Abstract
Background
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is characterized by red blood cell (RBC) destruction in response to oxidative stress. Although blood donors are not routinely screened for G6PD deficiency, the transfusion of stored G6PD-deficient RBCs may have serious adverse outcomes. By measuring G6PD enzyme activity of RBC units from a large metropolitan hospital transfusion service we sought to determine 1) the prevalence of G6PD-deficient RBC units, 2) if G6PD activity changes during storage, and 3) if G6PD activity in segments correlates with its activity in the bags.
Study Design and Methods
Quantitative G6PD activity was measured in 301 randomly selected packed RBC (pRBC) units and 73 D+C-E- (i.e. R0r or R0R0) pRBC units, all stored in additive solutions. G6PD deficiency was defined as activity <60% of the normal mean.
Results
The frequency of G6PD-deficient units in the general inventory was 0.3% (1/301) [95% CI <0.01%–2.1%]. In contrast, its frequency in D+C-E- pRBC units was 12.3% (9/73) [95% CI 6.4%–22.0%]. G6PD activity did not significantly change during the 42 day storage period, and G6PD activity measured in pRBC storage bags and attached segments correlated well (r=0.7–0.9, p≤0.001, Spearman rank correlation).
Conclusions
Although the frequency of G6PD-deficient pRBC units in the transfusion service general inventory was relatively low, it was significantly higher among a subset of R0r or R0R0 units. The latter are preferentially allocated for transfusion to patients with sickle cell disease to decrease the risk of RBC alloimmunization, possibly allowing more of these units to be inadvertently targeted to these patients.
Keywords: glucose-6-phosphate dehydrogenase deficiency, hemolysis, oxidative stress, sickle cell disease
INTRODUCTION
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is the most common enzyme deficiency, affecting approximately 400 million people world-wide. It manifests as red blood cell (RBC) destruction in response to oxidative stress, which can be precipitated by infection, and by the ingestion of certain medications and foods (e.g. certain anti-malarial medications, antibiotics, and fava beans). The prevalence of G6PD deficiency varies among populations and is most commonly found in individuals from sub-Saharan Africa, the Mediterranean region, and south-east Asia.1 Because G6PD deficiency has an X-linked inheritance, it is more common in males than females. In the United States, males of African descent have the highest prevalence of G6PD deficiency, approaching 10%.2
Although in most studies G6PD-deficient individuals have normal RBC survival at steady-state,3 this may vary based upon the G6PD variant present, and some individuals may have shortened RBC survival.4 In addition, normal subjects transfused with G6PD-deficient RBCs and subsequently given primaquine or nitrofurantoin (medications known to cause hemolysis in G6PD-deficient individuals), experienced both intra- and extra-vascular hemolysis.5,6 Finally, case reports and a prospective study provided evidence of hemolysis, and even death, in patients transfused with G6PD-deficient RBCs.7–9
It is not routine practice to screen blood donors for G6PD deficiency, and information about the frequency of G6PD deficiency among blood donors in the United States is lacking. However, the frequency of G6PD deficiency varies among the multiple ethnic groups residing in the United States.10–12 In addition, G6PD-deficient donor RBCs may store more poorly than normal RBCs, as evidenced by decreased post-transfusion recovery.13 Furthermore, irradiated G6PD-deficient RBCs have a significantly shortened half-life as compared to normal RBCs.14 The current study examines 1) the prevalence of G6PD-deficient RBC units in a large hospital transfusion service, 2) whether G6PD enzyme activity changes during storage, and 3) whether G6PD activity in segments correlates with its activity in the bags, thereby allowing testing of the segments to evaluate the G6PD activity of the transfused RBCs. In addition, a population of patients who may have a higher likelihood of being transfused with these units was identified.
MATERIALS AND METHODS
Blood Products
Packed RBC (pRBC) units were obtained from the Columbia University Medical Center-New York Presbyterian Hospital Blood Bank inventory. pRBCs were collected from volunteer donors, preserved in additive solution (AS-1, AS-3, or AS-5), purchased from local suppliers (e.g. the New York Blood Center or Metro), and stored under standard blood banking conditions for up to 42 days.
