Abstract
Progressive elevations of fibroblastic growth factor 23 [FGF23] in chronic kidney disease may reduce serum 25-hydroxyvitamin D [25(OH)] and 1,25-dihydroxyvitamin D [1,25(OH)2D] levels, via stimulation of 24-hydroxylase (Cyp24A1) mediated catabolism of these vitamin D metabolites. To test this possibility, we measured serum concentrations of 24,25-dihydroxyvitamin D [24,25(OH)2D], a product of Cyp24A1 hydroxylation of 25(OH)D, in the Col4α3 knockout mouse, a model of Alport syndrome-derived chronic kidney disease, and in patients with chronic kidney disease of variable severity. There was an inverse correlation between serum FGF23 and both 25(OH)D and 1,25(OH)2D in the mouse model but no significant relationship was observed in the cross-sectional patient cohort. The FGF23-dependent increase in Cyp24a1 mRNA expression in the mouse kidneys was consistent with the possibility that FGF23 induces vitamin D catabolism. There was, however, a reduction in serum 24,25(OH)2D levels, rather than the expected elevation, in both the mice and patients with chronic kidney disease. Low 25(OH)D and elevated FGF23 and parathyroid hormone levels were correlated with the reduced serum 24,25(OH)2D concentrations of these patients. Thus, we failed to find support for FGF23-mediated catabolism of vitamin D metabolites in chronic kidney disease assessed by 24,25(OH)2D levels.
Introduction
Chronic kidney disease (CKD) is associated with progressive elevations of serum PTH and FGF23 and reductions in 25(OH)D and 1,25(OH)2D concentrations. The pathogenesis of CKD has been viewed as arising from abnormalities of the PTH-Vitamin D axis, where reductions in 1,25(OH)2D leads to secondary increases in PTH 1. The discovery of the FGF23-vitamin D endocrine network, however, has challenged this conceptual framework, by implicating FGF23 as the initial hormonal adaptive response leading to phosphaturia, reduced circulating 1,25(OH)2D levels and secondary hyperparathyroidism 2–7.
FGF23 is a ~32 kDa secreted protein, predominately expressed in osteoblasts and osteocytes in bone 8–11, that principally targets receptor complexes consisting of FGFR 1, 3 or 4 and the transmembrane β glucuoronidase, α-Kl 12–15 located in the kidney. PTH and FGF23 have concordant effects to promote phosphaturia by suppressing phosphate co-transporters Npt2a and Npt2c expression, but have opposite effects on vitamin D metabolism, where PTH stimulates Cyp27b1-mediated conversion of 25(OH)D to 1,25(OH)2D, and FGF23 inhibits Cyp27b1 activity and stimulates Cyp24a1, the enzyme responsible for conversion of 25(OH)D and 1.25(OH)2D to inactive 24,25(OH)2D and 1,24,25(OH)3D metabolites, respectively 16–22. 1,25(OH)2D also stimulates FGF23 production in bone to create a bone-kidney endocrine feedback loop 23.
The primary role of FGF23 in the pathogenesis of abnormal vitamin D metabolism and pathogenesis of secondary hyperparathyroidism in CKD, however, remains unproven, in part because the contribution of FGF23-mediated suppression of Cyp27b1 and stimulation Cyp24a1 activity to the observed reductions in serum 25(OH)D and 1.25(OH)2D levels in CKD has not been defined. In addition, while FGF23-mediated suppression of Cyp27b1-mediated synthesis of 1,25(OH)2D is supported by concordant changes in FGF23, Cyp27b1 expression and reductions in serum 1,25(OH)2D levels in hereditary models of excess FGF23, the competing effects of PTH and FGF23 on Cyp27b1 expression and activity in CKD creates a more complex regulatory environment. Measurement of Cyp27b1 and Cyp24A1 enzymatic activity in a diseased kidney is technically challenging, but assays are available to measure both 1,25(OH)2D and 24,25(OH)2D, the end products of Cyp27b1 and Cyp24a1-mediated hydroxylation, respectively.
In the current study, to test the contributions of Cyp27b1 mediated suppression and Cyp24a1-mediated catabolism of vitamin D metabolites in CKD, we assessed serum 25(OH)D, 1,25(OH)2D and 24,25(OH)2D levels in both a mouse model of CKD 7 and in a cohort of humans with impaired renal function of varying severity. We predicted that circulating 24,25(OH)2D concentrations should be increased and 25(OH) and 1,25(OH2D decreased proportionate to the degree of renal insufficiency, if FGF23 stimulation of Cyp24a1 and inhibition of Cyp27b1 are contributing to the abnormalities of vitamin D metabolism in CKD.
Results
Serum Abnormalities in Col4a3−/− mice (Table 1)
Table 1.
Serum biochemistries of in mice.
| WT | Col4a3−/− | |
|---|---|---|
| BUN (mg/dL) | 19.9 ± 0.7 | 59.3 ± 9.2* |
| Serum creatinine (mg/dL) | 0.45 ± 0.03 | 0.83±0.16* |
| Serum PTH (pg/mL) | 34 ± 5 | 1827± 479* |
| Serum FGF23 (pg/mL) | 135± 10 | 1216± 199* |
| Serum PO4 (mg/dL) | 6.5 ± 0.5 | 9.5 ± 0.5* |
| Serum Ca (mg/dL) | 8.8 ± 0.4 | 9.3 ± 0.3 |
| Fractional excretion of PO4(%) | 4.7 ±1.6 | 14.8 ±2.7* |
| Fractional excretion of Ca (%) | 1.4 ± 0.3 | 13.7 ± 4.0 |
| Alkaline phosphatase (IU/L) | 67.0 ± 8.6 | 90.2 ± 10.9 |
Values are expressed as mean ± SEM from at least 13 mice per group. Comparisons were performed using one-way ANOVA and post-hoc Fisher test. P<0.05 vs: (*) WT. BUN, blood urea nitrogen; PTH, parathyroid hormone; FGF-23, fibroblast growth factor-23; PO4, phosphorous; Ca, calcium.
