Abstract
Leishmania vaccines that protect against needle challenge fail against the potency of a Leishmania-infected sand fly transmission. Here, we demonstrate that intradermal immunization of mice with 500 ng of the sand fly salivary recombinant protein LJM11 (rLJM11) from Lutzomyia longipalpis, in the absence of adjuvant, induces long-lasting immunity that results in ulcer-free protection against Leishmania major delivered by vector bites. This protection is antibody independent and abrogated by depletion of CD4+ T cells. Two weeks after challenge, early induction of IFN-γ specifically to rLJM11 correlates to diminished parasite replication in protected animals. At this time point, Leishmania-specific induction of IFN-γ in these mice is low in comparison with its high level in non-protected controls. We hypothesize that early control of parasites in a T-cell helper type 1 environment induced by immunity to LJM11 permits the slow development of Leishmania-specific immunity in the absence of open ulcers. Leishmania-specific immunity observed 5 weeks after infection in rLJM11-immunized mice shows a twofold increase over controls in the percentage of IFN-γ-producing CD4+ T cells. We propose LJM11 as an immunomodulator that drives an efficient and controlled protective immune response to a sand fly–transmitted Leishmania somewhat mimicking “leishmanization”-induced protective immunity but without its associated lesions.
Introduction
In vector-borne diseases, the contribution of vector saliva to pathogen establishment in mammalian hosts is largely ignored. Leishmania parasites are transmitted by phlebotomine sand flies and present as a wide range of clinical etiologies including visceral, mucocutaneous, diffuse, and cutaneous leishmaniasis (CL) (WHO, 2010). Every time an infected sand fly takes a blood meal, it deposits parasites and saliva into the skin. Sand fly saliva is composed of biologically active proteins (Valenzuela et al., 2004). Importantly, certain salivary proteins are immunogenic in different mammalian hosts, including humans (Valenzuela et al., 2001; Oliveira et al., 2006, 2008; Gomes et al., 2008; Collin et al., 2009; Teixeira et al., 2010).
In rodent models of infection, immunity to sand fly saliva or salivary molecules confers protection against both cutaneous and visceral leishmaniasis (Belkaid et al., 1998; Kamhawi et al., 2000; Valenzuela et al., 2001; Gomes et al., 2008; Oliveira et al., 2008; Collin et al., 2009). This protection results from the close proximity of salivary molecules to Leishmania at the site of an infective bite where immunity to a salivary molecule can adversely impact the parasites. Recently, it was shown that immunization with LJM11 DNA, encoding a 43.2-kDa salivary protein from Lutzomyia longipalpis, induced delayed-type hypersensitivity response in mice and protected against needle challenge comprising Leishmania major plus Lu. longipalpis saliva (Xu et al., 2011).
