Abstract
Although Attention Deficit/Hyperactivity Disorder (ADHD) has increasingly been studied in preschool-aged children, relatively few studies have provided a comprehensive evaluation of the factor structure and patterns of developmental changes in parent-reported ADHD symptomatology across the early childhood period. This study used confirmatory factor analyses to test for longitudinal measurement invariance of ADHD symptoms and semi-parametric finite mixture models to identify prototypic patterns of developmental changes in ADHD symptomatology from 3-5 years of age. Participants were 1155 children and their parents who participated in a prospective longitudinal study involving a representative sample of children who resided in six non-metropolitan counties in the United States. Results indicated that (1) ADHD symptomatology was best represented by a single latent factor that exhibited partial measurement invariance from 3-5 years of age, (2) 8.5% of children exhibited sustained high levels of ADHD symptoms from age 3-5 years, and (3) a variety of risk factors differentiated children with sustained high from those with sustained low levels of ADHD, relatively few (most notably caregiver education) were able to differentiate children with sustained high levels of ADHD symptoms from all other groups. Children who exhibit persistent ADHD symptomatology across the early childhood period may define a clinically important group for etiologic research and/or early intervention efforts.
Keywords: Attention Deficit/Hyperactivity Disorder, Early Childhood, Measurement Invariance, Semi-Parametric Finite Mixture Models, Developmental Change
Attention Deficit Hyperactivity Disorder (ADHD) is an early onset and chronic disorder, that involves developmentally inappropriate levels of inattentive and/or hyperactive-impulsive behaviors, that are observable in multiple settings, and that cause functional impairments in multiple domains of functioning (APA, 2000). Historically, the majority of research has involved school-aged children. Over the last 10-15 years, researchers have begun to systematically study ADHD in preschool-aged children (Byrne, DeWolfe, & Bawden, 1998; Connor, 2002; Egger, Kondo, & Angold, 2006; Wilens et al., 2002). The present study focused on the longitudinal measurement and developmental change of ADHD symptomatology in early childhood.
Although efforts to subtype ADHD youth as a function of their inattentive (IN) and/or hyperactive-impulsive (HI) symptomatology have undergone numerous changes (APA, 1980, 1987, 2000), confirmatory factor analyses (CFA) have consistently indicated that IN and HI behaviors are dissociable but correlated factors in elementary school-aged, but perhaps not preschool-aged, children around the world (Bauermeister, Canino, Polanczyk, & Rohde, 2010). The suggestion that the factor structure of ADHD may differ among preschoolers was based on the results of one large study (Hardy et al., 2007). Although Hardy and colleagues (2007) reported marginal and inconsistent fit for one-, two-, and three-factor models of ADHD symptoms, they did not formally test competing model structures. The first goal of the current study was to test the factor structure of ADHD symptoms in early childhood using a statistical approach that both accounted for the dichotomous nature of ADHD symptoms and that permitted formal comparisons between competing factor structures. Consistent with the larger literature, we hypothesized that a 2-factor model would provide optimal fit to the data.
A secondary goal involved testing whether the resulting factor structure of ADHD symptoms exhibited measurement invariance from ages 3 to 5 years. Although we are not familiar with previous studies that have examined the measurement invariance of ADHD symptoms across time, doing so is a necessary precondition to evaluating changes in mean level symptoms across time (Meredith, 1993; Meredith & Horn, 2001). We hypothesized that the factor structure of ADHD symptoms would be invariant across time.
Conventional lay wisdom holds that, as a group, preschool-aged children exhibit higher levels of ADHD behaviors than do school-aged children. This has raised attendant concerns regarding the potential over-identification of preschoolers as having ADHD. However, studies that comprehensively assess the full spectrum of DSM-IV ADHD symptoms (compared to a few isolated behaviors), preferably using structured assessments and/or multi-informant assessment protocols, have allayed these concerns (Byrne et al., 1998; Egger et al., 2006; Gimpel & Kuhn, 2000). In fact, the prevalence of ADHD among preschool-aged children is comparable to that observed in school-aged children (reviewed by Egger et al., 2006). Preschool-aged children who exhibit six or more IN and/or HI symptoms, especially across multiple settings, are markedly different from their typically developing preschool-aged peers and experience impairment in functioning in early childhood and adolescence (Egger & Angold, 2006; Lee, Lahey, Owens, & Hinshaw, 2008; Posner et al., 2007). Nonetheless, many preschoolers, especially 3 year-olds, who initially present with elevated ADHD symptoms demonstrate a pattern of remitting symptoms across time (Campbell, Breaux, Ewing, Szumowski, & Pierce, 1986; Harvey, Youngwirth, Thakar, & Errazuriz, 2009; Lavigne et al., 1998a; Tandon, Si, & Luby, 2011). The limited evidence to date indicates that psychiatric comorbidity (especially Oppositional Defiant Disorder), family history of disruptive behavior disorders, and family contextual factors all contribute to symptom persistence (Lavigne et al., 1998b; Tandon et al., 2011).
Prospective longitudinal studies that repeatedly assess ADHD behaviors across the preschool period provide one means for distinguishing those children with persistent ADHD symptoms from those children who exhibit time-limited elevations in ADHD symptoms that abate over time. At least four such studies have utilized semi-parametric finite mixture models (SPFMs) to describe developmental changes in ADHD behaviors across the early childhood period (Galera et al., 2011; Leblanc et al., 2008; Romano, Tremblay, Farhat, & Cote, 2006; Shaw, Lacourse, & Nagin, 2005). SPFMs differ from traditional growth curve models in that they can permit the functional form of change to vary across (latent) subgroups of youth. Hence, these models facilitate the identification of children who exhibit a persistent pattern of ADHD symptomatology across time (characterized by elevated intercepts and non-significant slopes) from those who exhibit alternate forms of change (e.g., remitters, who exhibit initially elevated symptoms that significantly decrease across time). Three Canadian studies, which involved unselected or representative samples and that used SPFMs to evaluate prospective changes in ADHD symptoms, indicated that 7-12% (two of the three studies converged at 7-8%) of children exhibited persistently elevated levels of ADHD behaviors across the preschool period (Galera et al., 2011; Leblanc et al., 2008; Romano et al., 2006). These results are similar to those from a clinically-informed project, which was published nearly three decades ago, that described 5-7% of children as having persistent attention problems from toddlerhood through school entry (Palfrey, Levine, Walker, & Sullivan, 1985). The Palfrey et al. (1985) study was interesting because it also identified another 8% of children as having elevated attention problems in early childhood that abated before kindergarten, which is consistent with diagnostic instability studies of ADHD. Perhaps due to the limited measurement of ADHD behaviors (parents rated 3-8 items on a 3-point Likert scale), none of the prospective longitudinal studies that utilized SPFM methods identified a group of children with remitting ADHD symptoms across time. A third objective of this study was to use the SPFM approach in conjunction with parent reports of all 18 DSM-IV ADHD symptoms in a representative sample of children to test for the identification of both persistent and remitting ADHD symptom trajectories from 3-5 years of age. We hypothesized that approximately 8% of the sample would be characterized by ADHD symptom persistence (stable, high levels), an approximately equal proportion, 8%, by symptom remittance (initially elevated but decreasing), with the remainder of the sample manifesting consistently low levels of ADHD symptoms across time.
The ability to differentiate persisting from remitting ADHD in early childhood is potentially clinically meaningful as it may help identify those children and families with the greatest need for early intervention services (Chacko, Wakschlag, Hill, Danis, & Espy, 2009; McGoey, Eckert, & DuPaul, 2002; Sonuga-Barke, Thompson, Abikoff, Klein, & Brotman, 2006). The fourth objective of the current study was to consider a wide-range of parent reported variables that may help predict persistent ADHD symptomatology. We used a combination of predictors from previous comparable studies (Galera et al., 2011; Romano et al., 2006), as well as earlier studies focused on risk factors for ADHD (Biederman et al., 1995; Das Banerjee, Middleton, & Faraone, 2007; Mick, Biederman, Faraone, Sayer, & Kleinman, 2002; St Sauver et al., 2004). We focused on predictors that were measured early in life (i.e., when the target child was approximately 2 months of age). Identifying early and easily measured risk factors has the greatest potential to facilitate early identification and intervention. Considering multiple risk factors together helped identify those risks that were uniquely predictive of persistent ADHD in early childhood.
