Abstract
Objective
To investigate whether the rate of weight gain is associated with cardiometabolic risk, independent of weight measured concurrently.
Study design
Healthy 7–17 year-old risperidone-treated patients (n=105, 88% male) had blood pressure, anthropometry, and laboratory tests performed. Growth history was extracted from medical records. The rate of change in age-sex-adjusted weight and body mass index (BMI) z-score after the initiation of risperidone was individually modeled. Multivariable linear regression analyses explored the association of the rate of weight (BMI) z score change with cardiometabolic outcomes, independent of last measured weight (BMI) z score.
Results
Following a mean of 1.9 years (sd=1.0) of risperidone treatment, the absolute increase in weight and BMI z-scores was 0.61 (sd=0.61) and 0.62 (sd=0.73), respectively. After controlling for the final weight z-score, the rate of change in weight z-score was significantly associated with final glucose (p<0.04), C-peptide (p<0.004), HOMA-IR (p<0.02), HDL cholesterol (p<0.0001), a metabolic syndrome score (p<0.005), adiponectin (p<0.04), and hsCRP (p<0.04). After controlling for the final BMI z-score, the rate of change in BMI z-score was associated with final HDL cholesterol (p<0.04), leptin (p<0.03), and adiponectin (p<0.04), with a suggestion of an association with final HOMA-IR (p<0.08).
Conclusions
The rate of weight gain in risperidone-treated children explains equally or more of the variance in certain cardiometabolic outcomes (e.g., HDL cholesterol: ΔR2= 11% vs. ΔR2= 8% and hsCRP: ΔR2= 9% vs. ΔR2= 5%) than the weight measured concurrently, and may serve as a treatment target.
Keywords: Antipsychotics, Risperidone, Weight Gain, Cardiometabolic Abnormalities, Children, Adolescents
Obesity has reached an epidemic level with established deleterious health effects [1]. In the general population, most obese individuals gain weight relatively slowly. Thus, studies have focused on the link between absolute body mass index (BMI) and cardiometabolic abnormalities and/or risk for cardiovascular disease. Little attention has been paid to the rate of weight gain, specifically whether the speed at which weight is gained affects cardiovascular risk factors, independent of absolute BMI. One exception is the Minneapolis Children’s Blood Pressure Study which found the rate of increase in weight during childhood and adolescence to be associated with higher insulin, lipids, and systolic blood pressure in young adulthood [2]. Importantly, however, the analysis did not adjust for the weight measured in young adulthood.
Additional circumstantial evidence highlighting the potential significance of the rate of weight gain comes from studies implicating rapid postnatal growth in later obesity and cardiometabolic abnormalities [3]. However, whether fetal growth restriction, postnatal “catch-up” growth, or their combination accounts for this association remains unclear [4, 5]. Moreover, these studies have largely sought to link changes in weight between two time points and cardiometabolic outcomes. They have not specifically focused on the rate of weight gain. Furthermore, whether it is the rapid growth itself or genetic/environmental triggers (e.g., a thrifty phenotype) that predispose to cardiometabolic sequelae later in life is also unknown.
“Iatrogenic” weight gain can provide a model to shed light on this issue especially when the increase in weight is potentially rapid and substantial. This is particularly true for atypical antipsychotics [6, 7]. Unlike the case with weight-inducing non-psychiatric medications, antipsychotics are prescribed in conditions not characterized by cardiometabolic abnormalities. Such an approach may identify novel mechanisms linking obesity and cardiovascular disease and also have therapeutic implications.
We used a sample of risperidone-treated children to explore whether the rate of weight gain, after starting the antipsychotic, is associated with later cardiometabolic abnormalities, independent of weight when the cardiometabolic outcomes were assessed (i.e., final weight). We hypothesized that, independent of final weight, children with rapid weight gain will exhibit a more disadvantageous metabolic profile and systemic inflammation compared with those who gain weight slowly.
Methods
The primary aim of the original study was to investigate the long-term safety of risperidone [6]. Briefly, 7 to 17 year-old patients treated with risperidone for at least six months were enrolled. Concurrent treatment, at enrollment, with other psychotropics, but not other antipsychotics, was allowed. Participants with neurological or medical conditions that could confound the metabolic assessments (e.g., hypothyroidism) were excluded as were pregnant females and those receiving hormonal contraception.
This study was approved by the local Institutional Review Board. Written assent was obtained from children ≤ 14 years of age and consent was obtained from adolescents and parents. As noted above, eligible participants were already on risperidone at study enrollment. Medical records were examined and all height and weight measurements were extracted. In addition, at enrollment, vital signs and anthropometric measurements were obtained following standard procedures as previously described [6]. To account for children’s natural growth, age-sex-specific z-scores for weight and BMI were determined [8]. Measurements made during the research visits were compared with those extracted from the medical records that had been collected during clinical encounters falling within a month of the research visit [mean interval=16 days (SD=9) for height (n=69) and 17 days (SD=9) for weight (n=97)]. The intra-class correlation coefficients for unadjusted height and weight as well as for their age-sex-adjusted z-scores were all above 0.97 (95% confidence interval (CI): 0.93–0.99). Reflecting clinical practice, the dataset contained more than twice as many weight as height, and therefore BMI, measurements.
