Abstract
Hair follicles are simple, accessible models for many developmental processes. Here, using mutant mice, we show that Bmpr2, a known receptor for bone morphogenetic proteins (Bmps), and Acvr2a, a known receptor for Bmps and activins, are individually redundant but together essential for multiple follicular traits. When Bmpr2/Acvr2a function is reduced in cutaneous epithelium, hair follicles undergo rapid cycles of hair generation and loss. Alopecia results from a failure to terminate hair development properly, as hair clubs never form, and follicular retraction is slowed. Hair regeneration is rapid due to premature activation of new hair-production programs. Hair shafts differentiate aberrantly due to impaired arrest of medullary-cell proliferation. When Bmpr2/Acvr2a function is reduced in melanocytes, gray hair develops, as melanosomes differentiate but fail to grow, resulting in organelle miniaturization. We conclude that Bmpr2 and Acvr2a normally play cell-type-specific, necessary roles in organelle biogenesis and the shutdown of developmental programs and cell division.
Keywords: anagen, catagen, telogen, terminal differentiation, melanosome, Bmp signaling
INTRODUCTION
Hair follicles are useful models for many aspects of mammalian morphogenesis, as throughout life they proceed through cycles of growth (anagen), regression (catagen), and rest (telogen), resulting in the periodic development of hair, repeated reshaping of follicular structure, and recapitulation of basic morphogenetic processes (recently reviewed in Schneider et al., 2009). During anagen, a follicle elongates into the dermis and develops its hair-producing components: the hair bulb, from which the hair shaft and inner root sheath (IRS) grow, and a transient stalk, in which the IRS and hair complete their differentiation. Within the hair bulb, the hair matrix (a vigorously proliferating epithelial cell population) generates the precursor cells of the hair and IRS, and the developing hair shaft acquires its pigmentation from melanocytes, which intermingle with the bulbar epithelial cells and transfer melanin to the precursors of the hair medulla and cortex. Once an anagen follicle reaches full maturity, a hair is grown for a programmed period of time, after which cell proliferation ceases in the hair bulb, and the follicle eliminates its hair-producing components, principally through apoptosis. During this catagen phase, the follicle retracts to approximately one-third its maximum length and concomitantly develops an anchoring club at the base of the hair shaft. Upon finishing its involution, the follicle holds the completed hair in place and becomes comparatively quiescent. In due course, this telogen phase ends and a new anagen is initiated, leading to the formation of a new hair and the repetition of each phase of the cycle.
The transforming growth factor β (TGFβ) superfamily is a set of secreted signaling proteins that regulates the development of virtually all mammalian tissues and whose members include the TGFβs, bone morphogenetic proteins (BMPs), and activins/inhibins (reviewed in Schmierer and Hill, 2007). Superfamily members produce most if not all of their effects by binding to two kinds of transmembrane receptors — type I and type II — both of which are serine/threonine kinases. To date, 7 type I receptors, 5 type II receptors, and at least 33 superfamily ligands have been identified in mammals (reviewed in Chang et al., 2002). In the canonical mechanism of superfamily signaling, the formation of ligand-receptor complexes leads the type II receptor to phosphorylate the type I receptor, which then phosphorylates receptor-activated Smad transcription factors, resulting ultimately in Smad regulation of gene expression (reviewed in Schmierer and Hill, 2007). In a variety of circumstances, signals are also transduced by noncanonical mechanisms, which include the Smad-independent activation of mitogen-activated protein kinases, Rho GTPases, or phosphatidylinositol-3-kinase (reviewed in Derynck and Zhang, 2003).
TGFβ superfamily signaling plays key roles in various aspects of hair follicle development and cycling (reviewed in Li et al., 2003). Tgfβ2 is required for the induction of hair follicle morphogenesis, whereas Tgfβ1 has an opposing effect and blocks hair follicle formation. Tgfβ1-3 all appear to play essential roles in the induction of catagen in hair follicles. Activin A, which is secreted by dermal-papilla cells, has an inhibitory effect on hair follicle morphogenesis and catagen entry, whereas follistatin, a secreted antagonist to activins and BMPs, is produced by hair follicle epithelial cells and blocks activin’s effects (Nakamura et al., 2003). BMPs, the largest subfamily of growth factors in the TGFβ superfamily, control hair follicle morphogenesis at many different stages (reviewed in Botchkarev and Sharov, 2004). At the initiation stage, BMPs function as an inhibitory signal, while noggin, a secreted BMP antagonist, neutralizes BMP activity and thereby stimulates the induction of secondary hair follicles (Botchkarev et al., 1999). In developing hair follicles, BMPs promote the differentiation of the inner root sheath and hair shaft (Andl et al., 2004; Kobielak et al., 2003; Ming Kwan et al., 2004; Yuhki et al., 2004) as well as cellular commitment to the sebaceous gland lineage (Guha et al., 2004). During postnatal follicular cycling, Bmp2 and Bmp4 inhibit the induction of anagen and keep follicular stem cells quiescent, thus maintaining telogen (Plikus et al., 2008).
The role of TGFβ superfamily signaling in hair pigmentation and melanocyte biology is not well understood. TGFβ1 inhibits growth and melanogenesis of cultured melanocytes and also induces apoptosis of melanoyctes in collagen gels (Alanko and Saksela, 2000). Similarly, activin induces growth inhibition and apoptosis in melanocyte cultures (Stove et al., 2004). In quail neural crest cultures, BMP4 prevents the specification of melanoblasts (Jin et al., 2001), the precursors of melanocytes, while BMP2 promotes melanosome differentiation and melanogenesis (Bilodeau et al., 2001). In human melanocyte cultures, BMP4 induces cell division but inhibits melanogenesis (Yaar et al., 2006), while BMP7 inhibits cell proliferation (Hsu et al., 2008).
Numerous murine lines have been generated to either overproduce or delete members of the TGFβ superfamily signaling pathway in cutaneous epithelium. Superfamily members whose activity was manipulated by mutation include: the ligands Bmp4 (Blessing et al., 1993), Bmp6 (Blessing et al., 1996), activin A (Nakamura et al., 2003), and Tgfβ1 (Sellheyer et al., 1993); the inhibitors noggin (Botchkarev et al., 1999; Guha et al., 2004; Kulessa et al., 2000; Plikus et al., 2004; Sharov et al., 2005; 2006), follistatin (Nakamura et al., 2003), and Smad7 (He et al., 2002; Klopcic et al., 2007); the type I receptors Bmpr1a (Andl et al., 2004; Kobielak et al., 2003; Ming Kwan et al., 2004; Yuhki et al., 2004) and Acvr1b (Qiu et al., 2011); and the transcription factor Smad 4 (Owens et al., 2008; Qiao et al., 2006; Yang et al., 2005). But to date, no study of type II receptor function in skin has been reported. Four type II receptors — Tgfbr2 (Tgfbeta receptor II), Bmpr2 (Bmp receptor, type II), Acvr2a (activin receptor IIa), and Acvr2b (activin receptor IIb) — have been detected in murine epidermis and hair follicles (He et al., 2002). Tgfbr2 was found in the outer root sheath (ORS), the outermost epithelial cylinder of the hair follicle, while the other three type II receptors were present in both the ORS and matrix cells. In this study, we used knockout mice to analyze the cutaneous functions of two type II receptors — Bmpr2 and Acvr2a. We show that these receptors play overlapping but necessary roles in the differentiation of the hair shaft, development of the hair club, timing and length of each phase of the hair cycle, and generation of pigmentation.
RESULTS
Type II Receptor Deficiency Causes Cyclic Alopecia
Germ line Bmpr2 knockouts exhibit early embryonic death (Beppu et al., 2000), preventing the study of Bmpr2 function in skin development. We thus employed a conditional knockout approach and ablated Bmpr2 from cutaneous epithelium using a floxed Bmpr2 allele (Bmpr2flox; Beppu et al., 2005) and K14-Cre transgene (Li et al., 2001). The floxed Bmpr2 allele contains loxP sites flanking exons 4 and 5, which encode the receptor’s transmembrane domain and part of its kinase domain. Accordingly, Cre-mediated recombination deletes a key portion of Bmpr2 and concomitantly yields a frameshift in the coding sequence, inactivating the receptor and generating a deletion/frameshift identical to that of the germ line Bmpr2 knockout. The K14-Cre transgene produces Cre in the progenitor cells of the epidermis and hair follicles (Li et al., 2001) and thus should ablate Bmpr2 throughout the cutaneous epithelium.
Surprisingly, K14-Cre;Bmpr2flox/flox mice were healthy, fertile, and normal to the naked eye. This absence of overt skin defects did not appear to result from a failure to ablate Bmpr2, as the transgene-derived Cre rendered the undeleted (floxed) Bmpr2 allele undetectable in cutaneous epithelial cells, converting the overwhelming majority of Bmpr2flox to the null allele (Fig. 1a shows a representative example of the recombination in skin cells carrying the K14-Cre transgene and Bmpr2flox allele). Likewise, K14-Cre;Bmpr2flox/− mice, which carry one copy of a germ line Bmpr2 null allele (Bmpr2−; Beppu et al., 2000), were identical to K14-Cre;Bmpr2flox/flox mice (data not shown), consistent with the results shown in Fig. 1a.