In sum, the current study tested the factor structure and longitudinal measurement invariance of parent-reported ADHD symptoms from ages 3-5 years, used group-based longitudinal models to describe prototypic patterns of developmental change in ADHD symptoms, and tested whether there were risk factors that were easily measured early in life that uniquely predicted persistent ADHD across the early childhood period. We hypothesized that parent-rated ADHD symptoms would be best represented by two latent factors (inattentive, hyperactive-impulsive), that symptoms would exhibit longitudinal measurement invariance, that trajectories of ADHD symptom change would generally follow three patterns (persistently low, persistently elevated, and initially elevated and remitting), and that well-established risks for ADHD—including child (e.g., male gender, low birth weight), parent (e.g., history of ADHD, prenatal substance use, post-natal depression), and household (e.g., poverty, household structure) variables—would differentiate children with persistently elevated symptoms from those with persistently low and/or remitting symptoms.
Method
Participants
The Family Life Project (FLP) was designed to study young children and their families who lived in two of the four major geographical areas of the United States with high poverty rates (Dill, 2001). Specifically, three counties in Eastern North Carolina (NC) and three counties in Central Pennsylvania (PA) were selected to be indicative of the Black South and Appalachia, respectively. The FLP adopted a developmental epidemiological design in which complex sampling procedures were employed to recruit a representative sample of 1292 children whose families resided in one of the six counties at the time of the child’s birth. Low-income families in both states and, in NC, African American families were over-sampled; however, through the use of weighted analyses, all of our inferences generalize back to the 6-county study area as if participants were selected using simple random sampling. Although space prohibits a full characterization of the sampling plan and study design, this information has been detailed elsewhere (Vernon-Feagans, Cox, & Investigators, in press).
The current study included children with parent-rated ADHD behaviors from age 3 (N = 1096), 4 (N = 1062), and/or 5-year (N = 1057) assessments (N = 1155 children participated in at least one of these three assessments, representing 89% of the total sample; among those participating, 6%, 8%, and 85% of children had one, two, or all three assessments of ADHD assessments). Some visits were conducted by phone for families who had moved beyond a 200 mile radius from the study counties. Families and children who were enrolled in the study but who did not participate in 3, 4, or 5 year assessments (N = 137) did not differ from study participants (N = 1155) with respect to state of residence (38% vs. 40% residing in PA, p = .58), sex of the child (56% vs. 50% male, p = .19), race of the child (39% vs. 43% African American, p = .34), living in a household that was recruited into the low income stratum (77% vs. 78% poor, p = .96), primary caregiver educational status at study enrollment (79% vs. 80% with a high school degree or GED, p = .67), household structure (58% vs. 53% single parent headed household, p = .21) or household size (15% vs. 19% households with 6+ residents, p = .31).
Procedures
Following hospital screening, participants who were selected and agreed to participate were formally enrolled into the study by completing a home visit when the target child was approximately 2 months old. Participating families completed additional home visits when their target child was approximately 7 and 15 months, as well as annually from 2-5 years of age. At each visit, parents and children completed a variety of standardized tasks, observational procedures, interviews and questionnaires. This study is based on parent-reported information, including risk factors drawn from the 2 month and 2 year assessments, as well as ADHD symptomatology for the target child at the 3 through 5 year assessments.
Measures
Outcome: Attention Deficit/Hyperactivity Disorder (ADHD) Symptom Rating Checklist (DuPaul et al., 1998)
The ADHD checklist includes the 18 DSM-IV symptoms for ADHD, each rated on a four point scale (0=not at all, 1=just a little, 2=pretty much, 3=very much). Following convention for the use of this instrument and others like it (see, for example, Pelham, Gnagy, Greenslade, & Milich, 1992), items that were rated as either “pretty much” or “very much” were considered an approximation for symptom endorsement. At the 3, 4, and 5 year assessments, primary caregivers rated their child’s current ADHD behaviors (no changes in symptom wording were made). IN and HI symptom counts had strong internal consistency (IN αs = .86, .86, and .87; HI αs = .83, .83, .85 at 3, 4, and 5 year assessments, respectively).
Risk Factor: Prenatal Exposure to Smoking, Alcohol & Drug Use
At the 2 month home visit, biological mothers of target children completed the pregnancy and delivery module of the Missouri Assessment of Genetics Interview for Children (MAGIC; Reich, Todd, Joyner, Neuman, & Heath, 2003). Reich and colleagues reported good short and long-term reliability for self-reports of pregnancy behaviors (Reich et al., 2003). Children whose biological mothers reported smoking cigarettes, drinking alcohol, or using illicit drugs (irrespective of duration, frequency, timing, or amount) were designated as having prenatal exposure to smoking, alcohol, or drug use (23%, 12%, and 2% of the total sample met these criteria, respectively).
Risk Factor: Low Birth Weight
As part of the 2 month interview (i.e., the first in-person visit with mothers following their recruitment from the delivery ward), mothers reported the target child’s birth weight in pounds and ounces. This weight was converted to grams and children weighing less than or equal to 2500 grams were designated low birth weight; 8% of the sample met this criterion.
Risk Factor: Caregiver Attention Deficit/Hyperactivity Disorder (ADHD) Symptom Rating Checklist (DuPaul et al., 1998)
At the 2 year assessment, primary caregivers who were biologically related to the target child retrospectively rated their own ADHD behaviors between ages 5-12 years. As described above, items that were rated as either “pretty much” or “very much” were considered an approximation for symptom endorsement. Retrospective accounts of Inattentive (IN), Hyperactive-Impulsive (HI), and Total symptoms exhibited good internal consistencies, αs = .87, .82, .89, respectively. Children whose biological primary caregiver retrospectively endorsed six or more IN and/or HI symptoms were considered to have a positive family history of ADHD; 5.5% of the sample met this criterion (N = 16, 1.5%, primary caregivers retrospectively reported 6 or more IN and HI symptoms; N = 14, 1.3%, reported 6 or more HI symptoms; N = 31, 2.8%, reported 6 or more IN symptoms).
Risk Factor: Brief Symptom Index 18 (BSI-18; Derogatis, 2000)
The BSI-18 was completed by primary caregivers at the 2 month home visit. The instrument is a short, sensitive, self-report screening index of internalizing symptomatology that is derived from the longer Brief Symptom Inventory (BSI: Derogatis & Melisaratos, 1983). The BSI-18 includes 18 items that are divided evenly across three dimensions: somaticism, anxiety, and depression. Primary caregivers who reported depression scores at the subclinical range and above (T >= 63), which included 8.4% of the total sample, were defined as at-risk for depression.
Risk Factor: Demographics
Biological mothers who were 18 years or younger at the time that they gave birth to the target child were considered teenage moms (5% of the total sample). Caregivers who had not completed a high school degree, including a GED, at the 2 month home visit were designated as having low education (20% of the sample).
Risk Factor: Household Size, Structure, and Poverty Level
As a part of the 2 month interview, the number of persons residing in the household was counted. We designated households with 6 or more persons as large (19% of the sample met this criterion). We also used the presence of single parent headed household (53% of the sample) as an indicator of household structure. Both were considered risks for ADHD (Biederman, Faraone, & Monuteaux, 2002; Biederman et al., 1995). Household poverty levels were defined by summing the income of anyone who resided (defined by sleeping at the household three or more nights per week) in the household and dividing it by the federal poverty threshold for a given family size to create the income/needs ratio. Consistent with the literature on poverty, households in which the income/needs ratio was less than or equal to 2.0 were designated as poor (65% of the sample).