Despite accumulating evidence regarding the immunogenicity of sand fly saliva, two questions vital to the concept of saliva-based vaccines remain unanswered. First, would immunity to a sand fly salivary protein be powerful enough against the virulence of vector-transmitted parasites (Rogers et al., 2004, 2006; Peters et al., 2008, 2009)? And if so, what are the immune correlates for this protection?
In this work, we demonstrate that adjuvantless immunization with rLJM11 modulates the mammalian host immune response to virulent vector-transmitted Leishmania parasites, resulting in ulcer-free protection against CL and establishing a vital role for CD4+ T cells in LJM11-mediated protection.
Results
Immunization with the recombinant salivary protein LJM11 (rLJM11) in the absence of adjuvant drives a robust T-cell helper type 1 (Th1) immune response
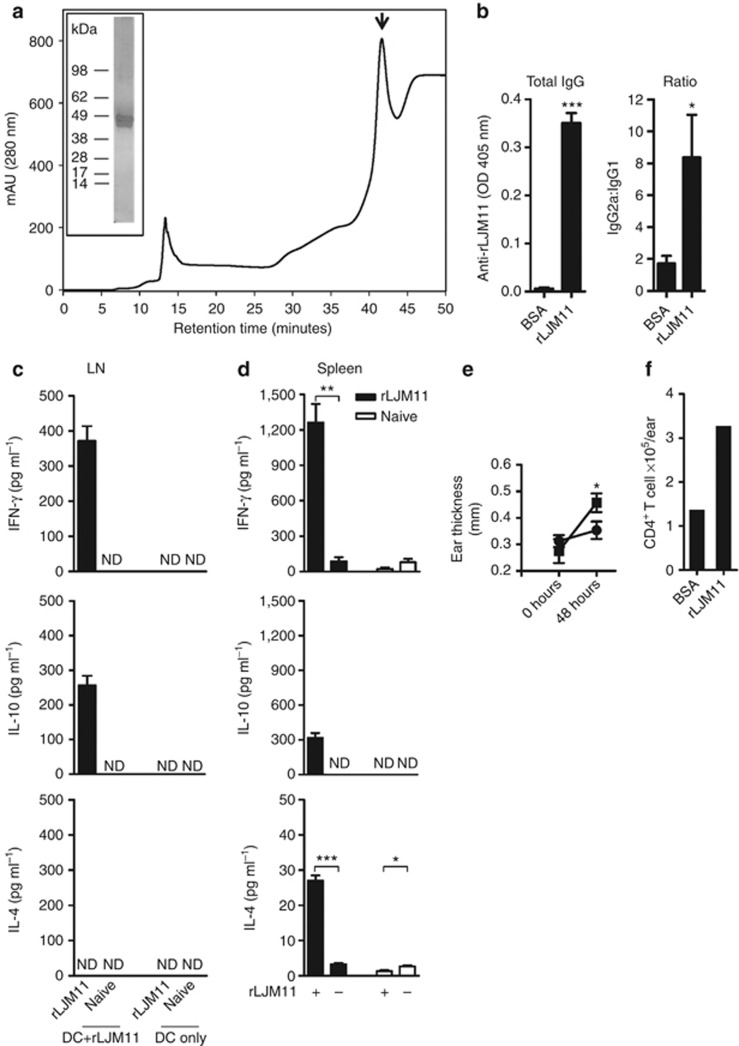
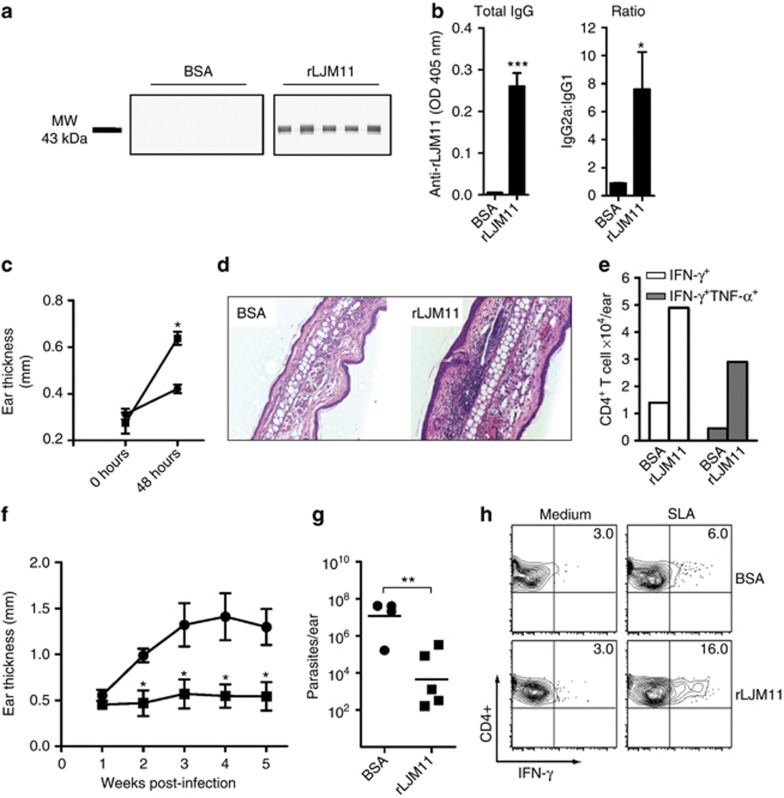
We produced rLJM11 in 293-HEK cells and purified it by high-pressure liquid chromatography (Figure 1a). The protein was soluble and had low endotoxin levels. Two weeks after the last immunization with 500 ng of rLJM11 in the absence of adjuvant, serum from rLJM11-immunized mice showed high levels of total IgG specific to rLJM11 with a high IgG2a:IgG1 ratio (Figure 1b). A robust rLJM11-specific cellular immune response characterized by IFN-γ production was observed upon in vitro stimulation of lymph nodes (LNs) and spleen cells with 20 μg of rLJM11 (Figure 1c and d). Following 3 days of stimulation with rLJM11, 371 and 1263 pg ml–1 of IFN-γ was produced by LN and spleen cells of immunized mice, respectively; IL-10 was also induced at a lower level, with 257 and 318 pg ml–1 detected from LNs and spleen cells, respectively; IL-4 was only detected from spleen cells (Figure 1d). Furthermore, exposure of rLJM11-immunized mice to uninfected sand fly bites showed a 4.5-fold increase (P<0.05) in differential ear thickness compared with the BSA-immunized group 48 hours after bite (Figure 1e). Recovery of cells from mouse ears revealed a 2.7-fold greater number of CD4+ T cells at the bite site of rLJM11-immunized mice compared with BSA-immunized animals (Figure 1f).
Figure 1.
Immunity generated by recombinant LJM11 salivary protein (rLJM11) is polarized toward a T-cell helper type 1 (Th1) response. (a) High-pressure liquid chromatography (HPLC) purification of rLJM11 (↓). Inset: silver-stained gel of purified rLJM11. (b–f) C57BL/6 mice were immunized with 500 ng of either rLJM11 or BSA in the left ear. (b) Total IgG levels against rLJM11 and the IgG2a:IgG1 ratio. IFN-γ, IL-10, and IL-4 production following in vitro stimulation of lymph node (LN, c) and (d) spleen cells with rLJM11. (e) Delayed-type hypersensitivity (DTH) response in mice immunized with rLJM11 (▪) or BSA (•). (f) The absolute number of CD4+ T cells per ear following sand fly challenge. *P<0.05; **P<0.01; ***P<0.001. Data are representative of three independent experiments (n=5). ND, not detected.