At study enrollment, pubertal stage was independently noted by a physician and the participants, with parental help when necessary [6]. Interrater agreement between the physician and self-rating was high (weighted kappa = 0.81, 95% CI: 0.74–0.88). In addition, a morning blood sample was obtained, after a 9-hr overnight fast, to measure glucose (Roche Diagnostics, Indianapolis, IN), total insulin (Diagnostic Products, Los Angeles, CA), C-peptide (Millipore, Billerica, MA), total cholesterol, HDL cholesterol (HDL), triglycerides (Roche Diagnostics, Indianapolis, IN), leptin and adiponectin (LINCO Research, Inc., St. Charles, MO), active ghrelin collected in EDTA tubes with aprotinin (LINCO Research, Inc., St. Charles, MO), and high-sensitivity C-reactive protein (hsCRP) (ALPCO Diagnostics, Windham, NH). The last four assays were added at a later point in the study, making them available for a subsample. LDL cholesterol was estimated following Friedewald’s equation [9]. Measurements for participants who were either not fasting or whose fasting status was missing (11%) were excluded from the analyses that related to the laboratory measures, except for hsCRP [10].
Data Analyses
Body fat was estimated using skinfold thickness measurements following Slaughter et al [11], and the homeostasis model assessment insulin resistance index (HOMA-IR) was estimated as: [insulin (μUI/ml) × glucose (mg/dl)]/405 [12]. Blood pressure measurements were converted to age-sex-height-adjusted z-scores [13]. The values of HOMA-IR, triglycerides, leptin, active ghrelin, and hsCRP were natural log (log) transformed to normalize their distributions. A metabolic syndrome score was constructed by first regressing waist circumference, mean arterial pressure, the negative of HDL, (log) triglycerides, and (log) HOMA-IR on age, tanner stage, sex, and race/ethnicity [14]. The standardized residuals from each of the five regression models were then summed to generate a metabolic syndrome score. One participant with hsCRP > 5 mg/l, representing acute inflammation [15], and one with a triglyceride concentration of 601 mg/dl were excluded from relevant analyses, although including these two participants did not appreciably alter the results.
We defined the date of initiation of risperidone as “baseline” and the enrollment date as “final” because this was when the cardiometabolic outcomes were measured. We estimated individual regression models of weight (or BMI) z-scores versus time. Because risperidone-induced weight gain plateaus, time was log transformed (This resulted in a better model fit compared with modeling time with a linear and quadratic term). Also, measurements recorded before age 5.5 years were excluded from the BMI analyses to avoid the phase of BMI “rebound” [8]. A standardized slope estimate was calculated by dividing the estimated slope by its standard error. The standardized and un-standardized slope estimates were highly correlated (Spearman r= 0.79 for weight slopes and r=0.82 for BMI slopes, p<0.0001). Nonetheless, we decided a priori to use the more conservative standardized slopes in the analyses because they are adjusted for variability in the number of observations (of weight and BMI z-scores: median=34 [interquartile range (IQR)=23] and 15 [IQR=11] observations, respectively) used to estimate the slope for each participant. The standardized slope for weight (or BMI) z-score was subsequently used as the index of rate of change and included as a predictor variable in multivariable linear regression analyses that modeled cardiometabolic outcomes, adjusting for final weight (BMI) z-score, age, and sex. The latter two were not included in models predicting body fat, systolic and diastolic blood pressure z-scores, and the metabolic syndrome score because they were already taken into account in computing these outcomes.
Antipsychotic-induced weight gain equally affects children across development [7]. Nonetheless, in a sensitivity analysis, we repeated the most relevant analyses in children in Tanner stage 1 at enrollment (n=48; Table I; available at www.jpeds.com), to exclude the possible confounding effect of the growth spurt and of the concurrent transient insulin-resistant state during puberty [16]. Additionally, we split the overall sample into fasting boys with negative or null rates of change in weight z score and those with positive rates. Then, within three groups of pubertal development (Tanner 1, Tanner 2/3, and Tanner 4/5), we matched boys with positive rates of change with those having non-positive rates on their final weight (or BMI) z-score. We calculated standardized between-group mean differences in the outcomes of interest [17]. Finally, we also substituted the change in weight (BMI) z score for the rate of change in the multivariable regression analyses to explore whether our findings would be better accounted for by the absolute change in weight (BMI) z score during risperidone treatment. All the statistical tests performed were two-tailed, using SAS version 9.2 for Windows (SAS Institute Inc., Cary, NC).
Table 1.