Quantitative Glucose-6-Phosphate Dehydrogenase Assay
The quantitative G6PD assay was performed at 30°C using the Trinity Biotech (Berkeley Heights, NJ) quantitative G6PD assay kit. G6PD activity was expressed as units of enzyme activity per gram of hemoglobin (U/g Hb). Assay reproducibility was determined by assaying 20 normal and G6PD-deficient control samples (which were obtained from Trinity Biotech) on the same day (intra-assay) or over 20 separate runs on multiple days (inter-assay) followed by calculation of the mean, standard deviation (SD), and coefficient of variation (CV).
Comparison of G6PD Activity in Storage Bags and Segments of pRBC Units
G6PD activity was measured in samples from storage bags and adjoining segments either from discarded pRBC units of varying ages that were used for erythrocytapheresis for sickle cell disease patients (n=37) or units that were purchased and repeatedly sampled over the storage period (n=16). To obtain RBCs from the storage bags, a sampling site coupler (Charter Medical, Lakewood, NJ) was attached to the port of each bag. Samples for testing were collected by swabbing the sampling site with 70% ethanol, followed by introduction of a sterile 18G needle (Becton Dickinson, Franklin Lakes, NJ).
Evaluation of G6PD Activity during Refrigerated Storage
Cross-sectional and longitudinal studies were used to evaluate G6PD activity during storage. G6PD activity was measured in a cross-sectional sample of segments from units selected at 7 day intervals of storage up to Day 42 (n=161). To evaluate changes in activity of single units during storage, G6PD activity was also repeatedly measured at 7 day intervals of storage from Day 5 or 6 (the age when the units were obtained) until Day 42 in the bags and segments of purchased RBC units that were not used for transfusion (n=16).
Statistics
The Spearman rank correlation was used for assessing the relatedness between variables. The Kruskal-Wallis one-way analysis of variance was used to compare G6PD levels throughout storage of red cells. The paired t test was used for comparing the means of 2 groups. To determine if a data set was normally distributed, the Kolmogorov-Smirnov normality test was used. Fisher’s exact test was used to determine the presence of a non-random association between two groups. Power and sample size PS software version 3.0.43 was used to provide sample size justification. The Fisher’s exact test was used to evaluate the null hypothesis that there is no difference between the proportion of G6PD deficient units in the D+C-E- and general inventories. Based on 1) an estimated proportion of G6PD deficiency of 10% and 0.3% in the D+C-E- and general inventories, respectively, 2) a type 1 error probability α=0.05, 3) a power of 0.90, and 4) a 1:4 allocation of D+C-E- to general inventory units, we determined the need to measure G6PD activity in at least 66 D+C-E- and 264 general inventory units.
RESULTS
G6PD Assay
