Abstract
Purpose
To highlight the rare yet devastating complication of cytomegalovirus (CMV) retinitis in a minimally immunosuppressed patient 8 years after liver transplantation for biliary atresia.
Case
A 22 year-old female status-post deceased donor liver transplant at age 13 secondary to biliary atresia receiving single agent immunosuppression presented with acute, unilateral, profound decrease in visual acuity. The patient was diagnosed to have acute onset unilateral CMV retinitis. Retinal exam uncovered classical appearance of retinal whitening and retinal hemorrhages with extensive macular involvement.
Conclusion
CMV retinitis can occur as a late complication following liver transplantation. Additionally, CMV retinal disease can occur in the absence of laboratory evidence of CMV infection and independent of additional clinical features suggesting CMV disease. Currently there is no standard of care regarding screening for CMV retinitis thus further research is needed to define the need for potential changes in current clinical practices and post-transplant screening protocols.
Keywords: Immunosuppression, Tacrolimus, Retina, Children, Ocular, Complications, Hepatic
Introduction
Cytomegalovirus (CMV) disease continues to be a well-known cause of morbidity and mortality in children who have received solid organ transplants (1). The risk of acquiring a viral illness after transplant is increased due to the emergence of immunosuppressive regimens that result in decreased T-cell mediated immunity. While prophylactic regimens to prevent infection during the period of peak immunosuppression have postponed its complications, delayed CMV disease continues to present a significant cause of morbidity (2). Late-onset CMV disease, defined as occurring after the discontinuation of antiviral prophylaxis, typically occurs between three to six months post-transplant, although it has been appreciated at more distant times as well (3). A well-documented complication of CMV, infection of the retina, is a rarely encountered end-organ disease after transplantation (4). We describe a case of a minimally immunosuppressed adolescent who developed unilateral CMV retinitis as an isolated complication greater than 8 years after transplantation.
Case Report
A 22 year-old female status-post deceased donor liver transplant at the age of 13 secondary to end stage liver disease for biliary atresia was being maintained on a tacrolimus trough goal of 3–8 ng/ml when she reported an acute onset, unilateral central scotoma in her right eye that she described as a “lights out” phenomenon. Her symptoms occurred suddenly while she was reading and was accompanied by a sensation of “tiredness and increased pressure” in the eye. She denied symptoms of conjunctivitis, trauma, fever, neck pain, headache, nausea, vomiting, abdominal pain, rash, or lymphadenopathy. When the symptoms failed to resolve spontaneously over the next week, she presented to an ophthalmologist who observed finger counting vision, unilateral disc edema and occlusive retinal vasculitis. A subsequent brain MRI was obtained that was negative for any appreciated intracranial process. Her symptoms persisted, a retinal specialist was consulted, and a diagnosis of CMV retinitis of the right eye on a background of systemic immunosuppression was made (Figure 1). A fluorescein angiogram revealed evidence of retinal vasculitis and secondary ischemic central retinal vein occlusion with macular infarction (Figure 2). No vitritis was present. She was then transferred to our tertiary care institution for subsequent evaluation and medical management.
Figure 1.