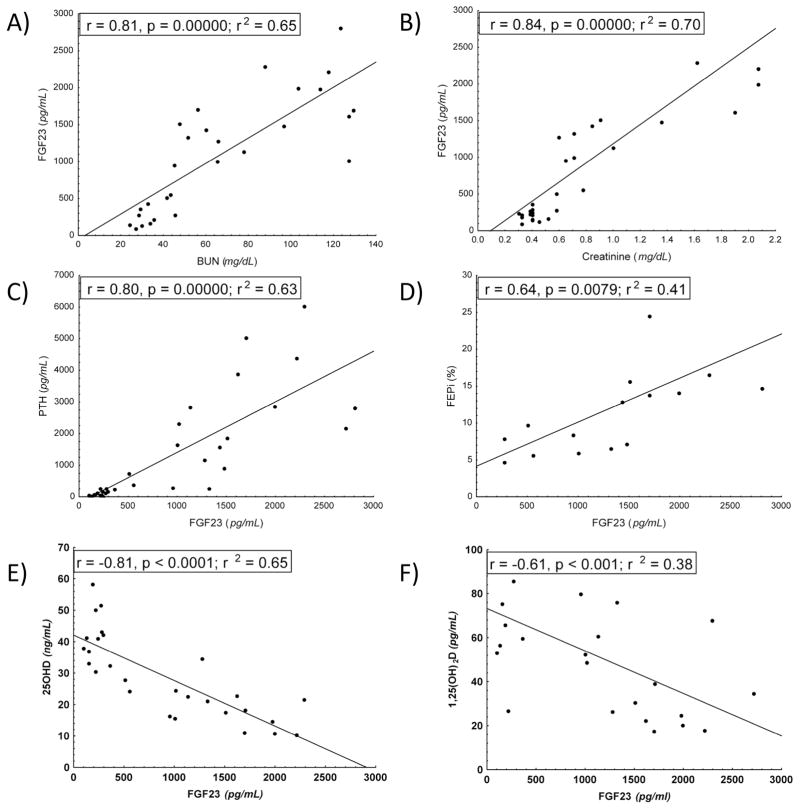
Consistent with our prior characterization of the Col4a3−/− model of CKD 7, we found that by 12-weeks of age, Col4a3−/− mice had significant elevations of serum BUN and creatinine concentrations. Col4a3−/− mice also had elevated serum phosphate levels and showed marked elevations of both circulating PTH and FGF23 concentrations compared to wild-type controls. The fractional excretion of phosphate was also increased ~3-fold in Col4a3−/− mice. We found that serum BUN and creatinine positively correlated with serum FGF23 levels (Fig 1A and B). In addition, serum FGF23 positively correlated with serum PTH concentrations (Fig 1C). The fractional excretion of phosphate also correlated with serum FGF23 levels (Fig 1D). Serum calcium and fractional excretion of calcium were also increased in Col4a3−/−, suggesting an advanced stage of hyperparathyroidism. Serum alkaline phosphatase was slightly increased in Col4a3−/− mice, likely reflecting the high turnover bone disease that has been previously characterized in greater detail in this CKD mouse model 7.
Figure 1. Pearson correlations between serum biochemical parameters in 12-week-old wild-type and Col4a3−/− mice.
Serum FGF23 was positively correlated with increments in serum BUN (A) and creatinine (B). Serum PTH and FGF23 were positively correlated (C). FGF23 was positively correlated with the fractional excretion of phosphate (D). Serum FGF23 levels were inversely correlated with serum 25(OH)D (E) and 1,25(OH)2D (E).
Abnormalities in vitamin D metabolism in Col4a3−/−
Next, we assessed serum vitamin D metabolite concentrations in Col4a3−/− and wild-type mice (Table 2). We found that serum 25(OH)D levels were significantly reduced in Col4a3−/− mice as were the serum 1,25(OH)2D levels, concordant with our prior findings in these mice 7. We have also found, that elevations in serum FGF23 were inversely correlated with both 25(OH)D and 1,25(OH)2D serum concentrations in Col4a3−/− mice (Fig 1E and F). In a multiple regression analysis, which included serum FGF23, PTH, 1,25(OH)2D, BUN, phosphate and calcium, only FGF23 predicted the changes in 25(OH)D (p=0.02, β= −1.54, std error, 0.46).
Table 2.
Serum vitamin D metabolite concentrations in mice.
| WT | Col4a3−/− | |
|---|---|---|
| 25(OH)D (ng/ml) | 44.1+3.2 | 24.8 ± 2.6* |
| 1,25(OH)2D (pg/ml) | 101± 19 | 70 ± 19* |
| 24,25(OH)2D (ng/mL) | 15.7 ± 1.0 | 4.1 ± 1.1* |
Values are expressed as mean ± SEM from at least 13 mice per group. Comparisons were performed using one-way ANOVA and post-hoc Fisher test. P<0.05 vs: (*) WT. 25(OH)D, 25-hydroxyvitamin D; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 24,25(OH)2D, 24,25-dihydroxyvitamin D.
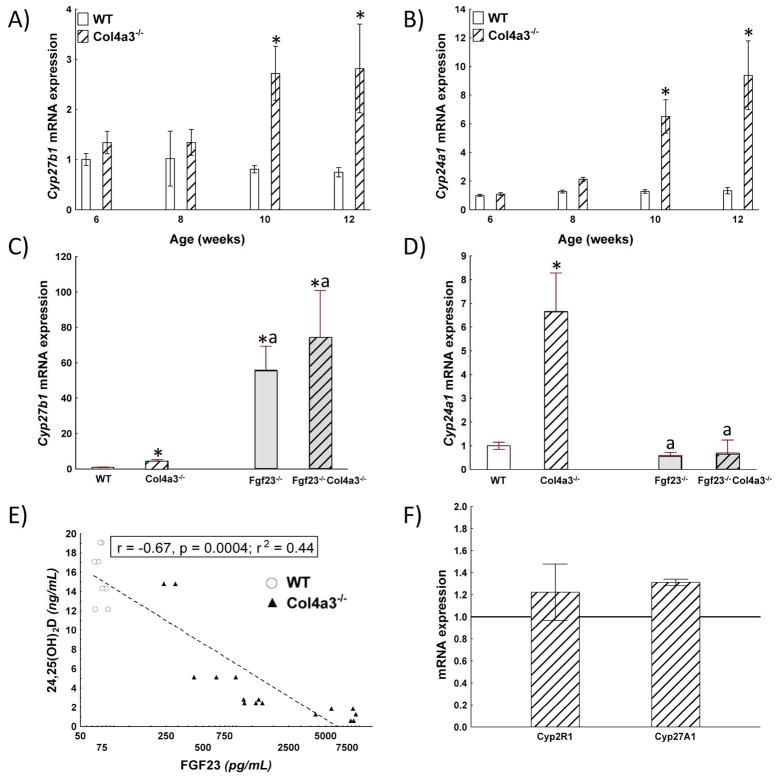
To explore mechanisms for the observed alterations in serum 25(OH)D, we measured the expression of Cyp27b1 in the kidney, the enzyme responsible for conversion of 25(OH)D to 1,25(OH)2D. The message levels of Cyp27b1 were not different between wild-type and Col4a3−/− at 6 and 8 weeks of age, but were increased ~3-fold in Col4a3−/− mice compared to controls (Fig 2A) by 10 wk-old animals and further increased with renal damage progression. Thus, Cyp27b1 mRNA may not reflect enzyme activity as previously reported 24. Thus, we found no evidence for a decrease in the expression of transcripts encoding the enzymes responsible for producing 25(OH) or 1,25(OH)2D.