Demographic and Clinical Characteristics of the Participants (n=105)
| Characteristic | Mean or N | SD or % |
|---|---|---|
| Age, years | 11.2 | 2.7 |
| Tanner Stage: I, II, III, IV, V | 48/16/13/21/7 | 46/15/12/20/7 |
| Anthropometry* | ||
| Baseline Weight z-score (n=80) | 0.1 | 1.1 |
| Final Weight, kg (n=105) | 44.8 | 17.0 |
| Final Weight z-score (n=105) | 0.6 | 1.1 |
| Baseline BMI z-score (n=79) | 0.1 | 1.1 |
| Final BMI, kg/m2 (n=105) | 20.0 | 3.8 |
| Final BMI z-score (n=105) | 0.7 | 1.0 |
| Final Percent Body Fat (n=97) | 20.4 | 8.9 |
| Final Waist Circumference, cm (n=98) | 70.2 | 12.7 |
| Final Blood Pressure (n=105) | ||
| Systolic Blood Pressure, mmHg | 107.4 | 11.5 |
| Systolic Blood Pressure z-score | 0.2 | 1.0 |
| Diastolic Blood Pressure, mmHg | 61.7 | 8.3 |
| Diastolic Blood Pressure z-score | −0.0 | 0.7 |
| Mean Arterial Pressure, mmHg | 77.0 | 8.4 |
| Pulse Pressure, mmHg | 45.7 | 9.5 |
| Heart Rate, bpm | 84.5 | 14.2 |
| Final Glucose Homeostasis† | ||
| Glucose, mg/dl (n=93) | 90.0 | 8.3 |
| Total Insulin, μUI/ml (n=92) | 6.7 | 4.8 |
| C-Peptide, ng/ml (n=46) | 1.7 | 0.6 |
| HOMA-IR (n=92) | 1.5 | 1.1 |
| Final Lipids† (n=92) | ||
| HDL Cholesterol, mg/dl | 57.7 | 13.9 |
| Total Cholesterol, mg/dl | 158.4 | 24.6 |
| LDL Cholesterol, mg/dl | 88.5 | 21.1 |
| Non-HDL Cholesterol, mg/dl | 100.9 | 25.1 |
| Triglycerides, mg/dl | 59.9 | 35.8 |
| Triglycerides/HDL Cholesterol Ratio | 1.2 | 0.9 |
| Final Metabolic Syndrome Score‡ (n=92) | −0.0 | 3.2 |
| Final Regulatory Hormones† | ||
| Leptin, ng/ml (n=92) | 5.6 | 5.2 |
| Adiponectin, μg/ml (n=46) | 15.0 | 7.0 |
| Active Ghrelin, pg/ml (n=44) | 237.0 | 150.5 |
| Final Inflammatory Marker† | ||
| High Sensitivity C-reactive Protein, mg/l (N=47) | 0.6 | 1.2 |
Legend for Table 1:
The measurements were made at study enrollment except for the baseline weight and BMI ones.
Only fasting samples are included, except for hsCRP. One participant with hsCRP > 5 mg/l and one with a triglyceride concentration of 601 mg/dl were excluded.
The metabolic syndrome score was generated following Eisenmann.
Results
Data were available to compute a weight or BMI z-score slope for 105 participants (88% males) (Table I). They had been taking risperidone for a mean of 1.9 years (range: 0.5 – 4.0), primarily to target disruptive behavior (83%). Polypharmacy was prevalent, with psychostimulants (71%), antidepressants (65%), and α2-agonists (32%) being most commonly co-prescribed.
Table I includes the means and standards deviations of the clinical and laboratory outcomes of interest. The mean absolute change between baseline and final weight z-score was 0.61 (sd=0.61, range: −1.19 to 1.94). Overall, 7 participants (7%) showed a reduction in their weight z-score, 32 (30%) had no change, and the majority (n=66, 63%) had an increase. Similarly, the mean absolute change in BMI z-score was 0.62 (sd=0.73, range: −1.48 to 2.56) with two participants (2%) showing a reduction in their BMI z-score, 50 (50%) having no significant change, and 48 (48%) exhibiting an increase.
Baseline weight and BMI z-scores were inversely related to the magnitude of change in these measures after risperidone was initiated (Pearson’s r= −0.33, p<0.003 and r= −0.44, p<0.0001, respectively). However, importantly, there was no significant association between baseline weight and BMI z-scores and the rate of change of either weight (r= −0.06, p>0.5) or BMI z-score (r= −0.16, p>0.1). As expected, the change in weight (BMI) z score was correlated with the rate of change (i.e., slope of weight (BMI) z score) (Spearman’s r= 0.68 and r= 0.55, p<0.0001, respectively).
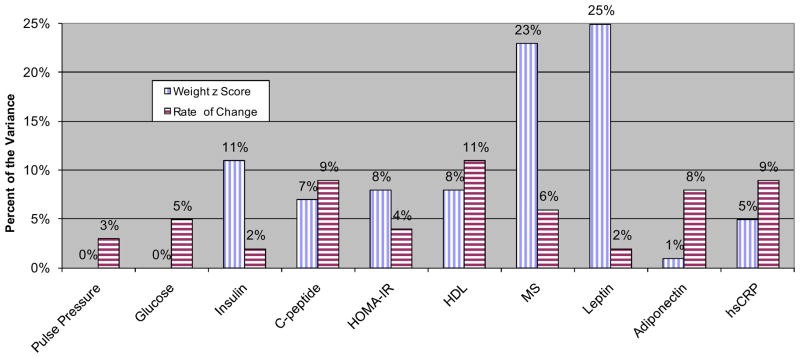
Table II includes the findings from the multivariable regression analyses exploring these associations. In some instances, the rate of weight gain was more strongly associated with the cardiometabolic outcomes than “final” weight (Figure).