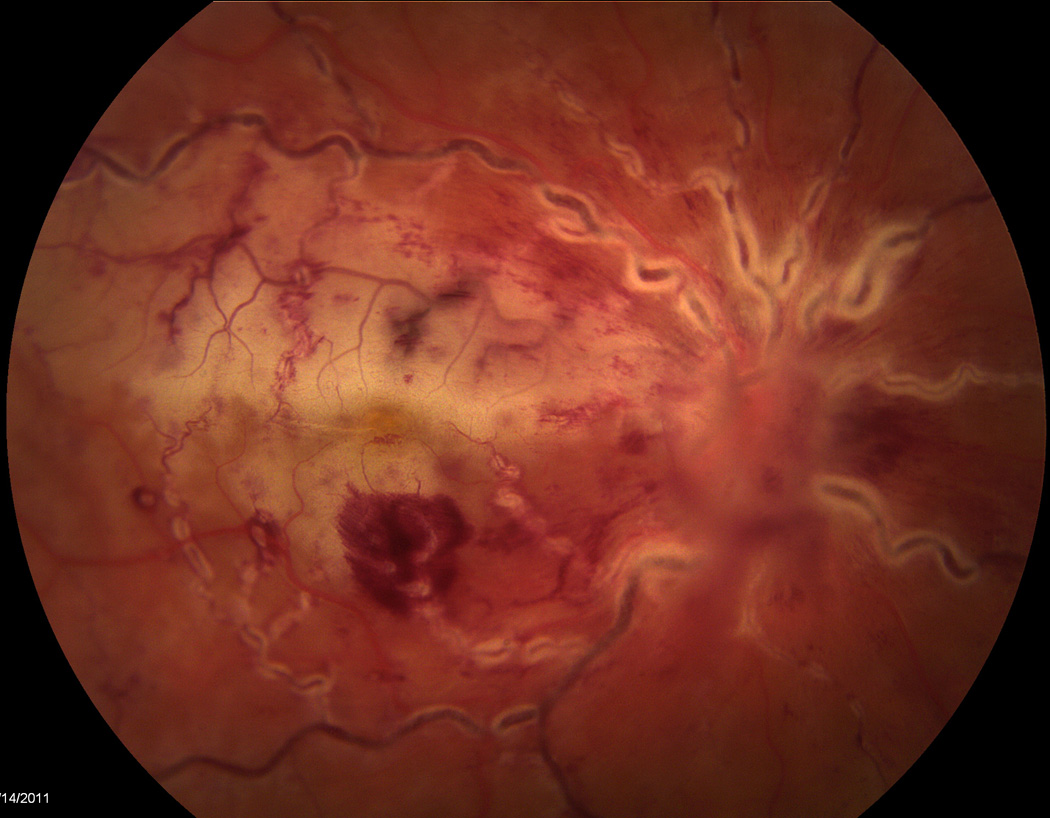
Figure 2.
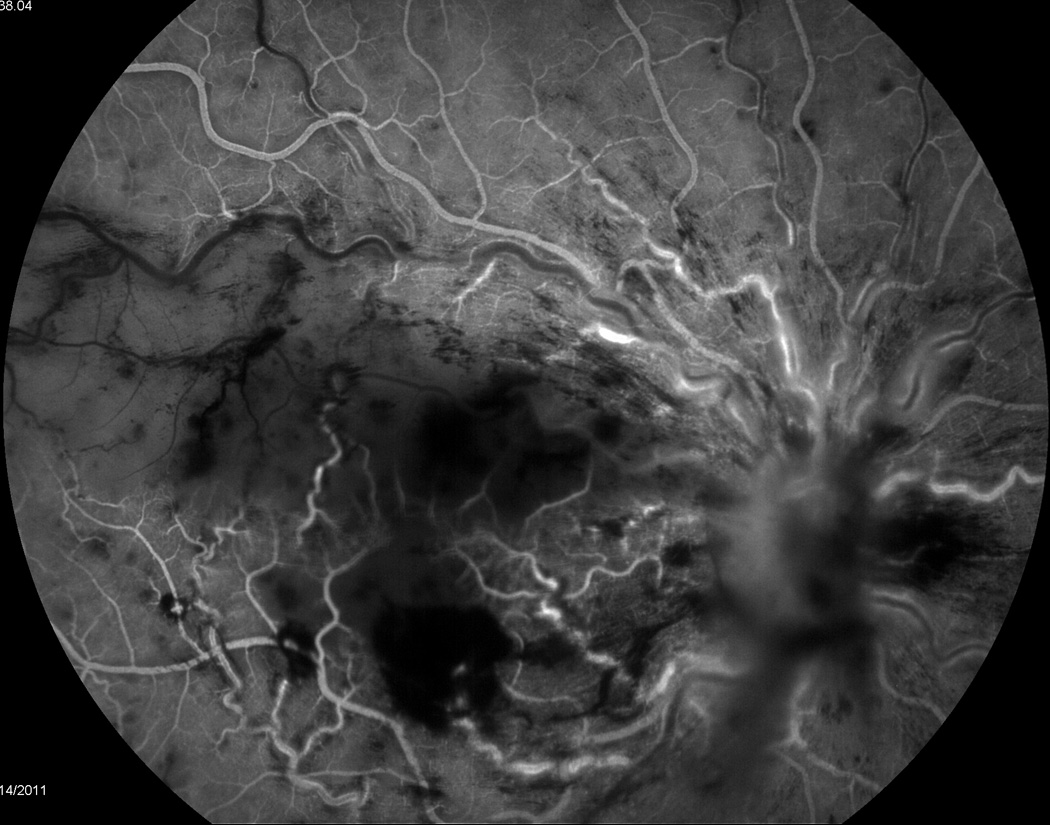
At the time of admission, a pediatric retinal specialist performed salvage treatment with 400 µg/0.1mL intravitreal ganciclovir and systemic treatment was initiated with 5 mg/kg intravenous ganciclovir. In addition, her immunosuppression mono-therapy of 2.5 mg twice a day tacrolimus was decreased to 1 mg twice daily. Serological laboratory data that was collected at the time of admission did not detect the presence of CMV by PCR quantitative assessment (0 copies/ml) with normal liver enzyme levels and complete blood count. Additionally, tacrolimus levels were noted to have been therapeutic as of one month prior to admission with a recorded medication trough of 5.0 ng/mL. At discharge, the patient was transitioned to 900 mg twice daily of oral valganciclovir with plan to complete a 6-week induction course followed by maintenance therapy with 900 mg of oral valganciclovir once daily. Unfortunately, given the findings of macular infarction appreciated at presentation, no visual acuity was recovered despite clinical resolution of retinitis, retinal vasculitis, and macular edema with adjuvant intravitreal bevacizumab injection.
Discussion
Cytomegalovirus (CMV) continues to be a well-recognized cause of infection in the transplant population, particularly because of the immunosuppressive medications that result in decreased T-cell immunity and subsequent increased risk of viral illnesses. Recent literature has stressed the utility of differentiating between CMV infection and CMV disease when considering treatment (5). While CMV infection accounts for viral presence in the variety of laboratory detection methods; CMV disease requires additional clinical observations, signs, and symptoms that correlate with a known viral infection prompting medication administration. The most common presentation of CMV disease is a mononucleosis-like syndrome with fever, malaise, and myalgias – with accompanying leukopenia and atypical lymphocytosis.(6)
