Abstract
Based on a lack of severe phenotype in human IgA deficiency syndromes, the role of IgA in controlling respiratory and gastrointestinal (GI) infections has not been clearly defined. C57BL/6 and BALB/c mice lacking IgA (IgA−/−) were developed and used to address this question. When exposed to a common GI virus, rotavirus, IgA−/− mice exhibited a substantial and significant delay in clearance of the initial infection compared to wild type mice. IgA−/− mice excreted rotavirus in stool up to three weeks after the initial exposure compared to ten days observed in wild type mice. Importantly, IgA−/− mice failed to develop protective immunity against multiple repeat exposures to the virus. All IgA−/− mice excreted virus in the stool upon re-exposure to rotavirus while wild type mice were completely protected against re-infection. These findings clearly indicate a critical role for IgA in the establishment of immunity against a GI viral pathogen.
INTRODUCTION
IgA is the most abundant antibody isotype found in the body 1 and plays an important role in the immune responses at mucosal surfaces such as the gastrointestinal (GI) tract, the respiratory tract, and the vaginal tract 2. It mediates its effector function through multiple mechanisms 3, including interactions with mucosal epithelial cells, binding to a receptor, and high and low affinity antigen binding. IgA clearly plays a multi-faceted role in mucosal immunity but whether IgA alone is critical to immunity against pathogens that invade and cause disease at mucosal surfaces remains unresolved. While some humans lacking IgA suffer from recurrent sinopulmonary and or GI infections 4 many exhibit no apparent phenotype 4, suggesting that IgA is not absolutely essential to combat mucosal pathogens.
To attempt to determine the significance of IgA production at the mucosal surface, IgA knockout mice were generated by deleting the IgA switch region and half of the constant region 5. These mice are of mixed 129xC57Bl/6 background (C57BL/6/129 IgA−/−) and lack IgA antibody secreting cells and thus have no detectable IgA in either serum or mucosal secretions 5. Presumably to compensate for the loss of IgA, the C57BL/6/129 IgA−/− mice have elevated levels of IgM and IgG compared to wild type mice 5. There have been limited studies examining whether the absence of IgA results in diminished immune responses to mucosal pathogens. Consistent with the evidence in humans that the lack of IgA does not have a devastating impact on mucosal immunity, infection of C57BL/6/129 IgA−/− mice with Herpes simplex virus (HSV), Helicobacter pylori, or influenza virus showed that IgA is not essential for clearance of or protection from these mucosal pathogens 6–8. However, IgA was essential to block non-enteropathogenic reovirus entry in Peyer’s patches 9. Presumably sites of infection and the pathogenesis of individual pathogens are expected to greatly influence the relative importance of IgA in protection.
Rotavirus is a common intestinal virus that causes acute diarrhea in humans and animals 10. Small intestinal absorptive epithelial cells are the primary site of virus replication and pathology 10. In humans and animals, intestinal rotavirus-specific IgA is a correlate of protection from rotavirus 10, which suggests that intestinal IgA may be essential in the immune response to rotavirus. To directly examine this possibility, we previously tested the outcome of rotavirus infection in C57BL/6/129 IgA−/− mice. We found that IgA was essentially dispensable for clearance of an initial rotavirus infection 11,12 and for protection from re-infection 11, the latter attributed to compensatory responses in IgM and IgG antibodies 11. This report, at the time, was consistent with the limited role of IgA in immune responses to influenza, HSV, and Helicobacter pylori 6–8.
Since our initial report, several other reports examined the role of either B cells or components of the IgA secretory pathway in rotavirus immunity and the conclusions from these studies are seemingly inconsistent with our initial conclusion that IgA is dispensable for rotavirus immunity. Mice lacking B cells (either JhD or μMt mice on C57Bl/6 background) are not protected from rotavirus re-infection 13,14 clearly suggesting antibody is an important player in the intestinal immune response to rotavirus. Similarly, we found that the JhD mice on a BALB/c background have a greatly impaired ability to clear a primary rotavirus infection 15, supporting a role for B cells in clearance of infection. The requirement for B cells does not directly prove that antibody or IgA is a critical factor, but IgA is certainly the predominant immunoglobulin produced in the intestine during rotavirus infection 10. Both rotavirus specific IgA and IgG have been implicated in protective immunity 12,16. Additional support for the importance of IgA to rotavirus immunity comes from studies in J-chain deficient mice which cannot transcytose IgA or IgM into the intestinal lumen. J chain−/− mice have difficulty clearing a primary rotavirus infection 17 and are not protected from re-infection 17,18. Therefore, rotavirus infection in mice lacking either B cells or secretory antibodies (IgA and IgM) at mucosal surfaces results in similar defects in clearance of rotavirus infection and protection from re-infection. However, because IgM is also affected by loss of B cells or J chain, these studies fail to definitively prove that IgA is critical for rotavirus immunity 19. As more information has been gathered on the important cells and effector molecules required for rotavirus immunity, we felt it was necessary to reexamine the conclusion that IgA is not required for immunity to rotavirus.
Genetic background can dramatically influence the susceptibility to rotavirus infection in the mouse model with BALB/c mice being 1000X more susceptible than C57BL/6 mice to the murine rotavirus strain ECwt 20. Since the phenotype of a mutation can be influenced by genetic background 21 and our previous data was collected in mice of a mixed genetic background 11, we backcrossed mice with the IgA mutation onto C57BL/6 and BALB/c genetic backgrounds (C57BL/6 IgA−/− and BALB/c IgA−/−) and reassessed the affects of clearance of rotavirus infection and protection from re-infection in each strain. Irrespective of genetic background, IgA−/− mice exhibited major defects in clearance of the initial rotavirus infection and development of protective immunity. These results clearly demonstrate the importance of intestinal IgA to timely clearance of an initial infection with rotavirus and the absolute necessity for IgA in the establishment of protective immunity against re-infection in the murine model of rotavirus.