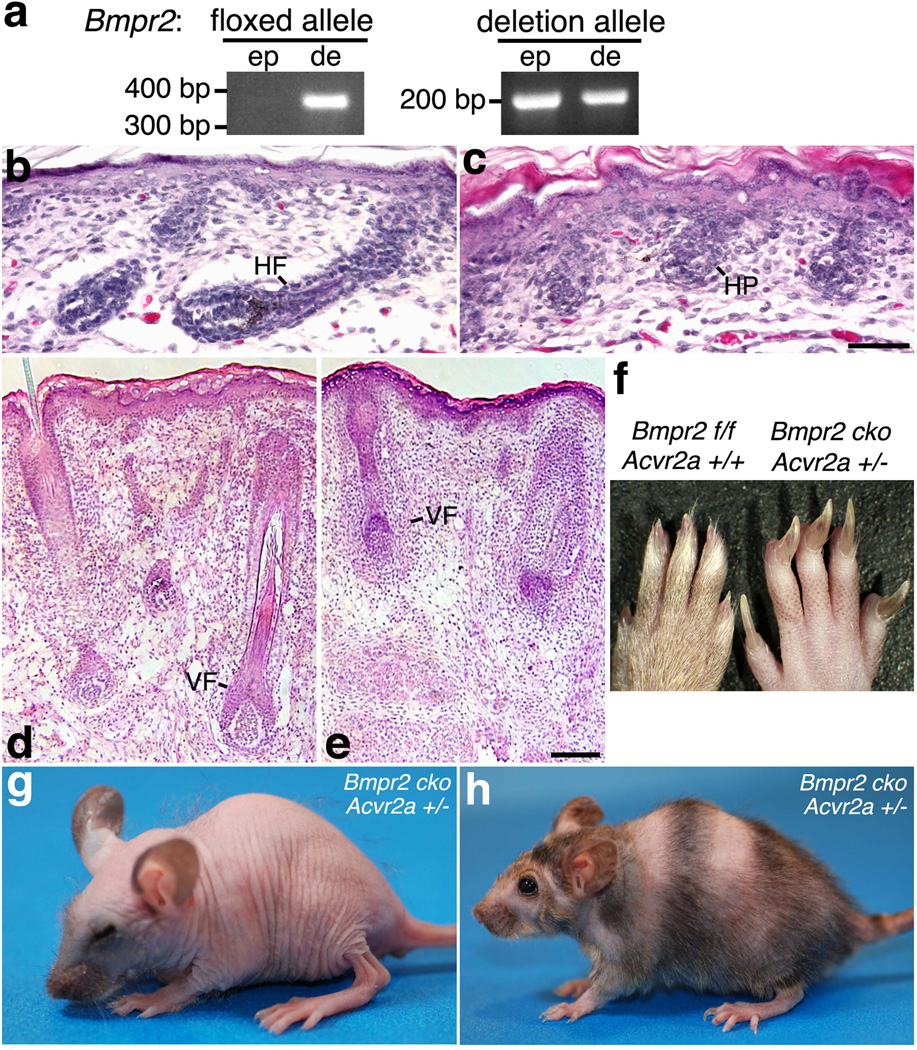
FIG. 1.
Bmpr2/Acvr2a double mutants exhibit defects in hair development and retention. (a) DNA was isolated from the epidermal (ep) or dermal (de) compartments of K14-Cre;Bmpr2flox/flox;Acvr2a+/− skin and assayed by PCR for the Bmpr2 floxed allele (no Cre-mediated recombination) and Bmpr2 deletion allele (generated by Cre-mediated recombination). Representative PCR products are shown on an agarose gel. The K14-Cre transgene yields the efficient ablation of Bmpr2, as the floxed alleles are replaced by deletion (null) alleles in cutaneous epithelial cells. (b–e) Skin sections of newborn control (b, d) and K14-Cre;Bmpr2flox/flox;Acvr2a−/−mice (c, e) are shown after staining with hematoxylin and eosin (H & E). Development of coat (c) and vibrissa (e) follicles is inhibited in the double mutant. HF, hair follicle; HP, hair peg; VF, vibrissa follicle. Scale bar for panels b–c, 50 µm; d–e, 100 µm. (f) Hind feet of 6-month-old normal (left) and Bmpr2/Acvr2a-deficient (right) mice are shown. Genotypes are indicated, with Bmpr2 f/f representing Bmpr2flox/flox and Bmpr2 cko (Bmpr2 conditional knockout) representing K14-Cre;Bmpr2flox/flox. Nails grow abnormally large in Bmpr2/Acvr2a deficient mice. (g, h) K14-Cre;Bmpr2flox/flox;Acvr2a+/−mice are shown at 23 days (g) and 7.5 months (h) of age. The hair coats of these double mutants undergo cycles of loss and re-growth throughout life.
As other type II receptors may compensate for the loss of Bmpr2, we generated type II receptor double mutants in which the conditional Bmpr2 deficiency was combined with germ line Acvr2a deficiency (the Acvr2a− allele; Song et al., 1999). The Acvr2a− allele (Fig. S1) contains a deletion in the sequence encoding the receptor’s kinase domain. This mutation, when homozygous, decreases the viability of a minority of animals but has not been previously associated with skin abnormalities. K14-Cre;Bmpr2flox/flox;Acvr2a−/− mice died around the time of birth with open eyes and a substantial impairment of hair-follicle morphogenesis. Whereas normal skin was already producing coat hair fibers and vibrissa, double-knockout skin was not generating either of these hair types, as coat follicles typically lacked hair bulbs (Fig. 1b–c), and vibrissa follicles, though possessing hair bulbs, were short (exhibiting less downgrowth into the dermis) and generally lacked terminally differentiating structures (such as the inner root sheath and hair shaft; Fig 1d–e) and terminal differentiation markers (such as trichohyalin; data not shown). Thus, the double-knockout hair follicles appeared to be slowed or arrested in their development. Death did not appear to result from a skin defect, such as a barrier defect, as the epidermis was intact and possessed all standard layers.
Given the early lethality of the Bmpr2/Acvr2a deficiency, we tested the effect of combining a lack of Bmpr2 (K14-Cre;Bmpr2flox/flox) with a reduced dose of functional Acvr2a (Acvr2a+/−). The resulting K14-Cre;Bmpr2flox/flox;Acvr2a+/− mice were healthy and fertile but exhibited fully penetrant, cyclical defects in the hair coat. Following birth, a dense coat developed, but a subset of hair fibers appeared thinner than those of controls (mice with Bmpr2flox/flox or Bmpr2flox/flox;Acvr2a+/− genotypes but lacking K14-Cre). Most strikingly, starting from approximately postnatal day 14 (P14), K14-Cre;Bmpr2flox/flox;Acvr2a+/− mice underwent the total, progressive loss of coat hair. Hair loss began on the face and then proceeded towards the tail so that the mice were completely bald by about three weeks of age (Fig. 1g). Shortly following the loss of all coat hair, a new hair coat formed in full once more, and this coat was quickly lost again after reaching its maximum length. These cycles of hair re-growth and loss were repeated throughout life and thus constituted a new example of ‘cyclic alopecia’ (Ma et al., 2003), a phenotype previously observed in Msx2 (Ma et al., 2003) and Sfn (Er) mutants (Hunsicker, 1960; Herron et al., 2005; Li et al., 2005). Adult K14-Cre;Bmpr2flox/flox;Acvr2a+/− mice exhibited hair growth and loss simultaneously, resulting in the appearance of stripes of coat hair and bald skin (Fig. 1h). On these striped adults, the patterns of hair growth resembled the simplified patterns described for Krt14-Nog transgenic mice (Plikus et al., 2008), whose hair follicles are grouped into larger, fewer domains of synchronized activity and which produce the Bmp inhibitor noggin from the keratin 14 promoter. Concomitant with their hair defects, the K14-Cre;Bmpr2flox/flox;Acvr2a+/− mice displayed excessive nail growth, which became apparent after two months of age (Fig. 1f). Thus, in contrast to the Bmpr2 and Acvr2a single mutants, Bmpr2 and Acvr2a double mutants developed prominent skin and nail abnormalities, with the K14-Cre;Bmpr2flox/flox;Acvr2a+/− mice exhibiting a particularly conspicuous oscillation between coat formation and loss. The alopecia appeared to arise at a specific point in the hair cycle, as hair was lost from juvenile mice in a head-to-tail progression, and hair follicles develop and initially cycle in a similar head-to-tail sequence. Likewise, the regionalized hair loss in adult mice was consistent with a defect specific to one hair-cycle point, as adults normally exhibit a more regionalized synchronicity of follicular cycling. The emergence of the alopecia and nail abnormalities in K14-Cre;Bmpr2flox/flox;Acvr2a+/− mice suggests that the Acvr2a− mutation, though typically recessive, reduces total Acvr2a activity when heterozygous. Overall, the results suggest that the Bmpr2 and Acvr2a receptors perform redundant functions but that these functions are essential for the development and maintenance of normal hair and nails.
