 months (two injections of 80mg triamcinolone and 10mg dexamethasone respectively). At subsequent follow up at 26 months, HbA1c had risen to 6.4% (figure 2). She was started on an oral anti-diabetic agent, sitagliptin, for 2 months; HbA1c improved to 5.6%, and all anti-diabetogenic therapy was discontinued. She has subsequently maintained HbA1c levels stable and consistently <6% off insulin and avoiding corticosteroids.
Figure 2.
Case #3 HbA1c, fasting, and peak stimulated glucose (from mixed meal tolerance test) over 3 years post-transplant. The arrows indicate three corticosteroid injections, which preceded the highest HbA1c, fasting glucose, and peak stimulated glucose for this subject. The subject remains insulin independent through 3 years of follow up.
Case #4 is a 19 y.o female who underwent TPIAT at the age of 14 years for chronic pancreatitis attributed to idiopathic disease and annular pancreas. She received a large islet mass of 11,576 IE/kg (approximately 8,000 IE/kg transplanted intraportal and the remaining islets in the peritoneal cavity). The patient achieved and maintained insulin independence, with HbA1c levels of 5.4–5.7%. At 3.2 years post-transplant, the patient received a single dose of dexamethasone (6 mg intravenously) for nausea related to an endoscopy procedure. Incidentally, she was scheduled for a routine oral glucose tolerance test (OGTT, 75 grams glucose load) the following morning. This test revealed significant insulin resistance; despite a robust insulin response (peak insulin level of 210 mU/L and C-peptide 15.3 ng/dL), the patient had profound post-prandial hyperglycemia, with blood sugar >400 mg/dL at 60 and 90 minutes after the glucose beverage (table 1). The patient’s HbA1c levels on the day of the test and repeat 5 weeks later were both 5.7%, suggesting that the hyperglycemia was specifically related to the corticosteroid and not indicative of the patient’s usual glycemic control. This patient had no long-term sequelae and remains insulin independent.
Table 1.
Results of oral glucose tolerance testing (75 grams) from case #4, performed 15 hours after receiving 6 mg of dexamethasone. Hyperglycemia was observed despite excellent secretory capacity of the intraportal islet graft, as documented by elevated insulin and C-peptide levels. The patient’s hemoglobin A1c was 5.7% at this time and several weeks later, suggesting that this profound post-prandial hyperglycemia was transient, related to corticosteroid-induced insulin resistance.
| Time (minutes) | Glucose (mg/dL) | Insulin (mU/L) | C-peptide* (ng/mL) |
|---|---|---|---|
| 0 | 100 | 35 | 3.9 |
| 30 | 291 | 71 | |
| 60 | 431 | 107 | |
| 90 | 410 | 210 | |
| 120 | 303 | 179 | 15.3 |
| 150 | 175 | 111 | |
| 180 | 111 | 63 | |
| 210 | 81 | 47 | |
| 240 | 73 | 38 |
C-peptide level was assayed at the fasting and 120 minute time points only
Discussion
High dose corticosteroids are commonly prescribed as anti-inflammatory agents for a number of conditions. As illustrated in the cases above, use of these agents in islet autotransplant recipients may cause transient or permanent compromise in glycemic control and warrants special consideration. IAT patients have a marginal islet mass, which may be unable to compensate for the profound insulin resistance induced by even brief exposure to high dose corticosteroids. Resulting hyperglycemia may mediate permanent damage through glucotoxicity. For this reason, we advocate that corticosteroids be avoided in IAT recipients when alternative therapies are available.
Glucocorticoids induce both skeletal muscle and hepatic insulin resistance, thereby reducing both post-prandial glucose uptake and the normal suppression of hepatic gluconeogenesis (7). The extent of insulin resistance can be profound and sufficient to induce diabetes in susceptible individuals. This is illustrated well in case #4. This patient received only a single dose of dexamethasone but shortly thereafter demonstrated profound post-prandial hyperglycemia despite the presence of relatively high insulin levels. Case #3 received just 3 isolated injections of corticosteroids, yet this was sufficient to elevate the hemoglobin A1c to 6.4%, significantly higher than any of her HbA1c levels before or since that time. Interestingly, there may also be an acute inhibitory effect of corticosteroids on insulin secretion, which would further impair the ability of the transplanted beta cells to compensate (7,8).
IAT recipients have a small islet mass compared to healthy individuals. For example, while an average adult pancreas may have 1 million islets (9), our IAT recipients receive 230,000 islet equivalents transplanted on average; some of the transplanted islets likely succumb to hypoxia and apoptosis, leaving an even smaller surviving islet mass (10). Thus, corticosteroid-induced insulin resistance places a great burden on these few islets, which are unable to compensate. The resultant hyperglycemia can lead to permanent damage to the beta cells, as is evidenced most clearly in case #1. This patient had a normal HbA1c and was insulin independent at more than 4 years after her transplant, but presented with rapid onset of symptomatic diabetes after prednisone treatment and continues to require an ambulatory insulin pump even after discontinuing steroids. Case #2 also had an irreversible decline in islet function documented shortly after corticosteroid administration, although modest hyperglycemia was present even prior to corticosteroid administration and may have contributed in this case.
Intraportal islets may be particularly susceptible to glucotoxicity. This is documented best in the early post-transplant period, during the time of islet engraftment. During islet engraftment, exposure to hyperglycemia greatly increases the proportion of apoptotic beta cells (6). Late hyperglycemia may also damage beta cells, by increasing oxidative stress and shifting beta cells towards a state of apoptosis, similar to that seen with type 2 diabetes mellitus (11). Elevations in circulating fatty acids induced by corticosteroids may cause further damage to the beta cells in the face of high glucose, a concept known as glucolipotoxicty (11).
