Abstract
Background
Differences in the breast cancer burden of African American compared with White American women are well-documented. Recent controversies have emerged regarding age-appropriate mammographic screening guidelines, and these surveillance recommendations may influence future breast cancer disparities. Our goal was to evaluate age-specific breast cancer stage distributions and incidence rates of triple-negative breast cancer (TNBC) in a population-based tumor registry.
Study Design
We analyzed California Cancer Registry (CCR) breast cancers diagnosed 1988 – 2006. Results were stratified by age and race/ethnicity, with White Americans identified as Non-Hispanic Whites (NHW) and African Americans as Non-Hispanic Blacks (NHB). Breast cancer stage distributions and TNBC incidence rates were also analyzed.
Results
A total of 375,761 invasive breast cancers were evaluated (including 276,938 in NHW and 21,681 in NHB) NHB and Hispanics tended to be younger than NHW (median ages 57; 54; and 64 years, respectively). Lifetime incidence rates were higher for NHW compared to NHB and Hispanics, but for women younger than 44 years incidence was highest among NHB. NHB also had higher incidence rates of Stage III and IV disease, and higher incidence of TNBC in all age categories.
Conclusions
Population-based data demonstrate that African American women have a more advanced stage distribution for breast cancer compared to White Americans, and higher incidence rates for TNBC. These patterns are observed for women age 40–49 years as well as older women, and suggest that mammographic screening for early detection of breast cancer will be particularly relevant for younger African American women.
Introduction
More than 200,000 women are diagnosed with breast cancer each year in the United States and approximately 40,000 are projected to die with this disease annually(1). Breast cancer mortality rates have declined over the past twenty years in the United States, and this improvement in outcome is largely explained by the combination of earlier detection with screening mammography coupled with utilization of more effective systemic therapy(2). Systemic therapy options for breast cancer are determined by the molecular marker expression of individual tumors. The best outcomes are therefore observed for cancers that are either detected early (i.e. when the distant organ micrometastatic risk is low) or that have a marker pattern indicating high likelihood of controlling micrometastases with targeted treatment such as endocrine therapy for hormone receptor-positivity and/or trastuzamab for HER2/neu overexpression. Conversely, tumors that are detected at advanced stages or that are negative for these markers are more likely to be associated with breast cancer mortality.
Population-based data regarding the breast cancer burden of different subsets of the American female population have revealed several interesting albeit incompletely-understood patterns. Most notably, African American women have a lower lifetime risk of breast cancer, but mortality rates are paradoxically higher when compared to White American women(3). The age-incidence curves also differ; among women younger than age 45 years the incidence rates are higher for African Americans compared to White Americans. Furthermore, frequency of breast tumors that are negative for the estrogen receptor (ER); the progesterone receptor (PR); and/or the HER2/neu marker is also increased among African American women(4).
Breast cancer screening with annual mammography beginning at age 40 years has been advocated since the mid-1990’s by organizations such as the American Cancer Society; the American College of Surgeons; and the National Comprehensive Cancer Network. This recommendation was challenged recently by the U.S. Preventive Services Task Force (USPSTF) which issued a published statement in November 2009 in support of deferring initiation of mammography screening until age 50 years(5). Presumably, this screening strategy could have a disproportionately adverse effect on women facing an increased risk of being diagnosed with early-onset breast cancer, advanced-stage disease, and/or biologically-more aggressive tumors. The goal of this project was to assess the potential implications of the USPSTF recommendation relative to African American women, by computing age-specific, population-based stage distributions for breast cancer as well as age-specific incidence rates of TNBC. For these analyses we relied upon data from the population-based California Cancer Registry (CCR). The CCR has provided valuable information regarding disparities in breast cancer related to racial/ethnic identity since 1988 (6–9).
Methods
An analysis of the California Cancer Registry (CCR) data from 1988 to 2006 was performed. Age-adjusted and age-specific invasive breast cancer incidence rates for the three major race/ethnic groups (Non-Hispanic White [NHW]; Non-Hispanic Black [NHB]; and Hispanic) were computed using data from the CCR. The CCR is a population-based cancer registry which has monitored cancer incidence and mortality in California since 1988. SEER*Stat 6.5.2 program was used to analyze the CCR database, including 95% confidence intervals(10). Statistical significance testing was based upon chi-squared comparisons for categorical variables and based upon student’s t-test to compare the mean values of continuous variables. P-values of less than 0.05 were considered statistically significant.
The CCR database for breast cancer was analyzed by American Joint Committee on Cancer (AJCC) seventh edition stage at diagnosis, stratified by age (categorized as <40 years, 40 to 49 years, 50 to 59 years, 60 to 74 years, and >=75 years) and then comparisons were evaluated with respect to the racial/ethnic subsets of the California population. Racial/ethnic identity was assigned by self-report as documented by the tumor registries contributing to the CCR program.