RESULTS
Clearance of rotavirus infection in mice coincides with the induction of fecal IgA
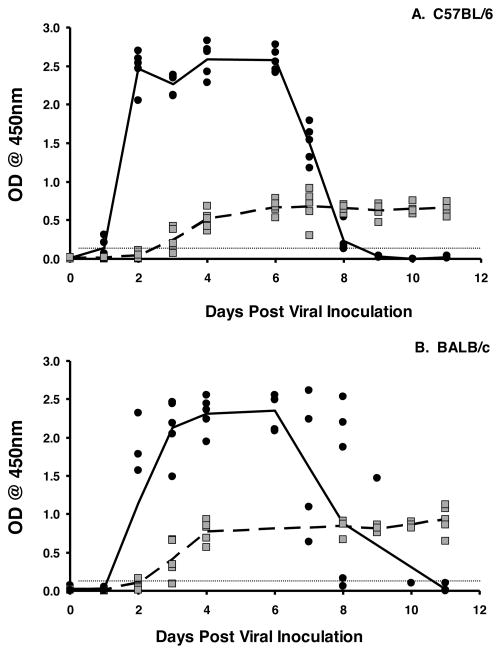
IgA has long been implicated as an important factor in protective immunity against rotavirus in humans and animal models of rotavirus infection and disease 10. To demonstrate that mice on two different genetic backgrounds exhibit a similar induction of intestinal IgA following exposure to a 103 ID50 dose of murine rotavirus, C57BL/6 and BALB/c mice were orally inoculated with rotavirus and stool assessed for the presence of rotavirus antigens or rotavirus-specific IgA by ELISA. As expected, rotavirus was detected in stool of mice from both genetic backgrounds by two days following virus exposure and was no longer detectable by ten days (Fig. 1). Rotavirus-specific IgA in stool was detected in both strains as early as three days following viral inoculation (Fig. 1). As previously reported 22, the appearance of rotavirus specific IgA in the stool preceded the disappearance of rotavirus from the fecal isolates. These findings indicate that induction of rotavirus specific IgA is similar on both genetic backgrounds and suggest that intestinal IgA is an important factor in the resolution of rotavirus infection.
Figure 1. Induction of fecal IgA coincides with clearance of rotavirus infection in mice.
C57BL/6 (A) and BALB/c mice (B) mice were orally inoculated with 103 ID50 of ECwt rotavirus on day 0. Fecal pellets were collected every day and analyzed for the presence of the VP6 middle capsid protein by ELISA (mean values shown by solid line) or the presence of rotavirus-specific IgA (mean value shown by dotted line). Each symbol represents the OD of either antigen (black circles) or antibody (grey squares) present in the stool of an individual mouse. Horizontal line indicates limit of detection of the assay.
Clearance of rotavirus infection in mice lacking IgA on a homologous genetic background is significantly delayed
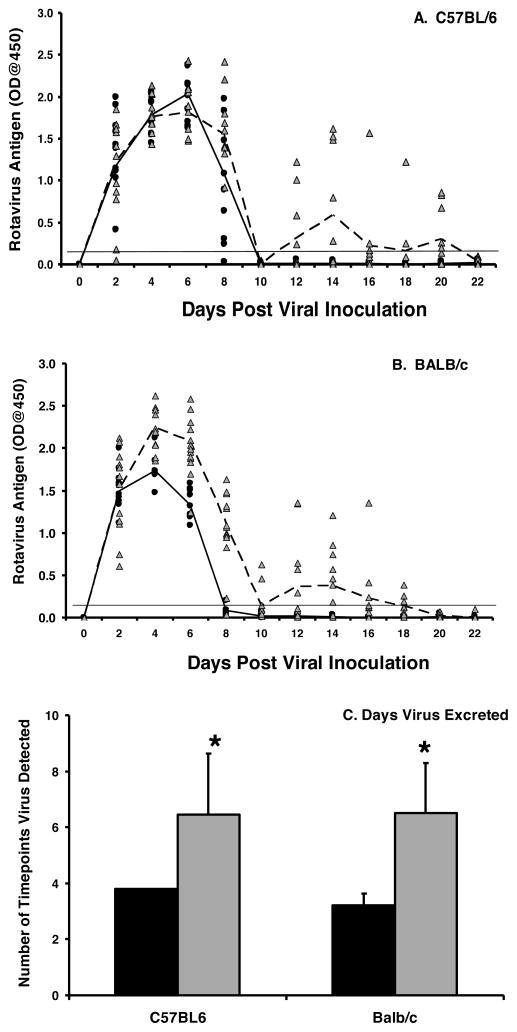
To re-examine whether IgA was important for clearance of a rotavirus infection, C57BL/6 and BALB/c IgA−/− mice were orally inoculated with ECwt. The kinetics of virus shedding between wild type and IgA−/− strains were initially similar at onset of shedding and in the subsequent sharp decline in shedding that occurred between 6–8 days following virus inoculation (Fig. 2A, B). While all wild type mice of both backgrounds resolved infection by 10 days, all IgA−/− mice continued to shed virus through 20–22 days following inoculation albeit at reduced levels compared to peak shedding (Fig. 2A, B). There was a significant difference in the mean number of time points that rotavirus was detectable in the stool (Fig. 2C) between C57BL/6 IgA−/− and wild type mice (6.4 versus 3.8 time points; p=0.003) and BALB/c IgA−/− and wild type mice (6.5 versus 3.2 time points; p=0.000). The endpoint titer of virus excreted in stool at the peak of viral shedding (day 4 for BALB/c strains and day 6 for C57BL/6 strains) was determined by limiting dilution. The geometric mean antigen titer was 2.6–3 fold higher in the IgA−/− mice but was not significantly different from the respective wild type controls (Table 1). The IgA−/− mice did not appear to have enhanced susceptibility to rotavirus infection compared to wild type mice (data not shown); however loss of IgA clearly affected the ability of mice to resolve rotavirus infection.
