Abstract
Despite the high prevalence and enormous public health implications of chronic kidney disease (CKD), the factors responsible for its development and progression remain incompletely understood. To date, only a few studies have attempted to objectively characterize sleep in CKD patients prior to kidney failure, but emerging evidence suggests a high prevalence of sleep disorders, particularly obstructive sleep apnea. Laboratory and epidemiologic studies have shown that insufficient sleep and poor sleep quality promote the development and exacerbate the severity of three important risk factors for CKD, namely hypertension, type 2 diabetes, and obesity. In addition, sleep disturbances might have a direct effect on CKD through chronobiological alterations in the renin-angiotensin-aldosterone system and sympathetic nervous system activation. The negative impact of sleep disorders on vascular compliance and endothelial function may also have also have a deleterious effect on CKD. Sleep disturbances may therefore represent a novel risk factor for the development and progression of CKD. Optimizing sleep duration and quality and treating sleep disorders may reduce the severity and delay the progression of CKD.
Index words: Sleep disorders, obstructive sleep apnea, chronic kidney disease
The prevalence of chronic kidney disease (CKD) has markedly increased over the past 10 years and this alarming trend is likely to continue.1 Indeed, type 2 diabetes and hypertension are the two best established risk factors for CKD and the prevalence of both conditions is rapidly increasing, driven by the epidemic of obesity. Despite the public health implications of CKD, we have an incomplete understanding of the factors responsible for its development and progression. There is a wide inter-individual variability in the progression of CKD that remains poorly understood. Obesity, dyslipidemia, inflammation, and cigarette smoking are known risk factors2 but they only partially account for individual differences in CKD progression.
In recent years, a limited number of studies have attempted to characterize sleep in predialysis CKD, and the emerging evidence suggests a high prevalence of sleep disorders, in particular sleep-disturbed breathing. There is substantial evidence indicating that insufficient sleep and poor sleep quality promote the development and exacerbate the severity of three important risk factors for CKD, namely type 2 diabetes, hypertension and obesity. Thus, sleep disturbances may play an indirect role in the development and progression of CKD. The plausibility of a direct adverse impact of sleep disturbances on CKD is supported by the fact that, under normal physiologic conditions, key hormones that regulate body fluid balance and blood pressure (BP) are exquisitely modulated by the sleep-wake cycle.3–5 Furthermore, insufficient sleep and poor sleep quality, including obstructive sleep apnea (OSA), are associated with an elevation of daytime sympathetic nervous system activity, which is likely to have an adverse impact on CKD. Finally, there is an increasing body of evidence suggesting that OSA may contribute directly to the development of CKD and accelerate its progression.
The present review summarizes the evidence supporting a role for sleep disturbances as both direct and indirect non-traditional risk factors for the development and progression of CKD. Figure 1 illustrates the conceptual framework that guided this review.
Figure 1.
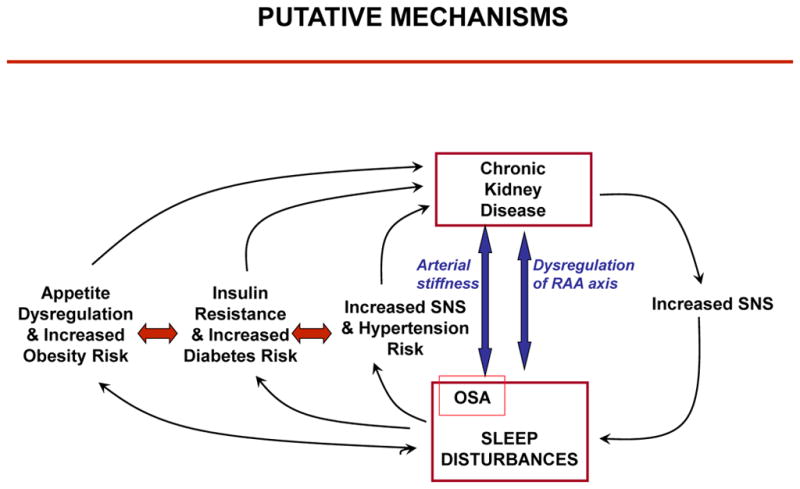
Putative mechanisms linking sleep disturbances and progression of CKD. Abbreviations: SNS, sympathetic nervous system activity; OSA, obstructive sleep apnea.
ASSESSMENT OF SLEEP AND SLEEP DISORDERS
The temporal organization of sleeping and waking over the 24-hour cycle is controlled in part by an internal circadian clock and in part by a homeostatic mechanism that increases the pressure for sleep in proportion to the duration of prior wakefulness. Sleep itself oscillates between two separate states of markedly different brain activity, known respectively as Rapid Eye Movement (REM) sleep and non-REM (NREM) sleep. Normal sleep is characterized by a 90-min oscillation between NREM and REM stages. NREM sleep is divided into 4 stages. Stages 3 and 4 of NREM sleep are the deepest stages and are termed Slow Wave Sleep (SWS). Muscle tone inhibition and oneiric activity are characteristics of REM sleep.
The gold standard of assessing sleep is polysomnography (PSG), a method that combines an all-night recording of multiple-lead electroencephalogram (EEG) with measures of muscle tone and eye movements. The recordings are visually scored using standardized criteria to categorize whether the individual is awake or in stages 1, 2 3, 4 or REM sleep over each 30-sec interval.
Objective estimations of sleep duration and fragmentation may be obtained under ambulatory conditions by wrist actigraphy. Actigraphy monitors are devices the size of a wristwatch that use sensitive omnidirectional accelerometers to count wrist movements. Wrist actigraphy does not provide an assessment of sleep architecture, as it does not differentiate REM from NREM sleep.
Lastly, a number of validated questionnaires to assess sleep duration and quality have been developed. The most commonly used is the Pittsburgh Sleep Quality questionnaire.6 The summary measure is the Pittsburgh Sleep Quality Index (PSQI). A global PSQI score above 5 is indicative of clinically significant poor sleep quality.
The most common form of sleep disturbance is insufficient sleep due to behavioral bedtime curtailment, an endemic condition in modern society. Self-reported sleep duration in Americans has decreased by 1.5 to 2 hours between 1960 and the beginning of the 21st century. In 2006, more than 30% of adults between 30 and 64 years of age reported sleep durations under 6 hours per night.7 This curtailment of sleep is a sleep disorder in and of itself in the sense that it produces both daytime and nighttime alterations of physiological systems.
Insomnia is the most common clinically recognized sleep disorder; the diagnostic criterion is a subjective complaint of difficulty in initiating or maintaining sleep for at least four weeks, to such an extent that daytime functioning is impaired. Prevalence of insomnia ranges from 7–50%,8 and is associated with increased age, chronic medical illnesses, and psychological disorders.