Figure 2. Regulation of vitamin D metabolism in 12-week-old wild-type and Col4a3−/− mice.
A,B) Time-course expression of A) Cyp27b1 and B) Cyp24A in the kidney of WT and Col4a3−/− mice. Both Cyp27b1 and Cyp24a1 message expression were age-dependently increased in Col4a3−/− mice compared to wild-type controls. C, D) Expression of C) Cyp27b1 and D) Cyp24A in the kidney of 12 week-old WT, Col4a3−/− Fgf23−/− and compound Fgf23−/− ol4a3−/− mice. N≥4, p<0.05, * vs. WT, a vs. Col4a3−/−. mRNA expression normalized by GAPDH and relative to values in wild-type controls. E) Relationship between serum FGF23 and 24,25(OH)2D levels. There was a significant inverse correlation between serum FGF23 and serum 24,25(OH)2D levels. (circles, wild-type, triangles, Col4a3−/− mice) F) Expression of Cyp2R1 and cyp27A1 in the liver. mRNA expression normalized by GAPDH and relative to values in wild-type controls. There was no significant difference in expression of the enzymes that generate 25(OH)D in Col4a3−/− mice compared to wild-type mice.
To further investigate the possibility of increased catabolism as an explanation for reduction in circulating vitamin D metabolites, we assessed the expression of Cyp24a1 message levels, the enzyme that converts 25(OH)D and 1,25(OH)2D into 24-hydroxylated derivatives that are converted to calcitroic acid. Similar to Cyp27b1, Cyp24a1 expression was not different between wild-type and Col4a3vmice at 6 and 8 weeks of age, but was increased in the kidneys of Col4a3−/− mice compared to wild-type controls at 10 and 12 weeks-of-age (Fig 2B). The relative increase in Cyp24a1 (~10 fold) was greater than Cyp27b1 (~3 fold) resulting in a higher ratio of Cyp24a1/Cyp27b1 in Col4a3−/− mice by 10 weeks-of-age. To determine the contribution of FGF23 to the alterations in Cyp27b1 and Cyp24a1 message, we examined expression of Cyp27b1 and Cyp24a1 message in the kidney after genetic ablation of FGF23 in Col4a3−/− mice. We found that compared to Col4a3−/− mice, that loss of FGF23 in compound Fgf23−/− Col4a3−/− mice dramatically increased Cyp27b1 expression, indicating that FGF23, was responsible for maintaining Cyp27b1 at a relative low-level (Fig 2C). More importantly, in the same setting, ablation of FGF23 restored Cyp24a1 mRNA levels (Fig 2D), proving evidence that FGF23 is likely responsible for up-regulation of this enzyme.
To seek additional evidence for enhanced vitamin D catabolism, we measured circulating 24,25(OH)2D levels in Col4a3−/− and wild-type mice as an index of Cyp24a1 activity. Surprisingly, we found that 24,25(OH)2D levels were significantly lower in Col4a3−/− mice than wild-type controls (Table 2). In addition, we found a strong negative correlation between serum FGF23 and serum 24,25(OH)2D levels in pooled data from Col4a3−/− and wild-type mice (Fig 2E). These results are opposite to those anticipated if serum 24,25(OH)2D concentrations are an indicator of FGF23-mediated Cyp24a1 hydroxylation of 25(OH)D.
Finally, we measured in Col3a4−/− and wild-type mice the message expression in the liver of two enzymes responsible for 25(OH)D production, Cyp2r1 and Cyp27a1. We found that both transcripts were slightly, but not significantly, increased in Col3a4−/− mice compared to wild-type controls (Fig 2F).
Relationships between FGF23, PTH, and vitamin D metabolism in humans
We stratified age- and disease matched male patients with low 25(OH)D levels into two groups based on an eGFR threshold of < 60 ml/min/1.73 m2 (Table 3). The eGFR, urine albumin creatinine ratio, serum FGF23, serum PTH and fractional excretion of phosphate values were significantly greater in the group with eGFR<60 ml/min/1.73 m2 compared to the patients with eGFR>60 ml/min/1.73 m2. As expected based on the selection criteria, we observed no difference in serum 25(OH)D levels in the two groups, both of which had average values below the lower limit of normal. Serum 1,25(OH)2D concentrations, however, were in the normal range and did not differ between the two groups.
Table 3.
Serum biochemical parameters in patients grouped by eGFR.
| eGFR ≥ 60 (ml/min/1.73 m2) (n = 14) |
eGFR < 60 (ml/min/1.73 m2) (n = 14) |
P value | |
|---|---|---|---|
| African American (%) | 55 | 44 | 0.6946 |
| Diabetes mellitus (%) | 42 | 42 | 1 |
| Age (years) | 62.57 ± 6.46 | 65.71 ± 8.49 | 0.1587 |
| eGFR (ml/min/1.73 m2) | 85.97 ± 14.66 | 35.65 ± 6.4 | <0.0001 |
| BMI (kg/m2) | 30.64 ± 5.54 | 28.88 ± 5.87 | 0.4229 |
| Urine ACR (mg/g) | 0.13 ± 0.16 | 1.79 ± 2.37 | 0.0093 |
| Serum FGF23 (pg/ml) | 80.92 ± 35.42 | 152 ± 34.81 | <0.0001 |
| Serum 25(OH)D (ng/ml) | 11.6 ± 4 | 14.6 ± 5 | 0.0677 |
| Serum 1,25(OH)2D (pg/ml) | 44 ± 19 | 43 ± 19 | 0.8329 |
| Serum 24,25(OH)2D (ng/ml) | 1.24 ± 1 | 0.65 ± 0.5 | 0.0683 |
| Serum PTH (pg/ml) | 58 ± 28 | 167 ± 117 | 0.0007 |
| Serum alkaline phosphatase (IU/L) | 84.71 ± 25.71 | 95.57 ± 23.94 | 0.7304 |
| Fractional excretion of calcium | 0.69 ± 0.65 | 1.15 ± 1.85 | 0.2140 |
| Fractional excretion of PO4 | 12.26 ± 4.03 | 29.92 ± 8.63 | <0.0001 |
Categorical variables are presented as percentage and continuous variables as mean ± SD. Fisher’s exact test was used for the variable African American, Chi-square test for the variable DM. Unpaired t-test was used for the variables FGF-23, serum 25(OH)D, and BMI, Mann–Whitney U test was used for the rest of the continuous variables. eGFR, estimated glomerular filtration rate; BMI, body mass index; ACR, albumin creatinine ratio; FGF-23, fibroblast growth factor-23; 25(OH)D, 25-hydroxyvitamin D; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 24,25(OH)2D, 24,25-dihydroxyvitamin D; PTH, parathyroid hormone, PO4; phosphorous.