Staging information and positive versus negative hormone receptor status (estrogen receptor, progesterone receptor) was also assigned according to the information contributed by the CCR registrars. For HER2/neu expression, positive or negative status was based upon documented immunohistochemistry and/or FISH staining. Statistics for TNBC incidence rates were limited to cases diagnosed beginning 2004. Documentation of HER2/neu status was felt to be more uniform and complete during this latter interval, when clinical trial results regarding effectiveness of adjuvant trastuzamab for HER2/neu-overexpressing breast cancer were more widely-available and this component of molecular marker information therefore became more relevant on a routine basis for all women diagnosed with invasive breast cancer (11, 12).
Results
A total of 375,761 women were newly-diagnosed with invasive breast cancer as reported in the California Cancer Registry from 1988 – 2006. Of these, NHW accounted for 278,241 (74%); NHB accounted for 21,716 (5.8%); and Hispanics 45,523 (12.1%).
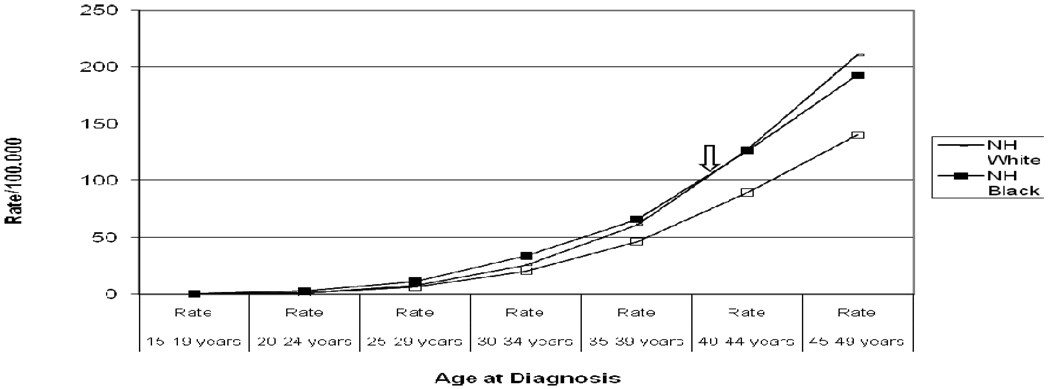
The median age at diagnosis was 64, 57, and 54 years for the NHW, NHB, and Hispanic patients, respectively. The median age at diagnosis for patients with unknown racial/ethnic background was 61.5 years. As demonstrated by the age-incidence rates shown in Table 1 and the age-incidence curves depicted in Figure 1, the risk of breast cancer rises with increasing age for all of the racial/ethnic subsets evaluated. Figure 1 also shows that among women younger than age 44 years, population-based incidence rates of breast cancer are highest for NHB; for women older than 44 years, incidence rates are highest for NHW. Incidence rates are lowest for Hispanic women in all age categories. Incidence rates of Stages 1 and 2 breast cancer were lower for NHB compared with NHW in all age categories, but incidence rates for Stages 3 and 4 disease were higher for NHB. Breast cancer incidence rates by any stage were generally lower for Hispanics compared with NHW in all age categories.
Table 1.
California Cancer Registry breast cancer incidence rates by stage and age category among Non-Hispanic Whites, Non-Hispanic Blacks, and Hispanic women diagnosed 1988–2006. Rates are per 100,000 and age-adjusted to the US 2000 Std population (19 age groups – Census P25-1130); Confidence intervals (Tiwari mod) are 95% for rates). Based on the January 2010 release of the CCR incidence data, NCHS estimates, CI = confidence interval.