OSA is an increasingly prevalent disorder that involves repetitive upper airway closures (apneas) or partial collapses (hypopneas) causing intermittent hypoxia and transient arousals that restore airflow but lead to sleep fragmentation and poor sleep quality. A clinical diagnosis of OSA is made when the number of apneas and hypopneas per hour (apnea-hypopnea index [AHI]) is ≥5. The major risk factor for OSA is obesity. In one study that used PSG to quantify the prevalence of sleep apnea in morbidly obese patients, 98% of the 51 participants had clinically diagnosable sleep apnea, while 33% had severe sleep apnea.9 The treatment of choice for OSA is the administration of continuous positive airway pressure (CPAP), a non-pharmacological intervention that is highly efficacious in eliminating obstructive events.
Periodic limb movement disorder is a sleep disorder characterized by involuntary movements of the legs (or, less frequently, arms) that result in sleep fragmentation and may cause excessive daytime sleepiness.
PREVALENCE OF SLEEP DISORDERS IN CKD
There is ample evidence indicating a greater prevalence of sleep disorders in patients with end-stage renal disease (ESRD) than in people with normal kidney function.10;11 In contrast, research on sleep disorders in earlier stages of CKD is a nascent field of inquiry, with the majority of studies relying on self-reported surveys rather than objective sleep measures. As a result, it is difficult to ascertain the prevalence of sleep disorders in non-dialysis-dependent CKD. Table 1 provides a summary of existing studies divided into three categories based on the methodology with which sleep disorders were diagnosed.
Table 1.
Summary of Representative Sleep Studies in Non-dialysis-dependent CKD
| Study | Participants | CKD Stage | Results |
|---|---|---|---|
| Diagnosed via questionnaire | |||
| Iliescu et al, 200467 | 120 CKD | 2–5 | prevalence of disordered sleep by PSQa: 53%; no association with CCr |
| Kurella et al, 200568 | 78 CKD; 78 HD | 3–4 | prevalence of disturbed sleep maintenance by KDQOLb: 14–27% in CKD, 34% in HD |
| Cohen et al, 200712 | 92 CKD; 61 non-CKD | 2–5 | prevalence of disordered sleep by PSQ: 55% in CKD (NS difference with non-CKD) |
| Sabbatini et al, 200869 | 78 CKD | 3–5 | prevalence of disordered sleep by PSQ: 50%; sleep quality worsened as eGFR declined over time, but association NS after adjusting for confounders |
| De Santo et al, 200870 | 124 CKD | 2–3 | prevalence of insomnia by Sleep Disorders Questionnaire: 57% |
| Kumar et al, 201071 | 673 CKD | 3–5 | prevalence of poor sleep quality by KDQOL: 57%; no association with eGFR |
| De Santo et al, 201013 | 220 CKD; 220 Hep C | 1–3 | prevalence of disordered sleep by PSQ: 85% in CKD, 60% in Hep C |
| Plantinga et al, 201172 | 1805 CKD; 7305 non-CKD | 1–4 | Prevalence of short sleep duration (≤6 h) by NHANESf: 43% in CKD1–2, 31% in CKD3–4, 37% in non-CKD |
| Diagnosed via wrist actigraphy | |||
| Barmar et al, 200914 | 36 CKD; 51 HD | 4–5 | Reduced sleep duration and efficiency in both CKD and HD but worse in HD |
| Agarwal et al,201115 | 145 CKD; 116 HD; 19 Non- CKD | 3 | Lower sleep efficiency and higher sleep fragmentation in CKD vs non-CKD |
| Diagnosed via PSG | |||
| Kimmel et al, 198973 | 8 CKD; 20 HD | 5 | prevalence of sleep apnea: 73% in patients with sleep apnea symptoms (16/22) |
| Parker et al, 200574 | 8 CKD; 16 HD | 4–5 | Reduced total sleep time and efficiency in CKD and HD but worse in HD |
| Markou et al, 200617 | 35 CKD | 3–5 | prevalence of sleep apnea: 54%; of periodic limb movements: 29%; no association between AHIc and CCr |
| Fleischmann et al, 201016 | 18 CKD; 140 non-CKDe | 3 | prevalence of sleep apnead : 94% in CKD, 83% in non-CKD (NS) |
| Roumelioti et al,201175 | 89 CKD; 75 HD; 224 non-CKD | 4–5 | Risk of severe sleep-disordered breathing significantly higher in CKD and HD vs non-CKD |
PSQ= Pittsburgh Sleep Questionnaire, threshold for disordered sleep is PSQ index > 5.
KDQOL=Kidney Disease Quality of Life.
AHI= Apnea-hypopnea index ≥5
Sleep apnea defined as the respiratory disturbance index (RDI) > 5.
non-CKD patients had been referred for sleep study
the NHANES sleep quality questionnaire
Abbreviations: CCr, creatinine clearance; CKD, chronic kidney disease; Hep C, hepatitis C; HD, hemodialysis; NS, nonsignificant; PSG, polysomnography
Studies based on questionnaires have provided widely different estimates of the prevalence of sleep disorders in CKD patients, ranging from 14% to 85% (Table 1). Furthermore, studies comparing CKD to non-CKD patients have reported inconsistent findings. For example, a study by Cohen et al12 found no significant difference between the sleep of 92 CKD patients and 51 general medical outpatients assessed by the PSQI. In contrast, a study by De Santo et al13 comparing 220 non-CKD patients with hepatitis C with 220 CKD patients found that 59.5% of hepatitis C controls suffered from poor sleep quality based on the PSQI versus84.6% of CKD patients. These somewhat disparate results call for a more objective, quantifiable and uniform methodology to estimate the prevalence of sleep disorders in CKD.
Few studies have used actigraphy to evaluate sleep disorders in non-dialysis-dependent CKD. Barmar et al14 measured sleep using actigraphy in 36 non-dialysis-dependent patients with stages 4–5 of CKD and 51 hemodialysis patients. These investigators found that both groups had short and fragmented sleep and that both subjective and objective measures were worse in hemodialysis patients than in the CKD patients who were not undergoing hemodialysis. Agarwal et al15 reported significantly lower sleep efficiency and higher sleep fragmentation in patients with CKD compared with those without CKD after adjusting for clinically relevant variables.
While PSG provides the most objective assessment of sleep to date, there has been no large systematic evaluation of sleep in CKD patients based on PSG. Fleischmann et al16 examined 158 CKD patients who were already suspected of having SA and, using PSG as an objective measure, were able to document very high rates of SA (80% to 94%). A study by Markou et al17 examined 35 patients with non-dialysis-dependent CKD and found a prevalence of sleep-distrubed breathing of 54.3% and of periodic limb movement disorder of nearly 30%. Using diagnostic codes to identify cases of SA, Sim et al18 reported a higher risk for SA in patients with estimated glomerular filtration rate (eGFR) of 45–89 ml/min/1.73 m2 as compared to patients with eGFR >90 ml/min/1.73m2. In a cross-sectional analysis of registry data, Iseki et al19 reported that 30.5% of patients with sleep-related breathing disorders have CKD.