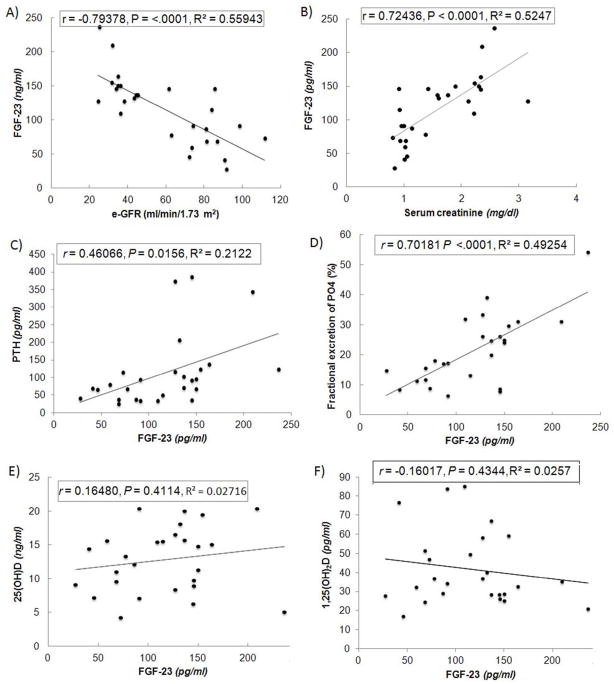
We found a strong negative correlation between eGFR and FGF23 (Fig 3A) and a positive correlation between serum creatinine and FGF23 (Fig 3B), as well as a strong positive correlation between serum FGF23 and PTH levels (Fig 3C). We also observed a positive correlation between FGF23 and fractional excretion of phosphate (Fig 3D). Unlike the mouse model of CKD, however, we did not find a significant correlation between FGF23 and either 25(OH)D (Fig 3E) or 1,25(OH)2D levels (Fig 3F) in the cohort of humans subjects, possibly due to the low circulating 25(OH)D levels used to select these patients. When we examined patients with FGF-23 levels >100 ng/ml (i.e., level typical of more advanced CKD patients), FGF23 did negatively correlate with baseline 1,25(OH)2D levels (r= 0.56, P = 0.02, n = 14).
Figure 3. Regression analysis in human subjects with varying degrees of renal impairment.
FGF23 is inversely correlated with eGFR (A). and positively correlated with serum creatinine (B). Serum PTH and FGF23 are positively correlated (C). FGF23 is also positively correlated with the fractional excretion of phosphate (D). Neither 25(OH)D (E) or 1,25(OH)2D (F) are significantly correlated with FGF23.
Similar to the findings in the mouse CKD model, however, serum 24,25(OH)2D levels were significantly lower in patients with eGFR<60 ml/min/1.73 m2 compared to patients with eGFR≥60 ml/min/1.73 m2. Indeed the serum 24,25 (OH)2D levels were 2-fold lower in the CKD group compared to the group with “normal” renal function (i.e., 0.65 vs 1.24 ng/ml).
Overall, the serum 24,25(OH)2D levels, 1,25(OH)2D, and FGF23 were 15-, 2-, and 1.6- fold higher, and PTH ~50% lower, respectively, in wild-type mice compared to humans with eGFR ≥ 60 ml/min/1.73 m2. In the setting of impaired renal function, these same parameters were 8-, 1.6-, 8- and 11-fold higher, respectively, in the Col4a3−/− mice with CKD compared to subjects with eGFR<60 ml/min/1.73 m2. Compared to controls, the reduction in the 24,25 (OH)2D levels was 4-fold greater and the elevation in FGF23 and PTH were ~4-fold higher observed the mouse model of CKD than the respective changes in humans with CKD, suggesting that the mouse model is more sensitive alterations in mineral metabolism markers. This finding is similar to the accentuated alterations in FGF23 and vitamin D metabolism observed in Hyp mice compared to human patients with X-linked Hypophosphatemic Rickets 25.
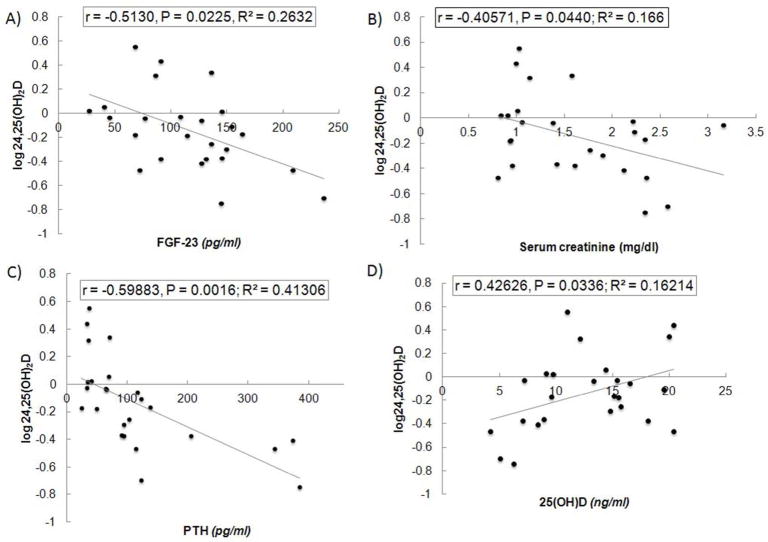
To determine predictors of serum 24,25(OH)2D levels in the human subjects, we performed univariate (Table 4, Fig 4) and multivariable regression analysis (Table 5). Serum PTH, 25(OH)D, FGF-23, creatinine and urinary fractional excretion of phosphorous were significant predictors of serum 24,25(OH)2D levels in simple regression analysis (Table 4). An inverse relationship was present between FGF23, PTH and creatinine and 24,25(OH)D2 levels (Fig 4). In contrast, a positive association was observed between serum 25(OH)D and 24,25(OH)2D levels, suggesting that low substrate leads to low 24,25(OH)2D levels. These predictor variables along with the interaction terms between them were utilized in multiple regression model. Collinearity statistics for all of the predictor variables entered in the multiple linear regression analysis indicated that in all cases, tolerance values were >0.7 and the VIF were <2. This excludes any serious problem with multicollinearity.
Table 4.