| Stage | Age (years) | Non Hispanic White | Non Hispanic Black | Hispanic | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rate | Lower CI |
Upper CI |
Count | Rate | Lower CI |
Upper CI |
Count | Rate | Lower CI |
Upper CI |
Count | ||
| I | <40 | 3.6 | 3.5 | 3.7 | 3,299 | 2.9 | 2.6 | 3.2 | 393 | 1.8 | 1.6 | 1.9 | 967 |
| 40–49 | 60.7 | 59.7 | 61.7 | 14,969 | 40.1 | 37.8 | 42.4 | 1,194 | 30.3 | 29.2 | 31.4 | 3,043 | |
| 50–59 | 130.5 | 128.9 | 132.2 | 24,576 | 75.5 | 71.7 | 79.4 | 1,508 | 60 | 58.1 | 62 | 3,545 | |
| 60–74 | 221.9 | 219.9 | 223.9 | 46,806 | 122.5 | 117.4 | 127.6 | 2,245 | 106.3 | 103.4 | 109.3 | 5,114 | |
| ≥75 | 210.6 | 208.2 | 213 | 29,303 | 115.6 | 108.6 | 122.9 | 1,013 | 100 | 95.7 | 104.6 | 1,972 | |
| II | <40 | 5.6 | 5.4 | 5.7 | 5,166 | 6.3 | 5.9 | 6.8 | 860 | 4.5 | 4.3 | 4.6 | 2,502 |
| 40–49 | 65.9 | 64.9 | 67 | 16,238 | 63.4 | 60.6 | 66.4 | 1,895 | 47.9 | 46.5 | 49.2 | 4,839 | |
| 50–59 | 104.5 | 103.1 | 106 | 19,622 | 95.1 | 90.8 | 99.5 | 1,897 | 68.2 | 66.1 | 70.4 | 4,033 | |
| 60–74 | 138.6 | 137 | 140.2 | 29,170 | 111 | 106.2 | 116 | 2,033 | 84.7 | 82.1 | 87.3 | 4,122 | |
| ≥75 | 134.1 | 132.2 | 136.1 | 18,855 | 111.2 | 104.3 | 118.4 | 976 | 86.5 | 82.5 | 90.8 | 1,698 | |
| III | <40 | 1.2 | 1.2 | 1.3 | 1,131 | 1.9 | 1.7 | 2.1 | 255 | 1.4 | 1.3 | 1.5 | 795 |
| 40–49 | 13.1 | 12.6 | 13.5 | 3,213 | 18.5 | 17 | 20.1 | 552 | 12.8 | 12.1 | 13.5 | 1,298 | |
| 50–59 | 20 | 19.3 | 20.6 | 3,745 | 26.7 | 24.5 | 29.1 | 532 | 18.4 | 17.3 | 19.5 | 1,087 | |
| 60–74 | 21.7 | 21.1 | 22.4 | 4,566 | 26.9 | 24.6 | 29.4 | 496 | 18.6 | 17.4 | 19.8 | 908 | |
| ≥75 | 26.2 | 25.4 | 27.1 | 3,745 | 34.9 | 31.1 | 39 | 307 | 20.3 | 18.3 | 22.4 | 396 | |
| IV | <40 | 0.5 | 0.4 | 0.5 | 433 | 0.9 | 0.8 | 1.1 | 124 | 0.5 | 0.4 | 0.5 | 275 |
| 40–49 | 5.5 | 5.2 | 5.8 | 1,350 | 8.8 | 7.8 | 10 | 263 | 4.4 | 4 | 4.8 | 440 | |
| 50–59 | 11.4 | 10.9 | 11.9 | 2,149 | 17.3 | 15.5 | 19.2 | 345 | 8 | 7.3 | 8.7 | 472 | |
| 60–74 | 17.3 | 16.8 | 17.9 | 3,648 | 23.5 | 21.4 | 25.9 | 431 | 12.5 | 11.5 | 13.5 | 605 | |
| ≥75 | 19 | 18.3 | 19.7 | 2,695 | 25.6 | 22.4 | 29.2 | 225 | 12.8 | 11.2 | 14.4 | 249 | |
| NA/unstaged | <40 | 2 | 1.9 | 2.1 | 1,817 | 3 | 2.7 | 3.3 | 406 | 1.7 | 1.6 | 1.8 | 973 |
| 40–49 | 21.4 | 20.8 | 22 | 5,266 | 27 | 25.2 | 28.9 | 806 | 16.4 | 15.6 | 17.2 | 1,659 | |
| 50–59 | 37 | 36.2 | 37.9 | 6,961 | 43.3 | 40.4 | 46.3 | 864 | 26.3 | 25 | 27.6 | 1,553 | |
| 60–74 | 69.8 | 68.7 | 70.9 | 14,740 | 68 | 64.3 | 72 | 1,238 | 39 | 37.3 | 40.8 | 1,889 | |
| ≥75 | 102.5 | 100.9 | 104.2 | 14,778 | 97 | 90.9 | 104 | 858 | 56.2 | 52.9 | 59.7 | 1,089 | |
Figure 1.