The heterogeneity of these studies and the variability in the findings indicate that additional research on the prevalence of sleep disorders in CKD is needed. Nonetheless, the evidence summarized in Table 1 points to a greater prevalence of sleep disorders in patients with CKD than in the general population. These associations may be bidirectional (Figure 1). It is possible that kidney disease may actually promote the development of sleep disturbances. Excessive fluid volume, with a potential shift to the neck during recumbency, has been suggested as a potential mechanism promoting OSA in CKD.20 Depression may be another factor in the pathway between CKD and poor sleep quality since patients with CKD and patients with sleep disorders both have an increased risk for depressive symptoms.21;22 It is possible that there may be a cyclical effect whereby CKD negatively affects sleep, and sleep disorders negatively affect CKD.
DIRECT IMPACT OF SLEEP DISORDERS ON CKD
Overview
There are two lines of evidence in support of an adverse effect of sleep disorders on the development and progression of CKD. First, sleep has a major impact on key regulators of kidney function and BP and therefore, reduced sleep duration and quality could potentially have a negative impact on kidney function. Second, recent evidence points at OSA as an independent risk factor for CKD, with endothelial dysfunction and arterial stiffness in the causal pathway.
Sleep Disturbances and the Autonomic Nervous System
Reduced sympathetic activity and increased vagal tone during normal sleep, particularly during NREM sleep, are responsible for the nocturnal dipping of BP associated with sleep. In non-CKD patients, it is well recognized that sleep loss and sleep disorders, in particular sleep-disturbed breathing with its nocturnal hypoxemia and sleep fragmentation, lead to sympathetic nervous system stimulation and attenuation of the sleep-induced decrease in BP.23–25 Furthermore, studies using microneurography have shown that OSA is associated with elevated daytime sympathetic nervous system activity, which can be corrected by successful CPAP treatment.23 The impact of sleep disorders on the autonomic nervous system in CKD patients has not been extensively evaluated. A recent PSG study including stage 4–5 CKD as well as HD patients demonstrated that both groups of participants were unable to increase cardiac vagal tone during the transition from wakefulness to NREM sleep.26 It is well documented that hyperactivation of the sympathetic nervous system is often present in patients with CKD and it has been postulated that this may be a risk factor for CKD progression due to its effects on BP and renal hemodynamics.27 It is reasonable to speculate that further activation of the sympathetic nervous system due to sleep disorders would exacerbate this risk in patients with CKD.
Sleep and the Renin-Angiotensin-Aldosterone System
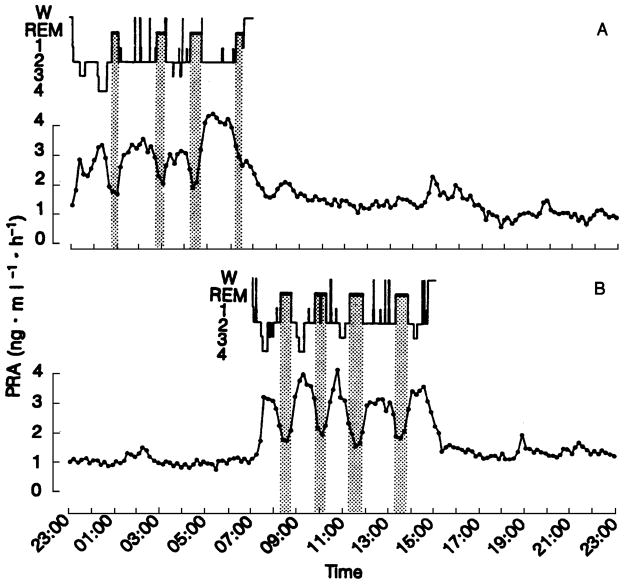
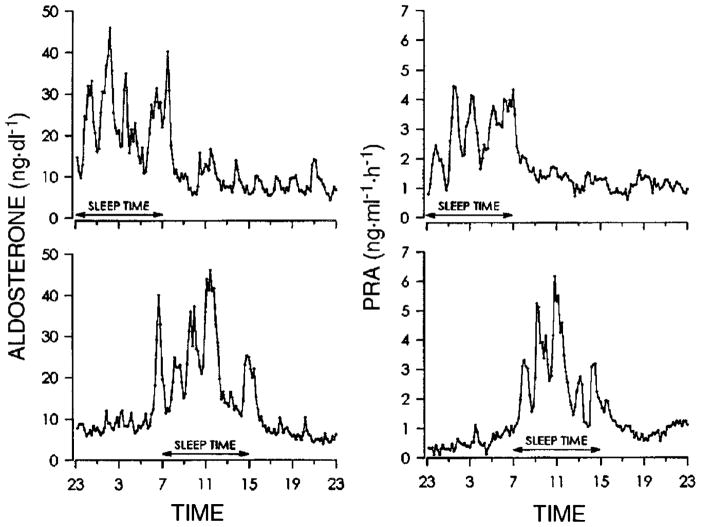
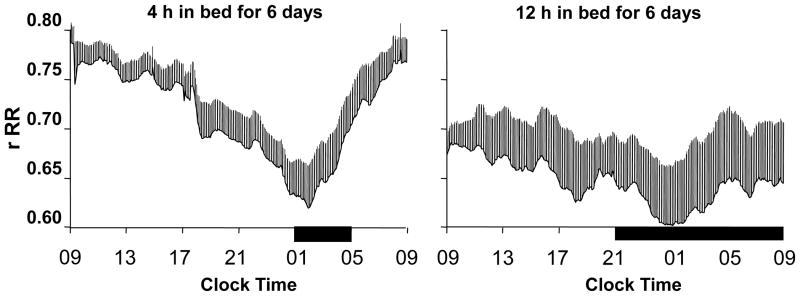
The 24-hour sleep/wake cycle in humans is intricately linked with homeostatic BP regulation.28–31 As described above, normal sleep is accompanied by the so-called “dipping” of systolic and diastolic BP. A study by Charloux et al29 that continuously recordedthe arterial BP of healthy volunteers using PSG identified this decrease in BP as the event that initiates a robust nocturnal elevation of plasma renin activity (PRA). Once sleep is initiated, sympatho-vagal balance is exquisitely modulated by the NREM-REM cycle, which is best quantified by assessing oscillations of EEG slow-wave activity using spectral analysis (Figure 2).28 This oscillation drives an oscillation in PRA (Figure 3, upper panel)3 and aldosterone (Figure 4, upper panel)4 during sleep, with higher levels of PRA and aldosterone during SWS than during REM sleep. Both plasma renin and aldosterone exhibit these reproducible 24-hour variations, which are influenced by the timing, quantity and quality of sleep.3;4 As shown in Figures 3 and 4 (lower panels), both PRA and aldosterone levels increase during nighttime and daytime sleep, and do not increase during a night of total sleep deprivation. Figure 5 shows the 24-hour profile of cardiac sympatho-vagal balance in healthy young participants after 6 days of bedtime restriction to 4 hours per night as compared to a fully rested condition.25 It can be seen that sympathovagal balance is markedly elevated during most of the waking period, while the period of reduced activity during sleep is much shorter. These alterations are likely to have a direct adverse effect on the regulation of the renin-angiotensin-aldosterone system during wakefulness as well as during sleep. When sleep is shallow and fragmented, the nocturnal dipping of BP is absent or dampened and the sleep-related increase of renin and aldosterone are similarly affected.