Univariate analysis of serum 24,25(OH)2D.
| Predictor variables | β | SE β | P value |
|---|---|---|---|
| Age (years) | −0.0054 | 0.00857 | 0.5285 |
| BMI (kg/m2) | −0.0016 | 0.01145 | 0.8899 |
| Serum PTH (pg/ml) | −0.0018 | 0.00052 | 0.0016 |
| Diabetes mellitus | 0.09148 | 0.13541 | 0.5061 |
| Serum FGF-23 (pg/ml) | −0.0029 | 0.00121 | 0.0225 |
| Serum 25(OH)D (ng/ml) | 0.02796 | 0.01237 | 0.0336 |
| Serum 1,25(OH)2D (pg/ml) | 0.00416 | 0.0347 | 0.2437 |
| Serum Ca (mg/dl) | 0.37953 | 0.22884 | 0.1108 |
| Serum PO4 (mg/dl) | 0.06079 | 0.09631 | 0.5344 |
| Serum creatinine (mg/dl) | −0.1981 | 0.09307 | 0.0442 |
| eGFR (ml/min/1.73 m2) | −0.0390 | 0.00228 | 0.1009 |
| Urine ACR(mg/g) | −0.0199 | 0.03495 | 0.5734 |
| Fractional excretion of PO4 (%) | −0.01204 | 0.00549 | 0.0392 |
| Fractional excretion of Ca (%) | −0.02414 | 0.04732 | 0.6149 |
24,25(OH)2D, 24,25-dihydroxyvitamin D; BMI, body mass index; PTH, parathyroid hormone; FGF-23, fibroblast growth factor-23; 25(OH)D, 25-hydroxyvitamin D; 1,25(OH)2D, 1,25-dihydroxyvitamin D; eGFR, estimated glomerular filtration rate; ACR, albumin creatinine ratio; PO4; phosphorous; Ca, calcium.
Fig 4. Determinants of serum 24,25(OH)2D levels in human subjects.
Serum 24,25(OH)2D was positively correlated with 25(OH)D (A) and inversely related to serum PTH (B), FGF23 (C), serum creatinine (D) and fractional excretion of phosphate (D).
Table 5.
Multivariable analysis of serum 24,25(OH)2D
| Predictor variables | β | SE β | P value |
|---|---|---|---|
| Serum PTH (pg/ml) | −0.0012 | 0.00049 | 0.0185 |
| Serum FGF-23 (pg/ml) | −0.0023 | 0.00121 | 0.0349 |
| Serum 25(OH)D (ng/ml) | 0.03157 | 0.01237 | 0.0027 |
24,25(OH)2D, 24,25-dihydroxyvitamin D; PTH, parathyroid hormone; FGF-23, fibroblast growth factor-23; 25(OH)D, 25-hydroxyvitamin D.
The final model predicting serum 24,25(OH)2D level consisted of FGF-23, PTH, and 25(OH)D without any interaction terms (Table 5). These variables together explained 55% of the variations in serum 24,25(OH)2D level (R2adj = 0.5548), and the overall relationship was significant F(3, 24) = 10.97, P = 0.0002. With other variables held constant, serum 24,25(OH)2D level is negatively related to FGF-23 (t21 = −2.26, P = 0.0349) and PTH (t21 = −2.55, P = 0.0185), and positively related to 25(OH)D (t21 = 3.40, P = 0.0027). The final model hold the following equation: estimated 24,25(OH)2D = −0.14307 + 0.03157 × 25(OH)D − 0.00126 × PTH − 0.00236 × FGF-23. The model suggested that a rise in serum PTH and FGF23 by 10 pg/mL would decrease 24,25(OH)2D levels by 1% and 2% respectively. On the other hand a 10 ng/mL rise in serum 25(OH)D level would increase 24,25(OH)2D level by 32%.
Discussion
In the current studies, we measured serum concentrations of 24,25(OH)2D levels and assessed the relationships between serum 24,25(OH)2D and FGF23, PTH, 25(OH)D, and 1,25(OH)2D concentrations in both mice and humans with CKD to determine if FGF23 stimulates the catabolism of 25(OH)D and 1,25(OH)2D in CKD2, 26, 27. Our initial findings supported a potential role of FGF23 in the reductions of 1,25(OH)2D as well as 25(OH)D in CKD. First, the ratio of Cyp24a1 to Cyp27b1 message is increased in the kidney of Colf4a3−/− mice. Second, serum FGF23 levels were inversely correlated with both serum 25(OH)D and 1,25(OH)2D in Col4a3−/− mice, consistent with FGF23-meidated degradation of these metabolites. Interestingly, we did observe an inverse correlation between serum FGF23 and 1,25(OH)D, but not 25(OH)D levels, in the human subjects examined in this study. However, others have observed a correlation between decreased eGFR and reduced 25(OH)D and increased FGF23 concentrations 28, 29. We were unable to find direct evidence, however, for increased Cyp24a1 activity in Col4a3−/− mice or the human subjects with CKD. Indeed, serum 24,25(OH)2D levels, the initial product of Cyp24a1 mediated hydroxylation of 25(OH)D, were suppressed in Col4a3−/− mice and human subjects with CKD, rather than elevated.
The most conservative interpretation of these findings is that reductions in serum 25(OH)D and 1,25(OH)2D in CKD are not caused by FGF23-mediated stimulation of Cyp24a1 catabolism. In this scenario, the increase in expression of Cyp24a1 message level in the kidney does not represent an increase in enzymatic activity. One possible explanation may be that elevated PTH decreases Cyp24a1 mRNA stability 30. Consistent with this possibility, the message expression of Cyp27b1 also does not correlate with enzymatic activity in other states of combined PTH and FGF23 excess24. If so, low circulating levels 1,25(OH)2D and 24,25(OH)2D in CKD might result from a decreased total enzymatic activity in the diseased kidney, which is the principal source of circulating 24,25(OH)2D 31. Alternatively, a more speculative conclusion is that Cyp24a1 activity is increased in CKD in response to increased serum FGF23, but that serum 24,25(OH)2D is not elevated due to its rapid clearance and conversion to calcitroic acid 32. If so, 24,25(OH)2D may not be a marker of increased Cyp24a1 activity in CKD. Consistent with this possibility, chronic 1,25(OH)2D administration to rats in vivo increases the in vitro conversion of 25(OH)D to 24,25(OH)2D, but at the same time decreases circulating 24,25(OH)2D levels 33. Further studies will be needed to distinguish between these two interpretations.