Table 2.
Results for the multivariable regression analyses exploring the association between the slope of change in weight or BMI z-score and cardiometabolic risk factors while controlling for age, sex, and final weight or BMI z-score.
| Outcome | Weight Z-score Analysis | BMI Z-score Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | Sex | Weight | Slope | ΔR2 | Age | Sex | BMI | Slope | ΔR2 | |
| β Estimate | β Estimate | β Estimate | β Estimate | β Estimate | β Estimate | β Estimate | β Estimate | |||
| (95% CI) | (95% CI) | (95.% CI) | (95% CI) | (95% CI) | (95% CI) | (95.% CI) | (95% CI) | |||
| Final Anthropometry | ||||||||||
| Percent Fat | -- | -- | 5.45 | 0.36 ‡ | 0.02 | -- | -- | 5.58 | 0.26 | 0.01 |
| (4.25, 6.65) | (−0.03, 0.75) | (4.38, 6.78) | (−0.12, 0.64) | |||||||
| Waist circumference | 2.92 | 1.22 | 8.26 | 0.13 | 0 | 3.16 | 1.2 | 8.1 | 0.07 | 0.00 |
| (2.53, 3.32) | (−1.88, 4.32) | (7.32, 9.20) | (−0.19, 0.44) | (2.72, 3.59) | (−2.31, 4.71) | (7.02, 9.18) | (−0.28, 0.42) | |||
| Final Blood Pressure (BP) | ||||||||||
| Systolic BP | -- | -- | −0.02 | 0.03 | 0.01 | -- | -- | 0.12 | −0.02 | 0.00 |
| (−0.21, 0.17) | (−0.03, 0.09) | (−0.08, 0.32) | (−0.08, 0.04) | |||||||
| Diastolic BP | -- | -- | −0.01 | −0.02 | 0.01 | -- | -- | 0.06 | −0.03 | 0.02 |
| (−0.14, 0.13) | (−0.06, 0.03) | (−0.08, 0.20) | (−0.08, 0.01) | |||||||
| Mean Arterial Pressure | 0.89 | 2.18 | 0.95 | 0.03 | 0 | 1.00 | 1.86 | 1.5 | −0.25 | 0.01 |
| (0.29, 1.49) | (−2.67, 7.04) | (−0.57, 2.46) | (−0.46, 0.52) | (0.38, 1.61) | (−3.24, 6.95) | (−0.11, 3.10) | (−0.75, 0.26) | |||
| Pulse Pressure | 1.00 | −0.17 | 0.57 | 0.53 ‡ | 0.03 | 1.11 | −0.86 | 1.00 | 0.19 | 0.00 |
| (0.35, 1.66) | (−5.48, 5.15) | (−1.09, 2.23) | (−0.01, 1.06) | (0.44–1.78) | (−6.40, 4.68) | (−0.74, 2.74) | (−0.36, 0.73) | |||
| Heart Rate | −1.36 | 1.59 | 0.08 | 0.67 | 0.02 | −1.66 | 3.7 | −0.09 | 0.64 | 0.02 |
| (−2.40, −0.32) | (−6.65, 9.82) | (−2.49, 2.65) | (−0.15, 1.50) | (−2.70, −0.63) | (4.67, 12.07) | (−2.73, 2.55) | (−0.18, 1.47) | |||
| Final Glucose Homeostasis | ||||||||||
| Glucose | 0.21 | −2.22 | −0.43 | 0.55 | 0.05 | 0.28 | −2.26 | −0.12 | 0.4 | 0.02 |
| (−0.47, 0.89) | (−8.04, 3.60) | (2.03, 1.17) | (0.03, 1.08) | (−0.43, 0.99) | (−8.63, 4.10) | (−1.86, 1.63) | (−0.15, 0.95) | |||
| Total Insulin | 0.75 | 0.94 | 1.5 | 0.23 | 0.02 | 0.83 | 1.93 | 1.62 | 0.11 | 0.01 |
| (0.43, 1.08) | (−1.82, 3.71) | (0.73, 2.27) | (−0.02, 0.49) | (0.50, 1.17) | (−1.10, 4.97) | (0.78, 2.45) | (−0.16, 0.37) | |||
| C-peptide | 0.13 | 0.03 | 0.16 | 0.05 | 0.09 | 0.12 | 0.15 | 0.16 | 0.02 | 0.01 |
| (0.08, 0.18) | (−0.38, 0.45) | (0.04, 0.27) | (0.02, 0.09) | (0.07, 0.17) | (.027, 0.56) | (0.05, 0.28) | (−0.02, 0.06) | |||
| HOMA-IR (log) | 0.11 | 0.23 | 0.17 | 0.04 | 0.04 | 0.12 | 0.44 | 0.18 | 0.03 ‡ | 0.03 |
| (0.07, 0.16) | (−0.14, .061) | (0.07, 0.28) | (0.01, 0.08) | (0.08, 0.17) | (0.03, 0.85) | (0.07, 0.30) | (−0.00, 0.07) | |||