Analytic strategy
The first two research questions were addressed by estimating a series of confirmatory factor analyses (CFA) involving ADHD symptoms. Model evaluations were based on a combination of chi square test statistics as well as fit statistics. Models with a comparative fit index (CFI) >= .95 and a root mean squared error of approximation (RMSEA) index < .05 were indicative of good overall fit (Browne & Cudeck, 1993; Hu & Bentler, 1999). Competing models were evaluated using nested chi square difference tests and changes in CFIs, where significant chi square tests and changes greater than or equal to .02 were indicative of changes in model fit (Cheung & Rensvold, 2002; Satorra & Bentler, 2001). All CFA models were fit using Mplus version 6.1 (Muthén & Muthén, 1998-2010) using the weighted least squares with mean and variance adjustment (WLSMV) estimator and the delta parameterization method. The WLSMV estimator uses pairwise deletion to accommodate missing data. All CFA models took into account the complex sampling design, including stratification and over-sampling of low income and, in NC, African American families.
The third research question was addressed using semi-parametric finite mixture (SPFM) models as implemented by PROC TRAJ in SAS® version 9.2 (Jones, Nagin, & Roeder, 2001; Nagin, 1999). Zero-inflated Poisson distributions took into account asymmetric distributions of ADHD symptom counts. By using SPFMs, our results were directly comparable to three previous studies that used this same approach with representative or unselected samples (Galera et al., 2011; Leblanc et al., 2008; Romano et al., 2006). Consistent with previous studies, we relied on the Bayesian Information Criterion (BIC) to inform the optimal number of groups and model trimming. All SPFMs took into account the complex sampling design, including stratification and over-sampling of low income and, in NC, African American families. SPFMs use full information maximum likelihood estimation to accommodate missing data.
The fourth research question was addressed by using posterior probabilities from SPFMs to assign children to the group-based trajectory group that was most likely given their observed data. Multinomial regression (MNR) models, as implemented by PROC LOGISTIC in SAS® version 9.2, were used to test whether risk factors distinguished membership in trajectory groups, with an emphasis on distinguishing children with a persistently elevated symptom pattern from the other groups. MNR models did not take into account the sampling design because the models that were used to assign group membership (SPFMs) did.
Results
Descriptive Statistics
Frequencies for all parent-rated 18 ADHD symptoms, as well as IN and HI symptom counts, at ages 3-5 years are summarized in Table 1. On average, children exhibited a total of four ADHD symptoms at age 3 years (1.7 IN symptoms, 2.5 HI symptoms) with an approximately ½ of a symptom reduction each subsequent year (an average of three symptoms by age 5 years). Hyperactive-impulsive and inattentive symptoms both exhibited comparable reductions across time.
Table 1.
Descriptive statistics for ADHD Symptoms (Counts)
| Assessment
|
|||
|---|---|---|---|
| 3 Year | 4 Year | 5 year | |
|
| |||
| Symptom Description | % | % | % |
| 1. Makes careless mistakes. | 17.1 | 12.5 | 10.0 |
| 2. Fidgets, squirms in seat. | 34.2 | 26.3 | 20.8 |
| 3. Difficulty sustaining attention | 23.1 | 16.6 | 15.2 |
| 4. Leaves seat | 28.3 | 21.9 | 16.1 |
| 5. Does not seem to listen | 23.2 | 15.2 | 12.3 |
| 6. Runs about or climbs excessively | 21.4 | 17.4 | 12.5 |
| 7. Does not follow through on instructions | 16.9 | 10.9 | 8.9 |
| 8. Difficulty playing quietly | 14.9 | 14.3 | 10.3 |
| 9. Difficulty organizing tasks | 13.2 | 12.1 | 8.4 |
| 10. Acts as if “driven by a motor” | 37.8 | 39.5 | 31.6 |
| 11. Avoids tasks of sustained mental effort | 12.7 | 10.9 | 9.0 |
| 12. Talks excessively | 41.2 | 46.3 | 42.0 |
| 13. Loses things | 17.1 | 15.4 | 13.0 |
| 14. Blurts out answers | 13.8 | 14.9 | 16.2 |
| 15. Easily distracted | 30.3 | 28.8 | 25.0 |
| 16. Difficulty awaiting turn | 31.0 | 25.4 | 22.1 |
| 17. Forgetful in daily activities | 11.5 | 10.2 | 10.2 |
| 18. Interrupts or intrudes | 28.7 | 29.5 | 22.9 |
|
| |||
| Symptom Count | M(SD) | M(SD) | M(SD) |
|
| |||
| Inattentive (odd numbered items) | 1.7 (2.4) | 1.3 (2.2) | 1.1 (2.1) |
| Hyperactive-Impulsive (even numbered items) | 2.5 (2.5) | 2.4 (2.5) | 1.9 (2.4) |
Note: Ns = 1096, 1062 and 1066 at ages 3-5, respectively; M = mean; SD = standard deviation
Factor Structure of ADHD Symptomatology
The first research question addressed the factor structure of ADHD symptoms. One-, two- (inattentive vs. hyperactive-impulsive), and three-factor (inattentive, hyperactive, impulsive) models were fit to ADHD symptoms at the age 3, 4, and 5 year assessments. A synopsis of model fit and comparisons appears in Table 2. Four points are noteworthy. First, in terms of fit statistics, all three models provided excellent fit to the data (all CFI >=.96, all RMSEA <= .05). Second, a two-factor model did not result in a statistically significant improvement in model fit relative to a one-factor model at any assessment. Third, although a three-factor model provided an improvement beyond the one- and two-factor models at all three assessments, the correlations between latent factors in the three-factor models were very large (φIN, HYP = .99, .98, .98; φIN, IMP = .93, .93, .94; φHYP, IMP = .88, .89, .90 at 3, 4, and 5 year assessments, respectively). Fourth, the same pattern of results was observed at each of the three assessments. Given virtually no changes in model fit statics, combined with the substantial statistical power for chi square difference tests (due to the large sample size), inter-correlations between factors that approached unity, and the uniformity of results across all three assessments, we concluded that a one-factor model provided the most parsimonious representation of ADHD symptoms at each assessment.
Table 2.
Synopsis of Confirmatory Factor Models Informing the Structure of ADHD Symptoms at Each Assessment
| Age | Factors | χ2 | df | CFI | RMSEA | Comparison of Factors | Δχ2 | Δdf | Δp |
|---|---|---|---|---|---|---|---|---|---|
| 3 years | 1 | 510.2 | 135 | 0.97 | 0.05 | 1 vs. 2 | 1.0 | 1 | 0.3173 |
| 2 | 511.7 | 134 | 0.97 | 0.05 | 2 vs. 3 | 23.8 | 2 | < .0001 | |
| 3 | 492.6 | 132 | 0.97 | 0.05 | 1 vs. 3 | 24.1 | 3 | < .0001 | |
|
| |||||||||
| 4 years | 1 | 351.3 | 135 | 0.98 | 0.04 | 1 vs. 2 | 2.1 | 1 | 0.1485 |
| 2 | 351.4 | 134 | 0.98 | 0.04 | 2 vs. 3 | 17.4 | 2 | 0.0002 | |
| 3 | 335.3 | 132 | 0.96 | 0.04 | 1 vs. 3 | 19.2 | 3 | 0.0003 | |
|
| |||||||||
| 5 years | 1 | 319.9 | 135 | 0.99 | 0.04 | 1 vs. 2 | 3.3 | 1 | 0.0705 |
| 2 | 319.0 | 134 | 0.99 | 0.04 | 2 vs. 3 | 20.6 | 2 | < .0001 | |
| 3 | 297.9 | 132 | 0.99 | 0.03 | 1 vs. 3 | 21.3 | 3 | 0.0001 | |
Note. All tests of perfect fit were significant at p < .0001; the columns indicating changes in chi-square (Δχ2), degrees of freedom (Δdf), and probability (Δp) refer to tests between models that imposed a different number of factors (e.g., the last four columns of the first row of the table provide a formal comparison of whether the 2-factor model provides a statistically significant improvement in fit relative to the 1-factor model for the age 3 data).