Figure 2.
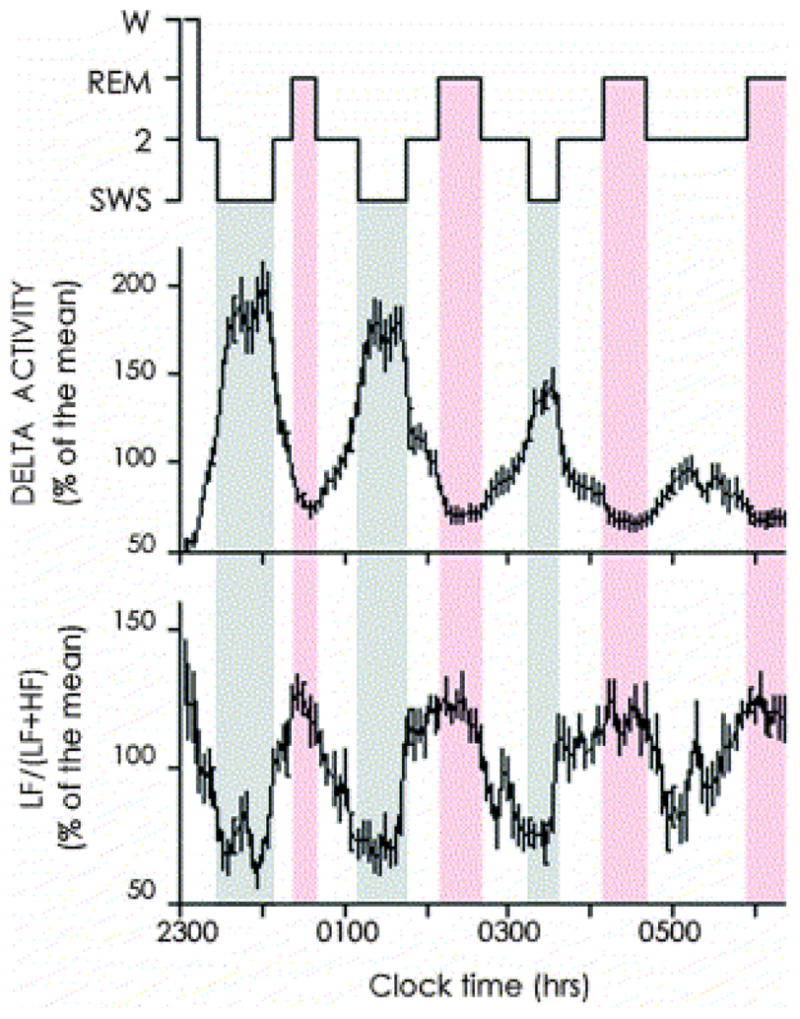
Inverse relationship between the intensity of NREM sleep, assessed by EEG spectral activity in the delta frequency band (top), and cardiac sympatho-vagal balance, assessed via spectral analysis of heart rate variability. Recordings were obtained in healthy young adults. Values reported are mean ± standard error of the mean. Reproduced from Brandenberger et al28 with permission from the International Federation of Clinical Neurophysiology
Figure 3.
24-hour profile of plasma renin activity (PRA) in an apparently healthy young adult studied once with sleep at the usual nocturnal time (A) and once with sleep deprivation during the night and recovery sleep in the daytime (B). The shaded area indicates REM stages . Reprinted from Brandenberger et al3 with permission from Wolters Kluwer Health.
Figure 4.
24-hour profile of plasma aldosterone levels (left) and plasma renin activity (PRA; right) in an apparently healthy young adult studied once with sleep at the usual nocturnal time (upper panels) and once with sleep deprivation during the night and recovery sleep in the daytime (lower panels). Reproduced from Charloux et al4 with permission from the American Physiological Society.4
Figure 5.
Impact of sleep duration on cardiac sympathovagal balance. Mean (+standard error of the mean) 24-hour profiles of sympathovagal balance (estimated at 5-min intervals using the coefficient of autocorrelation of successive cardiac interbeat intervals rRR; E panels), when time in bed is 4 hours for 6 consecutive nights (left; mean total sleep time during night 6) or 12 hours for 6 consecutive nights (right; mean total sleep time during night 6). Particpants were 11 young healthy men. The black bars represent the sleep periods. Reproduced from Spiegel et al25 with permission from the Endocrine Society.25
A study by Sayk et al31 showed that experimental SWS suppression reduced nocturnal BP dipping in healthy young adults. Thus, reduced sleep quality may decrease the normal nocturnal rise of PRA and aldosterone even if sleep duration is not affected, a fact of significant importance given the prevalence of poor sleep quality in patients with CKD. The relationship between sleep fragmentation caused by OSA and its subsequent effects on PRA have been well demonstrated in a study by Follenius et al,5 in which participants with OSA were treated with CPAP and the subsequent fluctuations in PRA and aldosterone levels were measured. CPAP treatment was found to increase the nighttime PRA and aldosterone levels significantly, demonstrating that OSA was preventing the normal nocturnal regulation of these hormones. This chronobiological alteration in the activity of the renin-angiotensin-aldosterone system could play a role in CKD progression. Experimental models of kidney disease have shown that glomerular capillary hypertension, which is maintained largely by angiotensin-dependent mechanisms, ultimately leads to glomerular scarring and nephron dropout.32
OSA as a Direct Contributor to CKD Development and Progression
Specific features of OSA that are not present in other sleep disorders include intermittent hypoxia and re-oxygenation, nocturnal BP surges and nocturnal sympathetic activation. The cycles of hypoxia and re-oxygenation stimulate the formation of reactive oxygen species that promote inflammation and systemic endothelial dysfunction.33;34 Endothelial dysfunction, inflammation, and oxidative stress all have adverse effects on kidney function.35 A recent 6-year prospective study showed that endothelial dysfunction and inflammation predict the development of arterial stiffness in individuals without CKD.36 Multiple studies have shown associations between OSA and arterial stiffness37;38 and demonstrated that CPAP treatment of OSA can decrease arterial stiffness.37 The association between OSA and arterial stiffness may have potential implications in CKD progression. A recent publication from the Multi-Ethnic Study of Atherosclerosis (MESA) reported that lower arterial elasticity was linearly and independently associated with faster kidney function decline.39 It is speculated that reduced elasticity of major arteries can result in microvascular damage and impair renal hemodynamics, and hence leads to compromised kidney function.