Age-specific breast cancer incidence rates in women under 50 years at age of diagnosis by Race/Ethnicity for California Cancer Registry. Note: Crossover in age-incidence curves between NH Whites and NH Blacks occurs in 40–44 year interval (arrow), with higher incidence rates among younger NH Blacks. NH = Non-Hispanic
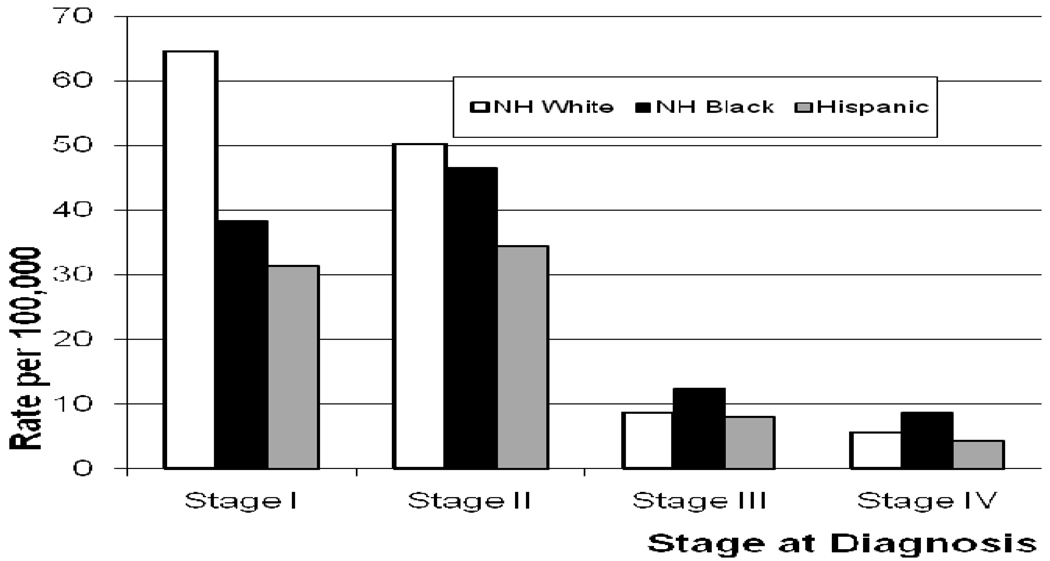
Figure 2 shows the incidence rates by stage for the study population subsets, and demonstrates the shift toward more frequent detection of advanced-stage disease (stages III and IV) for NHB compared to NHW and Hispanics.
Figure 2.
Age adjusted incidence rates of female breast cancer by race/ethnicity and stage at diagnosis from the California Cancer Registry. NH = Non-Hispanic
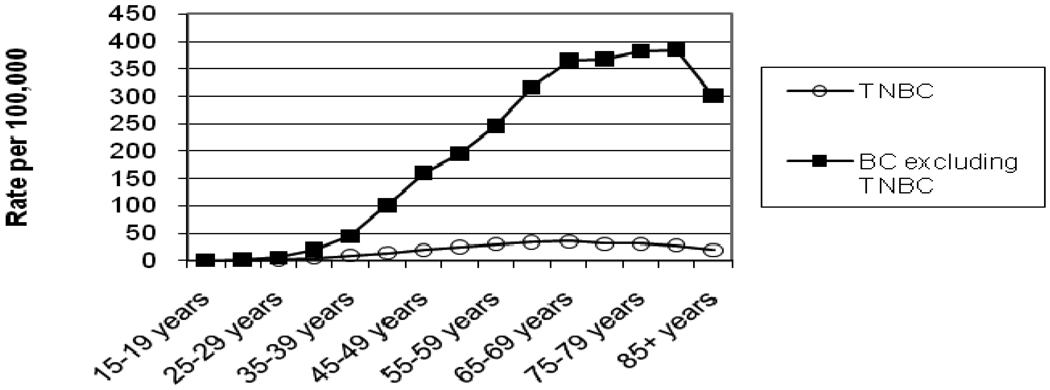
As shown in Figure 3, most of the rising breast cancer incidence rates associated with increasing age is a consequence of increasing risk for non-triple negative breast cancer (TNBC). Incidence rates for TNBC are less than 50 cases per 100,000 population (all race-ethnic groups combined) in all age categories, but these rates slowly rise with age and plateau beyond the fifth decade of life.
Figure 3.
Triple Negative Breast cancer (TNBC) compared with all other breast cancers (BC); Incidence rates per 100,000 by age from the California Cancer Registry.