The relative risk for the development of CMV disease is based on the serological status of the donor as well as recipient at the time of transplant – with the highest risk occurring when the donor is seropositive and the recipient seronegative for CMV (7). Our patient was known to be at high risk for the development of CMV disease as she was a CMV seronegative recipient of a seropositive deceased donor liver. On review, her CMV IgG antibody was noted to be positive four years after her transplant; however her CMV PCR serologies had always remained negative. Following her transplant, she had received standard prophylactic treatment with two weeks of intravenous ganciclovir followed by transition to oral ganciclovir for a total of 12 weeks of prophylactic therapy. Her immunosuppression regimen consisted of monotherapy with tacrolimus prior to her presentation with goal medication levels ranging from 3–8 ng/mL. Tacrolimus levels had been therapeutic for several years prior to her presentation. No formal ophthalmologic assessment had been made since her transplant, which raises the question of whether screening for CMV retinitis, despite its clinical rarity, should be considered in the post-transplant population.
Standard post-transplant CMV prophylaxis regimens include ganciclovir and/or oral valganciclovir. Twelve weeks of CMV prophylaxis is typically administered to bridge the period where the patient is maximally immunosuppressed and therefore most vulnerable for viral infection. However, there is no universal agreement regarding the required length of treatment and recent literature suggests that a longer period of prophylaxis may be safer and more efficacious in certain populations.(8) Complicating management further is the ill-defined role of preemptive therapy when the presence of CMV infection without clinical evidence of CMV disease would prompt initiation of antiviral therapy.