| Final Lipids | ||||||||||
| HDL Cholesterol | −0.92 | 1.45 | −3.73 | −1.43 | 0.11 | −1.36 | −1.63 | −4.14 | −0.86 | 0.04 |
| (−1.90, 0.07) | (−6.85, 9.75) | (−6.01, −1.44) | (−2.18, −0.68) | (−2.43, −0.29) | (−11.16, 7.91) | (−6.74, −1.53) | (−1.69, −0.04) | |||
| Total Cholesterol | 0.6 | 13.31 | −0.26 | −1.25 | 0.03 | 0.36 | 11.45 | 1.96 | −0.94 | 0.02 |
| (−1.43, 2.62) | (−4.08, 30.70) | (−5.10, 4.58) | (−2.83, 0.33) | (−1.67, 2.39) | (−6.83, 29.73) | (−3.07, 6.99) | (−2.52, 0.64) | |||
| LDL Cholesterol | −0.21 | 7.74 | 1.11 | −0.01 | 0.00 | −0.05 | 8.38 | 2.59 | −0.06 | 0.00 |
| (−2.01, 1.59) | (−7.48, 22.96) | (−3.08, 5.31) | (−1.39, 1.37) | (−1.91, 1.80) | (−8.15, 24.91) | (−1.93, 7.11) | (−1.49, 1.36) | |||
| Non-HDL Cholesterol | 1.41 | 11.59 | 3.44 | 0.21 | 0.00 | 1.58 | 12.75 | 6.17 | −0.06 | 0.00 |
| (−0.69, 3.51) | (−6.13, 29.32) | (−1.48, 8.37) | (−1.40, 1.82) | (−0.52, 3.68) | (−5.95, 31.45) | (1.04, 11.31) | (−1.68, 1.55) | |||
| Triglycerides (log) | 0.08 | 0.12 | 0.13 | 0.01 | 0.00 | 0.08 | 0.25 | 0.15 | 0.01 | 0.00 |
| (0.04, 0.12) | (−0.25, 0.49) | (0.03, 0.24) | (−0.02, 0.04) | (0.04, 0.13) | (−0.14, 0.64) | (0.04, 0.26) | (−0.03, 0.04) | |||
| Triglycerides/HDL Cholesterol Ratio | 0.12 | 0.36 | 0.24 | 0.06 | 0.05 | 0.13 | 0.57 | 0.31 | 0.01 | 0.00 |
| (0.05, 0.19) | (−0.22, 0.95) | (0.08, 0.41) | (0.01, 0.12) | (0.06, 0.20) | (−0.06, 1.19) | (0.14, 0.48) | (−0.04, 0.07) | |||
| Final Metabolic Syndrome Score | -- | -- | 1.46 | 0.23 | 0.06 | -- | -- | 1.52 | 0.1 | 0.01 |
| (0.95, 1.97) | (0.07, 0.39) | (0.99, 2.06) | (−0.07, 0.26) | |||||||
| Final Regulatory Hormones | ||||||||||
| Leptin (log) | 0.03 | 0.55 | 0.41 | 0.04 ‡ | 0.02 | 0.05 | 0.84 | 0.45 | 0.05 | 0.04 |
| (−0.03, 0.09) | (0.06, 1.04) | (0.27, 0.54) | (−0.01, 0.09) | (−0.01, 0.10) | (0.35, 1.33) | (0.31, 0.58) | (0.01, 0.09) | |||
| Adiponectin | −1.07 | −2.54 | −0.56 | −0.54 | 0.08 | −1.14 | −4.96 | −0.99 | −0.62 | 0.08 |
| (−1.75, −0.39) | (−8.38, 3.30) | (−2.17, 1.04) | (−1.04, −0.04) | (−1.86, −0.42) | (−11.04, 1.12) | (−2.66, 0.69) | (−1.21, −0.03) | |||
| Active Ghrelin (log) | −0.10 | −0.25 | 0.00 | 0.00 | 0.00 | −0.10 | −0.24 | −0.04 | −0.01 | 0.00 |
| (−0.17, −0.03) | (−0.83, 0.33) | (−0.16, 0.16) | (−0.05, 0.05) | (−0.17, −0.02) | (−0.84, 0.37) | (−0.21, 0.13) | (−0.07, 0.05) | |||
| Final Inflammatory Marker | ||||||||||
| High Sensitivity C- reactive Protein (log) | 0.03 | −0.33 | 0.23 | 0.10 | 0.09 | 0.05 | −0.08 | 0.28 | 0.05 | 0.02 |
| (−0.10, 0.15) | (−1.42, 0.77) | (−0.07, 0.53) | (0.01, 0.20) | (−0.09, 0.19) | (−1.32, 1.15) | (−0.05, 0.61) | (−0.06, 0.17) | |||
Legend for Table 2:
The measurements were all made at study enrollment except for the baseline weight and BMI ones.
Only fasting samples are included, except for hsCRP. One participant with hsCRP > 5 mg/l and one with a triglyceride concentration of 601 mg/dl were excluded.