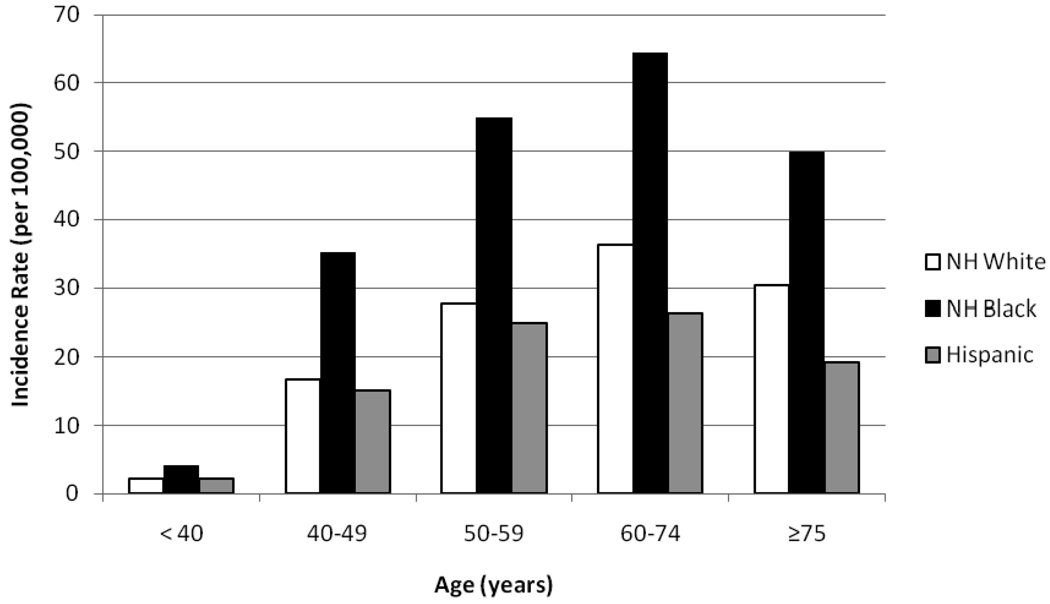
Table 2 and Figure 4 demonstrate the increased risk of TNBC for NHB women in all age categories. Incidence of TNBC rise more steeply with increasing age for NHB compared to NHW and Hispanics, with these incidence rates approximately twofold higher for NHB compared to either of the other subsets in the 40–49 and 50–59 year-old age categories. The TNBC incidence rates peak at 64.4 per 100,000 NHB in the 60–74 year-old age category.
Table 2.
Triple Negative Breast Cancer incidence rates per 100,000 by age and race from the California Cancer Registry, (rates are per 100,000 US 2000 Std population, Confidence intervals [Tiwari mod] are 95% for rates). NHB = Non-Hispanic Black, NHW = Non-Hispanic White. CI = confidence interval.
| Age (years) | NHB | NHW | Hispanic | |||
|---|---|---|---|---|---|---|
| Incidence Rate | 95% CI | Incidence Rate | 95% CI | Incidence Rate | 95% CI | |
| 0–39 | 4.1 +/−0.4 | 3.4–5.0 | 2.2 +/−0.1 | 2.0–2.5 | 2.1 +/−0.1 | 1.9–2.3 |
| 40–49 | 35.1 +/−2.2 | 31–39.6 | 16.8 +/−0.6 | 15.7–17.9 | 15.1 +/−0.7 | 13.7–16.5 |
| 50–59 | 54.9 +/−3.2 | 48.9–61.5 | 27.7 +/−0.8 | 26.2–29.2 | 24.9 +/−1.1 | 22.7–27.2 |
| 60–74 | 64.4 +/−3.8 | 57.1–72.4 | 36.3 +/−0.9 | 34.6–38.2 | 26.4 +/−1.4 | 23.8–29.3 |
| ≥75 | 49.9 +/−4.8 | 40.9–60.4 | 30.3 +/−1.0 | 28.3–32.3 | 19.3 +/−1.7 | 16.1−23.0 |
Figure 4.
California Cancer Registry, Triple Negative Breast Cancer incidence rates by age and race [rates are per 100,000 and age-adjusted to the 2000 US Std Population (19 age groups - Census P25-1130) standard; Confidence intervals (Tiwari mod) are 95% for rates]. NH = Non-Hispanic
Discussion
Recent challenges to the traditional recommendations that American women initiate annual screening mammography at age 40 years have the potential for exerting a disproportionately adverse effect on African American women because of the well-documented younger age distribution for breast cancer in this population subset. Early detection of breast cancer is the most powerful determinant of outcome, and this will be particularly relevant for tumors expressing phenotypes that cannot be controlled with targeted agents such as endocrine therapy and/or trastuzamab. African American women have higher mortality rates from breast cancer, and this is at least partially explained by the fact that they tend to present with more advanced stages compared to White American women. They therefore represent a community that has been the focus of many breast cancer awareness and screening/early detection programs. Our study provides further evidence of the need to continue intensive breast cancer surveillance among African American women age 40–49 years. By studying data from the California Cancer Registry, we found higher population-based incidence rates of locally-advanced breast cancer and triple-negative breast cancer among African American women.
Although the lifetime incidence rates of breast cancer are higher for White American compared to African American women, the Surveillance, Epidemiology and End Results (SEER) Program documents that for women younger than age 45 years, population-based incidence rates are higher for African Americans(13). Several investigators have demonstrated that the frequency of TNBC is higher for African American compared to White American breast cancer patients (14–17). Carey et al (18) furthermore demonstrated that the risk of triple-negative breast cancer is particularly high among premenopausal African American women, based upon analysis of the Carolina Breast Cancer Study. By reporting on age- and race/ethnicity- specific patterns of disease in the California Cancer Registry, our study provides powerful population-based evidence regarding the importance of aggressive screening for early detection of breast cancer in young African American women.