Intra- and inter-assay reproducibility was assessed by testing normal and G6PD-deficient controls 20 times. The intra-assay CV for the normal and deficient controls was 4% (mean 7.0 U/g Hb, SD 0.3) and 18% (mean 0.34 U/g Hb, SD 0.06), respectively. The inter-assay CV for the normal and deficient controls was 4% (mean 7.9 U/g Hb, SD 0.3) and 85% (mean 0.13, SD 0.11), respectively.
Comparison of G6PD Activity in pRBC Storage Bags and Attached Segments
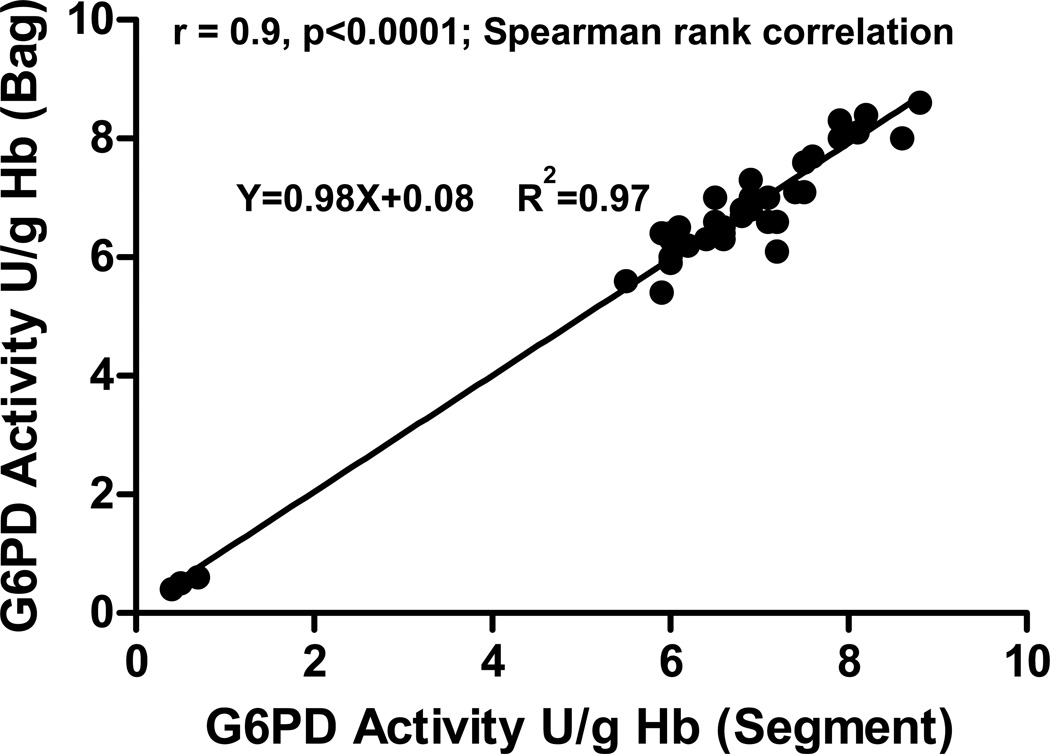
Because of possible differences in the cellular environment in the pRBC unit and the attached segments, the correlation between the G6PD enzyme activities in both sources was determined. Using a cross-sectional study design, 37 discarded pRBC units of varying storage duration, which were used for erythrocytapheresis procedures, were obtained along with their respective segments. There was an excellent correlation (r=0.9, p<0.0001, Spearman rank correlation) between the G6PD activity measured in the RBC units and the associated segments (Figure 1). In addition, as shown in Figure 1, the low G6PD activity detected in three of these pRBC units correlated with low G6PD activity in the associated segments. In addition, this correlation remained throughout the 42-day storage period, when serially evaluated in 16 purchased pRBC units that were specifically obtained for this purpose. From Day 5–6 to Day 42 of storage, the correlation was r=0.7–0.9, p≤0.001, Spearman rank correlation (data not shown).
Figure 1. Correlation of G6PD activity measured in pRBC storage bags and attached segments.
RBC G6PD activity was measured in the storage bags and the attached segments from discarded pRBC units (n=37) that were obtained following erythrocytapheresis procedures. The storage age of these units ranged from 5 to 27 days. Seventeen of these units were known to have a phenotype of D+, C-, E-, and 3 of these 17 units were G6PD-deficient.
G6PD Activity in pRBCs During Refrigerated Storage
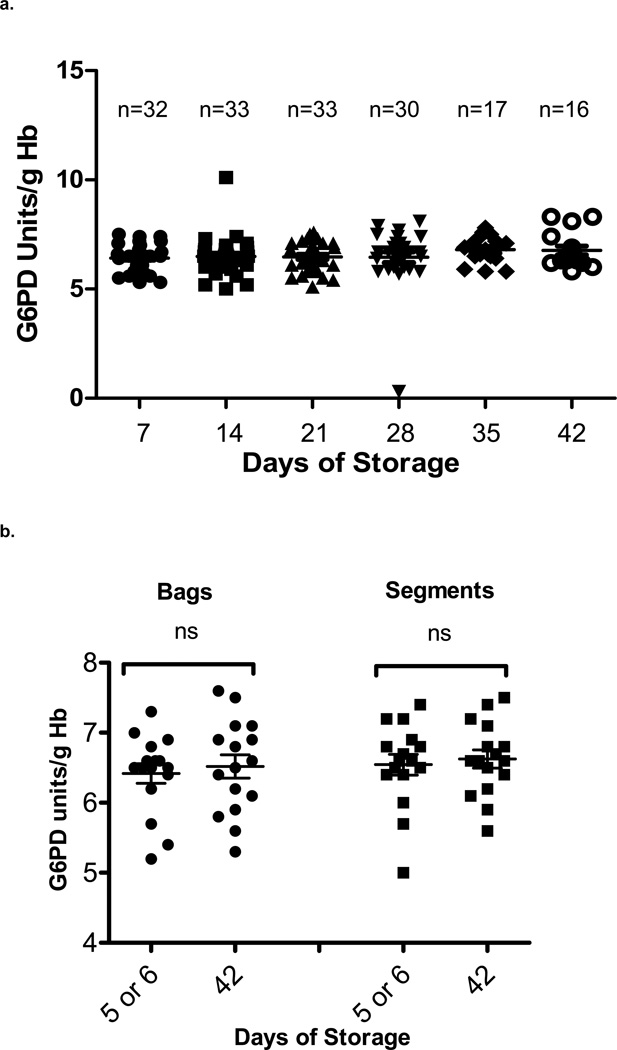
To determine if G6PD activity changed during refrigerated storage under standard blood banking conditions, G6PD activity was measured in a cross-sectional sample of units in the general inventory that were randomly selected at 7 day intervals of storage. G6PD activity was measured in segments from 161 different pRBC units randomly selected at Day 7 until Day 42 of storage (Day 7, n=32; Day 14, n=33; Day 21, n=33; Day 28, n=30; Day 35, n=17; Day 42, n=16). As shown in Figure 2a, there was no statistically significant difference in the mean G6PD activity measured at each time-point (p=0.4, Kruskal-Wallis test). Similar results were obtained when G6PD activity was measured longitudinally at 7 day intervals in 16 expressly-purchased pRBC units that were each obtained on Day 5–6 of storage and sampled repetitively until outdate at Day 42 (Figure 2b). There was no significant difference in G6PD activity between storage Day 5–6 and storage Day 42 in either bags (p=0.3, paired t test) or segments (p=0.2, paired t test).