A secondary research question was whether the measurement properties of the ADHD symptom ratings could take on identical values across the three assessments. To the extent that this was true, we were assured that mean level changes in symptoms across time reflected true changes and were not due to differential measurement characteristics. A series of longitudinal CFA models were estimated which imposed increasingly restrictive parameter constraints on ADHD symptoms across time (i.e., requiring that symptom thresholds and factor loadings take on identical values across time). A detailed description of model parameterization and model fit is provided in a supplementary document (see eTable 1 and supporting text). The results of these models demonstrated that all 18 ADHD symptoms could take on equal item thresholds and that 12 of the 18 ADHD symptoms could take on equal factor loadings across the three assessments, without significantly degrading model fit. Collectively, these results provided evidence of the partial measurement invariance of ADHD symptoms from age 3-5 years (Byrne, Shavelson, & Muthén, 1989). The final longitudinal CFA model indicated substantial stability of ADHD symptomatology across time, φ3-4 years = .71, φ3-5 years = .69, and φ4-5 years = .85, all ps < .05.
Developmental Changes in ADHD Symptomatology
The third research question tested for heterogeneity in the developmental course of ADHD symptoms using semi-parametric finite mixture models (SPFMs). A detailed description of model parameterization and trimming is provided in a supplementary document (see eTable 2 and supporting text). A seven-class solution was determined to provide the best fit to the data. Inspection of parameter estimates and posterior probabilities for classes indicated a single persistently high ADHD group (persisters; 8.4% of the sample; class 7 in Figure 1), two groups with initially elevated ADHD symptoms that remitted over time (remitters; combined 16.4% of the sample, classes 3 and 4 in Figure 1), a small group with initially low ADHD symptoms that increased over time (increasers; 3.5% of the sample, class 6 in Figure 1), and three groups characterized by persistently low (or mildly decreasing) ADHD symptoms over time (stable low; combined 71.7% of the sample, classes 1, 2 and 5 in Figure 1). The reported percentages of children characterized by each trajectory profile represent weighted estimates that take the stratification and over-sampling of low income and, in NC, African American families into account (i.e., they represent population-based estimates of the proportion of children in each trajectory group).
Figure 1.
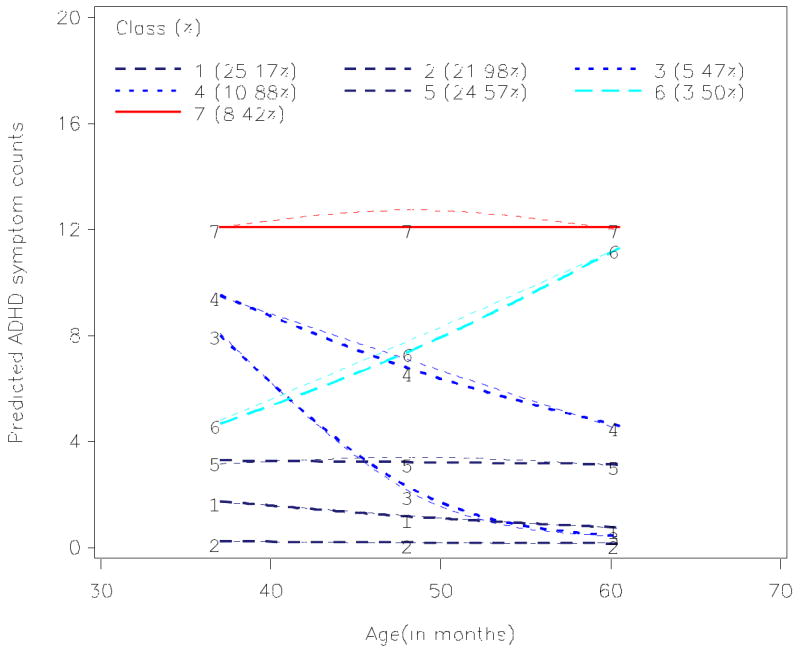
Model implied and observed ADHD symptom trajectories
Note: Observed means are represented by thin dashed lines.
Children in the persistently elevated group exhibited Ms = 12.2, 12.8, and 12.1 total ADHD symptoms at the 3-5 year assessments. Children in the combined remitting trajectory groups exhibited Ms = 9.7, 5.8, and 3.2 total ADHD symptoms at the 3-5 year assessments. Children in the increasing trajectory group exhibited Ms = 4.3, 7.7, and 12.3 total ADHD symptoms at the 3-5 year assessments. Children in the combined stable low trajectories exhibited Ms = 1.8, 1.8, and 1.5 total ADHD symptoms at the 3-5 year assessments.
Predictors of Membership in Trajectory Groups
Posterior probabilities were used to assign children into the trajectory class that most likely gave rise to their observed data, and trajectory class membership was then regressed on risk factors. Given the primary interest in understanding the risk factors that differentiated children with persistent ADHD symptoms from others, classes were collapsed to create four outcome groups that were described above (stable low, increasers, remitters, persisters). Descriptive and test statistics comparing the four trajectory groups on the 13 risk factors are summarized in Table 3. When considered alone a number of household (poverty, single parent headed household), child (low birth weight), and primary caregiver (high school degree/GED, prenatal smoking and drug use, postnatal depression, childhood history of ADHD) risk factors were identified as potentially important variables (i.e., at least two groups differed from each other).
Table 3.
Descriptive statistics and univariate tests for predictors of group membership
| Variable | Group
|
Comparison | ||||||
|---|---|---|---|---|---|---|---|---|
| Total (N = 1155) | Persister (N = 105) | Stable Low (N = 823) | Remitter (N = 194) | Increaser (N = 33) | ||||
|
| ||||||||
| % | % | % | % | % | χ2 (df = 3) | prob | BH critical | |
| Poor | 65.4 | 80.0 | 60.3 | 75.8 | 84.9 | 32.98 | < .0001 | 0.0058* |
| Teen Mom | 5.4 | 9.5 | 4.6 | 5.7 | 9.1 | 5.42 | 0.1436 | 0.0231 |
| Male | 50.2 | 53.3 | 49.1 | 52.1 | 57.6 | 1.81 | 0.6136 | 0.0250 |
| Spouse in HH | 52.7 | 77.1 | 47.1 | 59.3 | 75.8 | 45.76 | < .0001 | 0.0038* |
| PC has HS/GED | 80.4 | 56.2 | 85.1 | 74.7 | 72.7 | 55.42 | < .0001 | 0.0019* |
| HH Size ≥ 6 | 18.9 | 24.8 | 17.0 | 22.2 | 27.3 | 7.14 | 0.0677 | 0.0192 |
| LBW | 8.2 | 17.1 | 6.9 | 8.8 | 6.1 | 13.23 | 0.0042 | 0.0135* |
| Prenatal Alcohol | 12.3 | 10.5 | 11.5 | 14.4 | 24.2 | 6.02 | 0.1108 | 0.0212 |
| Prenatal Drugs | 2.2 | 1.9 | 2.2 | 1.0 | 9.1 | 8.65 | 0.0343† | 0.0173 |
| Prenatal Smoking | 23.3 | 31.4 | 20.9 | 27.8 | 33.3 | 10.57 | 0.0143 | 0.0154* |
| PC Depression | 8.2 | 19.8 | 6.1 | 10.5 | 12.1 | 24.86 | < .0001† | 0.0096* |
| Bio-mom IN | 4.1 | 12.9 | 2.5 | 5.6 | 10.0 | 27.22 | < .0001† | 0.0077* |
| Bio-Mom HI | 2.6 | 5.4 | 1.4 | 5.6 | 6.7 | 15.15 | 0.0017† | 0.0115* |
Note. BH = Benjamini-Hochberg.
significant after BH correction.
Pearson χ2 test may not be valid due to sparseness of cell counts.