Type II Receptors and the Hair Cycle
To determine the causes of the hair defects of the K14-Cre;Bmpr2flox/flox;Acvr2a+/− mice, the histology of back skin was examined at different stages of follicular hair production, regression, and quiescence. During the earlier stages of follicular morphogenesis (e.g., P0), the skin of K14-Cre;Bmpr2flox/flox;Acvr2a+/− mice was indistinguishable from that of normal (control) littermates and developed a normal number of hair follicles (data not shown). These hair follicles subsequently reached maturity and produced hair (Fig. 2a–b) but exhibited defects in hair shaft differentiation (see below). In many double-mutant hair follicles, hair production terminated prematurely, and catagen started early, as narrowing, amelanotic hair bulbs (signs of catagen) were observed as early as P15, a time when controls continued to grow hair (Fig. 2c–d). By P16–17, all double mutant hair follicles entered catagen, but their progression through this phase of the hair cycle was slow. Whereas normal hair follicles began catagen at P16–18 and completed their involution in two days, double-mutant hair follicles were still regressing at P20 (Fig. 2e–f), as they possessed extended epithelial strands (the involuting remnants of the outer root sheath and hair bulb). These slowly regressing epithelial strands often appeared abnormally curled (Fig. 2f), and concomitant with the impaired follicular involution, hair shafts disappeared from the skin. At this time point, normal skin, in contrast, exhibited firmly anchored hair shafts in fully retracted follicles (Fig. 2e). Thus, the alopecia of the Bmpr2/Acvr2a double mutants resulted from a disruption of catagen, showing that Bmpr2/Acvr2a function is necessary for proper catagen timing and progression.
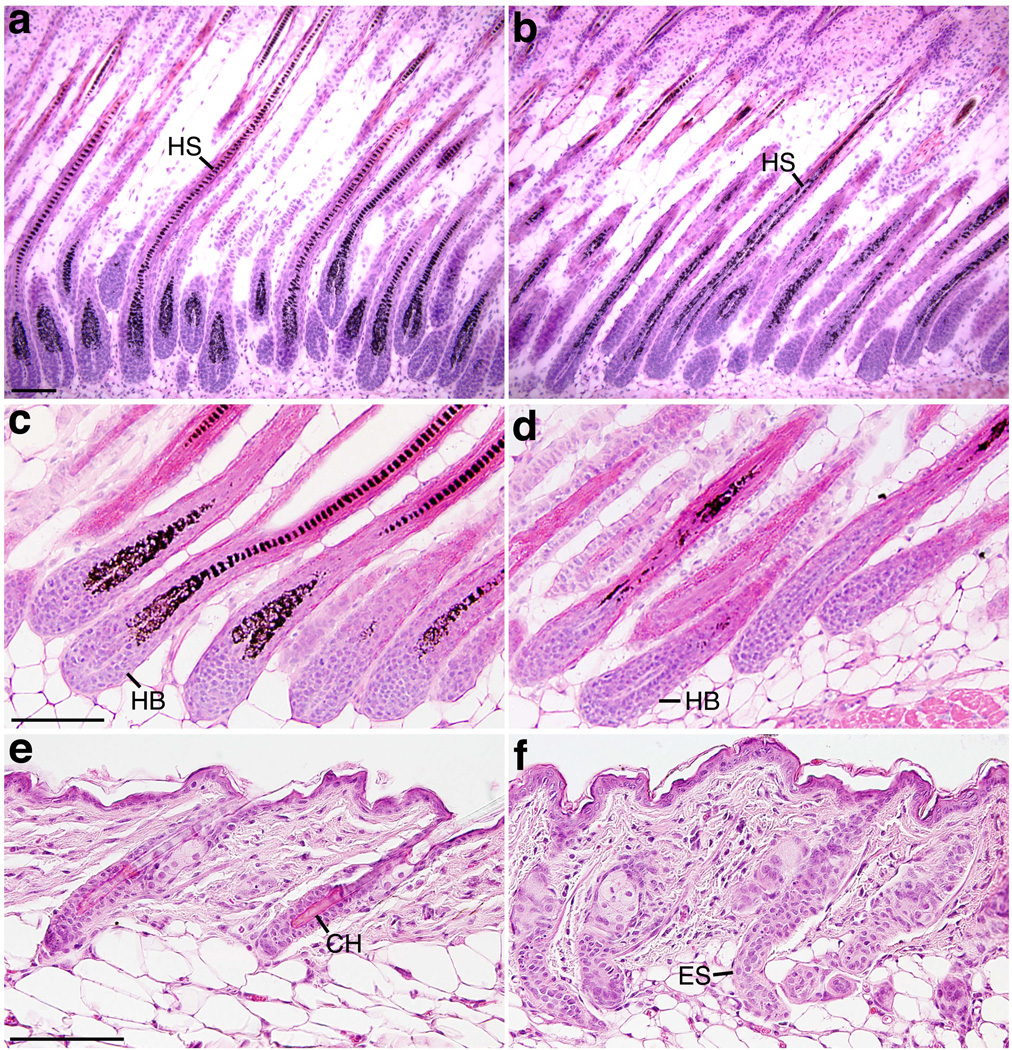
FIG. 2.
Bmpr2/Acvr2a double mutants display aberrant catagens. Skin sections of control (a, c, e) and K14-Cre;Bmpr2flox/flox;Acvr2a+/− (b, d, f) mice of different ages are shown after staining with H & E. (a, b) P11. (c, d) P15. (e, f) P20. Bmpr2/Acvr2a double mutants experience premature yet prolonged catagens. HS, hair shaft; HB, hair bulb; CH, club hair; ES, epithelial strand. Scale bars, 50 µm.
Upon completing regression, the double-mutant hair follicles quickly initiated anagen, and following a brief telogen, normal follicles did the same, leading to the production of new hair shafts in control and K14-Cre;Bmpr2flox/flox;Acvr2a+/− mice (Fig. 3a–b). This anagen concomitantly caused the double-mutant epidermis to become thicker than that of control animals (Fig. 3a–b, insets). Similar to the first period of hair production, this second period of hair production was shorter in double mutants than in controls (Fig. 3c–d), as the visibly-detectable component of this anagen averaged 10.7 days (± 0.7; n = 9) in K14-Cre;Bmpr2flox/flox;Acvr2a+/− mice versus 12.4 days (± 0.9; n =9) in normal mice. Thus, the decrease in Bmpr2/Acvr2a function shifted the anagen/catagen transition, leading to the premature end of anagen and induction of catagen.
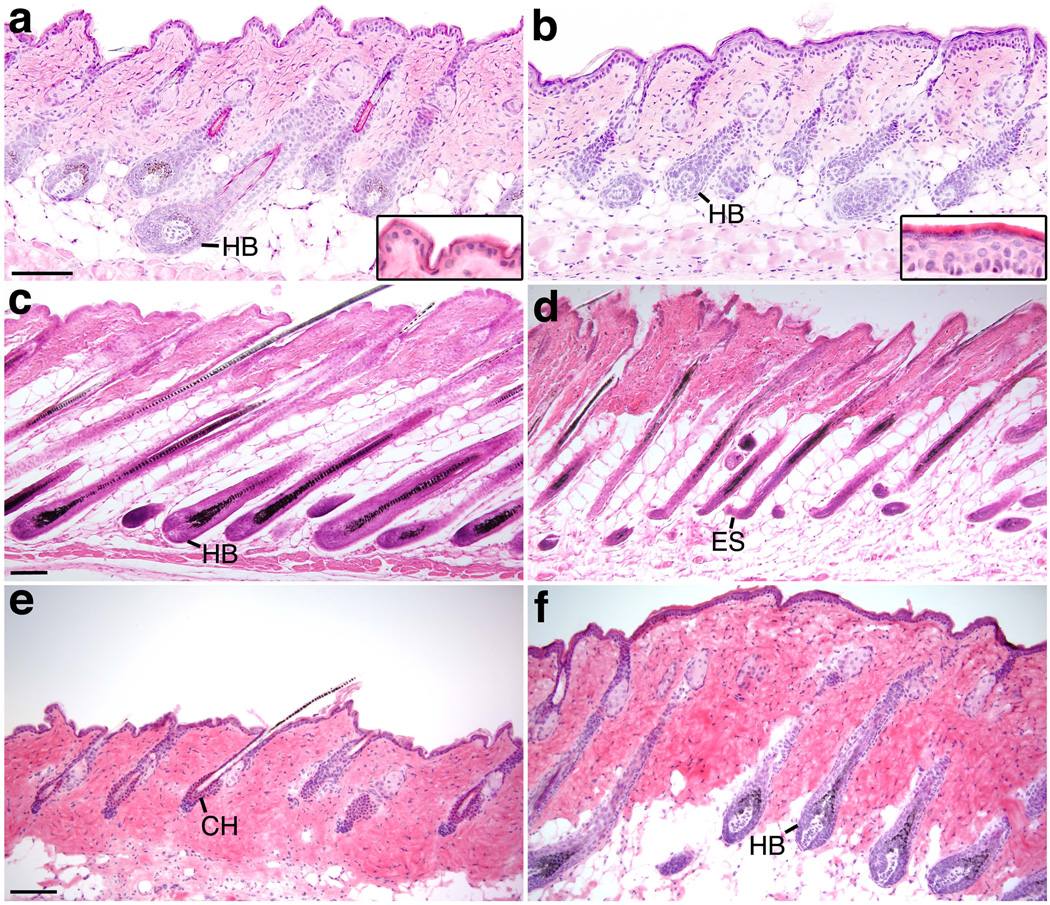
FIG. 3.