Immunity generated by rLJM11 confers ulcer-free protection against Leishmania transmitted by sand fly bites
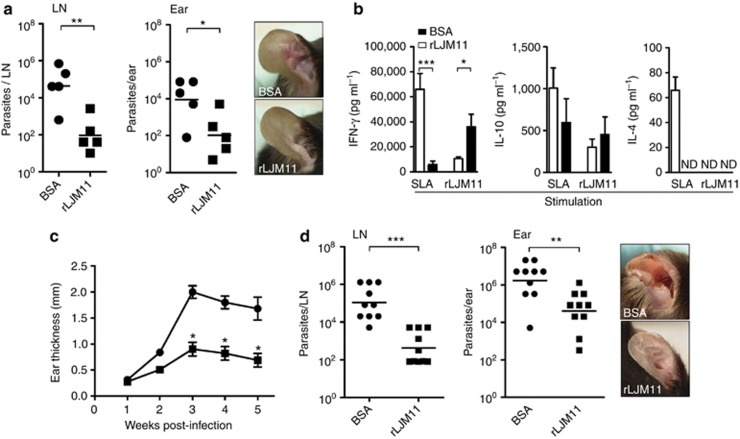
Two weeks after challenge with L. major–infected Lu. longipalpis sand flies, mice immunized with rLJM11 controlled the infection, maintaining a significantly smaller number of parasites in LNs (P<0.01) and ears (P<0.05) compared with the BSA-immunized group (Figure 2a). The lower number of parasites correlated with the absence of lesions in rLJM11-immunized mice compared with BSA-immunized animals (Figure 2a, panel). On stimulation with rLJM11, LN cells recovered from rLJM11-immunized mice 2 weeks after challenge showed a significant increase in IFN-γ production (P<0.05) compared with BSA-immunized animals (Figure 2b), indicative of a specific Th1 cellular immune response to the salivary protein. IL-4 was not detected following stimulation with rLJM11 in either rLJM11-immunized or control mice. Surprisingly, when LN cells were stimulated with soluble Leishmania antigen to test for specific parasite immune responses, we observed a greater amount of IFN-γ in BSA-immunized mice compared with rLJM11-immunized mice—the group that controlled parasite infection (Figure 2b). No significant differences were observed for IL-10 between these two groups, but IL-4 levels were greater (although in relatively small amounts) in BSA-immunized animals compared with rLJM11-immunized animals (Figure 2b). Protection in rLJM11-immunized mice was maintained up to 5 weeks after challenge (Figure 2c), when the study had to be terminated owing to extensive lesions observed in ears of the BSA-immunized group (Figure 2d, panel). Furthermore, the number of parasites was significantly lower in the LNs (P<0.001) and ears (P<0.01) of rLJM11-immunized mice compared with BSA-immunized mice 5 weeks after challenge (Figure 2d). It is important to note that the number of parasites detected in rLJM11-immunized mice, although considerable, did not manifest as open ulcers where none of the animals showed lesions throughout the follow-up period (Figure 3d, panel).
Figure 2.
Immunization with rLJM11 protects mice against Leishmania major–infected Lutzomyia longipalpis vector challenge. (a) Parasite load from lymph nodes (LNs) or ears of rLJM11- or BSA-immunized mice 2 weeks after sand fly challenge. Panel: cutaneous lesions at this time point. (b) ELISA measurements of IFN-γ, IL-10, and IL-4 from LN cells of BSA- or rLJM11-immunized mice after stimulation with soluble Leishmania antigen (SLA) or rLJM11. (c) Lesion thickness in animals immunized with rLJM11 (▪) or BSA (•) following challenge with 10 infected sand flies. (d) Parasite load 5 weeks after infected sand fly challenge in the LN and ears of BSA- or rLJM11-immunized mice. *P<0.05; **P<0.01; ***P<0.001. Cumulative data of two independent experiments are shown (n=5). ND, not detected.
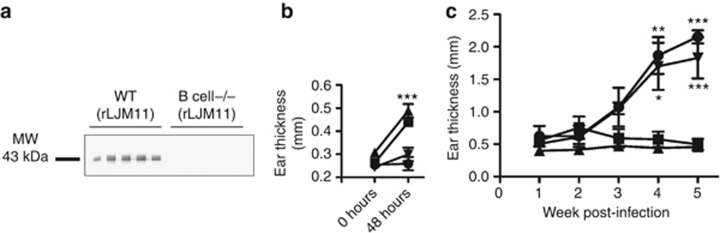
Figure 3.
LJM11 immunization protects B-cell−/− mice against infected sand fly challenge. B-cell−/− and wild-type (WT) mice were immunized with rLJM11 or BSA in the left ear. (a) Western blot against rLJM11 in rLJM11-immunized mice. (b, c) Mice challenged with infected flies in the right ear 2 weeks after immunization. (b) Delayed-type hypersensitivity (DTH) response 48 hours after challenge in rLJM11-immunized WT (▪) or B-cell−/− (▴) mice and in BSA-immunized WT (•) or B-cell−/− (▾) mice. (c) Lesion thickness in rLJM11-immunized WT (▪) or B-cell−/− (▴) mice and BSA-immunized WT (•) or B-cell−/− (▾) mice. *P<0.05; **P<0.01; ***P<0.001. Data are representative of two independent experiments (n=5).