Figure 2. IgA is important for clearance of rotavirus infection.
C57BL/6 (A) and BALB/c (B) mice were orally inoculated with 103 ID50 of ECwt rotavirus on day 0. Fecal pellets were collected every other day and analyzed for the presence of the VP6 middle capsid protein by ELISA. Each symbol represents the OD of VP6 present in an individual wild type (black circles) or IgA−/− (grey triangles) mouse. The mean OD values for the groups are indicated for wild type (solid line) and IgA−/− (dotted line) mice. Horizontal line indicates limit of detection of the assay. C, the mean number of time points antigen was detected in fecal samples for each group. Black bar, wild type; grey bar, IgA−/−. Each bar represents the mean for each group+SD (n=10–12 mice/group). *, p<0.05 by Kruskal-Wallis equality of populations rank test compared to wild type mice.
Table 1.
Geometric Mean Titer (Range) of Rotavirus Antigen in 10% (wt/v) Stool on Peak Day of Virus Shedding at Indicated Inoculation1.
|
|
||||
|---|---|---|---|---|
| Mouse | Primary | Secondary
|
Tertiary | |
| Strain (n=5) | 6 wk | 12 wk | 12 wk | |
| C57BL/6 | 460 (200–1600) | 0 | 0 | 0 |
| C57BL/6 IgA−/− | 1213 (400–3200) | 139 (5–2560) | 106 (5–2560) | 6 (5–10) |
| BALB/c | 696 (200–1600) | 0 | 0 | 0 |
| BALB/c IgA−/− | 2111 (800–25600) | 17 (5–2560) | 6 (5–10) | 6 (5–10) |
Abbreviations: wt/v, weight/volume; wk, week;
At all inoculations mice were administered 103 ID50 ECwt.
Mice lacking IgA are not protected from re-infection with rotavirus
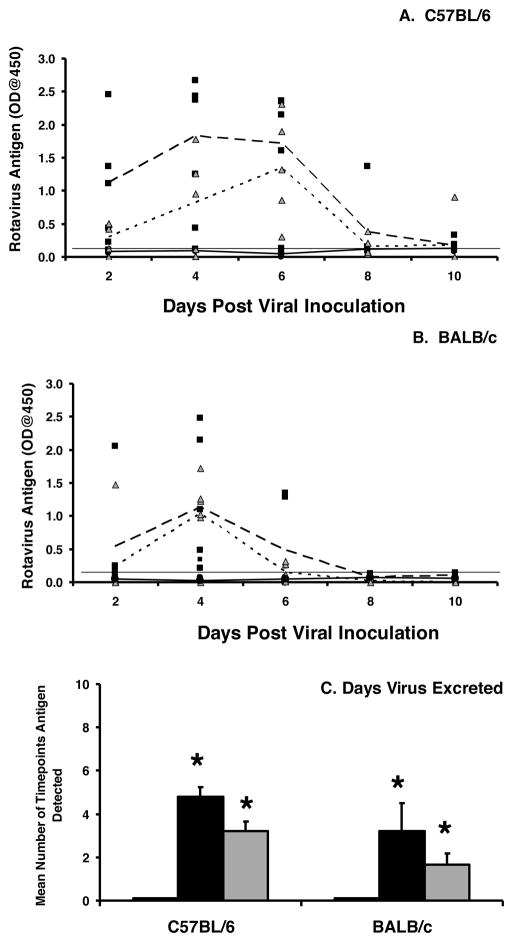
To determine whether the deletion of IgA also altered protection from re-infection, C57BL/6 and BALB/c IgA−/− and wild type mice previously infected with rotavirus were re-inoculated with rotavirus six weeks later. Susceptibility to re-infection was assessed based on viral excretion in stool. All C57BL/6 and BALB/c IgA−/− mice were susceptible to re-infection and excreted substantial levels of rotavirus in the stool for multiple days (Fig. 3A, B). In contrast, all the wild type mice were completely resistant to re-infection. The mean number of time points virus was present in the stool (Fig. 3C) was significantly higher in the knockout compared to the wild type mice (C57BL/6: 4.8 versus 0, p=0.004; BALB/c: 4.8 versus 0, p=0.005). The C57BL/6 IgA−/− mice exhibited an 8 fold higher geometric mean antigen at peak day of viral excretion (day 4 for both genetic strains) compared to BALB/c IgA−/− mice (Table 1) but the difference was not significant.
Figure 3. IgA is required for protection from a secondary rotavirus infection.
C57BL/6 (A) and BALB/c (B) wild type or IgA−/− mice were orally inoculated with 103 ID50 of ECwt rotavirus and received a secondary inoculation with 103 ID50 ECwt either six weeks or twelve weeks later. Fecal pellets were collected every other day and analyzed for the presence of the VP6 middle capsid protein by ELISA. Each symbol represents the OD obtained from an individual wild type mouse (black circles) or IgA−/− mouse at six weeks (black squares) or 12 weeks (grey triangles). The mean antigen OD values for the groups are indicated for wild type (solid line) and IgA−/− at either six weeks (large dotted line) or twelve weeks (small dotted line). Horizontal line indicates limit of detection of the assay. C, the mean number of time points antigen was detected in fecal samples for each group. White bar, wild type mice, six or twelve week challenge (no antigen shedding at either time point); black bar, IgA−/− mice, six week challenge; grey bar, IgA−/− mice, twelve week challenge. Each bar represents the mean for each group+SD (n=5–6 mice/group). *, p<0.05 by Kruskal-Wallis equality of populations rank test compared to wild type mice.