Multinomial regression models were estimated with the persister group serving as the reference against which the three other groups were compared. Type III tests provided an omnibus test of unique contribution of each risk factor. Individual regression coefficients represented pairwise comparisons of the effect of each risk factor for each trajectory group relative to membership in the persister group (instances in which the type III test was statistically significant but none of the pairwise coefficients were— e.g., household poverty—indicate that two of the non-persister groups differ from each other). As summarized in Table 4, the omnibus tests for household poverty, household structure (single parent headed household), primary caregiver education, prenatal exposure to drugs, primary caregiver postnatal depression and retrospective report of childhood ADHD were all statistically significant. In addition, pairwise but not omnibus tests were significant for LBW and prenatal alcohol exposure. Results are summarized with respect to pairwise comparisons.
Table 4.
Multinomial Regression Results with Persistent ADHD as Reference Group.
| Parameter | Type III χ2(3) |
Low vs. Persister | Increaser vs. Persister | Remitter vs. Persister | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Est. | OR | 95% CI | Est. | OR | 95% CI | Est. | OR | 95% CI | |||||
| Intercept | 2.22*** | 0.53 | -2.62** | ||||||||||
| Poor | 8.01* | -0.22 | 0.8 | 0.4 | 1.5 | 0.29 | 1.3 | 0.7 | 2.6 | 0.71 | 2.0 | 0.6 | 6.8 |
| Teen Mom | 1.66 | 0.57 | 1.8 | 0.6 | 5.0 | 0.42 | 1.5 | 0.5 | 5.0 | 1.00 | 2.7 | 0.5 | 14.9 |
| Male | 4.23 | -0.35 | 0.7 | 0.4 | 1.1 | -0.14 | 0.9 | 0.5 | 1.4 | 0.10 | 1.1 | 0.5 | 2.5 |
| Single Parent HH | 9.62* | -0.78** | 0.5 | 0.3 | 0.8 | -0.61 | 0.5 | 0.3 | 1.0 | -0.03 | 1.0 | 0.3 | 2.8 |
| PC has HS/GED | 23.74*** | 1.28*** | 3.6 | 2.1 | 6.1 | 0.70* | 2.0 | 1.1 | 3.7 | 1.16* | 3.2 | 1.1 | 9.1 |
| HH Size ≥ 6 | 0.75 | 0.12 | 1.1 | 0.6 | 2.0 | 0.17 | 1.2 | 0.6 | 2.2 | 0.40 | 1.5 | 0.6 | 3.9 |
| LBW | 4.79 | -0.72* | 0.5 | 0.3 | 1.0 | -0.55 | 0.6 | 0.3 | 1.3 | -0.99 | 0.4 | 0.1 | 1.8 |
| Prenatal Alcohol | 5.47 | 0.49 | 1.6 | 0.8 | 3.5 | 0.74 | 2.1 | 0.9 | 4.8 | 1.20* | 3.3 | 1.1 | 10.3 |
| Prenatal Drugs | 8.35* | 2.15* | 8.6 | 1.0 | 75.4 | 0.00 | 1.0 | 0.1 | 17.7 | 2.68* | 14.6 | 1.2 | 179.1 |
| Prenatal Smoking | 0.91 | -0.19 | 0.8 | 0.5 | 1.4 | -0.05 | 1.0 | 0.5 | 1.7 | -0.05 | 1.0 | 0.4 | 2.4 |
| PC Depression | 18.10** | -1.34*** | 0.3 | 0.1 | 0.5 | -0.73* | 0.5 | 0.2 | 1.0 | -0.91 | 0.4 | 0.1 | 1.4 |
| Bio-mom IN | 11.46** | -1.55** | 0.2 | 0.1 | 0.5 | -1.29* | 0.3 | 0.1 | 0.8 | -0.48 | 0.6 | 0.1 | 3.0 |
| Bio-Mom HI | 8.61* | -0.66 | 0.5 | 0.1 | 2.2 | 0.78 | 2.2 | 0.5 | 9.3 | 0.23 | 1.3 | 0.2 | 10.3 |
p < .05,
p< .01
p <.0001
Stable Low vs. Persisters
Relative to children in the persister group, children in the stable low group were less likely to reside in a single parent headed household (OR = 0.5), more likely to have a primary caregiver with at least a high school degree (OR = 3.6), less likely to be born with low birth weight (OR = 0.5), more likely to have been exposed to prenatal drugs (OR = 8.6), and less likely to have had a primary caregiver who endorsed post-partum depression (OR = 0.3) or a childhood history of 6 or more inattentive symptoms (OR = 0.2), all ps < .05.
Increaser vs. Persisters
Relative to children in the persister group, children in the increasing symptom group were more likely to have a primary caregiver with at least a high school degree (OR = 2.0), and less likely to have had a primary caregiver who endorsed post-partum depression (OR = 0.5) or a childhood history of 6 or more inattentive symptoms (OR = 0.3), all ps < .05
Remitters vs. Persisters
Relative to children in the persister group, children in the remitter trajectory group were more likely to have a primary caregiver with at least a high school degree (OR = 3.2), were more likely to have prenatal exposure to alcohol (OR = 3.3) and drugs (OR = 14.6), ps < .05.
Discussion
This study tested the factor structure, patterns of developmental change, and predictors of persistently elevated ADHD symptoms in early childhood. ADHD symptoms were best represented by a single factor that exhibited partial measurement invariance across time. Whereas nearly 70% of children never exhibited elevated ADHD symptoms during early childhood, 30% did. However, only 8% of children exhibited persistent ADHD from age 3 through 5 years. As elaborated below, children characterized by persistently elevated ADHD exhibited a variety of risk factors that differentiated them most prominently from children with stable low levels of ADHD and less so from children with time-limited elevations in ADHD symptoms.
Contrary to our hypotheses, ADHD symptoms were most parsimoniously represented by a single latent factor. Two-factor models did not improve the fit of the observed data relative to a one-factor model. Although three-factor models provided a statistically improved fit relative to a one-factor model, fit indices did not indicate improved fit; moreover, the correlations between factors in the three-factor model approached unity (typically > .90), which raised questions about the practical utility of differentiating all three dimensions of behavior. We consider four possible explanations for the apparent unidimensionality of ADHD symptoms in early childhood. The first concerns children’s social ecologies. The classroom structure and behavioral expectations made of 3-5 year olds differs appreciably from that typical of middle childhood. The shift away from more exclusively child-directed towards parent and teacher-directed activities, including an increasing focus on academically oriented activities, may result in attentional difficulties becoming more evident to parents in middle versus early childhood. Second, early childhood is characterized by changes in the neural networks that support emerging cognitive control processes (Durston et al., 2006; Durston et al., 2002). These changes may result in phenotypic changes that facilitate parents and/or teachers ability to better differentiate IN from HI symptomatology in middle versus early childhood. Third, the apparent unidimensionality of ADHD symptoms may be due to the fact that IN and HI symptoms were alternated on the ADHD checklist (see Table 1 for ordering of items). Asking parents to rate all of the IN and HI symptoms in succession may have reduced the magnitude of the estimated correlation between IN and HI factors. Fourth, our conclusions about the dimensionality of ADHD symptoms are based exclusively on parent reports. Follow-up studies of this sample that include both parent- and teacher-rated ADHD symptoms in middle childhood will help resolve when in development IN and HI can be reliability differentiated.
Longitudinal CFA models demonstrated that the unidimensional factor structure of ADHD symptoms that was separately observed at age 3-, 4-, and 5-year assessments exhibited partial measurement invariance. That is, the measurement properties of symptom ratings did not appreciably change from age 3-5 years. This helped rule out changes in measurement as an explanation for changes in mean levels of ADHD symptoms across time. Although the latent stability of ADHD symptomatology was large (i.e., > .70 between successive years), this was undoubtedly due, in part, to the large number of children who were consistently rated as having very low levels of symptomatology.
One of the strengths of this study was the assessment of the full range of DSM-IV ADHD symptoms across time, which permitted a full characterization of symptom trajectory profiles. Consistent with previous studies, 8% of children exhibited persistently elevated levels of ADHD symptomatology. This subgroup represents a potentially interesting group of children with respect to etiologic research and is likely at greatest risk to meet full diagnostic criteria for ADHD at the transition to formal schooling.