Immunization with rLJM11 protects B-cell−/− mice against L. major–infected sand fly bites
To determine the importance of antibodies to LJM11-mediated protection, we challenged B-cell–deficient mice (B-cell−/− mice) immunized with either rLJM11 or BSA with L. major–infected Lu. longipalpis sand flies. As expected, B-cell−/− mice did not generate antibodies following immunization with rLJM11 (Figure 3a). Furthermore, rLJM11-immunized mice maintained the ability to induce a cellular immune response comparable to that of wild-type rLJM11-immunized mice demonstrated by a 3.2-fold increase (P<0.001) in differential ear thickness compared with BSA-immunized B-cell−/− mice 48 hours after challenge with infected sand flies (Figure 3b). Importantly, rLJM11-immunized B-cell−/− mice controlled L. major infection at the same magnitude as wild-type rLJM11-immunized mice (Figure 3c). It is noteworthy that ears of rLJM11-immunized B-cell−/− mice were intact, with no tissue damage compared with those of the B-cell−/− BSA- or wild-type BSA-immunized groups, which presented typical CL lesions (data not shown). These data establish that antibodies are not required for LJM11-induced protection against vector-transmitted CL.
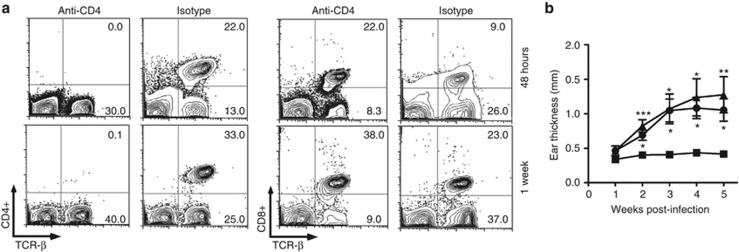
Depletion of CD4+ T cells abrogates protection from L. major conferred by immunity to rLJM11
To determine the importance of CD4+ T cells in rLJM11-induced protection, we depleted CD4+ T cells from rLJM11-immunized mice 2 days before sand fly transmission and on a weekly basis thereafter. CD4+ T cells were absent from depleted mice as demonstrated at 48 hours and 1 week after exposure to L. major–infected Lu. longipalpis bites (Figure 4a). It is noteworthy that CD8+ T cells were unaffected by depletion of CD4+ T cells (Figure 4a). Following challenge with L. major–infected Lu. longipalpis, animals immunized with rLJM11 and treated with a control isotype antibody controlled the infection throughout the follow-up period (Figure 4b). In contrast, the protection was lost in rLJM11-immunized animals treated with anti-CD4 antibody, producing a disease outcome similar to that in animals immunized with BSA (Figure 4b).
Figure 4.
Depletion of CD4+ T cells abrogates protection from sand fly–transmitted Leishmania in rLJM11-immunized mice. Mice were immunized with rLJM11 or BSA in the left ear. Forty-eight hours before infected challenge and weekly thereafter, rLJM11-immunized mice were treated with anti-CD4 (GK 1.5) or isotype (IgG2b). (a) CD4+ and CD8+ T cells in peripheral blood 48 hours and 1 week following challenge with infected sand flies. The frequency of cells is indicated on the corners of contour plots. (b) Lesion size of animals immunized with rLJM11 and treated with anti-CD4 (▴) or isotype (▪) and in BSA-immunized mice (•). *P<0.05; **P<0.01; ***P<0.001. Representative (a) and cumulative (b) data are shown (n=5).
Adjuvantless immunization with rLJM11 generates long-term immunity that protects mice against challenge by L. major–infected Lu. longipalpis sand flies
To investigate the capacity of rLJM11 to generate long-term immunity, we evaluated the status of the immune response 5 months after the last immunization. LJM11-specific IgG antibody response was sustained in rLJM11-immunized mice (Figure 5a and b) and maintained a high IgG2a:IgG1 ratio (Figure 5b). More important was the fact that the cellular immune response of rLJM11-immunized mice was not compromised 5 months after the last immunization (Figure 5c). Examination of histologic sections of mouse ears 48 hours following challenge with infected flies showed an increase in ear thickness of rLJM11-immunized mice compared with BSA controls. A distinct cellular recruitment with intense lymphocytic infiltration characteristic of a delayed-type hypersensitivity response was observed at the bite site in rLJM11-immunized mice (Figure 5d). Cells recovered ex vivo from the site of bite in rLJM11-immunized mice showed a considerable increase in the frequency of CD4+ T cells producing either IFN-γ alone (single producers) or both IFN-γ and tumor necrosis factor-α (double producers) compared with controls (Figure 5e). The salient question remains whether long-term memory to rLJM11 is robust enough to confer protection against vector-transmitted CL. Figure 5f shows that rLJM11-immunized mice challenged with L. major–infected Lu. longipalpis sand flies 5 months after the last immunization display a solid protection from disease throughout the study period. Furthermore, the number of parasites 5 weeks after infection was significantly lower in ears of rLJM11-immunized mice, showing over a 3-log reduction (P<0.01) compared with the BSA-immunized group (Figure 5g). In addition, when cells were recovered from infected ears 5 weeks after infection and stimulated with soluble Leishmania antigen, 16% of CD4+ T cells produced IFN-γ in rLJM11-immunized mice compared with only 6% in control mice (Figure 5h). These data suggest that following exposure to parasites, rLJM11-immunized animals develop Leishmania-specific immunity in the absence of ulcers.