Figure 2. G6PD activity during RBC storage.
a. Cross-sectional study of G6PD activity in 161 randomly-selected pRBC units in the general inventory. G6PD activity was measured in the attached segments of units selected for the following storage intervals: Day 7 (n=32), 14 (n=33), 21 (n=33), 28 (n=30), 35 (n=17), and 42 (n=16). Mean activities at each time-point were compared using the Kruskal-Wallis test. There was no significant difference in G6PD activity during the storage period examined (p=0.4). One unit selected at 28 days of storage was identified as G6PD deficient. b. Longitudinal study of G6PD activity in 16 purchased pRBC units (bags and segments), serially measuring activities beginning at Day 5 or 6 of storage and ending at Day 42. There was no significant difference in G6PD activity between day 5 or 6 and day 42 of storage in bags (p=0.3, paired t test) or segments (p=0.2, paired t test). ns=not significant.
Frequency of G6PD-Deficient pRBC Units in the General Inventory
To determine the reference range for G6PD activity in pRBC units, measurements were performed using 301 pRBC segments. Because the data obtained had a non-Gaussian distribution (p<0.0001, Kolmogorov-Smirnov normality test), a non-parametric percentile method was used to define the limits of the reference range. The mean, median, and standard deviation for G6PD activity of pRBC units in our transfusion service were 6.5 U/g Hb, 6.5 U/g Hb, and 0.8, respectively. The reference range for G6PD activity in the pRBC units was calculated as 5.3–8.0 U/g Hb (2.5–97.5 percentiles). G6PD activity that was less than 60% of the activity of the normal mean, 6.5 U/g Hb, was used to define G6PD deficiency; this is in accordance with the WHO classification, whereby G6PD variants having less than 60% activity are considered deficient.15 Therefore, pRBC units were considered deficient when the G6PD activity was <3.9 U/g Hb. Of the 301 pRBC units that were evaluated, only one unit was determined to be G6PD deficient with an activity of 0.3 U/g Hb, resulting in a frequency of 1/301 or 0.3% [95% CI <0.01%–2.1%] of G6PD-deficient pRBC units in the general inventory.
Frequency of G6PD-Deficient pRBC Units in the R0r or R0R0 inventory
In the United States, the prevalence of G6PD deficiency is highest in males of African descent. Moreover, the D-antigen positive, C- and E-antigen negative (i.e. R0r or R0R0) phenotype is common among donors of African descent. Thus, we hypothesized that the frequency of G6PD-deficient units would be increased in pRBCs of this phenotype. To test this hypothesis, G6PD activity was measured in 73 units with the D+C-E- phenotype. It was observed that 12.3% (9/73) [95% CI 6.4%–22.0%] of these units were G6PD deficient; a frequency significantly greater than the 0.3% observed in the general inventory (p<0.0001, Fisher’s exact test). Of the 9 G6PD-deficient units identified, 7 had activities between 0.3 and 0.6 U/g Hb, and 2 units had activities of 2.1 and 2.5 U/g Hb, respectively.