Bmpr2/Acvr2a double mutants display aberrant anagens and telogens. Skin sections of control (a, c, e) and K14-Cre;Bmpr2flox/flox;Acvr2a+/− (b, d, f) mice of different ages are shown after staining with H & E. (a, b) P24. Insets show higher magnification images of the epidermis. (c, d) P35. (e, f) P50. Bmpr2/Acvr2a double mutants experience shortened anagens and telogens. HB, hair bulb; ES, epithelial strand; CH, club hair. Scale bars, 50 µm.
Coat follicles normally exhibit a long second telogen, and while subsequent telogens vary in length, they are substantially longer than other phases of the hair cycle (Plikus et al., 2008 and references therein). In contrast, the coat follicles of K14-Cre;Bmpr2flox/flox;Acvr2a+/−mice always displayed short telogens, as new hair was produced soon after the preceding hair fibers were lost. For example, at P50 the coat follicles of normal mice were still in the second telogen, while those of K14-Cre;Bmpr2flox/flox;Acvr2a+/− mice had left this telogen and uniformly re-entered anagen (Fig. 3e–f). Thus, the reduction of Bmpr2/Acvr2a function caused hair follicles to enter anagen precociously, which in turn led to a greater number of hair cycles.
Type II Receptors and the Development of the Hair Shaft and Club
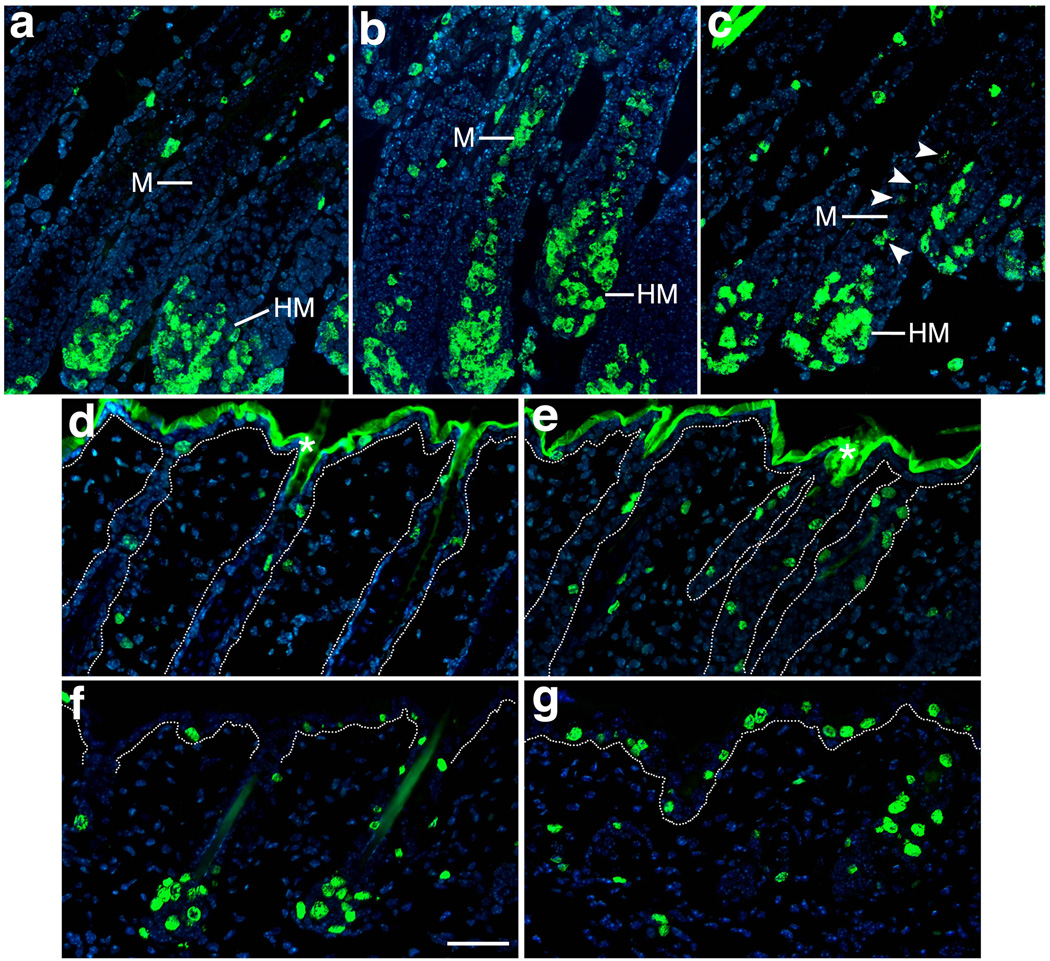
As K14-Cre;Bmpr2flox/flox;Acvr2a+/− mice always generate a grossly abnormal hair coat, the histology of their back skin was examined during hair production. The double mutants possessed all of the follicular compartments found in normal controls (including sebaceous glands with no obvious defects) but generated abnormal medullas (Fig. 4a–b), the central and typically thickest cylinder of murine hair shafts. Whereas medullary cells normally form one or more well-organized columns and arrange their pigment into a ladder-like pattern, the medullary cells of the double mutants appeared disorganized, and their ladder-like pattern of pigmentation was lost (Fig. 4a–b). In K14-Cre;Bmpr2flox/flox mice, the hair medulla occasionally appeared disorganized too, though these defects were less severe than those of the Bmpr2/Acvr2a double mutants (Fig. 4c). To assess the mutations’ effects on cell differentiation, skin sections were stained by immunofluorescence for trichohyalin, a marker of the hair medulla and IRS (Fietz et al., 1993). In the IRS, trichohyalin appeared unaffected by the Bmpr2 and Acvr2a mutations (Fig. 4d–f), suggesting that IRS cells differentiated appropriately in the single (K14-Cre;Bmpr2flox/flox) and double (K14-Cre;Bmpr2flox/flox;Acvr2a+/−) mutants. In contrast, medullary trichohyalin was clearly reduced in the K14-Cre;Bmpr2flox/flox mice and greatly diminished in the K14-Cre;Bmpr2flox/flox;Acvr2a+/− mice (Fig. 4d–f), suggesting that medullary cells fail to differentiate properly in both mutants. As the abnormalities in trichohyalin levels and medulla structure were more severe in the double mutant than in the single mutant, the results suggest that Bmpr2 and Acvr2a make additive or synergistic contributions to the differentiation of the hair medulla.
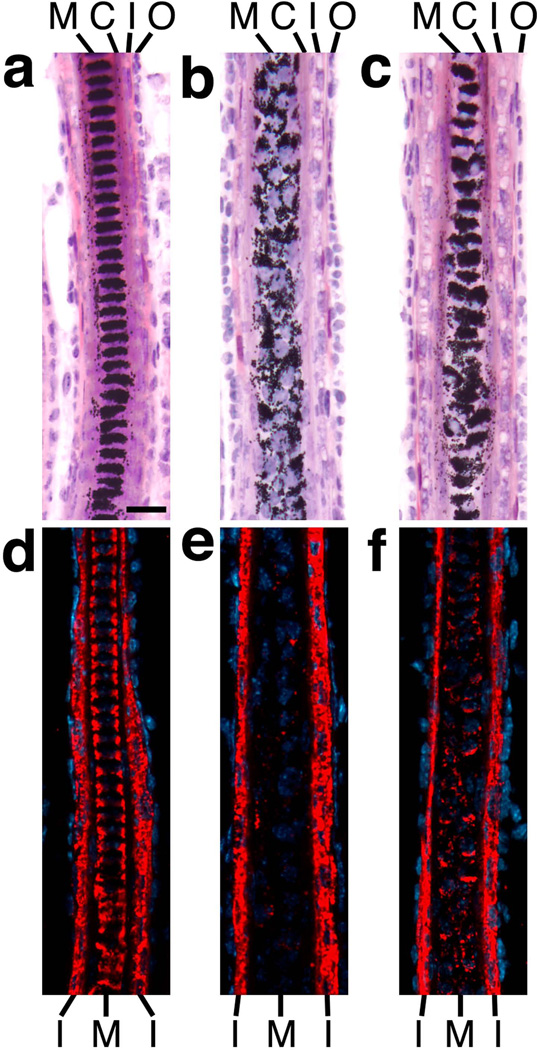
FIG. 4.
Mutations in Bmpr2 and Acvr2a cause differentiation defects in hair medulla. Skin sections of P11 control (a, d), K14-Cre;Bmpr2flox/flox;Acvr2a+/− (b, e) and K14-Cre;Bmpr2flox/flox (c, f) mice are shown after staining by H & E (a–c) or immunofluorescence (d–f) using antibodies to trichohyalin (red; DNA is stained in blue). A lack of type II receptor function leads to structural disorganization and trichohyalin deficiency in the hair medulla (M). C, cortex; I, IRS; O, ORS. Scale bar, 20 µm.