Limited 25(OH)D substrate might also influence the production of 24,25(OH)2D 34. In multiple regression analysis in human subjects, low 25(OH)D was a strong predictor of reductions in 24,25(OH)2D. We found no alterations in hepatic enzymes that generate 25(OH)D. Interestingly, serum 24,25(OH)2D levels were markedly higher in wild-type mice than humans with normal renal function. This may be due to genetic differences between mice and humans. Alternatively, the relatively high dietary content of vitamin D in rodent food may account for the higher 24,25(OH)2D levels in both CKD and wild-type mice compared to humans with CKD and preserved renal function. Finally, reductions in serum 25(OH)D as a function of CKD severity might also result from the failure of a diseased kidney to recycle 25(OH)D from the glomerular ultrafiltrate back into proximal tubular cells for 1,25(OH)2D synthesis, and back into the circulation to maintain serum 25(OH)D levels.
The finding of low levels of 24,25(OH)2D in CKD has also been found in patients with CKD by Ishimura et al 34 and in the Seattle Kidney Study cohort (Ian H. DeBoer, personal communication). The clinical significance of low serum 24,25(OH)2D concentrations is not clear. 24,25(OH)2D may have a role in bone mineralization and fracture repair 35. The low 24,25(OH)2D was associated with increased mortality in the Seattle Kidney Study cohort, but this could also reflect the effects of FGF23, which was a determinant of 24,25(OH)2D levels and is associated with increased mortality in CKD 36.
Our results suggest a complex interplay between elevated PTH and FGF23 on Cyp27b1 functions in CKD. In this regard, we found that the kidney expression of Cyp27b1 was 4-fold increase in the Col4a3−/− CKD model, possibly reflecting actions of elevated PTH to stimulate Cyp27b1 expression, but Cyp27b1 was increased over 50-fold after ablation of FGF23 in Col4a3−/− mice. In spite of increased PTH, a negative correlation was observed between FGF23 and 1,25(OH)2D levels in Col4a3−/− mice, consistent with the possibility that FGF23 and PTH have opposite effects on the production of 1,25(OH)2D. Other reports indicate that the stimulatory effects of PTH may be offset by the suppressive effects of FGF23 on Cyp27b1 enzymatic activity at the posttranscriptional levels 7, 24, 26. The importance of FGF23 on vitamin D metabolism is supported by the observation that treatment of a rat model of anti-GBM nephritis with a neutralizing anti-FGF23 antibody increases serum 1,25(OH)2D levels and Cyp27b1 expression and reduces Cyp24a1 expression 27.
Our findings suggest that FGF23 and PTH have concordant effects on phosphate homeostasis, since FGF23 is positively correlated with parathyroid hormone in both a homogenous mouse model of CKD and a more diverse group of human subjects with varying degrees of renal impairment. This positive association between PTH and FGF23 is consistent with other reports showing a strong association between elevated FGF23 levels and the severity of HPT in CKD and other disorders 13, 37, 38. The mechanism of FGF23 and PTH co-regulation are unclear, and both direct effects of PTH to stimulate FGF23 expression in bone, indirect effects of PTH to stimulate FGF23 through PTH-mediated increase in 1,25(OH)2D levels, and FGF23 effects to suppress PTH directly or to indirectly stimulate PTH due to FGF23-mediated suppression of 1,25(OH)2D have been proposed 39.
Our study has several limitations. With regard to the mouse studies, we lack measurements of Cyp27b1 or Cyp24a1 enzymatic activity and important genetic and dietary differences in these models may limit extrapolating findings to humans. Observations in the mouse CKD model also highlight the conflicting regulatory networks, where initial FGF23-mediated suppression of 1,25(OH)2D may lead to progressive elevations in PTH, which in turn may further stimulate both FGF23 and 1,25(OH)2D levels. Further studies are needed to assess the interdependent regulation of PTH, FGF23 and 1,25(OH)2D in CKD. With regards to the human studies, the sample size is small, the patient population is heterogeneous, and were preselected based on low 25(OH)D levels that may have lead to a failure to observe relationships between FGF23, PTH and vitamin D metabolites that are functionally important. However, the number of patients examined is insufficient for the accurate assessment of all the potential predictors of 24,25(OH)2D levels. Our data in both mice and human models of CKD fail to explain the reductions in 25(OH)D levels, which was measured by immunoassay, which may also detect other vitamin D metabolites40. Perhaps assessment of 25(OH)D by HPLC or free 25(OH)D might provide different results. We need additional assessments of pathways leading to 25(OH)D production as well as vitamin D catabolic pathways, such as 1,24,25(OH)3D levels, 24,25(OH)2D levels after treatment with vitamin D analogues and calcitroic acid (1α-hydroxy-23-carboxy-24,25,26,27-tetranorvitamin D) production41.
In summary, we found that the measurements of 24,25(OH)2D levels fail to evaluate the contribution of FGF23-driven increases in 24-hydroxylase in 25(OH)D catabolism in CKD. Indeed, circulating 24,25(OH)2D levels were not elevated in either mice or humans with CKD. The significance of this unexpected negative finding remains to be established.
Methods
Animals and genotyping
All mice were maintained on a standard diet (7912, Harlan Teklad, Madison, WI, USA). Animal care and protocols were in accordance with the guidelines established by the University of Tennessee Institutional Animal Care and Use Committee as detailed in the “Guide for Care and Use of Laboratory Animals,” prepared by the Institute on Laboratory Animal Resources, National Research Council (Department of Health & Human Services Publication NIH 86-23, National Academy Press, 1996).
Heterozygous Col4a3+/− mice were obtained from Jackson Laboratories ( Westgrove, PA, USA). Ear biopsies were collected to genotype the mice. REDExtract-N-Amp Tissue PCR Kit (Sigma-Aldrich, St. Louis, MO, USA) was used for DNA extraction and PCR amplification. Mice were genotyped for col4a3 mutation and PCR was repeated in all mice after sacrifice to exclude artifacts and ensure the correct genotype 9.
Serum biochemistry
Serum samples from mice were collected by intracardiac exsanguination. Calcium was measured using a Calcium CPC Liquicolor Kit (Stanbio Laboratories, Boerne, TX, USA) and phosphorus was measured using the phosphomolybdylate-ascorbic acid method 9. Parathyroid hormone (PTH) levels were measured using the Mouse Intact PTH ELISA kit (Immutopics, Carlsbad, CA, USA). 1,25(OH)2D and 25OHD levels were measured using the vitamin D EIA Kits (Immunodiagnostic Systems, Fountain Hills, AZ, USA).
FGF23 levels were measured in both mice and humans using the FGF23 ELISA kit (Kainos Laboratories, Tokyo, Japan). 24,25(OH)2D was measured in both mice and human samples by Heartland Assays LLC (Ames, Iowa, USA) according to previously published procedures 31, 42. 25(OH)D levels were measured in humans by immunochemical illuminometric assay, albumin by bromcresol green assay and creatinine by isotope dilution mass spectrometry traceable Jaffé method from Roche (compensated Jaffé, Roche Diagnostics, Mannheim, Germany).