To examine whether establishment of protective immunity against rotavirus exposure was merely delayed in the absence of IgA production, C57BL/6 or BALB/c IgA−/− and wild type mice were challenged with rotavirus at twelve, instead of six, weeks following the initial viral exposure. As expected 22, all wild type mice were protected against reinfection and did not excrete any detectable levels of rotavirus in stool. However, all IgA−/− mice excreted rotavirus in the stool (Fig. 3A, B) and for a significantly greater number of time points (Fig. 3C) compared to wild type mice (C57BL/6: 3.2 versus 0, p=0.004; BALB/c: 1.7 versus 0, p=0.003). The C57BL/6 IgA−/− mice had a 17.6 fold higher geometric mean titer of viral antigen on the day of peak viral shedding following challenge compared to BALB/c IgA−/− mice (day 4 for both strains) but the difference was not significance (Table 1). There was no difference in the geometric mean titer of virus excreted at 6 weeks compared to 12 weeks in either genetic background (Table 1). These results indicate that IgA is critical for the establishment of protective immunity against rotavirus infection.
IgA is essential for protection against repeated rotavirus infections
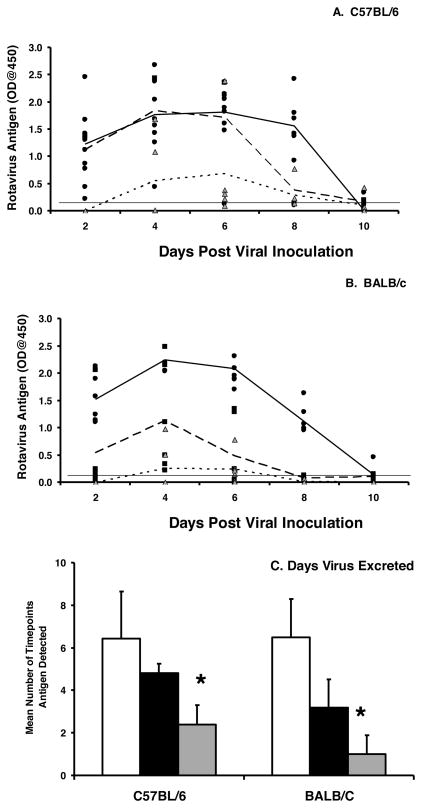
To determine whether IgA−/− mice were susceptible to repeated infections, C57BL/6 and BALB/c IgA−/− and wild type mice were inoculated with rotavirus three times (0, 6, and 12 weeks). Both C57BL/6 and BALB/c IgA−/− mice remained susceptible to repeated infection (Fig. 4A, B) unlike wild type mice that were fully protected following initial infection (data not shown). There was no difference in the geometric mean titer of virus excreted at peak viral excretion (4 days) between the first and second challenge (Table 1). However, rotavirus was excreted for significantly fewer time points (Fig. 4C) by all IgA−/− mice following the third infection (12 weeks) compared to the second infection (6 weeks) (C57BL/6: 4.8 versus 2.4, p=0.006; BALB/c: 2.4 versus 1.0, p=0.015). There was no difference in the mean number of time points positive for rotavirus at 12 weeks independent of a second inoculation at 6 weeks (C57BL/6: 2.4 versus 3.2, p=0.340; BALB/c: 1.0 versus 1.7, p=0.162). There was also no difference in geometric mean titer of virus excreted at peak viral infection (4 days) between the first and second challenge (Table 1). In contrast to wild type mice, none of the C57BL/6 or the BALB/c IgA−/− mice establish complete protective immunity to rotavirus infection even after multiple virus exposures.
Figure 4. Mice lacking IgA are susceptible to multiple infections with rotavirus.
Naïve C57BL/6 IgA−/− (A) or BALB/c IgA−/− (B) mice were orally inoculated with 103 ID50 of ECwt rotavirus (mean value, solid line) followed by an identical second inoculation six weeks (mean value, large dotted line) and third inoculation twelve weeks later (mean value, small dotted line). Fecal pellets were collected every other day and analyzed for the presence of the VP6 middle capsid protein by ELISA. Each symbol is the OD, representative of the amount of VP6 present in a sample from an individual IgA−/− mouse following the first (black circles), second (black squares, six weeks), and third (grey triangles, 12 weeks) inoculations. The mean antigen OD values for the groups are indicated following first (solid line), second (large dotted line), or third (small dotted line) inoculation. Horizontal line indicates the limit of detection of the assay. C, the mean number of time points antigen was detected in fecal samples for each group. White bars, initial infection, black bars, six week inoculation, grey bars, twelve week inoculation. Each bar represents the mean for each group+SD (n=5–6 mice per group). *, p<0.05 by Kruskal-Wallis equality of populations rank test compared mice receiving a challenge at 6 weeks.