A broad differential is important when evaluating retinitis is the transplant population. Given that current immunosupression regimens may predispose patients to any viral infection – etiologies beyond CMV, such as herpes-simplex virus (HSV) and varicella zoster virus (VZV), are important to consider. HSV retinitis may present as acute retinal necrosis with retinal vasculitis but usually involves the peripheral retina circumferentially before migrating posteriorly towards the macula. VZV retinitis may present as progressive outer retinal necrosis, but the early macular involvement is usually bilateral and not associated with retinal vasculitis. More often it occurs with optic neuritis and profound drop in acuity (light perception or worse). Often severe immune suppression is present in cases of HSV and VZV retinitis. When CMV retinitis is suspected, the physical findings are distinct and include opacification of the retina with areas of hemorrhage, exudate, and necrosis. Additionally, periphlebitis and even frosted branch angitis may be appreciated. Our patient demonstrated these classical features of CMV retinitis (9).
Retinitis associated with CMV, though well recognized, is rarely encountered after transplantation. A recent single-center retrospective review of all transplant patients (solid organ and hematopoietic stem cell) over a 14-year period reported only 14 cases of CMV retinitis in 9 different patients. (4) Other investigators have assessed the risk of developing CMV retinitis specifically following liver transplant and found that only 0.1% of over 12,000 reviewed patients developed CMV associated retinal disease.(10) When symptomatic, patients present more than 90% of the time with some form of ocular manifestation.(11) However CMV may produce an asymptomatic retinitis(12), particularly when the disease is isolated to the peripheral retina.(13) Response to treatment appears to inversely correlate with the severity of disease at the time of presentation, suggesting that early detection and initiation of treatment may be beneficial (4). When compared to other forms of post-transplant CMV-associated organ disease, the diagnosis of retinitis is often made further out from transplant, with most reports appreciating disease occurrence within one year (4, 11). However, CMV retinitis has been noted to develop significantly later following transplant – one report documented CMV retinitis more than two years after liver transplant (14). Current literature suggests that the slow progression of the disease process, combined with the fact that patients can be asymptomatic, contributes to this delayed presentation. In regards to screening, most centers advocate some form of monitoring for the development of CMV infection following transplant, usually with serological assessment utilizing quantitative or qualitative PCR techniques. However, specific screening for the development of CMV associated ocular disease is not the standard of care. Currently there have been no studies that have addressed this concern. Further inquiries into the benefit of ophthalmological evaluations in the transplant population are needed. Cost-benefit analyses assessing the utility of pre-transplant ophthalmology referrals in order to establish baseline exams as well as post transplant screening protocols will help direct future management of this rare, yet almost uniformly permanent and often progressive complication of the transplant population.
In conclusion, this case demonstrates the importance of retained vigilance in the evaluation of the transplant population in regards to complications of the chronic use of immunosuppressive medications and the continued risk of developing opportunistic infections. In particular, it raises the important question as to whether regular ophthalmologic examinations would be beneficial in identifying early CMV associated retina disease in the liver transplant population. While some authors suggest that quantitation of peripheral blood CMV DNA may be a useful for surveillance of recurrence of CMV retinitis after its initial detection(15), the diagnosis of CMV retinitis continues to be clinically based on ophthalmologic examination by a specialist. Finally, CMV retinitis can be localized infection without any systemic signs or blood PCR positivity - as in our patient.
Acknowledgments
Grant Support:
This work was supported by NIH/NIDDK K08 DK084310-01.
Abbreviations
- CMV
Cytomegalovirus
- PCR
Polymerase Chain Reaction
Footnotes
Disclosures: All authors have nothing to disclose.
Writing Assistance: None
Author Contributions: JS, RS, WFB, RK – All authors participated in acquisition of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content.