The expanded coverage of ADHD symptoms in this study likely contributed to the empirical identification of children characterized by remitting symptoms from age 3-5 years. The presence of a remitting group was consistent with a small literature that has examined the diagnostic stability of ADHD in early childhood. The presence of a remitting group highlights the fact that although the majority of preschoolers will never exhibit markedly elevated levels of ADHD symptoms, among those who do at early ages some of them will appear to “outgrow” these symptoms. Although beyond the scope of this investigation, an open question is whether the reductions in ADHD symptoms among remitters co-occur with the acquisition of improved regulatory abilities. Limited evidence supports this speculation (von Stauffenberg & Campbell, 2007). Future studies involving this sample will consider whether reductions in ADHD symptoms are related to corresponding improvements in executive functions.
A small proportion of children (3%) were characterized as having an increasing symptom profile. This group was unexpected. It is unclear whether the pattern of increasing ADHD is a real phenomenon versus a statistical artifact (Bauer & Curran, 2003). Moreover, the small number of children characterized by increasing ADHD symptoms resulted in under-powered tests of whether/how this group differed from the other groups. Descriptively, children characterized by increasing ADHD symptoms exhibited higher rates of prenatal alcohol exposure relative to all of the other groups (though prenatal alcohol exposure did not statistically differentiate this group from persisters). This is interesting in light of evidence that prenatal exposure to alcohol may help to define a subtype of ADHD youth with unique patterns of neurocognitive function (Burden et al., 2010; Vaurio, Riley, & Mattson, 2008). Future analyses that follow this group of children into school will help inform the validity of this increasing symptom profile.
Multinomial regression models indicated that children characterized by persistently elevated ADHD symptoms differed from children with stable low levels of ADHD symptoms on a variety of well-known household (single versus two-parent), caregiver (education, postnatal depression, retrospective report of childhood history of ADHD, prenatal substance use), and child (low birth weight) risk factors. Although early risk factors differentiated persistent ADHD from stable low levels, they did a relatively poor job of differentiating children with persistent versus time-limited (remitters, increasers) elevations in ADHD. The only risk factor that differentiated persisters from all three other groups was maternal education. Previous studies have identified low maternal education as a risk factor for ADHD (Palfrey et al., 1985; St Sauver et al., 2004). Low parental education likely serves as a proxy for a variety of factors. For example, parents with low education may not provide as much cognitive stimulation to their children, which has been found to be associated with persistently elevated ADHD symptoms across middle childhood into adolescence (Jester et al., 2005). Consistent with this speculation, Romano and colleagues (2006) did not report independent effects of low maternal education of risk for persistent ADHD; however, their models included more proximal indicators of both positive and hostile dimensions of parenting behaviors. It is also noteworthy that parental education was a unique predictor while household poverty was not. Low caregiver education may serve as a proxy for low cognitive ability, which represents unmeasured biological risk to children that is independent of caregiver behaviors.
Maternal self-reported history of ADHD symptoms (specifically inattention) and postnatal depression differentiated persisters from children in stable low and increasing, but not remitting, symptom profiles. Similar to low caregiver education, the processes through which this risk occurs remain under-studied and may represent some combination of genetic liability (in case of parental history of ADHD) and/or impairments in caregiving behaviors (in case of depression). It is important to point out that the inferences drawn from the multinomial regression models are often at odds with what one would conclude had they considered each risk factor in isolation. For example, whereas caregiver history of ADHD (inattention) differentiated children in the persisting from increasing groups (12.9% vs. 10.0%) it did not differentiate persisters from remitters despite larger differences descriptively (12.9% vs. 5.6%). This reflects the fact that bivariate associations fail to take into account the correlation structure among multiple risk factors when they are considered together.
Finally, a number of well-known risk factors did not exert any unique effects on ADHD symptom profiles. The failure to observe sex differences may have to do with the sampling plan, as the sex discrepancy is more evident in clinic than community samples. The failure to observe differences related to prenatal smoking may be due to the fact that the risk of prenatal smoking on ADHD is conditional on specific dopaminergic genotypes (Becker, El-Faddagh, Schmidt, Esser, & Laucht, 2008; Kahn, Khoury, Nichols, & Lanphear, 2003; Neuman et al., 2007). The failure for household poverty or size to differentiated persisters from other groups may have to do with the inclusion of a broader range of social risks including household structure (single-parent headed household) and caregiver education.
This study is characterized by at least four limitations. First, we relied exclusively on parent reported ADHD behaviors. In middle childhood, teachers are considered a better informant of ADHD behaviors than are parents, and combined reports provide the best approximation to diagnostic status and are most sensitive to detecting clinical change (Biederman, Faraone, Monuteaux, & Grossbard, 2004; Power et al., 1998; Sprafkin, Gadow, & Nolan, 2001; Tripp, Schaughency, & Clarke, 2006). Extending these analyses to include teacher reported ADHD in elementary school is an important future task. Second, this sample consists of families residing in low-wealth, non-metropolitan areas. It is unclear whether the risks of ADHD differ along a continuum of rural to urban settings. Third, we focused exclusively on early and easily measured risk factors of ADHD. More detailed consideration of specific developmental processes related to emerging child regulatory functioning (e.g., infant cognition; caregiver scaffolding to enhance emerging regulatory competence) will likely be necessary in order to better delineate differences between preschoolers with persistent versus time limited elevations in ADHD symptoms. Fourth, a ubiquitous concern of studies using SPFM methods is the possibility that the results of groups are reified (Bauer, 2007). From our vantage, these models serve a potentially “useful fiction” in that they facilitated the empirical identification of a priori assumed groups (remitters, persisters). Nonetheless, it is important to acknowledge that membership in trajectory groups is probabilistic, that posterior probabilities do not necessarily provide a bright line criterion for group assignment, and that it is remarkably easy to reify groups even if the absence of strong theory (e.g., increasers).
Despite the growing demand for the treatment of ADHD in preschool-aged children, a number of basic issues including the presentation, developmental course, and optimal treatment of preschool ADHD remain understudied (Dopfner, Rothenberger, & Sonuga-Barke, 2004). Moreover, it is likely that there are multiple, distinct developmental pathways through which children arrive at a diagnosis of ADHD (Sonuga-Barke, Auerbach, Campbell, Daley, & Thompson, 2005). Efforts to delineate the heterogeneity in the functional form of changes in ADHD symptomatology across time, as well as the risk factors that predict and developmental processes that shape changes in ADHD across time have the potential to dramatically improve early intervention activities that seek to prevent emerging ADHD and/or reduce its negative sequelae.