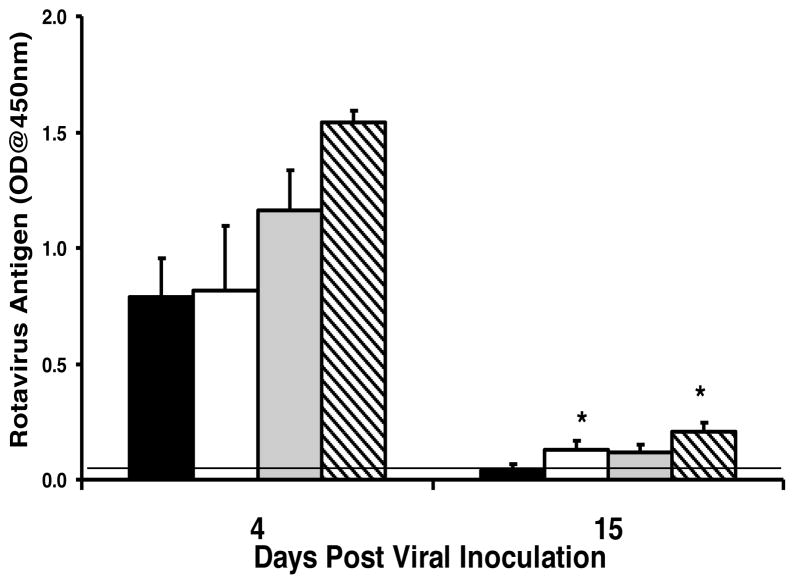
IgA plays a minimal role in clearance of rotavirus from the systemic circulation
Rotavirus infection is not limited to the intestine and also involves viremia and antigenemia 23. To determine whether the lack of IgA had any effect on rotavirus antigenemia, C57BL/6 and BALB/c IgA−/− or wild type mice were orally administered rotavirus and antigenemia assessed at the peak of viral excretion in stool (four days) or when virus is no longer detectable in wild type animals but still present in the IgA−/− animals (fifteen days). Both wild type mice and IgA−/− mice had high and equivalent levels of rotavirus antigenemia four days following initial infection (Fig. 5). There were small but significant differences between wild type and IgA−/− mice in the levels of antigenemia at fifteen days post inoculation, but the levels were dramatically reduced compared to the levels present at 4 days following infection (Fig. 5). In contrast to what was observed in stool, virus was not detected in the blood of any wild type or IgA−/− mice following a second (6 week) or third (12 week) rotavirus inoculation (data not shown). These results suggest that IgA plays a minor role in the clearance of a primary rotavirus infection from systemic circulation and is not required for protection against rotavirus antigenemia following re-exposure to the virus. In addition, the high levels of rotavirus-specific serum antibody induced in all infected IgA−/− mice following primary infection (data not shown) did not protect these mice from rotavirus re-infection of the intestine.
Figure 5. IgA is not critical for clearance of rotavirus antigenemia.
Mice were orally inoculated with 103 ID50 of ECwt rotavirus on day 0. Serum was collected either 4 or 15 days later and analyzed for the presence of the VP6 middle capsid protein by ELISA. C57BL/6 (black bar), C57BL/6 IgA−/− (white bar), BALB/c (grey bar), and BALB/c IgA−/− (hatched bar). Each bar represents the mean OD of VP6 present in 5–6 mice + SD. *, p<0.05 by Kruskal-Wallis equality of populations rank test compared to wild type mice.
Mice lacking IgA do not develop detectable compensatory rotavirus-specific antibody responses following rotavirus infection
To examine whether rotavirus-specific intestinal IgG or IgM is induced in IgA−/− mice, C57BL/6 and BALB/c IgA−/− and wild type mice were inoculated with rotavirus and fifteen days later, total rotavirus-specific (IgA, IgG, and IgM) in stool was quantified by ELISA 24. None of the C57BL/6 or the BALB/c IgA−/− mice had detectable rotavirus-specific total antibody (Fig. 6). Similar results were obtained in stool at six weeks and twelve weeks following infection with the virus (data not shown). These findings indicate that, in addition to not producing rotavirus-specific IgA, these mice also failed to produce detectable levels of rotavirus-specific IgM or IgG.
Figure 6. Mice lacking IgA do not develop detectable compensatory rotavirus-specific IgG or IgM fecal antibody.
Mice were orally inoculated with 103 ID50 of ECwt rotavirus on day 0. Fecal pellets were analyzed for the presence of rotavirus specific total antibody (IgA, IgM, and IgG) 15 days following viral exposure by ELISA. Each bar represents the geometric mean titer 4–6 mice + SD. *, p<0.05 by Mann Whitney U. Horizontal line indicates the limit of detection of the assay.
DISCUSSION
We find that loss of IgA seriously impacted rotavirus immunity. Clearance of a primary rotavirus infection was significantly and similarly delayed in C57BL/6 and BALB/c IgA−/− mice compared to wild type mice. While wild type mice previously infected with rotavirus were completely protected from infection, none of the IgA−/− mice were protected from a second infection either 6 or 12 weeks following the initial infection. Complete protective immunity was not induced in IgA−/− mice by repeated exposure to rotavirus infection. These results clearly demonstrate the critical importance of IgA in rotavirus intestinal immunity.
Our data also provide the first direct experimental evidence that pathogen-specific IgA is essential for the development of protective intestinal immunity to an enteropathogen. Indisputable in vivo experimental proof under normal physiological conditions that IgA alone is critical to immunity against pathogens that invade and cause disease at any mucosal surfaces has been lacking. In fact, IgA has only previously been shown to be critical to prevent non-enteropathogenic reovirus invasion of small intestinal Peyer’s patches but is otherwise dispensable to reovirus immunity 9. Some studies with enteric bacterial pathogens or toxins or viruses in mice deficient in secretory antibodies (IgM and IgA) indicate that these antibodies are not important in protecting the intestine from infection 25 while others support a possible role for IgA 16,25–27. The latter studies do not prove that IgA alone is critical to immunity because there was a deficiency in both IgM and IgA 25,26. Therefore, these plus numerous in vitro and passive immunization studies, correlations between IgA induction and pathogen clearance, as well as the preeminence of IgA in the intestine provide supportive, but not conclusive, proof that IgA is critical to protect mucosal surfaces from pathogens. Indeed, previous critical proof of concept studies in IgA deficient animals failed to support this view 6–8,28. This paradox might be explained by several non-mutually exclusive caveats, including (i) the presence of redundant or compensatory immune effectors, such as IgM 28, (ii) IgA being a component of but not truly critical to immunity or to the pathogens studied, or (iii) IgA playing a different or more subtle role in protective immunity than was assessed. In contrast, we show that intestinal IgA is functionally important to timely clearance of a primary infection and is absolutely essential to and can provide complete protection of the intestine from rotavirus reinfection.