Figure 5.
rLJM11 immunization induces protective long-lasting immunity against vector-transmitted Leishmania. Mice were immunized with rLJM11 (▪) or BSA (•) in the left ear and investigated 5 months later. (a) Western blot of rLJM11. (b) Total rLJM11-specific IgG and IgG2a:IgG1 ratio. (c) Delayed-type hypersensitivity (DTH) response 48 hours after uninfected bites on the right ear. (d–h) Mice were challenged with infected bites. (d) Hematoxylin and eosin (H&E)-stained ear sections (400 × ). (e) Absolute number of CD4+ T cells per ear producing IFN-γ and tumor necrosis factor (TNF)-α 48 hours after challenge. (f) Lesion thickness. (g) Parasite load 5 weeks after challenge. (h) Frequency of CD4+IFN-γ+ cells 5 weeks after stimulation with soluble Leishmania antigen (SLA). ***P<0.001; **P<0.01; *P<0.05. Data are representative of two independent experiments (n=5).
Discussion
A vaccine that protects mice against needle challenge of L. major fails when challenged by sand fly–transmitted parasites (Rogers et al., 2004, 2006; Peters et al., 2009). This highlights the virulence of vector-initiated Leishmania infections and the need to identify new approaches or antigens to combat this neglected disease (Rogers et al., 2004, 2006; Bethony et al., 2011). Here, we demonstrate that immunization with a defined salivary protein from Lu. longipalpis, LJM11, in the absence of adjuvant modulates the host immune response to Leishmania parasites, resulting in protection against challenge with infected sand flies.
Throughout the study, we used a recent field isolate of L. major transmitted by vector bites, a highly virulent challenge. This highlights both the potency of the protection observed in rLJM11-immunized mice and the unusual severity of lesions in control groups. The potency of rLJM11 immunity is underscored by ulcer-free protection from CL observed in mice challenged up to 5 months following the last immunization. As a result of the lesion severity in control groups, mice were killed 5 weeks after infection. At this time point, live parasites were still observed in rLJM11-immunized mice despite the absence of ulcers. Further studies with longer follow-up periods are needed to assess the duration of LJM11-mediated protection from CL. Reassuringly, mice vaccinated with complementary DNA encoding LJM11 and challenged by needle injection of parasite were protected up to 12 weeks after infection (Xu et al., 2011). Although immunization with rLJM11 may not mimic the outcome of vaccination with LJM11 DNA, we previously demonstrated that immunization with the sand fly salivary gland homogenate, in which the dominant Th1-inducing protein is LJM11, in the absence of adjuvant, produced a sustained protection against CL 12 weeks after challenge (Xu et al., 2011). This argues in favor of long-lasting immunity by rLJM11-induced immunity in the absence of adjuvant.
The cornerstone of LJM11-mediated immunity is cellular, where CD4+T cells producing IFN-γ or both IFN-γ and tumor necrosis factor-α were prominent in the ears and draining LN of rLJM11-immunized mice challenged with infected sand flies. Induction of multifunctional T cells that have been implicated in enhanced immunity to CL (Darrah et al., 2007) further emphasizes the significance of anti-saliva immunity in protection from leishmaniasis. The vital role of CD4+ T cells in LJM11-mediated protection is underlined by the abrogation of protection on depletion of these cells. Despite the fact that we cannot distinguish saliva-specific from Leishmania-specific CD4+ T cells in this study, we can conclude that the mechanism of saliva-mediated protection is not independent from CD4+ T cells. If anything, this finding suggests that saliva-mediated protection is intricately connected to the development of Leishmania-specific immunity. It is noteworthy that in the absence of CD4+ T cells CD8+ T cells do not have a major role in saliva-mediated protection from CL. Furthermore, antibodies are not required, suggesting that the protective effect is not related to antibody neutralization of LJM11.
Importantly, this protection was observed following immunization with a small amount (500 ng) of rLJM11 in the absence of adjuvant. LJM11 belongs to the Yellow family of proteins found exclusively in insects and only from sand fly saliva in its secreted form. The crystal structure of LJM11 displays a strong positive charge on one of its surfaces (Xu et al., 2011). The induction of long-term immunity by this protein in the absence of adjuvant is remarkable and represents an important feature in its consideration as a vaccine candidate. Part of the immunogenicity of this molecule may be due to its exclusive presence in sand fly saliva, making it quite foreign to the mammalian immune system. In addition, this molecule may be acting as a self-adjuvanting protein via its positively charged face that may promote interaction with cells of the innate immune system.