IAT studies in canines have clearly documented the adverse effect of prednisone. A short course of prednisone given early or late in the post-transplant period induced hyperglycemia and accelerated islet graft failure in dogs. However, this effect could be mitigated by administering insulin during the period of steroid therapy (12–14). Interestingly, one of the key advances in cadaveric donor islet allo-transplantation for type 1 diabetes was removing corticosteroids from the immunosuppression regimen (15), and current alloislet transplant protocols avoid corticosteroids (16).
Although we advocate avoiding corticosteroids when possible, there may be rare circumstances in which corticosteroid therapy is medically necessary. In these cases, we encourage the involvement of the patient’s endocrinologist and transplant care team. During the time of corticosteroid administration, insulin can be started or dose increased empirically to avoid glycemic stress on the islets. Patients should monitor blood sugars closely (for example, pre-meal and 2 hours after a meal). The goal is to aggressively maintain blood sugars in a normal or near-normal range, to prevent glucotoxicity and beta cell exhaustion. Insulin can be weaned down or off after the completion of steroid therapy.
Conclusions
In summary, these cases illustrate hyperglycemia in IAT recipients receiving corticosteroid therapy. In some cases, this may lead to irreversible diabetes mellitus and thus corticosteroids should be used with caution. Based on these experiences and the available evidence, we recommend the following: 1) avoidance of corticosteroids whenever medically feasible; 2) when no alternatives exist, corticosteroids should be administered only under careful monitoring and in conjunction with a physician experienced in insulin management.
References
- 1.Blondet JJ, Carlson AM, Kobayashi T, Jie T, Bellin M, Hering BJ, et al. The role of total pancreatectomy and islet autotransplantation for chronic pancreatitis. Surg Clin North Am. 2007 Dec;87(6):1477–501. x. doi: 10.1016/j.suc.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi T, Manivel JC, Carlson AM, Bellin MD, Moran A, Freeman ML, et al. Correlation of histopathology, islet yield, and islet graft function after islet autotransplantation in chronic pancreatitis. Pancreas. 2011 Mar;40(2):193–199. doi: 10.1097/mpa.0b013e3181fa4916. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi T, Manivel JC, Bellin MD, Carlson AM, Moran A, Freeman ML, et al. Correlation of pancreatic histopathologic findings and islet yield in children with chronic pancreatitis undergoing total pancreatectomy and islet autotransplantation. Pancreas. 2010 Jan;39(1):57–63. doi: 10.1097/MPA.0b013e3181b8ff71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Islet Auto Transplants at the University of Cincinnati: A 5 Year Experience. Clinical and Experimental Islet Transplantation I. 2006 Jul 26;2006 [Google Scholar]
- 5.Sutherland DE, Gruessner AC, Carlson AM, Blondet JJ, Balamurugan AN, Reigstad KF, et al. Islet autotransplant outcomes after total pancreatectomy: a contrast to islet allograft outcomes. Transplantation. 2008 Dec 27;86(12):1799–1802. doi: 10.1097/TP.0b013e31819143ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biarnes M, Montolio M, Nacher V, Raurell M, Soler J, Montanya E. Beta-cell death and mass in syngeneically transplanted islets exposed to short- and long-term hyperglycemia. Diabetes. 2002 Jan;51(1):66–72. doi: 10.2337/diabetes.51.1.66. [DOI] [PubMed] [Google Scholar]
- 7.van Raalte DH, Ouwens DM, Diamant M. Novel insights into glucocorticoid-mediated diabetogenic effects: towards expansion of therapeutic options? Eur J Clin Invest. 2009 Feb;39(2):81–93. doi: 10.1111/j.1365-2362.2008.02067.x. [DOI] [PubMed] [Google Scholar]
- 8.Schacke H, Docke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002 Oct;96(1):23–43. doi: 10.1016/s0163-7258(02)00297-8. [DOI] [PubMed] [Google Scholar]
- 9.Kim SK, Hebrok M. Intercellular signals regulating pancreas development and function. Genes Dev. 2001 Jan 15;15(2):111–127. doi: 10.1101/gad.859401. [DOI] [PubMed] [Google Scholar]
- 10.Boker A, Rothenberg L, Hernandez C, Kenyon NS, Ricordi C, Alejandro R. Human islet transplantation: update. World J Surg. 2001 Apr;25(4):481–486. doi: 10.1007/s002680020341. [DOI] [PubMed] [Google Scholar]
- 11.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008 May;29(3):351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rilo HL, Carroll PB, Zeng YJ, Fontes P, Demetris J, Ricordi C. Acceleration of chronic failure of intrahepatic canine islet autografts by a short course of prednisone. Transplantation. 1994 Jan;57(2):181–187. doi: 10.1097/00007890-199401001-00004. [DOI] [PubMed] [Google Scholar]
- 13.Kaufman DB, Morel P, Condie R, Field MJ, Rooney M, Tzardis P, et al. Beneficial and detrimental effects of RBC-adsorbed antilymphocyte globulin and prednisone on purified canine islet autograft and allograft function. Transplantation. 1991 Jan;51(1):37–42. doi: 10.1097/00007890-199101000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Morel P, Kaufman DB, Field MJ, Lloveras JK, Matas AJ, Sutherland DE. Detrimental effect of prednisone on canine islet autograft function. Transplant Proc. 1992 Jun;24(3):1048–1050. [PubMed] [Google Scholar]
- 15.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000 Jul 27;343(4):230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 16. [Accessed June 1, 2011.]; http://www.isletstudy.org/