Our current finding that IgA is critical for intestinal immunity to rotavirus are consistent with findings in JhD, μMT, J-chain−/−, all of which lack intestinal secretory antibody (IgA and IgM) and the backpack transfer of IgA that protected against rotavirus infection 13–18. Taken together, all of these previous studies suggested but did not prove the necessity of IgA to rotavirus immunity and were inconsistent with our previous finding in C57BL/6/129 IgA−/− mice 11. One difference between the studies is the genetic background of the IgA−/− mice. Based on SNP analysis, the C57BL/6 and BALB/c IgA−/− are >99 % B6 or >98% BALB/c, respectively except for the Igh gene locus on chromosome 12. In contrast, the C57BL/6/129 IgA−/− mice used previously exhibited an approximately even distribution of 129-related and C57BL/6 related genes. It is possible that such as large representation of 129 genes may have influenced the results of our previous examination of rotavirus infection in the IgA−/− mice. Alternatively it is possible that other confounding factors, such as the intestinal microbiota 29 or concurrent infections with Helicobacter and murine norovirus, all of which can indirectly influence intestinal immunity 30 inadvertently affected the rotavirus immune response leading to the erroneous conclusion that IgA was not required for rotavirus immunity 11. In light of our new conclusions as to the importance of IgA in rotavirus immunity, perhaps a re-examination of the importance of IgA in the context of a more controlled genetic background is necessary for other mucosal pathogens, including Helicobacter, Herpes simplex, and influenza 6–8.
Because rotavirus is commonly present in the systemic circulation during intestinal infection 31, we examined whether loss of IgA impacted rotavirus systemic infection, clearance or protection. The levels of antigen in the blood were not elevated in the absence of IgA, which was consistent with the induction of wild type levels of serum antibody in the IgA−/− mice. Unlike the importance of IgA in rotavirus clearance and protective immunity in the intestine, IgA plays only a minimal role in clearance of the virus from the blood and is not essential for protective immunity against systemic virus upon re-exposure to the virus (Fig. 5). However, B cells are important for the clearance of virus from the systemic circulation 15. Together, these studies implicate either systemic IgG or IgM as the important B cell effector product in removal of systemic virus. This is not an unexpected conclusion as rotavirus infection induces substantially higher levels of serum IgG than IgA 22. In addition, IgG rather than IgA, is the most abundant antibody isotype in the serum 1, and thus probably plays a key role in clearance of rotavirus in the blood and in protection against antigenemia and viremia upon re-exposure.
The loss of IgA results in a wide range of clinical responses to pathogenic infections in humans 4 but to our knowledge the impact on rotavirus infections has not been examined. Based on our mouse studies, rotavirus infections in IgA deficient humans would be expected to be more prolonged or occur more frequently compared to IgA normal individuals. Disease severity might also be increased. Importantly, rotavirus vaccines might be less efficacious in humans with IgA deficiency syndromes, although the immune mechanism of protection is not known for either the RotaRix® or RotaTeq® vaccines. Alternatively, compensatory increases in rotavirus-specific secretory IgM in the IgA deficient human intestine might provide greater levels of protection than we observed in the mouse studies 28. Studies in children indicate that stool IgA correlates with susceptibility to rotavirus infection, as children whose IgA levels declined following an initial rotavirus infection were not protected from re-infection 32. Clearly, the importance of IgA to rotavirus immunity found in mice re-emphasizes the importance of considering stool IgA as a correlate of rotavirus protection in human studies.
We definitively demonstrate that IgA is important for timely clearance of rotavirus infection and is absolutely essential in protection from re-infection. Although IgA is the most abundant antibody isotype in the GI tract and is synthesized in quantities that far exceed the other isotypes 1, multiple studies in both animals and humans lacking or deficient in only IgA generally failed to prove that IgA was critical for protective immunity 4. We provide new evidence that proves the importance of IgA by demonstrating that the protective immune response to a common intestinal viral enteropathogen absolutely depends on the presence of IgA and lends needed credence to the important role of IgA in pathogen specific intestinal immunity. Our findings reinforce the need to pursue intestinal IgA as an area for exploitation in the development of highly efficacious mucosal vaccines to prevent GI infections. GI infections, including rotavirus, account for direct costs of over $7.3 billion dollars to the US healthcare industry and non-food and food borne gastroenteritis are the most common reasons for emergency room visits 33.
METHODS
IgA knockout mice
C57BL/6/129 IgA−/− mice were initially generated as described previously 5. C57BL/6/129 mice were backcrossed >8 generations to either the C57BL/6 or BALB/c backgrounds. Tail DNA from each resulting knockout strain was assessed at the DartMouse Speed Congenic Core Facility at Dartmouth Medical School (Hannover, NH). DartMouse uses the Illumina, Inc. (San Diego, CA) GoldenGate Genotyping Assay to interrogate 1449 SNPs spread throughout the genome. The raw SNP data were analyzed using DartMouse’s proprietary SNaP-Map™ and Map-Synth™ software, allowing the determination for each mouse of the genetic background at each SNP location. SNP analysis confirmed the backcrossing results and showed that while the Igh locus on chromosome 12 remained 129, there were few if any 129 gene segments detectable in other loci (<1% C57BL6 or <2% BALB/c). Both female and male mice of at least six weeks of age were used in experiments. Each experiment was performed a minimum of 3 separate times with 5 mice in each group. Mice were individually housed in microisolator cages, fed ad libitum, and a 24 hour random fecal sample collected every other day. Procedures were carried out within the provisions of the Guide for the Care and Use of Laboratory Animals, NIH Guide for Grants and Contracts and approval for the study was obtained from the Institutional Animal Care and Use Committee at Baylor College of Medicine. All animals were cared for under veterinary surveillance.