RT-PCR
Total RNAs were isolated using TRI-reagent and first-strand cDNA was synthesized from the kidney or liver RNAs according to previously published method 43. The iCycler iQ Real-Time PCR Detection System and iQ SYBR Green Supermix (Bio-Rad, Hercules, CA, USA) were used for real-time quantitative PCR analysis. The expression was normalized by glyceraldehyde-3-phosphate dehydrogenase (Gapdh) in the same sample and expressed as fold change of WT.
Human Subjects
The human cohort in the trial includes 53 patients with vitamin D deficiency [serum 25(OH) D level < 20 ng/ml] identified between April 2009 to July 2010. The study was approved by the institutional review board of Veterans Affairs Medical Center, Memphis, TN. Inclusion criteria for the study were the following: Men and women over the age of 18 years and serum 25(OH)D levels < 20 ng/ml. Exclusion criteria included: eGFR < 15 ml/min/1.73 m2 or chronic dialysis patients, receipt of any form of vitamin D supplementation, presence of cirrhosis, sarcoidosis, lymphoma, malabsorption syndrome, solid organ transplant, or hypoparathyroidism, use of medications known to alter vitamin D metabolism, including rifampin, corticosteroids, anti-epileptics, phosphate binders, and calcimimetics. Patients with CKD were matched with patients with normal renal function based on several matching variables in 1:1 basis. The matching variables were age (within 5 years), race, gender, and diabetes mellitus (DM). Fourteen patients with CKD were matched with 14 patients with normal renal function.
Urinary protein excretion was measured as a ratio of spot urine protein-creatinine (UPC) ratio or as spot urine albumin-creatinine ratio (ACR) [ACR = UPC(1.054) × 0.596]. The eGFR was calculated according to the 4-variable formula used in the Modification of Diet in Renal Disease Study. Body mass index (BMI) was calculated by person’s weight in kilograms divided by their height in square meters.
Statistical Analysis
For the mouse studies differences among the two groups were tested by Student T test using the Statistica software (Statsoft, Tulsa, OK, USA). The differences were considered statistically significant at p<0.05. Pearson correlations and multiple regression analysis were performed to examine the relationship between the measured serum parameters.
For the human subjects, continuous variables were presented as means and standard deviations (SD) and categorical variables as percentages, unless otherwise specified. Normally distributed continuous variables were compared using unpaired t-test and non-normally distributed continuous variables with Mann-Whitney U test. To evaluate the factors affecting the 24,25 (OH)2D level, univariate analyses were done using the following predictor variables: age, race, diabetes mellitus (DM), BMI, serum calcium, phosphorous, creatinine, FGF-23, PTH, 25(OH)D, 1,25(OH)2D, eGFR, and urine ACR level. The variable, 24,25(OH)2D was log transformed to attain normal distribution. All tests were 2-sided and P value < 0.05 was considered significant. Statistical analysis was conducted using SAS® version 9.2 (SAS Institute Inc., Cary, NC, U.S.A.).
Acknowledgments
This work was supported by NIH Grant RO1-AR45955 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Abbreviations
- 24,25(OH)2D
24,25-dihydroxyvitamin D
- 1,25(OH)2D
1,25-dihydroxyvitamin D
- calcitriol
1α,25-Dihydroxycholecalciferol
- 25(OH)D
25-hydroxyvitamin D
- calcidiol
25-hydroxychoelcalciferol
- cholecalciferol
vitamin D
- Cyp27b1
1α-hydroxylase
- Cyp24a1
24-hydroxylase
Footnotes
Disclosure Statement. Dr. Quarles is a consultant and speaker for Amgen. No other author has any interest to disclose.
References
- 1.Wetmore JB, Quarles LD. Calcimimetics or vitamin D analogs for suppressing parathyroid hormone in end-stage renal disease: time for a paradigm shift? Nature clinical practice. 2009;5:24–33. doi: 10.1038/ncpneph0977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quarles LD. The bone and beyond: ‘Dem bones’ are made for more than walking. Nat Med. 2011;17:428–430. doi: 10.1038/nm0411-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fliser D, Kollerits B, Neyer U, et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007;18:2600–2608. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 4.Gutierrez O, Isakova T, Rhee E, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 5.van Husen M, Fischer AK, Lehnhardt A, et al. Fibroblast growth factor 23 and bone metabolism in children with chronic kidney disease. Kidney Int. 2010;78:200–206. doi: 10.1038/ki.2010.107. [DOI] [PubMed] [Google Scholar]
- 6.Isakova T, Wahl P, Vargas GS, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79:1370–1378. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stubbs JR, He N, Idiculla A, et al. Longitudinal evaluation of FGF23 changes and mineral metabolism abnormalities in a mouse model of chronic kidney disease. J Bone Miner Res. 2011 doi: 10.1002/jbmr.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu S, Guo R, Simpson LG, et al. Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J Biol Chem. 2003;278:37419–37426. doi: 10.1074/jbc.M304544200. [DOI] [PubMed] [Google Scholar]
- 9.Liu S, Zhou J, Tang W, et al. Pathogenic role of Fgf23 in Hyp mice. Am J Physiol Endocrinol Metab. 2006;291:E38–49. doi: 10.1152/ajpendo.00008.2006. [DOI] [PubMed] [Google Scholar]
- 10.Stubbs J, Liu S, Quarles LD. Role of fibroblast growth factor 23 in phosphate homeostasis and pathogenesis of disordered mineral metabolism in chronic kidney disease. Seminars in dialysis. 2007;20:302–308. doi: 10.1111/j.1525-139X.2007.00308.x. [DOI] [PubMed] [Google Scholar]
- 11.Yamashita T, Yoshioka M, Itoh N. Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem Biophys Res Commun. 2000;277:494–498. doi: 10.1006/bbrc.2000.3696. [DOI] [PubMed] [Google Scholar]