Supplementary Material
Acknowledgments
Support for this research was provided by the National Institute of Child Health and Human Development grant P01 HD39667, with co-funding from the National Institute on Drug Abuse. The Family Life Project Key Investigators in the first phase of the study included Lynne Vernon-Feagans, Martha Cox, Clancy Blair, Peg Burchinal, Linda Burton, Keith Crnic, Ann Crouter, Patricia Garrett-Peters, Mark Greenberg, Stephanie Lanza, Roger Mills-Koonce, Cynthia Stifter, Emily Werner, and Michael Willoughby.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders Third Edition (DSM-III) Washington, DC: American Psychiatric Association; 1980. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders Third Edition Revised (DSM-IIIR) Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders Fourth Edition-Text Revision (DSM-IV-TR) Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Bauer DJ. Observations on the use of growth mixture models in psychological research. Multivariate Behavioral Research. 2007;42:757–786. [Google Scholar]
- Bauer DJ, Curran PJ. Distributional assumptions of growth mixture models: Implications for over-extraction of latent trajectory classes. Psychological Methods. 2003;8:338–363. doi: 10.1037/1082-989X.8.3.338. [DOI] [PubMed] [Google Scholar]
- Bauermeister JJ, Canino G, Polanczyk G, Rohde LA. ADHD across cultures: Is there evidence for a bidimensional organization of symptoms? Journal of Clinical Child and Adolescent Psychology. 2010;39:362–372. doi: 10.1080/15374411003691743. [DOI] [PubMed] [Google Scholar]
- Becker K, El-Faddagh M, Schmidt MH, Esser G, Laucht M. Interaction of dopamine transporter genotype with prenatal smoke exposure on ADHD symptoms. Journal of Pediatrics. 2008;152:263–269. doi: 10.1016/j.jpeds.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone S-V, Monuteaux M-C. Differential effect of environmental adversity by gender: Rutter’s Index of Adversity in a group of boys and girls with and without ADHD. American Journal of Psychiatry Sep. 2002;159:1556–1562. doi: 10.1176/appi.ajp.159.9.1556. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV, Monuteaux MC, Grossbard JR. How informative are parent reports of attention-deficit/hyperactivity disorder symptoms for assessing outcome in clinical trials of long-acting treatments? A pooled analysis of parents’ and teachers’ reports. Pediatrics. 2004;113:1667–1671. doi: 10.1542/peds.113.6.1667. [DOI] [PubMed] [Google Scholar]
- Biederman J, Milberger S, Faraone SV, Kiely K, Guite J, Mick E, Reed E, et al. Family-environment risk-factors for Attention-Deficit Hyperactivity Disorder - A test of Rutters indicators of adversity. Archives of General Psychiatry. 1995;52:464–470. doi: 10.1001/archpsyc.1995.03950180050007. [DOI] [PubMed] [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing Structural Equation Models. Newbury Park, CA: Sage Publications, Inc; 1993. pp. 137–162. [Google Scholar]
- Burden MJ, Jacobson JL, Westerlund A, Lundahl LH, Morrison A, Dodge NC, Jacobson SW, et al. An event-related potential study of response inhibition in ADHD with and without prenatal alcohol exposure. Alcoholism-Clinical and Experimental Research. 2010;34:617–627. doi: 10.1111/j.1530-0277.2009.01130.x. [DOI] [PubMed] [Google Scholar]
- Byrne BM, Shavelson RJ, Muthén B. Testing for the equivalence of factor covariance and mean structures: the issue of partial measurement invariance. Psychological Bulletin. 1989;105:456–466. [Google Scholar]
- Byrne JM, DeWolfe NA, Bawden HN. Asessment of Attention-Deficit Hyperactivity Disorder in preschoolers. Child Neuropsychology. 1998;4:49–66. doi: 10.1076/chin.9.2.142.14501. [DOI] [PubMed] [Google Scholar]
- Campbell SB, Breaux AM, Ewing LJ, Szumowski EK, Pierce EW. Parent-identified problem preschoolers - mother-child interaction during play at intake and 1-year follow-up. Journal of Abnormal Child Psychology. 1986;14:425–440. doi: 10.1007/BF00915436. [DOI] [PubMed] [Google Scholar]
- Chacko A, Wakschlag L, Hill C, Danis B, Espy KA. Viewing preschool disruptive behavior disorders and Attention-Deficit/Hyperactivity Disorder through a developmental lens: What we know and what we need to know. Child and Adolescent Psychiatric Clinics of North America. 2009;18:627–643. doi: 10.1016/j.chc.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung GW, Rensvold RB. Evaluating goodness-of-fit indexes for testing measurement invariance. Structural Equation Modeling. 2002;9:233–255. [Google Scholar]
- Connor D. Preschool attention deficit hyperactivity disorder: A review of prevalence, diagnosis, neurobiology, and stimulant treatment. Journal of Developmental & Behavioral Pediatrics. 2002;23:S1–S9. doi: 10.1097/00004703-200202001-00002. [DOI] [PubMed] [Google Scholar]
- Das Banerjee T, Middleton F, Faraone SV. Environmental risk factors for attention-deficit hyperactivity disorder. Acta Paediatrica. 2007;96:1269–1274. doi: 10.1111/j.1651-2227.2007.00430.x. [DOI] [PubMed] [Google Scholar]
- Derogatis L. Brief Symptom Inventory 18. Minneapolis: NCS Pearson, Inc; 2000. [Google Scholar]
- Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychological Medicine. 1983;13:595–605. [PubMed] [Google Scholar]
- Dill BT. Rediscovering rural America. In: Blau JR, editor. Blackwell companions to sociology. Malden: Blackwell Publishing; 2001. pp. 196–210. [Google Scholar]
- Dopfner M, Rothenberger A, Sonuga-Barke E. Areas for future investment in the field of ADHD: Preschoolers and clinical networks. European Child & Adolescent Psychiatry. 2004;13:I130–I135. doi: 10.1007/s00787-004-1012-8. [DOI] [PubMed] [Google Scholar]
- DuPaul GJ, Anastopoulos AD, Power TJ, Reid R, Ikeda MJ, McGoey KE. Parent ratings of Attention-Deficit/Hyperactivity Disorder symptoms: factor structure and normative data. Journal of Psychopathology and Behavioral Assessment. 1998;20:83–102. [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, Casey BJ. A shift from diffuse to focal cortical activity with development. Developmental Science. 2006;9:1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Durston S, Thomas KM, Yang YH, Ulug AM, Zimmerman RD, Casey BJ. A neural basis for the development of inhibitory control. Developmental Science. 2002;5:F9–F16. [Google Scholar]
- Egger HL, Angold A. Common emotional and behavioral disorders in preschool children: presentation, nosology, and epidemiology. Journal of Child Psychology and Psychiatry. 2006;47:313–337. doi: 10.1111/j.1469-7610.2006.01618.x. [DOI] [PubMed] [Google Scholar]
- Egger HL, Kondo D, Angold A. The epidemiology and diagnostic issues in preschool Attention-Deficit/Hyperactivity Disorder - A review. Infants and Young Children. 2006;19:109–122. [Google Scholar]
- Galera C, Cote SM, Bouvard MP, Pingault JB, Melchior M, Michel G, Boivin M, Tremblay RE. Early risk factors for hyperactivity-impulsivity and inattention trajectories from age 17 months to 8 years. Archives of General Psychiatry. 2011;68:1267–1275. doi: 10.1001/archgenpsychiatry.2011.138. [DOI] [PubMed] [Google Scholar]
- Gimpel G, Kuhn B. Maternal report of attention deficit hyperactivity disorder symptoms in preschool children. Child: Care, Health and Development. 2000;26:163–179. doi: 10.1046/j.1365-2214.2000.00126.x. [DOI] [PubMed] [Google Scholar]
- Hardy KK, Kollins SH, Murray DW, Riddle MA, Greenhill L, Cunningham C, Abikoff HB, McCracken JT, Vitiello B, Davise M, McGough JJ, Posner K, Skrobala AM, Swanson JM, Wigal SB, Ghuman JK, Chuang SZ. Factor structure of parent- and teacher-rated attention-deficit/hyperactivity disorder symptoms in the Preschoolers with Attention-Deficit/Hyperactivity Disorder Treatment Study (PATS) Journal of Child and Adolescent Psychopharmacology. 2007;17:621–633. doi: 10.1089/cap.2007.0073. [DOI] [PubMed] [Google Scholar]
- Harvey EA, Youngwirth SD, Thakar DA, Errazuriz PA. Predicting Attention Deficit/Hyperactivity Disorder and Oppositional Defiant disorder from preschool diagnostic assessments. Journal of Consulting and Clinical Psychology. 2009;77:349–354. doi: 10.1037/a0014638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L-t, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- Jester JM, Nigg JT, Adams K, Fitzgerald HE, Puttler LI, Wong MM, Zucker RA. Inattention/hyperactivity and aggression from early childhood to adolescence: Heterogeneity of trajectories and differential influence of family environment characteristics. Development and Psychopathology. 2005;17:99–125. doi: 10.1017/50954579405050066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociological Research and Methods. 2001;29:374–393. [Google Scholar]