Viral infection, sample collection, and processing
The murine strain of rotavirus, ECwt, was obtained from Harry Greenberg (Stanford University Medical School, Palo Alto, CA). Intestinal lysates were prepared from suckling mice inoculated orally with ECwt and the ID50 (50% of the infectious dose) titer of the virus in the lysate was determined in adult C57BL/6 and BALB/c mice as described previously 24. Wild type and C57BL/6 or BALB/c IgA−/− mice were orally inoculated with 103 ID50 in 100 ul of phosphate buffered saline on day 0 and days 42 or 84 or on all three days (0, 48, and 84). Following both initial and subsequent viral exposures, 24 hour randomized fecal pellets from individual mice were collected every other day starting on the day of inoculation. Pellets were resuspended as a 10% weight/volume solution in 10 mM Tris, 100 mM NaCl, 1 mM CaCl2, 0.05% Tween 20, 5 mM sodium azide, 1 mg of aprotinin/ml, 1 mM benzamidine, 10 mg of leupeptin/ml, 10 mg of pepstatin A/ml [pH 7.4]) and then were centrifuged at 15,000g to remove fecal solids. Processed fecal antibody samples were stored at −20°C. Serum was collected by either tail bleeding or from the inferior vena cava.
ELISA to measure rotavirus
The amount of rotavirus in either 10% weight/volume fecal pellet suspensions or undiluted serum following pretreatment with 0.05M EDTA was measured by ELISA as previously described 24. Briefly, 96-well polyvinylchloride plates were coated with 50 ul of a mouse monoclonal anti-VP6 antibody (6E7, raised against rotavirus strain SA11) and incubated overnight at room temperature. Hyperimmune guinea pig anti-serum to rotavirus (strain SA11) was used to detect antigen in samples followed by horseradish peroxidase (HRP)-conjugated goat anti-guinea pig IgG (Sigma-Aldrich Corp., St. Louis, MO). Values with an optical density (OD) @ 450 nm >0.100 were considered positive. To allow for quantitative comparison of the amount of rotavirus antigen excreted in stool at the peak of infection when ELISA OD readings were saturated (>1.00), stools were serially diluted two-fold, endpoint titers determined for individual samples, and geometric mean titers calculated for each group. The antigen titer was defined as the reciprocal of the highest dilution resulting in an OD greater than 0.100.
ELISA to measure anti-rotavirus IgA, IgG, and IgM in feces and serum
The ELISA to measure rotavirus-specific IgA, IgG, and IgM has been previously described 24. Briefly, plates were coated with an anti-SA11 hyperimmune guinea pig serum followed by incubation with a lysate of SA11 infected MA104 cells treated with 0.05M EDTA. Incubation of SA11 lysate was followed by incubation of either fecal suspensions or serum with an initial dilution of 1/2 or 1/50 respectively. The amount of antibody was quantified following incubation with a HRP-conjugated goat anti-mouse IgA, IgG, and IgM (Kirkegaard and Perry) diluted in buffer containing normal guinea pig serum. Values with an OD>0.100 were considered positive. The antibody titer was defined as the reciprocal of the highest dilution resulting in an OD greater than 0.100.
Statistical Analysis
Chi-squared testing (Fisher’s Exact) followed by Kruskal-Wallis equality-of-populations rank test was used to determine statistical significance between groups (n=5–12 mice/group). Antibody titers between groups were compared by the Kruskal-Wallis test to determine statistical significance followed by a one-sided Mann-Whitney U test to determine the p values of groups that had statistically significant differences. Statistical significance was concluded when p<0.05.
Acknowledgments
This work was supported in part by Public Health Service Grants NIH AI24998 (S.E.B, A.M, M.E.C), NIH AI07471 (A.M.) and NIH AI41715 and NIH AI83878 (S.L.S, D.W.M.) and NIH DK56338, which funds the Texas Medical Center Digestive Diseases Center. Technical assistance was provided by Samuel Hardeman, Lara Berghammer, and Mary Penn.
Footnotes
DISCLOSURE
The authors declare no conflict of interest.
References
- 1.Janeway C, Travers P, Walport M, Shlomckik J. Immunobiology: The Immune System in Health and Disease. Elsevier Science Ltd./Garland Publishing; London: 2001. [Google Scholar]
- 2.Mestecky J, Moro I, Underdown J. Mucosal Immunoglobins. In: Ogra P, et al., editors. Mucosal Immunology. Academic Press; San Diego: 1999. pp. 133–152. [Google Scholar]
- 3.Cerutti A, Chen K, Chorny A. Immunoglobulin responses at the mucosal interface. Annu Rev Immunol. 2011;29:273–293. doi: 10.1146/annurev-immunol-031210-101317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yel L. Selective IgA deficiency. J Clin Immunol. 2010;30:10–16. doi: 10.1007/s10875-009-9357-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harriman GR, et al. Targeted deletion of the IgA constant region in mice leads to IgA deficiency with alterations in expression of other ig isotypes. Journal of Immunology. 1999;162:2521–2529. [PubMed] [Google Scholar]
- 6.Blanchard TG, et al. Antibody-independent protective mucosal immunity to gastric helicobacter infection in mice. Cell Immunol. 1999;191:74–80. doi: 10.1006/cimm.1998.1421. [DOI] [PubMed] [Google Scholar]
- 7.Parr MB, Harriman GR, Parr EL. Immunity to vaginal HSV-2 infection in immunoglobulin A knockout mice. Immunology. 1998;95:208–213. doi: 10.1046/j.1365-2567.1998.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mbawuike IN, et al. Mucosal immunity to influenza without IgA: an IgA knockout mouse model. J Immunol. 1999;162:2530–2537. [PubMed] [Google Scholar]