As background for the updated screening guidelines presented by the U.S. Preventive Services Task Force (USPSTF) in November 2009(5) reviews of the data from the historic mammography screening trials were prepared by Nelson et al(19) and Mandelblatt et al on behalf of the Cancer Intervention and Surveillance Modeling Network (CISNET)(20). In summary, they found that for women age 40–49 years, approximately 1900 must be invited to mammographic screening in order to save one life; for women age 50–59 years, approximately 1300 must be invited; and for women age 60–69 years, approximately 400 must be invited. They also found that initiation of mammography screening at age 40 rather than age 50 years results in an average gain of 33 life years per 1000 women screened. CISNET therefore stated: “If the goal of a national screening program is to reduce mortality in the most efficient manner, then programs that screen biennially from age 50 years…are among the most efficient on the basis of the ratio of benefits to the number of screening mammograms. If the goal of a screening program is to efficiently maximize the number of life-years gained, then the preferred strategy would be to screen biennially starting at age 40 years.”(20) The USPSTF opted to advocate in favor of supporting an efficiency-based screening model rather than a longevity-based program, and they therefore issued the statement that “The USPSTF recommends against routine screening mammography in women aged 40–49 years… The USPSTF recommends biennial screening mammography for women between the ages of 50 and 74 years.”(5) CISNET furthermore commented that none of the mammography screening models were likely to capture differences in outcome among specific population subsets such as “black women who seem to have more disease at younger ages than white women”(20). The USPSTF recommendation statement did not comment on the potential impact of their screening recommendations on race-ethnicity-associated breast cancer disparities.
Our study serves to inform the discussion regarding relevance of mammography screening for African American women age 40–49 years. We found more advanced stage of disease at diagnosis in these younger African American women, and we also found higher incidence rates of TNBC for this population subset. Since mammography screening programs should improve early detection rates for breast cancer, and since early detection of TNBC is critical for improving its successful treatment, we believe that our study findings provide compelling evidence that screening mammography should be particularly important for young African American women. The USPSTF recommendation that routine screening mammography should not be initiated until age 50 years has the potential for widening the magnitude of breast cancer outcome disparities between African American and White American women.
The CCR data on race/ethnicity-associated frequency of TNBC is consistent with other studies, as shown in Table 3. The population-based incidence rates featured in our study serve to strengthen the validity of these observations. These rates indicate an inherently higher risk of TNBC for NHB/African American women, refuting the argument that the larger proportion of TNBC in African American women is an artifact of the “denominator” phenomenon (i.e. African American women appear to have more TNBC simply because they have fewer total breast cancers compared to White American women). Our data on women from California demonstrate increased population-based risk of TNBC for NHB women.
Table 3.
Published studies of ER-negative/PR-negative/HER2/neu-negative (TNBC) requency by race/ethnic identity.
| Dataset | Sample Size Dataset |
Proportion of of TNBC | |||
|---|---|---|---|---|---|
| AA | WA | P | |||
| Carey et al(18), 2006 | Carolina Breast Cancer Study | 97 premenopausal AA 164 premenopausal WA women |
39% | 16% | <0.001 |
| Morris et al(14), 2007 | Thomas Jefferson University Hosp pts SEER Program | 2230 Thomas Jefferson University Hosp pts 197,274 SEER pts |
21% | 10% | <0.001 |
| Lund et al(16), 2009 | Population-based Atlanta, Georgia cohort | 116 AA 360 WA pts |
47% | 22% | <0.001 |
| Lund et al(15), 2008 | Grady Hospital; Atlanta, Georgia | 167 AA 23 WA |
29% | 13% | 0.05 |
| Moran et al(17), 2008 | BCS pts from Yale University School of Medicine | 99 AA 968 WA |
21% | 8% | <0.001 |
| Parise et al (6), 2009 | California Cancer Registry | 3,743 AA 48,863 WA |
28% | 12% | NR |
| Stark et al(31) 2010 | Henry Ford Hospital, Detroit, Michigan | 1,008 WA 581 AA |
16% | 26% | <0.01 |
ER=estrogen receptor; PR=progesterone receptor; TNBC= triple-negative breast cancer; AA= African American; WA=White American; SEER= Surveillance, Epidemiology, and End Results Program; BCS= breast-conservation Surgery
Our study is limited by our unfortunate inability to correlate mammography screening information with the age- and race/ethnicity-specific breast cancer incidence rates. However data from the California Health Interview Survey (CHIS) indicate similar mammography utilization rates for African American and White American women, but somewhat lower rates for Hispanic/Latina women (unpublished data) and this finding mirrors national data on mammography utilization reported by the American Cancer Society(1). Although the data regarding effectiveness of mammography in detecting TNBC (compared to detection rates for non-TNBC) are limited, the available published studies indicate that frequency of mammographically-occult breast tumors is similar for TNBC and non-TNBC. TNBC does however, appear to be less-frequently associated with microcalcifications and is more likely to be identified as a mass or asymmetric density(21–24). Furthermore, Ma et al demonstrated that mammographic density (a common imaging finding among premenopausal women) is a risk factor for TNBC as well as for non-TNBC(25).