The metabolic syndrome score was generated following Eisenman.
Figure.
Percent of the variation in “final” cardiometabolic outcomes independently accounted for by “final” weight z score and the rate of change in weight z score during risperidone treatment. hsCRP: high-sensitivity C-reactive Protein. MS: Metabolic Syndrome Score.
We repeated the significant analyses in prepubertal children. Compared with the overall group, we found, in this subgroup, comparable estimates of regression coefficients for final (log) HOMA-IR, leptin, adiponectin, hsCRP, and, to a lesser extent, for HDL but not for the metabolic syndrome score (data not shown).
Across 23 pairs of boys matched on their final weight z-score, those with positive rates of change had a higher (log) HOMA-IR (Cohen’s d=0.64, p<0.04) and metabolic syndrome score (Cohen’s d=0.53, p<0.09). However, these differences failed to reach significance across 26 pairs matched on their final BMI z-score (Cohen’s d=0.44 and 0.32, respectively, p>0.10).
When we substituted absolute change in weight (BMI) z score for the rate of change in the multivariable regression analyses, they explained 2% of the variance in HOMA-IR and 0% of the variance in the metabolic syndrome score (all p values >0.1).
Discussion
This study showed that the rate of weight change during childhood/adolescence is associated with a potentially atherogenic profile, independent of weight itself. Of particular interest is that the rate of change in weight was equally or more predictive of some cardiometabolic outcomes than the concurrently measured weight (or BMI) z-score. These findings did not appear to be influenced by developmental stage.
The association between weight, metabolic syndrome components, and inflammation has been widely replicated in studies involving diverse populations [18]. We have previously shown that risperidone-treated overweight children/adolescents exhibit a number of cardiometabolic abnormalities [6]. However, unlike most forms of idiopathic obesity where weight accrues gradually, the change in weight observed with antipsychotic treatment is often dramatic, with most taking place over the first months of treatment, generally reaching a plateau within the first two years [6, 7, 19]. Remarkably, across a variety of cardiometabolic markers, we here found that the rate of weight (BMI) gain is of clinical significance. Most importantly, the rate appeared to be more predictive of certain outcomes compared with the final weight z-score, obtained when the cardiometabolic variables were measured (Table II and Figure). The findings for the rate of BMI z-score change were comparable, albeit weaker likely due to more imprecision in estimating the trajectory with fewer BMI measurements available per participant.
The mechanism by which rapid weight gain contributes to the emergence of an atherogenic phenotype is not known. We speculate that rapid weight gain overwhelms the capacity of lower-body adipose tissue to store the excess fat, which, as a result, “overflows” into the upper body where it further enlarges abdominal adipocytes, increasing insulin resistance [20–22]. Another potential mechanism includes a larger deposition of adipose tissue in the liver with accelerated weight gain [23].
Some have argued that suppression of thermogenesis, specifically in the adipose tissue, and the associated insulin resistance mediate a disproportionate increase in fat mass, compared with lean mass, during postnatal “catch-up” growth or during recovery from starvation and wasting [24]. Importantly, our sample had normal baseline weight/BMI z-scores, at the start of risperidone (Table I). Furthermore, when we restricted the regression analysis predicting (log) HOMA-IR to participants with a baseline weight z-score greater than −1.0, the rate of change in weight z-score still accounted for nearly half as much of the variance in HOMA-IR (4%, p<0.05) as final weight z-score (9%, p<0.004) (For metabolic syndrome score: 7% (p<0.02) and 26% (p<0.0001), respectively). We also failed to find, in this sample, an association between change in weight z-score between birth and two years of age on the one hand and the rate of weight gain on the other (Calarge et al., submitted). In addition, we found no association between the rate of weight (BMI) z-score change before risperidone was started and the rate of weight gain after it was started (Calarge et al., submitted). Furthermore, although low baseline weight and BMI z-scores were positively associated with the magnitude of weight gain during risperidone treatment, they were not associated with the rate of weight gain. Thus, we conclude that the “suppression of thermogenesis” hypothesis might apply to cases where unfavorable conditions result in weight restriction but does not necessarily explain the development of an atherogenic profile following accelerated weight gain in children of normal baseline weight.