DISCUSSION
G6PD activity measured in the pRBC storage bag correlated well with that in the attached segment. In addition, during the FDA-allowable storage period examined, the G6PD activity in pRBC units having normal G6PD activity did not change significantly. This establishes the validity of testing G6PD activity in pRBC segments regardless of storage time, both for the purposes of this study and as a general strategy. In the general population, the frequency of G6PD deficiency was 0.3%. Given the magnitude of RBC transfusion (approximately 15 million units a year in the USA), this predicts that ~45,000 units of G6PD-deficient RBCs may be transfused each year. Of particular note was the observation that when certain antigen-matched pRBC units were tested, which would be expected to be from donors of African descent, the frequency of G6PD-deficient units was 12.3%, which was significantly higher. These particular pRBC units are more likely to be transfused into patients with sickle cell disease, who may receive multiple such units in the setting of an RBC exchange transfusion. If accumulated evidence demonstrates an increased probability of transfusing G6PD-deficient pRBCs in certain patient populations, then screening for G6PD activity in segments prior to transfusion is a viable approach. Measurements of other analytes, such as alanine aminotransferase16 and cytokines,17 are also known to correlate well in storage bags and attached segments of whole blood and pRBC units, respectively.
G6PD activity during RBC storage under standard blood banking conditions was previously examined in both normal and G6PD-deficient RBCs. In one study, G6PD activity in G6PD-deficient RBCs decreased rapidly and became undetectable by Day 7 of storage.18 This may be due to the protein instability of the mutated enzyme variants.19 Studies of G6PD activity during storage of normal, non-deficient, RBCs had varying results, either demonstrating no decrease in activity20 or a decrease of up to 35%.21 The reasons for these differences are not clear; however, methodological variations in storage conditions and preservative solutions may play a role. In the current study, G6PD activity did not decrease between 5 and 42 days of storage in pRBC units with normal G6PD activity. One possible reason for not observing a decrease in G6PD activity may relate to the kinetics of the decrease of G6PD activity during storage; thus, Swarup-Mitra et al. reported that G6PD activity in normal RBCs decreases during the first week of storage and then remains unchanged.18 The earliest storage day on which we measured G6PD activity was Day 5, raising the possibility that an initial decrease in G6PD activity had occurred, but was not observed. Nonetheless, the reference range defined by quantifying enzyme activity in these pRBC units overlapped with that determined using freshly-obtained blood samples (data not shown) and no decrease in G6PD activity was seen when freshly obtained blood samples were stored at 4°C for 7–10 days (data not shown).
The prevalence of G6PD deficiency varies based on the population being considered; in the United States, it is most often found in individuals of African or Mediterranean descent.1 To the best of our knowledge, there are no published studies quantifying the prevalence of G6PD deficiency among healthy blood donors in the United States. An analysis of G6PD deficiency among United States military personnel estimated a prevalence of 0.3% among Caucasian subjects and 12.2% among African Americans.10 In addition, data from the Retrovirus Epidemiology in Blood Donors Study-II (REDS-II) indicate that approximately 87% of blood donors in the United States are Caucasian and approximately 6% are African American.22 These data are consistent with the frequency of G6PD-deficient pRBC units in both our general inventory and our D-antigen positive, and C- and E-antigen negative inventory. Our general hospital inventory most likely reflects the general donor population, which is mostly Caucasian, thereby explaining the low frequency of G6PD-deficient units (0.3%). The D-positive, C- and E-negative RBCs are expected to be derived from donors who are predominantly of African descent, and this is consistent with the frequency of 12.3% G6PD deficiency that we observed.