Early studies have suggested that patients with OSA have abnormalities in tubular function, leading to increased nocturnal natriuresis.40;41 However, these studies were small and reported inconsistent findings. In contrast, there is limited but convincing evidence that OSA influences renal hemodynamics and that treatment with CPAP may ameliorate these effects. Kinebuchi et al42 measured glomerular filtration rate (GFR) and renal plasma flow (RPF) in 27 patients with OSA before and after treatment with CPAP. After one week of treatment, GFR remained unchanged but RPF increased, which resulted in a small but significant decrease in filtration fraction. These findings are consistent with OSA-mediated vasoconstriction, which is attenuated by CPAP therapy.
A number of studies have investigated the link between OSA and proteinuria. Casserly et al43 examined protein-creatinine ratios in 148 individuals referred for PSG and found no association between severity of OSA and proteinuria. These negative findings were contradicted by Faulx et al,44 who performed an overnight PSG in 496 patients with mild-to-severe OSA and found that, after adjusting for body mass index (BMI), severe OSA (AHI≥30) was significantly associated with increased urine albumin excretion independently of GFR and of the presence of diabetes or hypertension. Glomerular endothelial dysfunction was proposed as the underlying mechanism for the elevated urinary albumin excretion. In another cross-sectional study, Tsioufis et al45 compared 62 hypertensive patients with OSA and 79 hypertensive patients without OSA matched for age, sex, smoking status, BMI and 24-hour pulse pressure, and found that OSA was associated with a 57% elevation of albuminuria and that AHI was a significant predictor of albuminuria. In a subsequent study, Agrawal et al46 performed PSG in 91 morbidly obese patients and found no differences in albuminuria in individuals with OSA (n=55) and without OSA (n=36), but detected a significant positive correlation between the severity of OSA and serum creatinine levels. Differences in these findings are likely related to differences in methodology and populations studied. Furthermore, it is difficult to assess the influence of OSA relative to that of obesity on CKD, despite statistical adjustment for BMI. Most likely, there is a complex interplay between these two factors and kidney function. Interestingly, however, an early small study47 reported decreases in proteinuria with OSA treatment.
Based on the multiple negative effects of OSA on the systemic and renal vasculature, it is reasonable to speculate that OSA may be an independent risk factor for CKD progression. However, this association needs to be evaluated in a prospective study.
INDIRECT IMPACT OF SLEEP DISORDERS ON CKD: EFFECTS ON KNOWN RISK FACTORS
Overview
Research on the relationship between sleep, cardio-metabolic risk and endocrine function has become a fast growing topic in the last 10 years. Insufficient sleep and/or poor sleep quality have been shown to promote the risks of hypertension, type 2 diabetes and obesity, suggesting that sleep disturbance may be an important indirect contributor to both the development and progression of CKD. The following summarizes the associations that have been found between sleep and cardio-metabolic function that may be important in CKD.
Sleep disturbances and hypertension
It is well established that hypertension is a major risk factor for the development and progression of CKD2 and that BP control, particularly with an angiotensin converting enzyme inhibitor or an angiotensin receptor blocker can attenuate the progression of CKD.48;49 Short sleep, poor sleep and OSA can all promote the risk of hypertension or exacerbate its severity. In the Coronary Artery Risk Development in Young Adults (CARDIA) Sleep Study,50 shorter sleep duration and lower sleep maintenance assessed by actigraphy significantly predicted higher daytime systolic and diastolic BP cross-sectionally, and there were more adverse changes in systolic and diastolic BP over five years after excluding patients taking anti-hypertensive medications and adjusting for age, race and sex. Short sleep duration also significantly predicted increased odds of incident hypertension (OR, 1.37; 95% CI, 1.05–1.78). In NHANES, short sleep duration by self-report also predicted the development of hypertension.51 Further, a recent prospective study in older men showed that those with low amounts of SWS were more likely to develop hypertension.52
Several studies have demonstrated that poor sleep quality is associated with a blunting of the nocturnal dip in BP in participants with normal kidney function.31;53 Correlations between objectively assessed sleep quality and BP dipping have actually been documented in CKD patients. Indeed, a study by Agarwal et al53 used wrist actigraphy monitoring to demonstrate that the sleep fragmentation associated with nocturia in patients with CKD (eGFR <60mL/min per 1.73 m2) was positively correlated with a blunted night-time BP drop; individuals with increased activity due to nocturia were shown to have a decreased dip in their night-time BP.
Lastly, OSA is a well-documented risk factor for hypertension. The prospective analysis of the Wisconsin Sleep Cohort Study showed an increased risk of developing hypertension nearly threefold higher for those with clinically significant OSA.54 In addition, OSA has been independently linked to specific cardiovascular outcomes such as stroke, myocardial ischemia, arrhythmias, cardiovascular events, and all-cause mortality.55 These findings suggest that sleep disorders, particularly OSA, might contribute to increased cardiovascular risk in patients with CKD.
Sleep Disturbances and glucose intolerance
A large body of laboratory and epidemiologic research on the relationship between sleep and glucose metabolism has demonstrated that sleep quality and quantity play an integral role in glucose regulation.56–60 Specifically, experimentally reduced sleep quantity or increased sleep fragmentation have both been shown to significantly increase insulin resistance without appropriate compensation by increased insulin release in healthy participants. In a study by Spiegel et al,56 healthy young men were restricted to 4 hours of sleep per night for 6 nights, which resulted in a decrease in glucose tolerance consistent with pre-diabetes. A recent meta-analysis of prospective studies examining the impact of short sleep duration or reduced sleep quality on the incidence of type 2 diabetes concluded that, after controlling for multiple confounders, short sleepers were 28% more likely to develop diabetes, whereas difficulty in initiating or maintaining sleep was associated with an even higher risk of diabetes.58 A subsequent analysis of the National Institutes of Health-American Association of Retired Persons Diet and Health Study, which involved nearly 175,000 individuals, found that the relative risk of developing type 2 diabetes over the 5- to 10-year follow-up period was 46% higher in those sleeping 5 hours or less than in those sleeping 7 to 8 hours.61 Furthermore, two prospective studies have found that the presence of OSA independently increases the risk of developing diabetes.59;60
There is also good evidence indicating that sleep disturbances exacerbate the severity of diabetes. A study by Knutson et al59 demonstrated that in diabetic patients, reduced self-reported sleep duration and quality are significant predictors of poorer glycemic control. More recently, OSA was found to affect 53% to 87% of patients with diabetes and a “dose-response” relationship between the severity of OSA and hemoglobin A1c levels was evidenced.60 Lastly, the presence of insomnia in diabetic patients was found to be associated with markedly higher blood glucose levels.62
In summary, multiple studies have suggested that poor sleep quantity and quality are significant risk factors for type 2 diabetes, an important consideration for CKD in light of the close association between diabetes and CKD. The current data also indicate that sleep disturbances have an adverse impact on glycemic control in diabetic patients and would thus exacerbate this frequent co-morbidity of CKD.