Another notable limitation of our study is the fact that the CCR (similar to the SEER Program) lacks detailed information on menstrual history and reproductive factors. Millikan et al(26) and other investigators(27) have suggested that childbearing patterns and lactation history may account for race/ethnicity-associated variation in breast cancer burden. Others have been unable to confirm these hypotheses (28). Yet other investigators have reported elevated risk of TNBC among contemporary female populations of continental Africa(29–31), suggesting that African ancestry may be associated with some heritable risk factor for TNBC(32). Our population-based California dataset is unable to address any of these issues.
In summary, our data from California (which appears to be representative of national data) demonstrates increased risk of advanced-stage breast cancer and triple-negative breast cancer in African American women compared to White American and Hispanic American women. These patterns are notably prominent for women younger than age fifty, suggesting that mammography screening to improve early detection of biologically-aggressive patterns of breast cancer will be particularly relevant for younger African American women, especially since overall breast cancer incidence rates are higher for African American women in the premenopausal age range.
Acknowledgement
The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N01-PC-54404 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement 1U58DP00807-01 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should they be inferred.
A special thanks is warranted to our coders, without whom the registry would not be possible. In particular we are grateful to Ellen Malek, CTR, for her wisdom and insight regarding the data, and its translation into the CCR.
Footnotes
Authors do not have any financial disclosures.
Contributor Information
Kathryn C. Amirikia, University of California San Francisco, Fresno.
Paul Mills, University of California San Francisco, Fresno.
Jason Bush, California State University, Fresno.
Lisa A Newman, University of Michigan.
References
- 1.American Cancer Society. Breast Cancer Facts & Figures 2009–2010. Atlanta, GA: American Cancer Society, Inc.; [Google Scholar]
- 2.Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353(17):1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society. Breast Cancer Facts & Figures 2009–2010. Atlanta GACS, Inc.; [Google Scholar]
- 4.Anderson WF, Chatterjee N, Ershler WB, Brawley OW. Estrogen receptor breast cancer phenotypes in the Surveillance, Epidemiology, and End Results database. Breast Cancer Res Treat. 2002;76(1):27–36. doi: 10.1023/a:1020299707510. [DOI] [PubMed] [Google Scholar]
- 5.Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716–726. doi: 10.7326/0003-4819-151-10-200911170-00008. W-236. [DOI] [PubMed] [Google Scholar]
- 6.Parise CA, Bauer KR, Caggiano V. Variation in breast cancer subtypes with age and race/ethnicity. Crit Rev Oncol Hematol. 2009 doi: 10.1016/j.critrevonc.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Parise CA, Bauer KR, Brown MM, Caggiano V. Breast cancer subtypes as defined by the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) among women with invasive breast cancer in California, 1999–2004. Breast J. 2009;15(6):593–602. doi: 10.1111/j.1524-4741.2009.00822.x. [DOI] [PubMed] [Google Scholar]
- 8.Brown M, Tsodikov A, Bauer KR, Parise CA, Caggiano V. The role of human epidermal growth factor receptor 2 in the survival of women with estrogen and progesterone receptor-negative, invasive breast cancer: the California Cancer Registry, 1999–2004. Cancer. 2008;112(4):737–747. doi: 10.1002/cncr.23243. [DOI] [PubMed] [Google Scholar]
- 9.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109(9):1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 10. In http://seer.cancer.gov/seerstat/
- 11.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr., Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 12.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 13.Horner MJRL, Krapcho M, Neyman N, Aminou R, Howlader N, Altekruse SF, Feuer EJ, Huang L, Mariotto A, Miller BA, Lewis DR, Eisner MP, Stinchcomb DG, Edwards BK, editors. SEER Cancer Statistics Review, 1975–2006, based on November 2008 SEER data submission, posted to the SEER web site, 2009. Bethesda, MD: National Cancer Institute; 2009. at http://seer.cancer.gov/csr/1975_2006/ [Google Scholar]