- Kahn RS, Khoury J, Nichols WC, Lanphear BP. Role of dopamine transporter genotype and maternal prenatal smoking in childhood hyperactive-impulsive, inattentive, and oppositional behaviors. Journal of Pediatrics. 2003;143:104–110. doi: 10.1016/S0022-3476(03)00208-7. [DOI] [PubMed] [Google Scholar]
- Lavigne JV, Arend R, Rosenbaum D, Binns HJ, Christoffel KK, Gibbons RD. Psychiatric disorders with onset in the preschool years: I. Stability of diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 1998a;37:1246–1254. doi: 10.1097/00004583-199812000-00007. [DOI] [PubMed] [Google Scholar]
- Lavigne JV, Arend R, Rosenbaum D, Binns HJ, Christoffel KK, Gibbons RD. Psychiatric disorders with onset in the preschool years: II. Correlates and predictors of stable case status. Journal of the American Academy of Child and Adolescent Psychiatry. 1998b;37:1255–1261. doi: 10.1097/00004583-199812000-00008. [DOI] [PubMed] [Google Scholar]
- Leblanc N, Boivin M, Dionne G, Brendgen M, Vitaro F, Tremblay RE, Perusse D. The development of hyperactive-impulsive behaviors during the preschool years: The predictive validity of parental assessments. Journal of Abnormal Child Psychology. 2008;36(7):977–987. doi: 10.1007/s10802-008-9227-7. [DOI] [PubMed] [Google Scholar]
- Lee SS, Lahey BB, Owens EB, Hinshaw SP. Few preschool boys and girls with ADHD are well-adjusted during adolescence. Journal of Abnormal Child Psychology. 2008;36:373–383. doi: 10.1007/s10802-007-9184-6. [DOI] [PubMed] [Google Scholar]
- McGoey K, Eckert T, DuPaul G. Early intervention for preschool-age children with ADHD: A literature review. Journal of Emotional and Behavioral Disorders. 2002;10:14–28. [Google Scholar]
- Meredith W. Measurement invariance, factor analysis and factorial invariance. Psychometrica. 1993;58:525–543. [Google Scholar]
- Meredith W, Horn J. The role of factorial invariance in modeling growth and change. In: Collins LM, Sayer AG, editors. New methods for the analysis of change. 1. Washington, DC: American Psychological Association; 2001. pp. 203–240. [Google Scholar]
- Mick E, Biederman J, Faraone SV, Sayer J, Kleinman S. Case-control study of attention-deficit hyperactivity disorder and maternal smoking, alcohol use and drug use during pregnancy. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(4):378–385. doi: 10.1097/00004583-200204000-00009. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus Users Guide. Sixth Edition. Los Angeles, CA: 1998-2010. [Google Scholar]
- Nagin DS. Analyzing developmental trajectories: A semi-parametric, group-based approach. Psychological Methods. 1999;4:139–157. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- Neuman RJ, Lobos E, Reich W, Henderson CA, Sun LW, Todd RD. Prenatal smoking exposure and dopaminergic genotypes interact to cause a severe ADHD subtype. Biological Psychiatry. 2007;61:1320–1328. doi: 10.1016/j.biopsych.2006.08.049. [DOI] [PubMed] [Google Scholar]
- Palfrey JS, Levine MD, Walker DK, Sullivan M. The emergence of attention deficits in early childhood: a prospective study. Journal of Developmental and Behavioral Pediatrics. 1985;6:339–348. [PubMed] [Google Scholar]
- Pelham WE, Gnagy EM, Greenslade KE, Milich R. Teacher ratings of DSM-III--R symptoms for the disruptive behavior disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 1992;31:210–218. doi: 10.1097/00004583-199203000-00006. [DOI] [PubMed] [Google Scholar]
- Posner K, Melvin GA, Murray DW, Gugga SS, Fisher P, Skrobala A, Cunningham C, Vitiello B, Abikoff HB, Ghuman JK, Kollins S, Wigal SB, McCracken JT, McGough JJ, Kastelic E, Boorady R, Davies M, Chuang SZ, Swanson JM, Riddle MA, Greenhill LL. Clinical presentation of Attention-Deficit/Hyperactivity Disorder in preschool children: The preschoolers with Attention-Deficit/Hyperactivity treatment study (PATS) Journal of Child and Adolescent Psychopharmacology. 2007;17:547–562. doi: 10.1089/cap.2007.0075. [DOI] [PubMed] [Google Scholar]
- Power TJ, Andrews TJ, Eiraldi RB, J DB, Ikeda MJ, DuPaul GJ, Landau S. Evaluating Attention Deficit/Hyperactivity Disorder using multiple informants: The incremental utility of combining teacher with parent reports. Psychological Assessment. 1998;10(3):250–260. [Google Scholar]
- Reich W, Todd RD, Joyner CA, Neuman RJ, Heath AC. Reliability and stability of mothers’ reports about their pregnancies with twins. Twin Research. 2003;6:85–88. doi: 10.1375/136905203321536209. [DOI] [PubMed] [Google Scholar]
- Romano E, Tremblay RE, Farhat A, Cote S. Development and prediction of hyperactive symptoms from 2 to 7 years in a population-based sample. Pediatrics. 2006;117:2101–2110. doi: 10.1542/peds.2005-0651. [DOI] [PubMed] [Google Scholar]
- Satorra A, Bentler PM. A scaled difference chi-square test statistic for moment structure analysis. Psychometrika. 2001;66:507–514. doi: 10.1007/s11336-009-9135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw DS, Lacourse E, Nagin DS. Developmental trajectories of conduct problems and hyperactivity from ages 2 to 10. Journal of Child Psychology and Psychiatry. 2005;46:931–942. doi: 10.1111/j.1469-7610.2004.00390.x. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Auerbach J, Campbell SB, Daley D, Thompson M. Varieties of preschool hyperactivity: multiple pathways from risk to disorder. Developmental Science. 2005;8:141–150. doi: 10.1111/j.1467-7687.2005.00401.x. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Thompson M, Abikoff H, Klein R, Brotman LM. Nonpharmacological interventions for preschoolers with ADHD - The case for specialized parent training. Infants and Young Children. 2006;19:142–153. [Google Scholar]
- Sprafkin J, Gadow KD, Nolan EE. The utility of a DSM-IV-referenced screening instrument for attention-deficit/hyperactivity disorder. Journal of Emotional and Behavioral Disorders. 2001;9:182–191. [Google Scholar]
- St Sauver JL, Barbaresi WJ, Katusic SK, Colligan RC, Weaver AL, Jacobsen SJ. Early life risk factors for attention-deficit/hyperactivity disorder: a population-based cohort study. Mayo Clinic Proceedings. 2004;79:1124–1131. [PubMed] [Google Scholar]
- Tandon M, Si XM, Luby J. Preschool onset Attention-Deficit/Hyperactivity Disorder: Course and predictors of stability over 24 months. Journal of Child and Adolescent Psychopharmacology. 2011;21:321–330. doi: 10.1089/cap.2010.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp G, Schaughency EA, Clarke B. Parent and teacher rating scales in the evaluation of Attention-Deficit/Hyperactivity Disorder: Contribution to diagnosis and differential diagnosis in clinically referred children. Journal of Developmental and Behavioral Pediatrics. 2006;27:209–218. doi: 10.1097/00004703-200606000-00006. [DOI] [PubMed] [Google Scholar]
- Vaurio L, Riley ER, Mattson SN. Differences in executive functioning in children with heavy prenatal alcohol exposure or attention-deficit/hyperactivity disorder. Journal of the International Neuropsychological Society. 2008;14:119–129. doi: 10.1017/S1355617708080144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon-Feagans L, Cox M Investigators, F. L. P. K. The Family Life Project: An epidemiological and developmental study of young children living in poor rural communities. Monographs of the Society for Research in Child Development. doi: 10.1111/mono.12046. in press. [DOI] [PubMed] [Google Scholar]
- von Stauffenberg C, Campbell SB. Predicting the early developmental course of symptoms of Attention Deficit/Hyperactivity Disorder. Journal of Applied Developmental Psychology. 2007;28:536–552. doi: 10.1016/j.appdev.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Biederman J, Brown S, Tanguay S, Monuteaux MC, Blake C, Spencer TJ. Psychiatric comorbidity and functioning in clinically referred preschool children and school-age youths with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:262–268. doi: 10.1097/00004583-200203000-00005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