BSA-immunized mice challenged 2 weeks after the last immunization showed severe lesions that contained a large number of viable parasites despite the presence of a high level of Leishmania-specific IFN-γ. The failure to control CL in the presence of such an elevated level of IFN-γ remains unexplained particularly in light of the low level of detectable IL-4. We cannot fully exclude the fact that the observed low levels of IL-4 may have contributed to disease progression. Alternatively, other regulatory molecules could be protecting the parasite and promoting disease. In comparison, the ulcer-free protection resulting from immunity to LJM11 in mice challenged 2 weeks after the last immunization is striking. The immune response in LNs 2 weeks after infection in these mice reflects a Th1 environment characterized by the induction of LJM11-specific IFN-γ in the absence of IL-4. We believe that, in contrast to the BSA group, parasite replication is controlled in rLJM11-immunized mice by a moderate induction of LJM11-specific IFN-γ. In a separate experiment, rLJM11-immunized mice challenged 5 months after the last vaccination maintained powerful protection from CL. Importantly, these mice demonstrated a robust Leishmania-specific immunity 5 weeks after infection. This is important for long-lasting protection of rLJM11-immunized mice.
Leishmanization—the inoculation of virulent parasites—results in strong and durable immunity (Handman, 2001). This involves the development of patent disease at the chosen site of inoculation followed by cure. Leishmanization was discontinued owing to several factors, including the development of adverse reactions in a proportion of “leishmanized” individuals and lack of reproducibility (Handman, 2001). Thus far, leishmanization, the gold standard for protection in humans, is represented by mice that have healed their lesions (Peters et al., 2009). Immunization with rLJM11 provides a better alternative in which rLJM11-immunized mice challenged by vector transmission of virulent L. major develop Leishmania-specific immunity characterized by persistence of live parasites in the absence of CL lesions.
In conclusion, this work reveals several new aspects pertinent to understanding the potency and mechanism of sand fly saliva–mediated protection from leishmaniasis. We demonstrate that (i) immunity to a single salivary molecule confers protection against vector-transmitted CL; (ii) adjuvantless immunity generated by such a molecule is long lasting, conferring undiminished protection in mice challenged 5 months after the last immunization; (iii) protection from CL as a result of saliva-induced immunity is cell mediated and dependent on CD4+ T cells; and (iv) anti-saliva immunity leads to the development of a controlled ulcer-free Leishmania-specific immunity.
Materials and Methods
Animals
C57BL/6 mice were from Charles River Laboratories (Wilmington, MA). B-cell–deficient [B−/−](B10.129S2(B6)-Igh-6〈tm1Cgn〉/j mice) were from Jackson Laboratory (Bar Harbor, ME).
Ethics statement
All animal procedures were reviewed and approved by the National Institute of Allergy and Infectious Diseases (NIAID) Animal Care and Use Committee.
Sand flies and preparation of salivary gland homogenate
Lu. longipalpis sand flies, Jacobina strain, were reared at the Laboratory of Malaria and Vector Research, NIAID, NIH. Salivary glands were dissected from 5- to 7-day-old females and stored in phosphate-buffered saline at −70 °C. Salivary glands were sonicated and centrifuged at 12,000 × g for 3 minutes.
Parasites
L. major promastigotes (WR 2885 strain) were cultured in Schneider's medium supplemented with 20% heat-inactivated fetal bovine serum, 2 mM L-glutamine, 100 U ml–1 penicillin, and 100 μl ml–1 streptomycin. WR 2885 strain was typed at the Walter Reed Army Institute of Research.
Production and purification of recombinant LJM11 protein (rLJM11)
LJM11 DNA plasmids with a histidine tag were sent to the Protein Expression Laboratory at NCI-Frederick (Frederick, MD) for expression in HEK-293F cells. Expressed protein was purified as previously described (Collin et al., 2009) and by using a GS2000SW molecular sieving column connected to a Dionex high-pressure liquid chromatography pump (Thermo Fischer Scientific, Waltham, MA). The protein was eluted using phosphate-buffered saline, pH 7.2, at 1 ml min–1 and detected at 280 nm. Purity of the eluted rLJM11 was verified by separation on a 4–12% NuPAGE gel followed by silver staining using SilverQuest (Life Technologies, Grand Island, NY). The endotoxin level of the recombinant protein was measured using the ToxinSensor Chromogenic LAL endotoxin assay kit (GenScript, Piscataway, NJ). The endotoxin level of used rLJM11 batches was below 20 EU ml–1.
Exposure of mice to uninfected sand flies
Female flies (5–7 days old) were starved of sugar overnight and placed in plastic vials covered at one end with a 0.25-mm nylon mesh. Mice exposure was performed as previously described (Kamhawi et al., 2000).
Immunization of mice
Mice were immunized intradermally in the left ear, three times at 2-week intervals, with 10 μl containing 500 ng of either rLJM11 (without adjuvant) or BSA in phosphate-buffered saline.
Sand fly infection and transmission of L. major to vaccinated mice
Procyclic parasites were washed with phosphate-buffered saline, centrifuged at 3,500 r.p.m. for 15 minutes, and counted. Infection of sand flies with Leishmania (3 × 106 ml–1) was performed as described previously (Kamhawi et al., 2000). The sand flies were used for transmission on days 12 or 13 after infection.