Sleep and Obesity
Closely linked with the aforementioned association between sleep and diabetes is that of sleep and obesity. There is increasing evidence that obesity may be an independent risk factor for CKD and its progression to kidney failure and the need for dialysis.63;64 In healthy lean young adults, curtailing sleep has an important impact on the neuroendocrine control of satiety and hunger, promoting excessive eating.25 Leptin, a hormone that is an important satiety factor in mammals, was found to be decreased in patients whose sleep had been shortened, while levels of ghrelin, a hunger hormone, were markedly increased; as a consequence, hunger ratings increased, suggesting that reduced sleep quantity could promote weight gain. Sleep restriction in middle-aged overweight adults was indeed shown to increase food intake from snacks.65 Further, when healthy volunteers who had had bedtimes restricted to 4 h per night for 5 nights were presented with ad libitum food, they ingested nearly 300 kcal more than when they had 8 h bedtimes.66
There is an abundance of epidemiologic studies showing an association between short sleep and elevated BMI, after controlling for multiple confounders. The impact of sleep quality on the risk of obesity has been explored in fewer studies, but all studies to date have had positive findings. The association between sleep and obesity is very relevant for CKD because of the high rate of obesity amongst patients with CKD and the emerging evidence that obesity, an inflammatory state, may in itself have an adverse impact on kidney function. Furthermore, obesity is the major risk factor for OSA and its cardio-vascular consequences, including the risk of hypertension and its deleterious effects on kidney function.
CONCLUSION
Research in the last decade has demonstrated that sleep disturbances promote the development and exacerbate the severity of three well-documented risk factors for CKD: diabetes, hypertension and obesity. Furthermore, a tight link between the renin-angiotensin-aldosterone axis and sleep has been demonstrated, and a plausible direct deleterious impact of OSA on kidney function has been proposed. Taken together, these findings strongly suggest that sleep disturbances may represent a novel risk factor for the progression of CKD. Identifying and treating sleep disorders may have a therapeutic benefit in CKD.
Acknowledgments
The authors thank Drs. Eve Van Cauter and Kristen L. Knutson for helpful advice and criticism and for a careful review of the manuscript.
Support:This work was supported by grants R01 DK-072231-91 and U01 DK060980- 04S2 (to Dr Lash).
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Yu HT. Progression of chronic renal failure. Arch Intern Med. 2003;163:1417–1429. doi: 10.1001/archinte.163.12.1417. [DOI] [PubMed] [Google Scholar]
- 3.Brandenberger G, Follenius M, Goichot B, Saini J, Spiegel K, Ehrhart J, Simon C. Twenty-four-hour profiles of plasma renin activity in relation to the sleep-wake cycle. J Hypertens. 1994;12:277–283. [PubMed] [Google Scholar]
- 4.Charloux A, Gronfier C, Lonsdorfer-Wolf E, Piquard F, Brandenberger G. Aldosterone release during the sleep-wake cycle in humans. Am J Physiol. 1999;276:E43–E49. doi: 10.1152/ajpendo.1999.276.1.E43. [DOI] [PubMed] [Google Scholar]
- 5.Follenius M, Krieger J, Krauth MO, Sforza F, Brandenberger G. Obstructive sleep apnea treatment: peripheral and central effects on plasma renin activity and aldosterone. Sleep. 1991;14:211–217. [PubMed] [Google Scholar]
- 6.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 7.QuickStats. Percentage of Adults Aged >18 Years Who Reported an Average of ≤6 Hours of Sleep per 24-Hour Period, by Sex and Age Group --- National Health Interview Survey, United States, 1985 and 2006. National Center for Health Statistics; 2008. [Google Scholar]
- 8.Buscemi N, Vandermeer B, Friesen C, Bialy L, Tubman M, Ospina M, Klassen TP, Witmans M. Manifestations and management of chronic insomnia in adults. Evid Rep Technol Assess. 2005 Summ;:1–10. doi: 10.1037/e439752005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valencia-Flores M, Orea A, Castano VA, Resendiz M, Rosales M, Rebollar V, Santiago V, Gallegos J, Campos RM, Gonzalez J, Oseguera J, Garcia-Ramos G, Bliwise DL. Prevalence of sleep apnea and electrocardiographic disturbances in morbidly obese patients. Obes Res. 2000;8:262–269. doi: 10.1038/oby.2000.31. [DOI] [PubMed] [Google Scholar]
- 10.Benz RL, Pressman MR, Hovick ET, Peterson DD. Potential novel predictors of mortality in end-stage renal disease patients with sleep disorders. Am J Kidney Dis. 2000;35:1052–1060. doi: 10.1016/s0272-6386(00)70039-4. [DOI] [PubMed] [Google Scholar]
- 11.Hanly P. Sleep apnea and daytime sleepiness in end-stage renal disease. Semin Dial. 2004;17:109–114. doi: 10.1111/j.0894-0959.2004.17206.x. [DOI] [PubMed] [Google Scholar]
- 12.Cohen SD, Patel SS, Khetpal P, Peterson RA, Kimmel PL. Pain, sleep disturbance, and quality of life in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2007;2:919–925. doi: 10.2215/CJN.00820207. [DOI] [PubMed] [Google Scholar]
- 13.De Santo RM, Bilancio G, Santoro D, Vecchi ML, Perna A, De Santo NG, Cirillo M. A longitudinal study of sleep disorders in early-stage chronic kidney disease. J Ren Nutr. 2010;20:S59–S63. doi: 10.1053/j.jrn.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Barmar B, Dang Q, Isquith D, Buysse D, Unruh M. Comparison of sleep/wake behavior in CKD stages 4 to 5 and hemodialysis populations using wrist actigraphy. Am J Kidney Dis. 2009;53:665–672. doi: 10.1053/j.ajkd.2008.10.045. [DOI] [PubMed] [Google Scholar]
- 15.Agarwal R, Light RP. Sleep and Activity in Chronic Kidney Disease: A Longitudinal Study. Clin J Am Soc Nephrol. 2011;6:1258–1265. doi: 10.2215/CJN.10581110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleischmann G, Fillafer G, Matterer H, Skrabal F, Kotanko P. Prevalence of chronic kidney disease in patients with suspected sleep apnoea. Nephrol Dial Transplant. 2010;25:181–186. doi: 10.1093/ndt/gfp403. [DOI] [PubMed] [Google Scholar]
- 17.Markou N, Kanakaki M, Myrianthefs P, Hadjiyanakos D, Vlassopoulos D, Damianos A, Siamopoulos K, Vasiliou M, Konstantopoulos S. Sleep-disordered breathing in nondialyzed patients with chronic renal failure. Lung. 2006;184:43–49. doi: 10.1007/s00408-005-2563-2. [DOI] [PubMed] [Google Scholar]