Our study suffers from several limitations. First, although antipsychotic treatment offers a model of rapid and significant weight gain, it is unknown whether our findings would apply to psychotropic-naïve populations. Although psychiatric conditions are less likely to confound the association between the rate of weight gain and cardiometabolic outcomes compared with other medical conditions, including “catch-up” growth, there is some evidence linking psychiatric disorders to excessive weight, cardiometabolic abnormalities, and inflammation [25, 26]. Although medications are extrinsic factors not directly related to the individual’s genetic background, their metabolism, efficacy, and potential to cause weight gain clearly are. This is relevant because the dose of psychotropics might influence the rate of weight gain [27]. Of note, after adjusting for the other covariates, the weighted average daily dose of risperidone was not significantly associated with HOMA-IR or the metabolic syndrome score, minimizing the likelihood of a direct effect of risperidone on insulin resistance. Second, although spanning several years of observation, this study was retrospective. Consequently, patients unresponsive to or intolerant of risperidone would have not been enrolled. However, our specific aim here was not to characterize the trajectory of weight gain or the nature of cardiometabolic abnormalities caused by risperidone. Our design clearly is not optimal for such an investigation. Rather, we took advantage of this convenience sample both because risperidone often causes rapid weight gain [6, 7] and because, compared with other antipsychotics, risperidone-related weight gain is thought to be most susceptible to the influence of patient- and drug-related factors during long-term treatment [28]. Specifically, we anticipated that polypharmacy and changes in the dose of risperidone (whether prescribed or due to partial adherence) over the course of treatment should optimize the ability to test our hypothesis because they will generate variability in the trajectory of weight gain across our participants [27]. A placebo-controlled randomized trial would avoid the shortcomings of an observational study like ours but will likely be difficult to conduct for longer than a few weeks for ethical, budgetary, and practical reasons. In addition, although most antipsychotic-induced weight gain generally occurs within the first months of treatment, such a short period of time may be insufficient to test our specific hypothesis.
Additional study limitations include the fact that, except for those obtained at study enrollment (the “final” measurement), all other anthropometric measurements were extracted from the medical records. However, like others [29], we found these weight and height measurements to be highly correlated with the corresponding research measurements, obtained within a month. Moreover, because our primary focus was the rate of change in weight (or BMI) z-score during the course of treatment, as opposed to single measurements, it is unlikely that minor deviations would have had major influences. Finally, it is important to replicate our findings in females and individuals from a diverse racial/ethnic background. Moreover, it is necessary to explore whether this finding is more prominent when the weight gain occurs during childhood as opposed to adulthood or in the context of polypharmacy, which was quite prevalent in our participants.
Our findings deserve further investigation because the metabolic syndrome profile tracks from childhood into adulthood and is associated with vascular abnormalities even in childhood [30]. Rapid weight gain might serve as a potential target for interventions to attenuate the long-term cardiovascular sequelae of obesity.
Acknowledgments
Funded by the Brain and Behavior Research Foundation, the National Institute of Mental Health (R21MH080968 and K23MH085005), and the National Center for Research Resources (RR0024979). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Aspects of this work have been presented at the 58th Annual Meeting of the American Academy of Child and Adolescent Psychiatry, October 2011, Toronto, Canada.
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Chadi A. Calarge, Email: chadi-calarge@uiowa.edu, The University of Iowa Carver College of Medicine, Departments of Psychiatry and Pediatrics, 500 Newton Road, Iowa City, IA 52242, Tel: 319-335-8771, Fax: 319-353-3003.
Diqiong Xie, The University of Iowa College of Public Health, Department of Psychiatry, 500 Newton Road, Iowa City, IA 52242
Jess G. Fiedorowicz, The University of Iowa Carver College of Medicine, Departments of Psychiatry and Internal Medicine, The University of Iowa College of Public Health, Department of Epidemiology, 200 Hawkins Drive, Iowa City, IA 52242
Trudy L. Burns, The University of Iowa College of Public Health, Department of Epidemiology, The University of Iowa Carver College of Medicine, Department of Pediatrics, 200 Hawkins Drive, Iowa City, IA 52242
William G. Haynes, The University of Iowa Carver College of Medicine, Department of Internal Medicine, 200 Hawkins Drive, Iowa City, IA 52242
References
- 1.Wormser D, Kaptoge S, Di Angelantonio E, Wood AM, Pennells L, Thompson A, et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377:1085–95. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sinaiko AR, Donahue RP, Jacobs DR, Jr, Prineas RJ. Relation of weight and rate of increase in weight during childhood and adolescence to body size, blood pressure, fasting insulin, and lipids in young adults. The Minneapolis Children’s Blood Pressure Study. Circulation. 1999;99:1471–6. doi: 10.1161/01.cir.99.11.1471. [DOI] [PubMed] [Google Scholar]