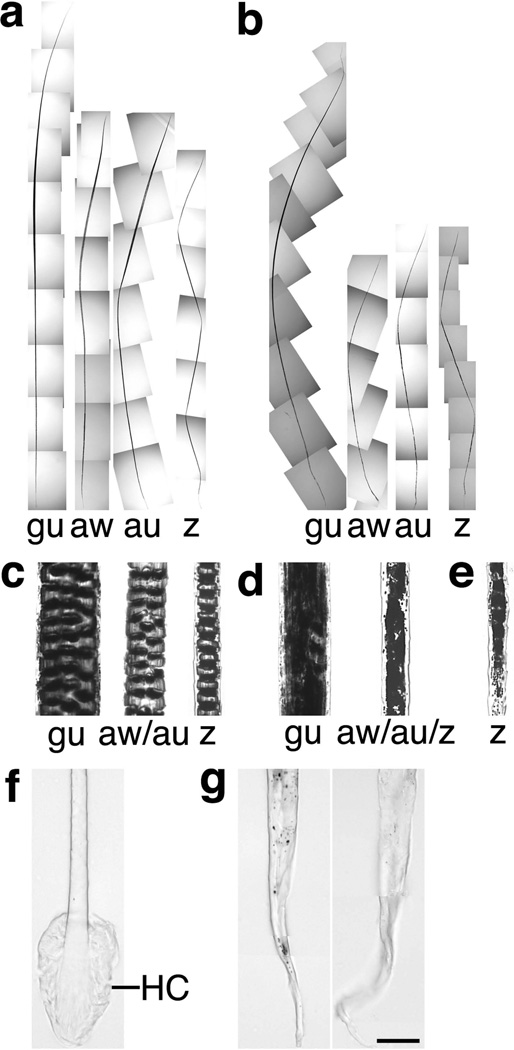
To assess the effects of Bmpr2 and Acvr2a on hair type and other macrostructural features of the hair shaft, hair was collected from the backs of control and receptor-mutant mice with a forceps. Hair fibers were harvested at P20, at which point the K14-Cre;Bmpr2flox/flox;Acvr2a+/− mice were in late catagen and had lost the coat hair from the anterior halves of their bodies but still possessed hair on the posterior halves. Murine hair coats normally contain four hair types — guard, awl, auchene, and zigzag — which differ in length, shape (kinks), or the number of columns of medullary cells. In particular, guard hairs are greatest in length, auchene and zigzag hairs possess kinks, and zigzag hairs have single (rather than multiple) columns of medullary cells. These four hair types were recognizable in the coats of K14-Cre;Bmpr2flox/flox;Acvr2a+/− mice, as the hairs exhibited defining differences in relative length and number of kinks (Fig. 5a–b). Nonetheless, the double-mutant hairs displayed several macrostructural abnormalities. In double-mutant hair coats, the nontylotrich hairs, i.e., the awl, auchene, and zigzag hairs (which develop later than guard hairs), were substantially shorter than normal (Fig. 5a–b), consistent with their skins’ premature entry into catagen, and the normally straightest hair types — guard and awl — were curved instead (Fig. 5a–b). Additionally, the double-mutant awl and auchene hairs lacked multiple columns of medullary cells and possessed instead a single column (Fig. 5c–d). As such, these awl/auchene hairs had zigzag-like medullas and widths equivalent to normal zigzag fibers, making them substantially thinner than normal awl/auchene hairs (which together normally comprise ~25% of the coat; Sundberg and Hogan, 1994). Consistent with the similar widths of nontylotrich hair shafts, the nontylotrich hair follicles exhibited no differences in the size of the dermal papillae or other follicular structures. In all double-mutant hair types, the medullary cells failed to form well-defined ladders of pigment bands, as the medullary pigment appeared amorphously clumped. The hair fibers of Bmpr2 single mutants (K14-Cre;Bmpr2flox/flox mice) at times displayed a similar clumping and disorganization of pigment in regions of the hair medulla (Fig. 5e, which shows a zigzag hair). Thus, Bmpr2 and Acvr2a are necessary for the development of hair shafts of proper length and structure.
FIG. 5.
Bmpr2/Acvr2a double mutants exhibit macrostructural defects in hair fibers. (a, b) Full-length views of intact coat hair from control (a) and K14-Cre;Bmpr2flox/flox;Acvr2a+/− (b) mice are shown. Examples of the four hair types — guard (gu), awl (aw), auchene (au), and zigzag (z) — are indicated. The double mutants display a shortening of the awl, auchene, and zigzag fibers and a curvature of the guard and awl fibers. (c–e) High magnification views of intact hair fibers from control (c), K14-Cre;Bmpr2flox/flox;Acvr2a+/− (d), and K14-Cre;Bmpr2flox/flox (e) mice are shown. Double-mutant awl and auchene hair follicles (d) fail to produce multiple columns of medullary cells, generating a single column instead. The hair medulla is structurally abnormal in all hair types of the double mutant (d) and in occasional hairs of the single mutant (e). (f–g) High magnification views of intact hair-shaft bases from control (f) and K14-Cre;Bmpr2flox/flox;Acvr2a+/− (g) mice are shown. The hair club (HC) develops on control hairs but not on double-mutant hairs, which exhibit tapered, wavy ends instead. Scale bar for panels c–g, 25 µm.
The final component of the hair to develop is the club, which forms from keratinized epithelial cells, envelops the base of the shaft, and interdigitates with the surrounding outer root sheath to hold the hair in place. Whereas control mice generated well-formed clubs, no club structures were observed in hair from K14-Cre;Bmpr2flox/flox;Acvr2a+/− mice (Fig. 5f–g). Instead, the double-mutant hair shafts possessed ends of variable length that were tapered, thin, and wavy. Identical observations were made when hair was collected from the double mutants with a comb rather than a forceps. Gentle combing easily dislodged double-mutant hair during late catagen, and these comb-collected hair fibers had the same tapered ends as those gathered with a forceps. Thus, hair shafts failed to acquire hair clubs in the Bmpr2/Acvr2a double mutants, showing that Bmpr2/Acvr2a function is essential for club differentiation and/or stability. As the club is necessary for the retention of a completed hair, the absence of the club is presumably the principal cause of the total hair loss of the double mutants.
Type II Receptors and Follicular Epithelial Cell Proliferation
As catagen includes the cessation of cell proliferation and the involution of the hair follicle via apoptosis, we examined the Bmpr2/Acvr2a mutants for defects in these processes. TUNEL assays did not reveal any clear differences between control and K14-Cre;Bmpr2flox/flox;Acvr2a+/− skin in apoptosis, as similar patterns of cell death were detected during catagen (data not shown). Thus, the Bmpr2 and Acvr2a mutations did not appear to disrupt the distribution of apoptotic cells.
To measure cell proliferation in the skin, BrdU labeling was performed at different ages. During periods of hair production, K14-Cre;Bmpr2flox/flox;Acvr2a+/− mice exhibited normal cell proliferation in most compartments of the skin, as multiplying cells were abundant in the hair matrix and scattered in the outer root sheath and epidermis (Fig. 6a–b and data not shown). However, the double mutants also possessed a compartment with aberrant cell division, the hair medulla, as columns of proliferating epithelial cells rose from the hair matrix along the central axis of the hair shaft, one-third or more of the distance to the skin’s surface (Fig. 6b). Normally, this region of the hair is comprised exclusively of cells undergoing terminal differentiation and lacking the ability to divide. In some hair follicles of Bmpr2 single mutants (i.e., K14-Cre;Bmpr2flox/flox mice), the differentiating medulla contained proliferating cells as well (Fig. 6c), though such cells were fewer in number than those of the double mutants. Thus, a lack of type II receptor function led to a failure of pre-medullary cells to withdraw from the cell cycle. During catagen, K14-Cre;Bmpr2flox/flox;Acvr2a+/− skin did not exhibit cell proliferation in an abnormal location but instead displayed an increase in proliferating cells in the ‘permanent’ segment of the ORS (the superficial ORS segment that survives catagen; Fig. 6d–e). Likewise, at the onset of anagen, K14-Cre;Bmpr2flox/flox;Acvr2a+/− mice exhibited substantially more proliferating cells in the epidermis than controls (Fig. 6f–g), consistent with the thick epidermis observed at this time in the double mutant (Fig. 3b). Thus, a lack of type II receptor function led to localized increases in cell proliferation, suggesting that Bmpr2 and Acvr2a normally promote the cessation of growth. This growth-inhibitory function appears necessary for the differentiation of the hair medulla.
FIG. 6.
Mutations in Bmpr2 and Acvr2a cause increases in cell proliferation. Skin sections of control (a, d, f), K14-Cre;Bmpr2flox/flox;Acvr2a+/− (b, e, g) and K14-Cre;Bmpr2flox/flox (c) mice were stained by immunofluorescence using antibodies to BrdU (green; DNA is stained in blue). (a–c) P11. Whereas BrdU-labeled (proliferating) cells are normally absent from the differentiating hair shaft (a), the hair medulla (M) possesses many BrdU-labeled cells in double-mutant hair follicles (b). To a lesser extent, single-mutant hair follicles (c) also exhibit medullary BrdU-labeling (marked by arrowheads). (d, e) P18. During catagen, BrdU-labeled cells are elevated in the superficial segments of double-mutant hair follicles. Asterisks denote background staining in the stratum corneum of the epidermis. (f, g) P21. At the start of a new anagen, BrdU-labeled cells are more prominent in the epidermis of the double mutant. Dotted lines in panels d–g indicate the epithelial-dermal boundary. HM, hair matrix. Scale bar, 50 µm.