- 12.Kurosu H, Ogawa Y, Miyoshi M, et al. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urakawa I, Yamazaki Y, Shimada T, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 14.Yu X, Ibrahimi OA, Goetz R, et al. Analysis of the biochemical mechanisms for the endocrine actions of fibroblast growth factor-23. Endocrinology. 2005;146:4647–4656. doi: 10.1210/en.2005-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li H, Martin AC, David V, et al. Compound Deletion of FGFR3 and FGFR4 Partially Rescues the Hyp Mouse Phenotype. Am J Physiol Endocrinol Metab. 2010 doi: 10.1152/ajpendo.00499.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimada T, Kakitani M, Yamazaki Y, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113:561–568. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimada T, Mizutani S, Muto T, et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A. 2001;98:6500–6505. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White KE, Carn G, Lorenz-Depiereux B, et al. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int. 2001;60:2079–2086. doi: 10.1046/j.1523-1755.2001.00064.x. [DOI] [PubMed] [Google Scholar]
- 19.Shimada T, Yamazaki Y, Takahashi M, et al. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Renal Physiol. 2005;289:F1088–1095. doi: 10.1152/ajprenal.00474.2004. [DOI] [PubMed] [Google Scholar]
- 20.Tomiyama K, Maeda R, Urakawa I, et al. Relevant use of Klotho in FGF19 subfamily signaling system in vivo. Proc Natl Acad Sci U S A. 2010;107:1666–1671. doi: 10.1073/pnas.0913986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beckman MJ, Tadikonda P, Werner E, et al. Human 25-hydroxyvitamin D3-24-hydroxylase, a multicatalytic enzyme. Biochemistry. 1996;35:8465–8472. doi: 10.1021/bi960658i. [DOI] [PubMed] [Google Scholar]
- 22.Hoenderop JG, Chon H, Gkika D, et al. Regulation of gene expression by dietary Ca2+ in kidneys of 25-hydroxyvitamin D3-1 alpha-hydroxylase knockout mice. Kidney Int. 2004;65:531–539. doi: 10.1111/j.1523-1755.2004.00402.x. [DOI] [PubMed] [Google Scholar]
- 23.Liu S, Tang W, Zhou J, et al. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol. 2006;17:1305–1315. doi: 10.1681/ASN.2005111185. [DOI] [PubMed] [Google Scholar]
- 24.Yuan B, Xing Y, Horst RL, et al. Evidence for abnormal translational regulation of renal 25-hydroxyvitamin D-1alpha-hydroxylase activity in the hyp-mouse. Endocrinology. 2004;145:3804–3812. doi: 10.1210/en.2004-0192. [DOI] [PubMed] [Google Scholar]
- 25.Weber TJ, Liu S, Indridason OS, et al. Serum FGF23 levels in normal and disordered phosphorus homeostasis. J Bone Miner Res. 2003;18:1227–1234. doi: 10.1359/jbmr.2003.18.7.1227. [DOI] [PubMed] [Google Scholar]
- 26.Helvig CF, Cuerrier D, Hosfield CM, et al. Dysregulation of renal vitamin D metabolism in the uremic rat. Kidney Int. 2010;78:463–472. doi: 10.1038/ki.2010.168. [DOI] [PubMed] [Google Scholar]
- 27.Hasegawa H, Nagano N, Urakawa I, et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int. 2010;78:975–980. doi: 10.1038/ki.2010.313. [DOI] [PubMed] [Google Scholar]
- 28.de Boer IH, Katz R, Chonchol M, et al. Serum 25-hydroxyvitamin D and change in estimated glomerular filtration rate. Clin J Am Soc Nephrol. 2011;6:2141–2149. doi: 10.2215/CJN.02640311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chonchol M, Cigolini M, Targher G. Association between 25-hydroxyvitamin D deficiency and cardiovascular disease in type 2 diabetic patients with mild kidney dysfunction. Nephrol Dial Transplant. 2008;23:269–274. doi: 10.1093/ndt/gfm537. [DOI] [PubMed] [Google Scholar]
- 30.Zierold C, Mings JA, DeLuca HF. Parathyroid hormone regulates 25-hydroxyvitamin D(3)-24-hydroxylase mRNA by altering its stability. Proc Natl Acad Sci U S A. 2001;98:13572–13576. doi: 10.1073/pnas.241516798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horst RL, Littledike ET, Gray RW, et al. Impaired 24,25-dihydroxyvitamin D production in anephric human and pig. J Clin Invest. 1981;67:274–280. doi: 10.1172/JCI110023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar R, Wiesner R, Scott M, et al. Physiology of 24,25-dihydroxyvitamin D3 in normal human subjects. Am J Physiol. 1982;243:E370–374. doi: 10.1152/ajpendo.1982.243.5.E370. [DOI] [PubMed] [Google Scholar]
- 33.Halloran BP, Bikle DD, Levens MJ, et al. Chronic 1,25-dihydroxyvitamin D3 administration in the rat reduces the serum concentration of 25-hydroxyvitamin D by increasing metabolic clearance rate. The Journal of clinical investigation. 1986;78:622–628. doi: 10.1172/JCI112619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishimura E, Nishizawa Y, Inaba M, et al. Serum levels of 1,25-dihydroxyvitamin D, 24,25-dihydroxyvitamin D, and 25-hydroxyvitamin D in nondialyzed patients with chronic renal failure. Kidney Int. 1999;55:1019–1027. doi: 10.1046/j.1523-1755.1999.0550031019.x. [DOI] [PubMed] [Google Scholar]
- 35.St-Arnaud R. CYP24A1-deficient mice as a tool to uncover a biological activity for vitamin D metabolites hydroxylated at position 24. J Steroid Biochem Mol Biol. 2010;121:254–256. doi: 10.1016/j.jsbmb.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Isakova T, Xie H, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305:2432–2439. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 38.Li SA, Watanabe M, Yamada H, et al. Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell structure and function. 2004;29:91–99. doi: 10.1247/csf.29.91. [DOI] [PubMed] [Google Scholar]
- 39.Haussler MR, Haussler CA, Whitfield GK, et al. The nuclear vitamin D receptor controls the expression of genes encoding factors which feed the “Fountain of Youth” to mediate healthful aging. J Steroid Biochem Mol Biol. 2010;121:88–97. doi: 10.1016/j.jsbmb.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lips P, Chapuy MC, Dawson-Hughes B, et al. An international comparison of serum 25-hydroxyvitamin D measurements. Osteoporos Int. 1999;9:394–397. doi: 10.1007/s001980050162. [DOI] [PubMed] [Google Scholar]
- 41.Inouye K, Sakaki T. Enzymatic studies on the key enzymes of vitamin D metabolism; 1 alpha-hydroxylase (CYP27B1) and 24-hydroxylase (CYP24) Biotechnol Annu Rev. 2001;7:179–194. doi: 10.1016/s1387-2656(01)07037-5. [DOI] [PubMed] [Google Scholar]
- 42.Horst RL, Shepard RM, Jorgensen NA, et al. The determination of 24,25-dihydroxyvitamin D and 25,26-dihydroxyvitamin D in plasma from normal and nephrectomized man. J Lab Clin Med. 1979;93:277–285. [PubMed] [Google Scholar]
- 43.Liu S, Zhou J, Tang W, et al. Pathogenic role of Fgf23 in Dmp1-null mice. Am J Physiol Endocrinol Metab. 2008;295:E254–261. doi: 10.1152/ajpendo.90201.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]