Measurement of the delayed-type hypersensitivity response and lesion size
Measurements were obtained using a Vernier caliper (Mitutoyo, Baltimore, MD). For delayed-type hypersensitivity responses, the differential ear thickness for each group of mice was measured from values of the mean ear thickness 48 hours after exposure to sand fly bites subtracted from the mean ear thickness of the same mice before exposure; for Leishmania lesions, the largest thickness of an ear lesion was recorded.
Histologic analysis
Mouse ears were fixed in 10% phosphate-buffered formalin and embedded in paraffin. Sections (5 μm) were stained with hematoxylin–eosin at Histoserv (Germantown, MD)
ELISA
ELISA plates (Immulon4-Thermo, Waltham, MA) were coated overnight at 4 °C with 2 μg ml–1 rLJM11. After washing and blocking with 4% BSA for 2 hours at room temperature, sera from immunized mice (1:50) were incubated for 1 hour at 37 °C. After washing, plates were incubated with alkaline phosphatase–conjugated anti-mouse IgG (Promega, Madison, WI), IgG1 (BD Biosciences, Sparks, MD), or IgG2a (BD Biosciences) antibody (1/1,000). The reaction was revealed using alkaline phosphate substrate (Promega). Absorbance was recorded at 405 nm.
Western blot analysis
Western blot analysis for rLJM11 (10 μg) was performed as previously described (Valenzuela et al., 2001).
Ear tissue preparation
Ear tissue was processed as previously described (Belkaid et al., 1998; Peters et al., 2009). For ex vivo experiments, 2 million cells were cultured for 4 hours with Brefeldin A (BD Golgi Plug; BD Pharmingen, Sparks, MD). For experiments involving overnight stimulation, 2 million cells were cultured with bone marrow–derived dendritic cells generated as previously described (Lutz et al., 1999) with or without Lu. longipalpis salivary gland homogenate (2 pair ml–1), rLJM11 (4 μg ml–1), or soluble Leishmania antigen (100 μg ml–1) at 37 °C and 5% CO2 for 18 hours. Brefeldin A was added during the last 4 hours of culture. Cell were then harvested and analyzed by flow cytometry.
Parasite quantification by limiting dilution assay
Parasite quantification was performed as previously described (Titus et al., 1985; Belkaid et al., 1998).
Flow cytometry
The following antibodies were used for cell staining: PerCP or FITC-labeled anti-CD4 (RM4-5 and GK1.5), PerCP-labeled anti-CD8 (53-6.7), antigen-presenting cell-labeled anti-TCR-β (H57-597), FITC-labeled anti-IFN-γ (XMG 1.2), and phycoerythrin-labeled anti-tumor necrosis factor-α (MP6-XT22). A minimum of 100,000 cells were acquired using a FACSCalibur cytometer (BD Biosciences). Data were analyzed with the Flow Jo software version 9.4.10 (Tree Star, Phoenix, AZ).
In vitro stimulation of LN cells with rLJM11-pulsed bone marrow–derived dendritic cells
At day 6, cultured bone marrow–derived dendritic cells were pulsed overnight with 10 μg ml–1 of rLJM11. The next day, pulsed and naive bone marrow–derived dendritic cells were cultured in the presence of LN cells from mice immunized with rLJM11 or from naive cells. Cells were incubated at 37 °C with 5% CO2. Supernatants were collected and analyzed by ELISA.
In vitro stimulation of spleen cells with rLJM11
Spleen cells (5 × 106 ml–1) were cultured in RPMI medium (Life Technologies) supplemented with 10% heat-inactivated fetal bovine serum, 100 U ml–1 of penicillin, 100 μg ml–1 of streptomycin, and 5 × 10−5 M of 2-mercaptoethanol (Sigma, St. Louis, MO) in the presence of 20 μg ml–1 of rLJM11. Cells were incubated at 37 °C with 5% CO2 for 72 hours. Supernatants were collected and analyzed by ELISA.
Cytokine ELISA
LN and spleen cells from rLJM11-immunized mice or naive mice were processed as mentioned above. After 72 hours of stimulation with rLJM11, IFN-γ, IL-10, and IL-4 levels were measured in supernatants using specific sandwich ELISA (BD Biosciences).
CD4+ T-cell depletion
Mice were injected intraperitoneally with 0.5 mg of anti-mouse CD4 (L3T4) mAb (GK 1.5 clone) or rat anti-mouse IgG2b isotype, both from eBioscience (San Diego, CA), 2 days before challenge and weekly until the end of the experiment.
Statistical analysis
The Student's two-tailed unpaired t-test was used for statistical analysis between two groups. Multiple groups were analyzed using one-way analysis of variance followed by Tukey's multiple-comparison test.