Type II Receptor Inactivation Causes Gray Hair
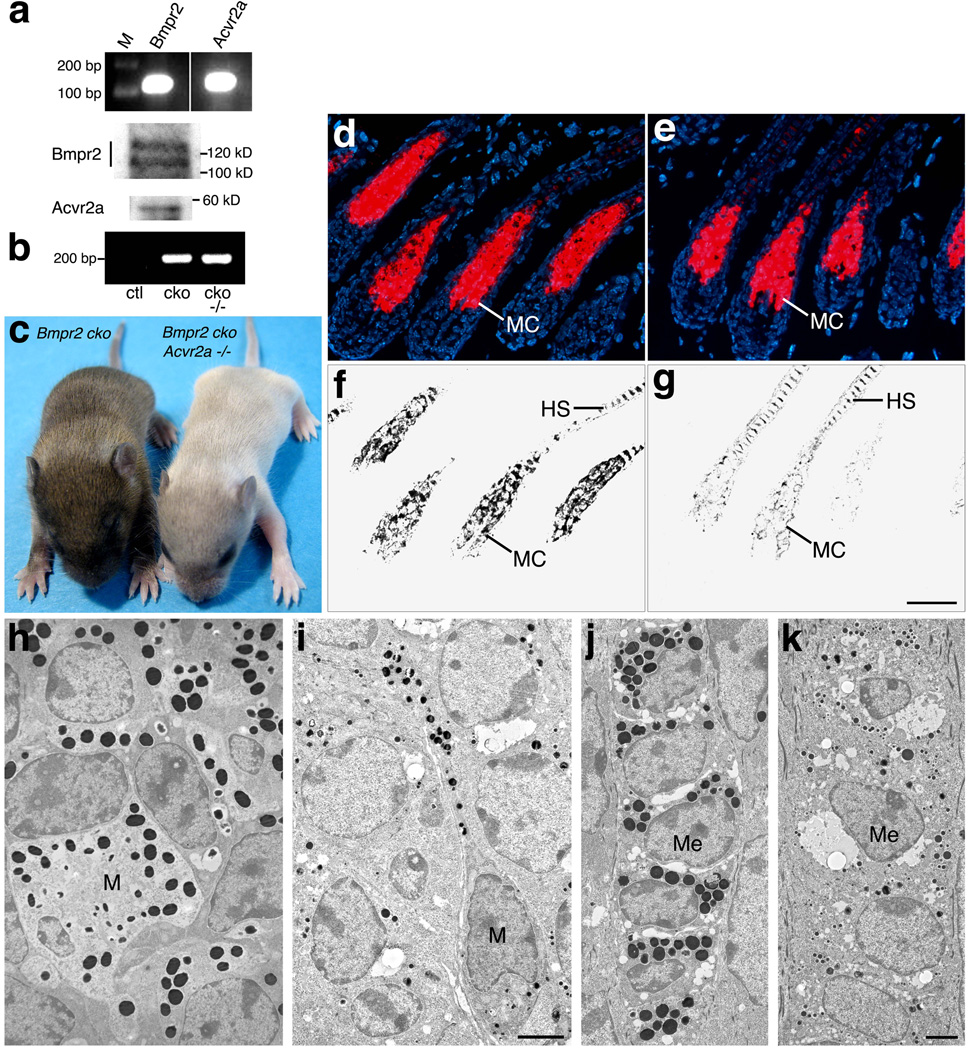
BMPR2 (Yaar et al., 2006) and ACVR2A (Stove et al., 2004) are expressed in human melanocytes, and this expression is conserved in murine melanocytes, as these cells possessed the Bmpr2 and Acvr2a transcripts and proteins (Fig. 7a). To determine the roles of type II receptors in pigment cells, mice were generated in which Bmpr2 was specifically ablated from the melanocyte lineage and Acvr2a was rendered wild-type (Acvr2a+/+), reduced in dosage (Acvr2a+/−), or functionally lacking (Acvr2a−/−). To delete Bmpr2 from melanocytes, the Bmpr2flox allele was converted to a null allele using Tyr::Cre (Fig. 7b), an X-linked transgene that produces Cre from the promoter for the melanogenic enzyme tyrosinase (Delmas et al., 2003).
FIG. 7.
Loss of Bmpr2 and Acvr2a in melanocytes inhibits melanosome maturation. (a) Bmpr2 and Acvr2a expression are detected in primary murine melanocytes by RT-PCR (upper panel) and immunoblotting (middle and lower panels). M, markers. (b) Tail DNA was probed by PCR for the Tyr::Cre-driven conversion of the Bmpr2flox allele to the deletion (null) allele. Representative PCR products from Bmpr2flox/flox;Acvr2a+/− (ctl, control), Tyr-Cre;Bmpr2flox/flox (cko, conditional knockout), and Tyr::Cre;Bmpr2flox/flox;Acvr2a−/− (cko −/−) mice are shown on an agarose gel. The Tyr::Cre transgene generates the Bmpr2 deletion allele. (c) Tyr::Cre;Bmpr2flox/flox(left; Bmpr2 cko) and Tyr::Cre;Bmpr2flox/flox;Acvr2a−/−(right) mice are shown at P10. The Bmpr2/Acvr2a double knockout develops gray hair during coat development. (d, e) Skin sections of P10 Tyr-Cre;Bmpr2flox/flox (d) and Tyr-Cre;Bmpr2flox/flox;Acvr2a−/− (e) mice are stained for melanocytes by the TTA assay (red; DNA is stained in blue). Double-mutant hair follicles develop a melanocyte cluster (MC) of normal size and location. (f, g) Bright-field views are shown of the sections in panels d and e above. Despite their robust melanocyte clusters (e), the double-mutant hair follicles generate low levels of melanin (g). (h–k) Skin sections from P10 Tyr-Cre;Bmpr2flox/flox(h, j) and Tyr-Cre;Bmpr2flox/flox;Acvr2a−/−(i, k) mice are shown in transmission electron micrographs. Melanosomes are visible in melanocytes (M; panels h, i) and medullary cells (ME; panels j, k) as black, spherical or ovoid bodies. The melanosomes of the double mutant are darkly pigmented but substantially reduced in size. HS, hair shaft. Scale bar for panels d–g, 50 µm; h–k, 0.1 µm.
Mice exhibited normal coat color as long as the melanocyte lineage possessed at least one fully functional allele of either Bmpr2 or Acvr2a. But when their genotype was Tyr::Cre;Bmpr2flox/flox;Acvr2a−/−, a dramatic loss of pigmentation was observed, as gray hair developed during the first period of hair production. Male pups developed uniformly gray coats (Fig. 7c), while female pups, which were hemizygous for Tyr::Cre, produced gray hair mosaically (not shown), consistent with the mosaic inactivation of the Tyr::Cre-carrying X chromosome. Thus, Bmpr2 and Acvr2a are redundant individually but together essential for the development of pigmented hair.
To determine the status of melanocytes in the gray-haired mice, skin sections were stained using tyramide-based tyrosinase assay (TTA; Han et al., 2002), a sensitive technique that detects active tyrosinase in situ and thereby identifies pigment cells, including melanoblasts and other relatively undifferentiated melanocyte progenitors. As shown in Fig. 7d–e, uniformly gray-haired mice exhibited the same TTA staining pattern as their normally pigmented littermates, suggesting that melanocytes were normal in location and approximately normal in number in gray-haired animals. Immunofluorescent staining for the melanocyte markers Tyrp1 and Mitf was likewise similar in normal and gray-haired skin samples, again consistent with a normal distribution of melanocytes in gray-haired skin (data not shown). Despite this normal melanocyte distribution but consistent with the hair coat’s hypopigmentation, gray-haired skin displayed substantially less melanin in the hair follicles than normal skin (Fig. 7f–g, which shows the same hair follicles as Fig. 7d–e). The decrease in melanin was evident in the hair bulb, where melanocytes form a conical cluster, as well as in the hair shaft. Thus, the lack of pigmentation did not result from a lack of melanocytes but from a failure of melanocytes to produce or accumulate melanin.
To identify the defects underlying this melanin deficiency, skin from normally pigmented and gray-haired mice was examined by transmission electron microscopy. The melanin deficiency was thus traced to an abnormality in the development of melanosomes, the melanocyte organelles responsible for melanin synthesis. As shown in Fig. 7h–i, the melanocytes of gray-haired skin produced ‘miniaturized’ melanosomes, i.e., melanosomes substantially smaller in size than those developed by normal melanocytes. This reduction in melanosome size was not accompanied by a substantial reduction in melanosome number or pigment-making ability, as the miniaturized melanosomes were numerous and filled with pigment, appearing about as heavily melanized as normal melanosomes. Thus, in gray-haired skin, the principal melanogenic defect appeared to be an impairment of melanosome growth, leading to the development of small melanosomes and the production/accumulation of less melanin per melanosome. Like the melanosomes of normal skin, the miniaturized melanosomes were transferred to epithelial cells of the hair shaft (Fig. 7j–k); but given each melanosome’s reduced melanin content, the hair shafts received substantially less pigment in total, resulting in their hypopigmentation and gray color. Thus, the lack of Bmpr2 and Acvr2a led to a failure of melanosomes to grow to their normal size, which in turn resulted in the production of small quantities of melanin. These results show that Bmpr2 and Acvr2a are necessary for proper melanosome biogenesis.