- 3.Monteiro PO, Victora CG. Rapid growth in infancy and childhood and obesity in later life--a systematic review. Obes Rev. 2005;6:143–54. doi: 10.1111/j.1467-789X.2005.00183.x. [DOI] [PubMed] [Google Scholar]
- 4.Ibanez L, Lopez-Bermejo A, Suarez L, Marcos MV, Diaz M, de Zegher F. Visceral adiposity without overweight in children born small for gestational age. J Clin Endocrinol Metab. 2008;93:2079–83. doi: 10.1210/jc.2007-2850. [DOI] [PubMed] [Google Scholar]
- 5.Law CM, Shiell AW, Newsome CA, Syddall HE, Shinebourne EA, Fayers PM, et al. Fetal, infant, and childhood growth and adult blood pressure: a longitudinal study from birth to 22 years of age. Circulation. 2002;105:1088–92. doi: 10.1161/hc0902.104677. [DOI] [PubMed] [Google Scholar]
- 6.Calarge CA, Acion L, Kuperman S, Tansey M, Schlechte JA. Weight gain and metabolic abnormalities during extended risperidone treatment in children and adolescents. J Child Adolesc Psychopharmacol. 2009a;19:101–9. doi: 10.1089/cap.2008.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302:1765–73. doi: 10.1001/jama.2009.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 9.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 10.Ockene IS, Matthews CE, Rifai N, Ridker PM, Reed G, Stanek E. Variability and classification accuracy of serial high-sensitivity C-reactive protein measurements in healthy adults. Clin Chem. 2001;47:444–50. [PubMed] [Google Scholar]
- 11.Slaughter MH, Lohman TG, Boileau RA, Horswill CA, Stillman RJ, Van Loan MD, et al. Skinfold equations for estimation of body fatness in children and youth. Hum Biol. 1988;60:709–23. [PubMed] [Google Scholar]
- 12.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 13.The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–76. [PubMed] [Google Scholar]
- 14.Eisenmann JC. On the use of a continuous metabolic syndrome score in pediatric research. Cardiovasc Diabetol. 2008;7:17. doi: 10.1186/1475-2840-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woodruff BA, Blanck HM, Slutsker L, Cookson ST, Larson MK, Duffield A, et al. Anaemia, iron status and vitamin A deficiency among adolescent refugees in Kenya and Nepal. Public Health Nutr. 2006;9:26–34. doi: 10.1079/phn2005825. [DOI] [PubMed] [Google Scholar]
- 16.Moran A, Jacobs DR, Jr, Steinberger J, Hong CP, Prineas R, Luepker R, et al. Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes. 1999;48:2039–44. doi: 10.2337/diabetes.48.10.2039. [DOI] [PubMed] [Google Scholar]
- 17.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale, NJ: Erlbaum Associates; 1988. [Google Scholar]
- 18.Daniels SR, Arnett DK, Eckel RH, Gidding SS, Hayman LL, Kumanyika S, et al. Overweight in children and adolescents: pathophysiology, consequences, prevention, and treatment. Circulation. 2005;111:1999–2012. doi: 10.1161/01.CIR.0000161369.71722.10. [DOI] [PubMed] [Google Scholar]
- 19.Reyes M, Croonenberghs J, Augustyns I, Eerdekens M. Long-term use of risperidone in children with disruptive behavior disorders and subaverage intelligence: efficacy, safety, and tolerability. J Child Adolesc Psychopharmacol. 2006b;16:260–72. doi: 10.1089/cap.2006.16.260. [DOI] [PubMed] [Google Scholar]
- 20.Tchoukalova YD, Votruba SB, Tchkonia T, Giorgadze N, Kirkland JL, Jensen MD. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc Natl Acad Sci U S A. 2010;107:18226–31. doi: 10.1073/pnas.1005259107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia. 2000;43:1498–506. doi: 10.1007/s001250051560. [DOI] [PubMed] [Google Scholar]
- 22.Sniderman AD, Bhopal R, Prabhakaran D, Sarrafzadegan N, Tchernof A. Why might South Asians be so susceptible to central obesity and its atherogenic consequences? The adipose tissue overflow hypothesis. Int J Epidemiol. 2007;36:220–5. doi: 10.1093/ije/dyl245. [DOI] [PubMed] [Google Scholar]
- 23.Perseghin G. Viewpoints on the way to a consensus session: where does insulin resistance start? The liver. Diabetes care. 2009;32 (Suppl 2):S164–7. doi: 10.2337/dc09-S303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dulloo AG. Regulation of fat storage via suppressed thermogenesis: a thrifty phenotype that predisposes individuals with catch-up growth to insulin resistance and obesity. Horm Res. 2006;65 (Suppl 3):90–7. doi: 10.1159/000091512. [DOI] [PubMed] [Google Scholar]
- 25.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–57. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 26.Cizza G. Major depressive disorder is a risk factor for low bone mass, central obesity, and other medical conditions. Dialogues Clin Neurosci. 2011;13:73–87. doi: 10.31887/DCNS.2011.13.1/gcizza. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calarge C, Xie D, Zimmerman B. Clinical Predictors of Weight Change During Long-Term Risperidone Treatment. 51st NCDEU Meeting; Boca Raton. p. FL2011. [Google Scholar]
- 28.Gentile S. Contributing factors to weight gain during long-term treatment with second-generation antipsychotics. A systematic appraisal and clinical implications. Obesity reviews: an official journal of the International Association for the Study of Obesity. 2009;10:527–42. doi: 10.1111/j.1467-789X.2009.00589.x. [DOI] [PubMed] [Google Scholar]
- 29.Arterburn D, Ichikawa L, Ludman EJ, Operskalski B, Linde JA, Anderson E, et al. Validity of Clinical Body Weight Measures as Substitutes for Missing Data in a Randomized Trial. Obes Res Clin Pract. 2008;2:277–81. doi: 10.1016/j.orcp.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mimoun E, Aggoun Y, Pousset M, Dubern B, Bougle D, Girardet JP, et al. Association of arterial stiffness and endothelial dysfunction with metabolic syndrome in obese children. The Journal of pediatrics. 2008;153:65–70. doi: 10.1016/j.jpeds.2007.12.048. [DOI] [PubMed] [Google Scholar]