- 14.Morris GJ, Naidu S, Topham AK, Guiles F, Xu Y, McCue P, et al. Differences in breast carcinoma characteristics in newly diagnosed African-American and Caucasian patients: a single-institution compilation compared with the National Cancer Institute's Surveillance, Epidemiology, and End Results database. Cancer. 2007;110(4):876–884. doi: 10.1002/cncr.22836. [DOI] [PubMed] [Google Scholar]
- 15.Lund MJ, Butler EN, Bumpers HL, Okoli J, Rizzo M, Hatchett N, et al. High prevalence of triple-negative tumors in an urban cancer center. Cancer. 2008;113(3):608–615. doi: 10.1002/cncr.23569. [DOI] [PubMed] [Google Scholar]
- 16.Lund MJ, Trivers KF, Porter PL, Coates RJ, Leyland-Jones B, Brawley OW, et al. Race and triple negative threats to breast cancer survival: a population-based study in Atlanta, GA. Breast Cancer Res Treat. 2009;113(2):357–370. doi: 10.1007/s10549-008-9926-3. [DOI] [PubMed] [Google Scholar]
- 17.Moran MS, Yang Q, Harris LN, Jones B, Tuck DP, Haffty BG. Long-term outcomes and clinicopathologic differences of African-American versus white patients treated with breast conservation therapy for early-stage breast cancer. Cancer. 2008;113(9):2565–2574. doi: 10.1002/cncr.23881. [DOI] [PubMed] [Google Scholar]
- 18.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. Jama. 2006;295(21):2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 19.Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151(10):727–737. W237–W242. doi: 10.1059/0003-4819-151-10-200911170-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mandelblatt JS, Cronin KA, Bailey S, Berry DA, de Koning HJ, Draisma G, et al. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009;151(10):738–747. doi: 10.1059/0003-4819-151-10-200911170-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang WT, Dryden M, Broglio K, Gilcrease M, Dawood S, Dempsey PJ, et al. Mammographic features of triple receptor-negative primary breast cancers in young premenopausal women. Breast Cancer Res Treat. 2008;111(3):405–410. doi: 10.1007/s10549-007-9810-6. [DOI] [PubMed] [Google Scholar]
- 22.Yang TJ, Yang Q, Haffty BG, Moran MS. Prognosis for mammographically occult, early-stage breast cancer patients treated with breast-conservation therapy. Int J Radiat Oncol Biol Phys. 76(1):79–84. doi: 10.1016/j.ijrobp.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 23.Kojima Y, Tsunoda H. Mammography and ultrasound features of triple-negative breast cancer. Breast Cancer. doi: 10.1007/s12282-010-0223-8. [DOI] [PubMed] [Google Scholar]
- 24.Ko ES, Lee BH, Kim HA, Noh WC, Kim MS, Lee SA. Triple-negative breast cancer: correlation between imaging and pathological findings. Eur Radiol. 20(5):1111–1117. doi: 10.1007/s00330-009-1656-3. [DOI] [PubMed] [Google Scholar]
- 25.Ma H, Luo J, Press MF, Wang Y, Bernstein L, Ursin G. Is There a Difference in the Association between Percent Mammographic Density and Subtypes of Breast Cancer? Luminal A and Triple-Negative Breast Cancer. Cancer Epidemiol Biomarkers Prev. 2009 doi: 10.1158/1055-9965.EPI-08-0805. [DOI] [PubMed] [Google Scholar]
- 26.Millikan RC, Newman B, Tse CK, Moorman PG, Conway K, Dressler LG, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109(1):123–139. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmer JR, Wise LA, Horton NJ, Adams-Campbell LL, Rosenberg L. Dual effect of parity on breast cancer risk in African-American women. J Natl Cancer Inst. 2003;95(6):478–483. doi: 10.1093/jnci/95.6.478. [DOI] [PubMed] [Google Scholar]
- 28.Ursin G, Bernstein L, Wang Y, Lord SJ, Deapen D, Liff JM, et al. Reproductive factors and risk of breast carcinoma in a study of white and African-American women. Cancer. 2004;101(2):353–362. doi: 10.1002/cncr.20373. [DOI] [PubMed] [Google Scholar]
- 29.Huo D, Ikpatt F, Khramtsov A, Dangou JM, Nanda R, Dignam J, et al. Population differences in breast cancer: survey in indigenous african women reveals over-representation of triple-negative breast cancer. J Clin Oncol. 2009;27(27):4515–4521. doi: 10.1200/JCO.2008.19.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bird PA, Hill AG, Houssami N. Poor hormone receptor expression in East African breast cancer: evidence of a biologically different disease? Ann Surg Oncol. 2008;15(7):1983–1988. doi: 10.1245/s10434-008-9900-7. [DOI] [PubMed] [Google Scholar]
- 31.Stark A, Kleer C, Martin IK, Awuah B, Nsiah-Asare A, Takyi V, et al. African Ancestry and Higher Prevalence of Triple Negative Breast Cancer: Findings from an International Study. Cancer. 2010 doi: 10.1002/cncr.25276. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fregene A, Newman LA. Breast cancer in sub-Saharan Africa: how does it relate to breast cancer in African-American women? Cancer. 2005;103(8):1540–1550. doi: 10.1002/cncr.20978. [DOI] [PubMed] [Google Scholar]