The risks of transfusing G6PD-deficient RBCs into patients are likely affected by various factors, including the degree of G6PD deficiency of the donor, the presence or absence of an oxidative stress in the recipient (such as an infection or a medication known to cause hemolysis of G6PD-deficient RBCs), the amount of RBCs transfused as a proportion of the blood volume of the recipient, and the storage interval of the donor RBCs.18 For example, stored RBCs from G6PD-deficient donors were shown to have a significantly lower 24-hour post-transfusion recovery as compared with normal RBCs.13 In addition, intra- and extravascular hemolysis were seen in normal recipients of G6PD-deficient RBCs who simultaneously received primaquine or nitrofurantoin, medications known to cause hemolysis in G6PD-deficient individuals.5,6 The outcomes of transfusion of G6PD-deficient RBCs into patients who are not receiving oxidizing medications are more variable, ranging from no evidence of decreased RBC recovery,23 to increases in bilirubin and lactate dehydrogenase,8 and, finally, to frank hemolysis with decreased hematocrit and hemolglobinuria.7 Therefore, it may be desirable to ensure that patients who may be subject to excessive oxidative stress do not receive RBCs from G6PD-deficient donors. This may be particularly true for individuals who are at risk for receiving multiple pRBC units from G6PD-deficient donors, such as patients with sickle cell disease.
In summary, the data presented demonstrate that, although the overall prevalence of G6PD-deficient RBC units is low in the general blood donor population of a large metropolitan transfusion service, there is a subset of patients, because of their requirement for antigen-matched units, who are more likely to receive a pRBC unit from a G6PD-deficient donor. For example, because of high rates of alloimmunization to RBC antigens, patients with sickle cell disease frequently require antigen-matched units from donors of presumed African descent. The consequences of transfusing G6PD-deficient RBCs to such patients are currently unknown. Additional studies are currently underway to determine the effect of G6PD-deficiency on the storage properties of RBCs, as well as to elucidate the physiological effects of transfusion of G6PD-deficient RBCs to specific patient populations. Screening for G6PD deficiency is not routine for healthy blood donors. However, as demonstrated in this study, G6PD activity of RBC units can be measured using the attached segment at any time during storage; this could help guide decisions about whether or not a specific unit should be transfused into a specific patient.
Acknowledgements
This work was supported by a grant from the National Institutes of Health (HL098014) to SLS.
We thank the staff of the Blood Bank at Columbia University Medical Center-New York Presbyterian Hospital and the members of the Laboratory of Transfusion Biology for their help with various aspects of this study. This work was supported by a grant from the National Institutes of Health (HL098014) to SLS.
Footnotes
Conflicts of Interest Disclosure: The authors declare that they have no conflicts of interest relevant to the manuscript submitted to TRANSFUSION.
References
- 1.Nkhoma ET, Poole C, Vannappagari V, et al. The global prevalence of glucose-6-phosphate dehydrogenase deficiency: a systematic review and meta-analysis. Blood Cells Mol Dis. 2009;42(3):267–278. doi: 10.1016/j.bcmd.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Calvert AF, Trimble GE. Glucose-6-phosphate dehydrogenase in an Afro-American population. Hum Hered. 1980;30(5):271–277. doi: 10.1159/000153143. [DOI] [PubMed] [Google Scholar]
- 3.Dern RJ, Beutler E, Alving AS. The hemolytic effect of primaquine. II. The natural course of the hemolytic anemia and the mechanism of its self-limited character. J Lab Clin Med. 1954;44(2):171–176. [PubMed] [Google Scholar]
- 4.Brewer GJ, Tarlov AR, Kellermeyer RW. The hemolytic effect of primaquine. XII. Shortened erythrocyte life span in primaquine-sensitive male Negroes in the absence of drug administration. J Lab Clin Med. 1961;58(2):217–224. [Google Scholar]