- 18.Sim JJ, Rasgon SA, Kujubu DA, Kumar VA, Liu IL, Shi JM, Pham TT, Derose SF. Sleep apnea in early and advanced chronic kidney disease: Kaiser Permanente Southern California cohort. Chest. 2009;135:710–716. doi: 10.1378/chest.08-2248. [DOI] [PubMed] [Google Scholar]
- 19.Iseki K, Tohyama K, Matsumoto T, Nakamura H. High Prevalence of chronic kidney disease among patients with sleep related breathing disorder (SRBD) Hypertens Res. 2008;31:249–255. doi: 10.1291/hypres.31.249. [DOI] [PubMed] [Google Scholar]
- 20.Mirrakhimov AE. Sleep Breath. 2011. Obstructive sleep apnea and kidney disease: is there any direct link? [DOI] [PubMed] [Google Scholar]
- 21.Hedayati SS, Finkelstein FO. Epidemiology, diagnosis, and management of depression in patients with CKD. Am J Kidney Dis. 2009;54:741–752. doi: 10.1053/j.ajkd.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wheaton AG, Perry GS, Chapman DP, Croft JB. Sleep Disordered Breathing and Depression among U.S. Adults: National Health and Nutrition Examination Survey, 2005–2008. Sleep. 2012;35:461–467. doi: 10.5665/sleep.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 25.Spiegel K, Leproult R, L'hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89:5762–5771. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 26.Roumelioti ME, Ranpuria R, Hall M, Hotchkiss JR, Chan CT, Unruh ML, Argyropoulos C. Abnormal nocturnal heart rate variability response among chronic kidney disease and dialysis patients during wakefulness and sleep. Nephrol Dial Transplant. 2010;25:3733–3741. doi: 10.1093/ndt/gfq234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neumann J, Ligtenberg G, Klein II, Koomans HA, Blankestijn PJ. Sympathetic hyperactivity in chronic kidney disease: Pathogenesis, clinical relevance, and treatment. Kidney International. 2004;65:1568–1576. doi: 10.1111/j.1523-1755.2004.00552.x. [DOI] [PubMed] [Google Scholar]
- 28.Brandenberger G, Ehrhart J, Piquard F, Simon C. Inverse coupling between ultradian oscillations in delta wave activity and heart rate variability during sleep. Clin Neurophysiol. 2001;112:992–996. doi: 10.1016/s1388-2457(01)00507-7. [DOI] [PubMed] [Google Scholar]
- 29.Charloux A, Piquard F, Ehrhart J, Mettauer B, Geny B, Simon C, Brandenberger G. Time-courses in renin and blood pressure during sleep in humans. J Sleep Res. 2002;11:73–79. doi: 10.1046/j.1365-2869.2002.00277.x. [DOI] [PubMed] [Google Scholar]
- 30.Degaute JP, van de BP, Linkowski P, Van Cauter E. Quantitative analysis of the 24-hour blood pressure and heart rate patterns in young men. Hypertension. 1991;18:199–210. doi: 10.1161/01.hyp.18.2.199. [DOI] [PubMed] [Google Scholar]
- 31.Sayk F, Teckentrup C, Becker C, Heutling D, Wellhoner P, Lehnert H, Dodt C. Effects of selective slow-wave sleep deprivation on nocturnal blood pressure dipping and daytime blood pressure regulation. Am J Physiol Regul Integr Comp Physiol. 2010;298:R191–R197. doi: 10.1152/ajpregu.00368.2009. [DOI] [PubMed] [Google Scholar]
- 32.Hostetter TH, Olson JL, Rennke HG, Venkatachalam MA, Brenner BM. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. J Am Soc Nephrol. 2001;12:1315–1325. [Google Scholar]
- 33.Lavie L. Oxidative stress--a unifying paradigm in obstructive sleep apnea and comorbidities. Prog Cardiovasc Dis. 2009;51:303–312. doi: 10.1016/j.pcad.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Yamauchi M, Kimura H. Oxidative stress in obstructive sleep apnea: putative pathways to the cardiovascular complications. Antioxid Redox Signal. 2008;10:755–768. doi: 10.1089/ars.2007.1946. [DOI] [PubMed] [Google Scholar]
- 35.Fassett RG, Venuthurupalli SK, Gobe GC, Coombes JS, Cooper MA, Hoy WE. Biomarkers in chronic kidney disease: a review. Kidney Int. 2011;80:806–821. doi: 10.1038/ki.2011.198. [DOI] [PubMed] [Google Scholar]
- 36.van Bussel BC, Schouten F, Henry RM, Schalkwijk CG, de Boer MR, Ferreira I, Smulders YM, Twisk JW, Stehouwer CD. Endothelial dysfunction and low-grade inflammation are associated with greater arterial stiffness over a 6-year period. Hypertension. 2011;58:588–595. doi: 10.1161/HYPERTENSIONAHA.111.174557. [DOI] [PubMed] [Google Scholar]
- 37.Doonan RJ, Scheffler P, Lalli M, Kimoff RJ, Petridou ET, Daskalopoulos ME, Daskalopoulou SS. Increased arterial stiffness in obstructive sleep apnea: a systematic review. Hypertens Res. 2011;34:23–32. doi: 10.1038/hr.2010.200. [DOI] [PubMed] [Google Scholar]
- 38.Tsioufis C, Thomopoulos K, Dimitriadis K, Amfilochiou A, Tousoulis D, Alchanatis M, Stefanadis C, Kallikazaros I. The incremental effect of obstructive sleep apnoea syndrome on arterial stiffness in newly diagnosed essential hypertensive subjects. J Hypertens. 2007;25:141–146. doi: 10.1097/HJH.0b013e32801092c1. [DOI] [PubMed] [Google Scholar]
- 39.Peralta CA, Jacobs DR, Jr, Katz R, Ix JH, Madero M, Duprez DA, Sarnak MJ, Criqui MH, Kramer HJ, Palmas W, Herrington D, Shlipak MG. Association of pulse pressure, arterial elasticity, and endothelial function with kidney function decline among adults with estimated GFR >60 mL/min/1.73 m(2): the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Kidney Dis. 2012;59:41–49. doi: 10.1053/j.ajkd.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krieger J, Imbs JL, Schmidt M, Kurtz D. Renal function in patients with obstructive sleep apnea. Effects of nasal continuous positive airway pressure. Arch Intern Med. 1988;148:1337–1340. [PubMed] [Google Scholar]
- 41.Rodenstein DO, D'Odemont JP, Pieters T, Aubert-Tulkens G. Diurnal and nocturnal diuresis and natriuresis in obstructive sleep apnea. Effects of nasal continuous positive airway pressure therapy. Am Rev Respir Dis. 1992;145:1367–1371. doi: 10.1164/ajrccm/145.6.1367. [DOI] [PubMed] [Google Scholar]
- 42.Kinebuchi S, Kazama JJ, Satoh M, Sakai K, Nakayama H, Yoshizawa H, Narita I, Suzuki E, Gejyo F. Short-term use of continuous positive airway pressure ameliorates glomerular hyperfiltration in patients with obstructive sleep apnoea syndrome. Clin Sci (Lond) 2004;107:317–322. doi: 10.1042/CS20040074. [DOI] [PubMed] [Google Scholar]
- 43.Casserly LF, Chow N, Ali S, Gottlieb DJ, Epstein LJ, Kaufman JS. Proteinuria in obstructive sleep apnea. Kidney Int. 2001;60:1484–1489. doi: 10.1046/j.1523-1755.2001.00952.x. [DOI] [PubMed] [Google Scholar]