DISCUSSION
Previous studies performed in vitro have suggested that the Bmp type II receptor binds Bmps exclusively whereas the activin type II receptors bind activins and Bmps (Attisano et al., 1992; Ebisawa et al., 1999; Hoodless et al., 1996; Liu et al., 1995; Mathews and Vale, 1991; Mathews et al., 1992; Nishitoh et al. 1996; Nohno et al., 1995; Rosenzweig et al., 1995; Yamashita et al., 1995). Similarly, studies performed in cell culture have shown that Bmpr2 and Acvr2a can transduce Bmp signals and that each receptor can partially compensate for the loss of the other (Lavery et al., 2008; Yu et al., 2005). Our results extend these findings, as the present study demonstrates for the first time that Bmpr2 and Acvr2a perform compensatory functions in animals and that this functional overlap occurs in multiple cell lineages, including epithelial and pigment cell lineages. In cutaneous epithelial cells, the effects of Bmpr2 and Acvr2a appear largely additive, as the defects of Bmpr2-deficient epithelia become progressively more severe as Acvr2a decreases (i.e., as genotypes change from Acvr2a+/+ to Acvr2a+/− to Acvr2a−/−). In contrast, melanocytes generate a normal coat color until they are deficient in both Bmpr2 and Acvr2a, at which point they exhibit substantial defects in melanosome development and largely fail to pigment the coat. Thus, type II receptor function is necessary for proper epithelial and melanocytic morphogenesis, and the precise level of receptor function required — and the threshold separating normal from abnormal development — appears to be cell-type or trait specific.
Based on several lines of evidence, we think it likely that the skin defects of the K14-Cre;Bmpr2flox/flox;Acvr2a+/− mice result primarily from a lack of Bmp signaling. Firstly, some of these defects, such as those of the hair medulla, are observed in milder form in K14-Cre;Bmpr2flox/flox mice, whose principal signaling abnormality is presumably a reduction in Bmp signaling. Secondly, K14-Cre;Bmpr2flox/+;Acvr2a−/−mice (generated by us in this study) do not display any of the defects found in K14-Cre;Bmpr2flox/flox;Acvr2a+/− mice, such as the abnormalities in follicular differentiation, cycling, club formation, or cell proliferation (data not shown). Thus, these processes seem to require the signaling of ligands that primarily use Bmpr2. Finally, when the Bmp type I receptor Bmpr1a is ablated from murine cutaneous epithelium, hair shafts fail to differentiate (Andl et al., 2004; Kobielak et al., 2003; Ming Kwan et al., 2004; Yuhki et al., 2004), a phenotype more severe than but analogous to the failure of the hair medulla to differentiate in the Bmpr2/Acvr2a double mutants. Thus, the phenotypes of the K14-Cre;Bmpr2flox/flox;Acvr2a+/− mice appear more closely associated with the impairment of Bmp function than activin function. If the phenotypes of the Tyr-Cre;Bmpr2flox/flox;Acvr2a−/− mice have a similar basis, then the defects in melanosome biogenesis also result primarily from a loss of Bmp signaling, though other explanations cannot be ruled out, such as synergistic effects of losses in activin and Bmp signaling.
Previous studies have suggested a key role for the Bmp pathway in the initiation of hair growth, as Bmp signaling was found to prevent the activation of stem cells in quiescent follicles (Kobielak et al., 2007; Plikus et al., 2008; Zhang et al., 2006) and hence to prevent the start of new anagens (Plikus et al., 2008). Our results support these findings. The K14-Cre;Bmpr2flox/flox;Acvr2a+/− mice display shortened telogens and premature re-entry into anagen, showing that Bmpr2 and Acvr2a are necessary for the proper timing of telogen-to-anagen transitions. As the effects of Bmpr2/Acvr2a deficiency on anagen entry closely resemble the effects of the Krt14-Nog transgene (Plikus et al., 2004; Plikus et al., 2008), the defects of the type II receptor mutant appear to result from a lack of Bmp signaling. Likewise, Bmp signaling has been implicated in other aspects of hair-cycle regulation. In particular, a deficiency of Bmpr1a in cutaneous epithelial cells abolished the hair cycle, as follicular progenitor cells exhibited sustained proliferation, ultimately forming cysts and tumors (Andl et al., 2004; Kobielak et al., 2003; Ming Kwan et al., 2004; Yuhki et al., 2004; Zhang et al., 2006). Additionally, mice carrying an NSE-Noggin transgene, which produces noggin in hair matrix cells, exhibited accelerated follicular cycling, due primarily to premature exit from anagen (Guha et al., 2004). As shown in the present study, the K14-Cre;Bmpr2flox/flox;Acvr2a+/− mice exhibit a shortened anagen, premature entry into catagen, and slowed progression through catagen, resulting in delayed entry into telogen. As such, Bmpr2 and Acvr2a function are necessary for the correct duration of anagen and catagen as well as telogen. In particular, as Bmpr2/Acvr2a deficiency accelerates entry into anagen and catagen, Bmp signaling seemingly antagonizes or inhibits inducers of both of these hair-cycle phases. In all, taking our study together with others, Bmp signaling appears to be a key determinant of the timing and time-span of every phase of the hair cycle and to regulate the transition from each phase to the next.
The type II receptor mutants of this study display defects during anagen that are similar in kind, but different in extent, to the anagen defects of other Bmp pathway mutants. As shown here, the ablation of Bmpr2 alone in epithelial cells (K14-Cre;Bmpr2flox/flox mice) affects the differentiation of one follicular cell lineage, namely, the medullary cell lineage, and this lineage exhibits more severe defects when the Bmpr2 ablation is coupled to a reduction in Acvr2a (K14-Cre;Bmpr2flox/flox;Acvr2a+/− mice). In comparison, Msx2-Noggin transgenic mice, which produce the Bmp inhibitor noggin from a promoter active in hair-shaft precursor cells, lack all three differentiated lineages of the hair (the medullary, cortical, and cuticular lineages; Kulessa et al., 2000). The epithelial ablation of Bmpr1a blocks the differentiation of six follicular lineages, namely, the three lineages of the hair shaft and three lineages of the inner root sheath (Henle's layer, Huxley's layer, and cuticle) (Andl et al., 2004; Kobielak et al., 2003; Ming Kwan et al., 2004; Yuhki et al., 2004).
One explanation for these differing phenotypes involves threshold effects: that is, the various lineages require different threshold levels of Bmp signaling to differentiate and the differing phenotypes reflect the differing extents to which this signaling is inhibited. A second explanation involves the complexity and likely specialization of Bmp signal transducers. Mammals possess at least 10 different Bmps (reviewed in Schmierer and Hill, 2007), and their signals can be transduced by 3 different type II receptors (Bmpr2, Acvr2a, and Acvr2b) and 3 different type I receptors (Bmpr1a, Bmpr1b, and Acvr1a) (Ebisawa et al., 1999; Fujii et al., 1999; Koenig et al., 1994; Lavery et al., 2008; Liu et al., 1995; Macias-Silva et al., 1998; Nishitoh et al., 1996; Nohno et al., 1995; Rosenzwieg et al., 1995; ten Dijke et al., 1994; Yamashita et al., 1995; Yu et al., 2005). These 6 receptors in turn can interact in a variety of combinations and form a variety of signaling complexes, as for example Acvr2a forms signaling complexes with all 3 Bmp-binding type I receptors (Lavery et al., 2008; Liu et al., 1995; Nishitoh et al., 1996; Yamashita et al., 1995; Yu et al., 2005). Moreover, each combination of receptors appears to bind a particular subset of Bmps preferentially (Ebisawa et al., 1999; Lavery et al., 2008; Macias-Silva et al., 1998; Nishitoh et al., 1996; Nohno et al., 1995; ten Dijke et al., 1994; Yu et al., 2005) and, upon binding these ligands, potentially activates a distinct set or subset of Bmp responses (Fujii et al., 1999; Piscione et al., 1997). As such, the differing phenotypes above may reflect differing downstream targets or outputs of the various receptors. In particular, a decrease in Bmpr2/Acvr2a function, rather than yield a general deficit in Bmp signaling, may instead produce a specific defect, such as an abnormality in the activity of a particular Smad or other (Smad-independent) factor. This specific defect may then specifically affect the differentiation of the hair medulla. Consistent with this idea, we note that, as judged by immunofluorescent staining, the K14-Cre;Bmpr2flox/flox and K14-Cre;Bmpr2flox/flox;Acvr2a+/− mice did not exhibit any obvious differences with wild-type mice in the total (combined) cutaneous levels of phosphorylated Bmp-effector Smads (Smad1/5/8; data not shown), which mediate Bmp effects in the canonical signaling mechanism. As no general phospho-Smad deficit was detected (despite the robust phenotypes of the K14-Cre;Bmpr2flox/flox;Acvr2a+/− mice), Bmpr2 and Acvr2a may preferentially promote a subset of Bmp responses that are especially critical for the differentiation of the hair medulla. As an additional explanation, consistent with the previous two, the differing phenotypes may reflect differing functional redundancies, as Bmpr2 is clearly redundant with Acvr2a (and perhaps with other type II receptors), while Bmpr1a is plainly less redundant, at least in cutaneous epithelial cells.