- 5.Tizianello A, Pannacciulli I, Ajmar F, Salvidio E. Sites of destruction of red cells in G-6-PD deficient Caucasians and in phenylhydrazine treated patients. Scand J Haematol. 1968;5(2):116–128. doi: 10.1111/j.1600-0609.1968.tb01726.x. [DOI] [PubMed] [Google Scholar]
- 6.Tizianello A, Pannacciulli I, Salvidio E, Gay A. Erythrocytic glucose-6-phosphate dehydrogenase deficiency as a problem in the selection of blood donors. Vox Sang. 1963;8:47–50. doi: 10.1111/j.1423-0410.1963.tb04148.x. [DOI] [PubMed] [Google Scholar]
- 7.Kumar P, Sarkar S, Narang A. Acute intravascular haemolysis following exchange transfusion with G-6-PD deficient blood. Eur J Pediatr. 1994;153(2):98–99. doi: 10.1007/BF01959216. [DOI] [PubMed] [Google Scholar]
- 8.Shalev O, Manny N, Sharon R. Posttransfusional hemolysis in recipients of glucose-6-phosphate dehydrogenase-deficient erythrocytes. Vox Sang. 1993;64(2):94–98. doi: 10.1111/j.1423-0410.1993.tb02525.x. [DOI] [PubMed] [Google Scholar]
- 9.Van Der Sar A, Schouten H, Boudier AM. Glucose-6-Phosphate Dehydrogenase Deficiency in Red Cells. Incidence in the Cura Cao Population, Its Clinical and Genetic Aspects. Enzymologia. 1964;27:289–310. [PubMed] [Google Scholar]
- 10.Chinevere TD, Murray CK, Grant E, Jr, et al. Prevalence of glucose-6-phosphate dehydrogenase deficiency in U.S. Army personnel. Mil Med. 2006;171(9):905–907. doi: 10.7205/milmed.171.9.905. [DOI] [PubMed] [Google Scholar]
- 11.Doeblin TD, Ingall GB, Pinkerton PH, et al. Genetic studies of the Seneca Indians: haptoglobins, transferrins, G-6-PD deficiency, hemoglobinopathy, color blindness, morphological traits and dermatoglyphics. Acta Genet Stat Med. 1968;18(3):251–260. doi: 10.1159/000152142. [DOI] [PubMed] [Google Scholar]
- 12.Jim RT. Survey for erythrocyte glucose-6-phosphate dehydrogenase deficiency in Hawaii. Acta Haematol. 1967;37(2):94–99. doi: 10.1159/000209056. [DOI] [PubMed] [Google Scholar]
- 13.Orlina AR, Josephson AM, McDonald BJ. The poststorage viability of glucose-6-phosphate dehydrogenase-deficient erythrocytes. J Lab Clin Med. 1970;75(6):930–936. [PubMed] [Google Scholar]
- 14.Westerman MP, Wald N, Diloy-Puray M. Irradiation shortens the survival time of red cells deficient in glucose-6-phosphate dehydrogenase. Radiat Res. 1980;81(3):473–477. [PubMed] [Google Scholar]
- 15.Glucose-6-phosphate dehydrogenase deficiency. WHO Working Group. Bull World Health Organ. 1989;67(6):601–611. [PMC free article] [PubMed] [Google Scholar]
- 16.Shulman G, Baker D, Stewart NC, Liles BA. Validity of testing alanine aminotransferase in refrigerated whole blood. Transfusion. 1988;28(5):444–445. doi: 10.1046/j.1537-2995.1988.28588337333.x. [DOI] [PubMed] [Google Scholar]
- 17.Weiskopf RB, Yau R, Sanchez R, et al. Microarray kit analysis of cytokines in blood product units and segments. Transfusion. 2009;49(11):2269–2275. doi: 10.1111/j.1537-2995.2009.02274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swarup-Mitra S, Datta MC, Ghosh SK, Chatterjea JB. Observations on erythrocytic glutathione (GSH) and related enzymes during in vitro storage of blood from haemolytic anaemia patients. Indian J Pathol Bacteriol. 1972;15(1):27–33. [PubMed] [Google Scholar]
- 19.Morelli A, Benatti U, Gaetani GF, De Flora A. Biochemical mechanisms of glucose-6-phosphate dehydrogenase deficiency. Proc Natl Acad Sci U S A. 1978;75(4):1979–1983. doi: 10.1073/pnas.75.4.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rocchigiani M, Pescaglini M, Sestini S, et al. Density increase and ageing of erythrocytes in stored blood. J Int Med Res. 1989;17(5):461–466. doi: 10.1177/030006058901700508. [DOI] [PubMed] [Google Scholar]
- 21.Korgun DK, Bilmen S, Yesilkaya A. Alterations in the erythrocyte antioxidant system of blood stored in blood bags. Res Commun Mol Pathol Pharmacol. 2001;109(5–6):357–363. [PubMed] [Google Scholar]
- 22.Murphy EL, Shaz B, Hillyer CD, et al. Minority and foreign-born representation among US blood donors: demographics and donation frequency for 2006. Transfusion. 2009;49(10):2221–2228. doi: 10.1111/j.1537-2995.2009.02271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCurdy PR, Morse EE. Glucose-6-phosphate dehydrogenase deficiency and blood transfusion. Vox Sang. 1975;28(3):230–237. doi: 10.1111/j.1423-0410.1975.tb02761.x. [DOI] [PubMed] [Google Scholar]