- 44.Faulx MD, Storfer-Isser A, Kirchner HL, Jenny NS, Tracy RP, Redline S. Obstructive sleep apnea is associated with increased urinary albumin excretion. Sleep. 2007;30:923–929. doi: 10.1093/sleep/30.7.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsioufis C, Thomopoulos C, Dimitriadis K, Amfilochiou A, Tsiachris D, Selima M, Petras D, Kallikazaros I, Stefanadis C. Association of obstructive sleep apnea with urinary albumin excretion in essential hypertension: a cross-sectional study. Am J Kidney Dis. 2008;52:285–293. doi: 10.1053/j.ajkd.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 46.Agrawal V, Vanhecke TE, Rai B, Franklin BA, Sangal RB, McCullough PA. Albuminuria and renal function in obese adults evaluated for obstructive sleep apnea. Nephron Clin Pract. 2009;113:c140–c147. doi: 10.1159/000232594. [DOI] [PubMed] [Google Scholar]
- 47.Sklar AH, Chaudhary BA. Reversible proteinuria in obstructive sleep apnea syndrome. Arch Intern Med. 1988;148:87–89. [PubMed] [Google Scholar]
- 48.Wright JT, Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP, Rostand SG. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 49.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 50.Knutson KL, Van Cauter E, Rathouz PJ, Yan LL, Hulley SB, Liu K, Lauderdale DS. Association between sleep and blood pressure in midlife: the CARDIA sleep study. Arch Intern Med. 2009;169:1055–1061. doi: 10.1001/archinternmed.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, Rundle AG, Zammit GK, Malaspina D. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–839. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 52.Fung MM, Peters K, Redline S, Ziegler MG, Ancoli-Israel S, Barrett-Connor E, Stone KL. Decreased slow wave sleep increases risk of developing hypertension in elderly men. Hypertension. 2011;58:596–603. doi: 10.1161/HYPERTENSIONAHA.111.174409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Agarwal R, Light RP, Bills JE, Hummel LA. Nocturia, nocturnal activity, and nondipping. Hypertension. 2009;54:646–651. doi: 10.1161/HYPERTENSIONAHA.109.135822. [DOI] [PubMed] [Google Scholar]
- 54.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 55.Selim B, Won C, Yaggi HK. Cardiovascular consequences of sleep apnea. Clin Chest Med. 2010;31:203–220. doi: 10.1016/j.ccm.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 56.Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol. 2009;5:253–261. doi: 10.1038/nrendo.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105:1044–1049. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33:414–420. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Knutson KL, Ryden AM, Mander BA, Van Cauter E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med. 2006;166:1768–1774. doi: 10.1001/archinte.166.16.1768. [DOI] [PubMed] [Google Scholar]
- 60.Aronsohn RS, Whitmore H, Van Cauter E, Tasali E. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. Am J Respir Crit Care Med. 2010;181:507–513. doi: 10.1164/rccm.200909-1423OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu Q, Song Y, Hollenbeck A, Blair A, Schatzkin A, Chen H. Day napping and short night sleeping are associated with higher risk of diabetes in older adults. Diabetes Care. 2010;33:78–83. doi: 10.2337/dc09-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Knutson KL, Van Cauter E, Zee P, Liu K, Lauderdale DS. Cross-sectional associations between measures of sleep and markers of glucose metabolism among subjects with and without diabetes: the Coronary Artery Risk Development in Young Adults (CARDIA) Sleep Study. Diabetes Care. 2011;34:1171–1176. doi: 10.2337/dc10-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kramer H, Luke A, Bidani A, Cao G, Cooper R, McGee D. Obesity and prevalent and incident CKD: the Hypertension Detection and Follow-Up Program. Am J Kidney Dis. 2005;46:587–594. doi: 10.1053/j.ajkd.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 64.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144:21–28. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 65.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89:126–133. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.St Onge MP, Roberts AL, Chen J, Kelleman M, O'Keeffe M, RoyChoudhury A, Jones PJ. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr. 2011;94:410–416. doi: 10.3945/ajcn.111.013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iliescu EA, Yeates KE, Holland DC. Quality of sleep in patients with chronic kidney disease. Nephrol Dial Transplant. 2004;19:95–99. doi: 10.1093/ndt/gfg423. [DOI] [PubMed] [Google Scholar]
- 68.Kurella M, Luan J, Lash JP, Chertow GM. Self-assessed sleep quality in chronic kidney disease. Int Urol Nephrol. 2005;37:159–165. doi: 10.1007/s11255-004-4654-z. [DOI] [PubMed] [Google Scholar]
- 69.Sabbatini M, Pisani A, Crispo A, Ragosta A, Gallo R, Pota A, Serio V, Tripepi G, Cianciaruso B. Sleep quality in patients with chronic renal failure: a 3-year longitudinal study. Sleep Med. 2008;9:240–246. doi: 10.1016/j.sleep.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 70.De Santo RM, Bartiromo M, Cesare CM, Cirillo M. Sleep disorders occur very early in chronic kidney disease. J Nephrol. 2008;21 (Suppl 13):S59–S65. [PubMed] [Google Scholar]
- 71.Kumar B, Tilea A, Gillespie BW, Zhang X, Kiser M, Eisele G, Finkelstein F, Kotanko P, Levin N, Rajagopalan S, Saran R. Significance of self-reported sleep quality (SQ) in chronic kidney disease (CKD): the Renal Research Institute (RRI)-CKD study. Clin Nephrol. 2010;73:104–114. doi: 10.5414/cnp73104. [DOI] [PubMed] [Google Scholar]
- 72.Plantinga L, Lee K, Inker LA, Saran R, Yee J, Gillespie B, Rolka D, Saydah S, Powe NR. Association of sleep-related problems with CKD in the United States, 2005–2008. Am J Kidney Dis. 2011;58:554–564. doi: 10.1053/j.ajkd.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 73.Kimmel PL, Miller G, Mendelson WB. Sleep apnea syndrome in chronic renal disease. Am J Med. 1989;86:308–314. doi: 10.1016/0002-9343(89)90301-x. [DOI] [PubMed] [Google Scholar]
- 74.Parker KP, Bliwise DL, Bailey JL, Rye DB. Polysomnographic measures of nocturnal sleep in patients on chronic, intermittent daytime haemodialysis vs those with chronic kidney disease. Nephrol Dial Transplant. 2005;20:1422–1428. doi: 10.1093/ndt/gfh816. [DOI] [PubMed] [Google Scholar]
- 75.Roumelioti ME, Buysse DJ, Sanders MH, Strollo P, Newman AB, Unruh ML. Sleep-Disordered Breathing and Excessive Daytime Sleepiness in Chronic Kidney Disease and Hemodialysis. Clin J Am Soc Nephrol. 2011;6:986–994. doi: 10.2215/CJN.05720710. [DOI] [PMC free article] [PubMed] [Google Scholar]