Acknowledgments
We thank Elvin Morales, Anika T. Haque, and Nathan Smith for their help with sand fly colonization. We thank David Sacks and Nathan Peters and members of the VMBS for critical comments and revision of the manuscript; Robert Gwadz and Thomas Wellems for continuous support; and NIAID intramural editor Brenda Rae Marshall for assistance. The study was funded by the Intramural Research Program of the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. As RG, FO, CM, DCG, SK, and JGV are government employees and this is a government work, the work is in the public domain in the United States. Notwithstanding any other agreements, the NIH reserves the right to provide the work to PubMed Central for display and use by the public, and PubMed Central may tag or modify the work consistent with its customary practices. You can establish rights outside of the United States subject to a government use license.
Glossary
- B-cell−/− mice
B-cell–deficient mice
- CL
cutaneous leishmaniasis
- LN
lymph node
- Th1
T-cell helper type 1
- TNF
tumor necrosis factor
The authors state no conflict of interest.
References
- Belkaid Y, Kamhawi S, Modi G, et al. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J Exp Med. 1998;188:1941–1953. doi: 10.1084/jem.188.10.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethony JM, Cole RN, Guo X, et al. Vaccines to combat the neglected tropical diseases. Immunol Rev. 2011;239:237–270. doi: 10.1111/j.1600-065X.2010.00976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin N, Gomes R, Teixeira C, et al. Sand fly salivary proteins induce strong cellular immunity in a natural reservoir of visceral leishmaniasis with adverse consequences for Leishmania. PLoS Pathogens. 2009;5:e1000441. doi: 10.1371/journal.ppat.1000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrah PA, Patel DT, De Luca PM, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- Gomes R, Teixeira C, Teixeira MJ, et al. Immunity to a salivary protein of a sand fly vector protects against the fatal outcome of visceral leishmaniasis in a hamster model. Proc Natl Acad Sci USA. 2008;105:7845–7850. doi: 10.1073/pnas.0712153105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E. Leishmaniasis: current status of vaccine development. Clin Microbiol Rev. 2001;14:229–243. doi: 10.1128/CMR.14.2.229-243.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamhawi S, Belkaid Y, Modi G, et al. Protection against cutaneous leishmaniasis resulting from bites of uninfected sand flies. Science. 2000;290:1351–1354. doi: 10.1126/science.290.5495.1351. [DOI] [PubMed] [Google Scholar]
- Lutz MB, Kukutsch N, Ogilvie AL, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- Oliveira F, Kamhawi S, Seitz AE, et al. From transcriptome to immunome: identification of DTH inducing proteins from a Phlebotomus ariasi salivary gland cDNA library. Vaccine. 2006;24:374–390. doi: 10.1016/j.vaccine.2005.07.085. [DOI] [PubMed] [Google Scholar]
- Oliveira F, Lawyer PG, Kamhawi S, et al. Immunity to distinct sand fly salivary proteins primes the anti-Leishmania immune response towards protection or exacerbation of disease. PLoS Neglected Tropical Dis. 2008;2:e226. doi: 10.1371/journal.pntd.0000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters NC, Egen JG, Secundino N, et al. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science. 2008;321:970–974. doi: 10.1126/science.1159194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters NC, Kimblin N, Secundino N, et al. Vector transmission of leishmania abrogates vaccine-induced protective immunity. PLoS Pathogens. 2009;5:e1000484. doi: 10.1371/journal.ppat.1000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers ME, Ilg T, Nikolaev AV, et al. Transmission of cutaneous leishmaniasis by sand flies is enhanced by regurgitation of fPPG. Nature. 2004;430:463–467. doi: 10.1038/nature02675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers ME, Sizova OV, Ferguson MA, et al. Synthetic glycovaccine protects against the bite of leishmania-infected sand flies. J Infect Dis. 2006;194:512–518. doi: 10.1086/505584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira C, Gomes R, Collin N, et al. Discovery of markers of exposure specific to bites of Lutzomyia longipalpis, the vector of Leishmania infantum chagasi in Latin America. PLoS Neglected Tropical Diseases. 2010;4:e638. doi: 10.1371/journal.pntd.0000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus RG, Marchand M, Boon T, et al. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 1985;7:545–555. doi: 10.1111/j.1365-3024.1985.tb00098.x. [DOI] [PubMed] [Google Scholar]
- Valenzuela JG, Belkaid Y, Garfield MK, et al. Toward a defined anti-Leishmania vaccine targeting vector antigens: characterization of a protective salivary protein. J Exp Med. 2001;194:331–342. doi: 10.1084/jem.194.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela JG, Garfield M, Rowton ED, et al. Identification of the most abundant secreted proteins from the salivary glands of the sand fly Lutzomyia longipalpis, vector of Leishmania chagasi. J Exp Biol. 2004;207 (Pt 21:3717–3729. doi: 10.1242/jeb.01185. [DOI] [PubMed] [Google Scholar]
- WHO 2010. WHO Technical Report Series. 〈 . 〈 www.WHO.int 〉
- Xu X, Oliveira F, Chang BW, et al. Structure and function of a “yellow” protein from saliva of the sand fly Lutzomyia longipalpis that confers protective immunity against Leishmania major infection. J Biol Chem. 2011;286:32383–32393. doi: 10.1074/jbc.M111.268904. [DOI] [PMC free article] [PubMed] [Google Scholar]