- 9.Silvey KJ, Hutchings AB, Vajdy M, Petzke MM, Neutra MR. Role of immunoglobulin A in protection against reovirus entry into Murine Peyer’s patches. J Virol. 2001;75:10870–10879. doi: 10.1128/JVI.75.22.10870-10879.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Estes MK, Kapikian AZ. In: Field’s Virology. Knipe DM, Howley PM, editors. Lippincott-Raven Publishers; Philadelphia: 2007. pp. 1917–1974. [Google Scholar]
- 11.O’Neal CM, Harriman GR, Conner ME. Protection of the villus epithelial cells of the small intestine from rotavirus infection does not require immunoglobin A. J Virol. 2000;74:4102–4109. doi: 10.1128/jvi.74.9.4102-4109.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuklin NA, et al. Protective intestinal anti-rotavirus B cell immunity is dependent on alpha 4 beta 7 integrin expression but does not require IgA antibody production. J Immunol. 2001;166:1894–1902. doi: 10.4049/jimmunol.166.3.1894. [DOI] [PubMed] [Google Scholar]
- 13.Franco MA, Greenberg HB. Role of B cells and cytotoxic T lymphocytes in clearance of and immunity to rotavirus infection in mice. J Virol. 1995;69:7800–7806. doi: 10.1128/jvi.69.12.7800-7806.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McNeal MM, Barone KS, Rae MN, Ward RL. Effector functions of antibody and CD8+ cells in resolution of rotavirus infection and protection against reinfection in mice. Virology. 1995;214:387–397. doi: 10.1006/viro.1995.0048. [DOI] [PubMed] [Google Scholar]
- 15.Marcelin G, Miller AD, Blutt SE, Conner ME. Immune mediators of rotavirus antigenemia clearance in mice. J Virol. 2011;85:7937–7941. doi: 10.1128/JVI.00844-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burns JW, Siadat-Pajouh M, Krishnaney AA, Greenberg HB. Protective effect of rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity. Science. 1996;272:104. doi: 10.1126/science.272.5258.104. [DOI] [PubMed] [Google Scholar]
- 17.McNeal MM, et al. Protection against rotavirus shedding after intranasal immunization of mice with a chimeric VP6 protein does not require intestinal IgA. Virology. 2006;346:338–347. doi: 10.1016/j.virol.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz-Cornil I, Benureau Y, Greenberg H, Hendrickson BA, Cohen J. Heterologous protection induced by the inner capsid proteins of rotavirus requires transcytosis of mucosal immunoglobulins. J Virol. 2002;76:8110–8117. doi: 10.1128/JVI.76.16.8110-8117.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brandtzaeg P, Prydz H. Direct evidence for an integrated function of J chain and secretory component in epithelial transport of immunoglobulins. Nature. 1984;311:71–73. doi: 10.1038/311071a0. [DOI] [PubMed] [Google Scholar]
- 20.Blutt SE, Warfield KL, O’Neal CM, Estes MK, Conner ME. Host, viral, and vaccine factors that determine protective efficacy induced by rotavirus and virus-like particles (VLPs) Vaccine. 2006;24:1170–1179. doi: 10.1016/j.vaccine.2005.08.090. [DOI] [PubMed] [Google Scholar]
- 21.Sanford LP, Kallapur S, Ormsby I, Doetschman T. Influence of genetic background on knockout mouse phenotypes. Methods Mol Biol. 2001;158:217–225. doi: 10.1385/1-59259-220-1:217. [DOI] [PubMed] [Google Scholar]
- 22.Franco MA, Greenberg HB. Immunity to homologous rotavirus infection in adult mice. Trends Microbiol. 2000;8:50–52. doi: 10.1016/s0966-842x(99)01682-0. [DOI] [PubMed] [Google Scholar]
- 23.Blutt SE, Conner ME. Rotavirus: to the gut and beyond! Current Opinion in Gastroenterology. 2007;23:39–43. doi: 10.1097/MOG.0b013e328011829d. [DOI] [PubMed] [Google Scholar]
- 24.O’Neal CM, Crawford SE, Estes MK, Conner ME. Rotavirus VLPs administered mucosally induce protective immunity. J Virol. 1997;71:8707–8717. doi: 10.1128/jvi.71.11.8707-8717.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uren TK, et al. Vaccine-induced protection against gastrointestinal bacterial infections in the absence of secretory antibodies. Eur J Immunol. 2005;35:180–188. doi: 10.1002/eji.200425492. [DOI] [PubMed] [Google Scholar]
- 26.Wijburg OL, et al. Innate secretory antibodies protect against natural Salmonella typhimurium infection. J Exp Med. 2006;203:21–26. doi: 10.1084/jem.20052093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silvey KJ, Hutchings AB, Vajdy M, Petzke MM, Neutra MR. Role of immunoglobulin A in protection against reovirus entry into Murine Peyer’s patches. J Virol. 2001;75:10870–10879. doi: 10.1128/JVI.75.22.10870-10879.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brandtzaeg P, et al. The clinical condition of IgA-deficient patients is related to the proportion of IgD- and IgM-producing cells in their nasal mucosa. Clin Exp Immunol. 1987;67:626–636. [PMC free article] [PubMed] [Google Scholar]
- 29.Reading NC, Kasper DL. The starting lineup: key microbial players in intestinal immunity and homeostasis. Front Microbiol. 2011;2:148. doi: 10.3389/fmicb.2011.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–2202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 31.Blutt SE, et al. Rotavirus antigenaemia and viraemia: a common event? Lancet. 2003;362:1445–1449. doi: 10.1016/S0140-6736(03)14687-9. [DOI] [PubMed] [Google Scholar]
- 32.Coulson BS, et al. Comparison of rotavirus immunoglobulin A coproconversion with other indices of rotavirus infection in a longitudinal study in childhood. J Clin Microbiol. 1990;28:1367–1374. doi: 10.1128/jcm.28.6.1367-1374.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandler RS, et al. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122:1500–1511. doi: 10.1053/gast.2002.32978. [DOI] [PubMed] [Google Scholar]