References
- 1.Martin JM, Danziger-Isakov LA. Cytomegalovirus risk, prevention, and management in pediatric solid organ transplantation. Pediatr Transplant. 2011;15:229–236. doi: 10.1111/j.1399-3046.2010.01454.x. [DOI] [PubMed] [Google Scholar]
- 2.Bedel AN, Hemmelgarn TS, Kohli R. Retrospective review of the incidence of cytomegalovirus infection and disease post liver transplantation in pediatric patients: Comparison of prophylactic oral ganciclovir versus oral valganciclovir. Liver Transpl. 2011 doi: 10.1002/lt.22471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paya C, Humar A, Dominguez E, et al. Efficacy and safety of valganciclovir vs. oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2004;4:611–620. doi: 10.1111/j.1600-6143.2004.00382.x. [DOI] [PubMed] [Google Scholar]
- 4.Eid AJ, Bakri SJ, Kijpittayarit S, Razonable RR. Clinical features and outcomes of cytomegalovirus retinitis after transplantation. Transplant infectious disease : an official journal of the Transplantation Society. 2008;10:13–18. doi: 10.1111/j.1399-3062.2007.00241.x. [DOI] [PubMed] [Google Scholar]
- 5.Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2002;34:1094–1097. doi: 10.1086/339329. [DOI] [PubMed] [Google Scholar]
- 6.Winston DJ, Wirin D, Shaked A, Busuttil RW. Randomised comparison of ganciclovir and high-dose acyclovir for long-term cytomegalovirus prophylaxis in liver-transplant recipients. Lancet. 1995;346:69–74. doi: 10.1016/s0140-6736(95)92110-9. [DOI] [PubMed] [Google Scholar]
- 7.Kotton CN, Kumar D, Caliendo AM, et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779–795. doi: 10.1097/TP.0b013e3181cee42f. [DOI] [PubMed] [Google Scholar]
- 8.Humar A, Lebranchu Y, Vincenti F, et al. The efficacy and safety of 200 days valganciclovir cytomegalovirus prophylaxis in high-risk kidney transplant recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10:1228–1237. doi: 10.1111/j.1600-6143.2010.03074.x. [DOI] [PubMed] [Google Scholar]
- 9.Committee SRW. Retina and Vitreous. American Academy of Ophthalmology’s Basic and Clinical Sciences Course. 2009/2010;Volume 12:203–214. [Google Scholar]
- 10.Egli A, Bergamin O, Mullhaupt B, Seebach JD, Mueller NJ, Hirsch HH. Cytomegalovirus-associated chorioretinitis after liver transplantation: case report and review of the literature. Transplant infectious disease : an official journal of the Transplantation Society. 2008;10:27–43. doi: 10.1111/j.1399-3062.2007.00285.x. [DOI] [PubMed] [Google Scholar]
- 11.Kuo IC, Kempen JH, Dunn JP, Vogelsang G, Jabs DA. Clinical characteristics and outcomes of cytomegalovirus retinitis in persons without human immunodeficiency virus infection. American journal of ophthalmology. 2004;138:338–346. doi: 10.1016/j.ajo.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 12.Fishburne BC, Mitrani AA, Davis JL. Cytomegalovirus retinitis after cardiac transplantation. American journal of ophthalmology. 1998;125:104–106. doi: 10.1016/s0002-9394(99)80245-1. [DOI] [PubMed] [Google Scholar]
- 13.Baumal CR, Levin AV, Kavalec CC, Petric M, Khan H, Read SE. Screening for cytomegalovirus retinitis in children. Archives of pediatrics & adolescent medicine. 1996;150:1186–1192. doi: 10.1001/archpedi.1996.02170360076013. [DOI] [PubMed] [Google Scholar]
- 14.Shibolet O, Ilan Y, Kalish Y, et al. Late cytomegalovirus disease following liver transplantation. Transplant international : official journal of the European Society for Organ Transplantation. 2003;16:861–865. doi: 10.1007/s00147-003-0643-x. [DOI] [PubMed] [Google Scholar]
- 15.Aw MM, Murugasu B, Tan AW, Seah CC, Balakrishnan V, Yap HK. Quantitation of peripheral blood cytomegalovirus DNA for monitoring recurrent cytomegalovirus retinitis in pediatric solid organ transplant recipients. Pediatr Transplant. 2000;4:100–106. doi: 10.1034/j.1399-3046.2000.00095.x. [DOI] [PubMed] [Google Scholar]