While failing to generate proper hair medullas, the K14-Cre;Bmpr2flox/flox;Acvr2a+/− mice also fail to produce hair clubs, and this absence presumably results from an impairment of club differentiation (similar to the impairment of medulla differentiation) and/or the abnormal activation of degradative processes, such as those thought to promote hair shedding (exogen; Milner et al., 2002). In conjunction with their differentiation defects, the K14-Cre;Bmpr2flox/flox and K14-Cre;Bmpr2flox/flox;Acvr2a+/− mice display ectopic cell proliferation in the hair medulla, suggesting that the aberrant differentiation of medullary cells is caused by a cell cycle abnormality. Analogous ectopic cell proliferation is observed in Msx2-Noggin mice at the central follicular locations where hair shafts should form (Kulessa et al., 2000). Thus, as the lack of Bmpr2/Acvr2a and a gain of noggin yield similar effects on cell division, the Bmp pathway appears to play an essential role in the inhibition of cell proliferation during hair shaft differentiation.
The K14-Cre;Bmpr2flox/flox;Acvr2a+/− mice exhibit cyclic alopecia (Ma et al., 2003), as each anagen produces dense coat hair, and each catagen brings the total loss of this hair. This type of alopecia has been observed in two other murine mutants: Msx2 null mutants (Msx2−/−), which like the Bmpr2/Acvr2a double mutants exhibit hair loss combined with premature entry into and slow progression through catagen (Ma et al., 2003), and repeated epilation mutants (SfnEr/+), which are heterozygous for a frameshift mutation in stratifin (14-3-3σ; Hunsicker, 1960; Herron et al., 2005; Li et al., 2005) and like the Bmpr2/Acvr2a double mutants lose hair in part due to defects in hair club formation (Xin et al., 2010). Additionally, loss-of-function mutations in Hr (hairless) lead to the total loss of a dense coat as well as excessive nail growth (similar to the nail phenotype observed in the K14-Cre;Bmpr2flox/flox;Acvr2a+/− mice), and while the lost hair does not re-grow, the alopecia results from multiple defects in catagen (reviewed in Panteleyev et al., 1999). Based on immunofluorescent staining, the Msx2, Sfn, and Hr proteins do not exhibit substantial changes in level or localization in the K14-Cre;Bmpr2flox/flox;Acvr2a+/− mice (data not shown), suggesting that other changes (in these or other factors) are responsible for the type II receptor phenotypes. Nonetheless, the similar phenotypes of the Sfn, Msx2, Hr, and Bmpr2/Acvr2a mutants suggest that these proteins are linked or synergistic regulators of the various events of catagen. One major event of catagen — the shortening of the hair follicle — is slowed in K14-Cre;Bmpr2flox/flox;Acvr2a+/− mice, yet the pattern of apoptosis, the process that produces the shortening, appears grossly normal and appropriately matched to follicular morphology, as judged by TUNEL staining. Potentially, the slowed shortening is explained by the increased cell proliferation observed in the follicle’s permanent segment during catagen (Fig. 6e): that is, excessive growth in the permanent segment drives cells ‘downward’ into the transient segment, thus replenishing the transient segment and slowing its regression. An alternative possibility is that the length of the cell death program itself increased: i.e., individual follicular cells take longer to die, or to be eliminated after they die, and the end result is slower regression. In all, the results suggest that Bmpr2/Acvr2a function is not essential for the induction of apoptosis but is important for other processes that affect the rate of follicular regression.
While necessary for the retention of hair, Bmpr2 and Acvr2a are also essential for the pigmentation of hair, as a gray coat color results from a deficiency of these receptors in melanocytes. This loss of pigmentation stems primarily from a miniaturization of the melanosomes, as the melanosomes fill with pigment but are substantially smaller than normal, leading to the accumulation of less melanin per organelle. This result suggests that melanosome growth is a process separable from melanosome differentiation (the acquisition of pigment-making capacity) and that signaling by Bmps, and perhaps activins, promotes melanosome growth. Overall, as miniaturized melanosomes result from Bmpr2/Acvr2a deficiency, the Bmpr2 and Acvr2a receptors are key determinants of melanosome size, providing insight into how cells regulate the development and dimensions of their organelles. As such, the epithelial and melanocytic phenotypes reported here highlight the different uses to which different cells apply the Bmp pathway.
METHODS
Mice
All alleles and transgenes used in this study — Bmpr2flox (Beppu et al., 2005), Bmpr2− (Beppu et al., 2000), Acvr2a− (Song et al., 1999), K14-Cre (Li et al., 2001), and Tyr::Cre (Delmas et al., 2003) — were described previously. All animal studies were conducted under IACUC-approved protocols.
Detection of Bmpr2 ablation and Acvr2a alleles
To detect the K14-Cre-driven conversion of the Bmpr2flox allele to a null allele, tail skins of one-month-old mice were heated for 1 min. in phosphate-buffered saline (PBS) at 60°C and transferred immediately to cold PBS (4°C). The epidermis was then scraped off the dermis with a scalpel. DNA was prepared separately from the epidermal and dermal compartments by heating samples in 50 mM NaOH (600 µl) at 95°C for 30 min., neutralizing with 1 M Tris-HCl, pH 8.0 (50 µl), and centrifuging for 5 min. at room temperature. The extracted DNA was then used for PCR analysis. Primers A and 6R were used to amplify a fragment of Bmpr2 floxed allele before Cre-mediated deletion; primers A and C were used to amplify a fragment of Bmpr2 sequence only after Cre-mediated deletion. The sequences of primers were: primer A, 5′-CAC ACC AGC CTT ATA CTC TAG ATA C-3′; primer C, 5′-TTA TTG TAA GTA CAC TGT TGC TGT C-3′; primer 6R, 5′-CAC ATA TCT GTT ATG AAA CTT GAG-3′ (Beppu et al., 2005).
To perform routine genotyping and to detect the Tyr::Cre-driven conversion of the Bmpr2flox allele to a null allele, tail DNA was probed by PCR; the conversion to the null allele was assayed using primers A and C above. To detect the Acvr2a− allele, the following primers were used: forward, 5′-TCA GGA CAT AGC GTT GGC TAC C-3′; reverse, 5′-GCT GAA GTA AGA GGA ACC TGC TC-3′. The forward primer recognizes a sequence within the neoR insertion of Acvr2a−, while the reverse primer recognizes a sequence downstream from the insertion; the resulting product thus spans the downstream insertion site. To detect the Acvr2a+ allele by PCR, the following primers were used: forward, 5′-GAA TTG GCT TCT CGT TGC ACT G-3′; reverse, 5′-CTC CAC CTA CAC CCT ACT TGA G-3′. These primers amplify a region that is deleted in the Acvr2a− allele (i.e., parts of exon 9 and intron 9). To detect the Cre transgenes, the following primers were used: forward, 5′-GGT CGA TGC AAC GAG TGA TGA GGT-3′; reverse, 5′-CAG CAT TGC TGT CAC TTG GTC GTG-3′.
Histological Analyses, Immunofluorescence, Tyrosinase Assays, Hair Fiber Analyses, Anagen Measurement, and Electron Microscopy
Back skin was removed from mice and either embedded in OCT and flash-frozen or fixed in 10% buffered formalin (Fisher Scientific) and embedded in paraffin. Frozen skin sections were fixed in 1:1 methanol:acetone at −20°C immediately after adherence to glass slides. For histological analyses, sections were stained with hematoxylin and eosin.
Immunofluorescent analyses were carried out as described (Weiner and Green, 1998) with little modification. Frozen sections were blocked in SuperBlock T20 PBS blocking buffer (Thermo Scientific), incubated sequentially with rabbit polyclonal anti-trichohyalin antibody (1:3000; the gift of Dr. George E. Rogers, University of Adelaide), goat-anti-rabbit IgG (1:2000; Santa Cruz Biotechnology), and streptavidin-CY3 conjugates (1:700, Sigma-Aldrich, Inc.), and counterstained with Hoechst dye 33258 (Fluka).
Tyramide-based tyrosinase assay (TTA) was performed as described (Han et al., 2002) on frozen sections.
Hair fibers were collected from back of mice with a forceps or comb and then mounted on glass slides with Cytoseal XYL mounting medium (Richard-Allan Scientific).
The length of anagen was evaluated by monitoring skin pigmentation according to the method of Plikus et al., 2008. To ensure the comparison of similar follicle types and body sites, measurements were made exclusively on skin located in the anterior dorsal region of the body, between the front shoulders and on or near the body’s midline (i.e., the ‘upper back’).
For electron microscopy, back skin was removed from mice, fixed in 4% methanol-free formaldehyde, and embedded in epon epoxy. Ultrathin sections were viewed in a transmission electron microscope.
Cell Proliferation Assays
5-bromo-2′-deoxyuridine (BrdU; Invitrogen) was diluted in PBS to 10 mg/ml and sterile filtered. BrdU solution was then injected intraperitoneally into mice at 100 µg/g body weight. After two hours, mice were sacrificed, and skin was removed from the back. Skin samples were then embedded in OCT compound, flash-frozen, sectioned, and fixed in methanol immediately after adherence to glass slides. To detect incorporated BrdU, sections were re-hydrated with PBS, denatured by treatment with freshly made 2N HCl for 30 min at room temperature, and then neutralized by three washes (5 min. each) with 0.1M sodium borate, pH 8.5. Sections were next washed three times with PBS, incubated with Alexa Fluor 488-conjugated anti-BrdU (murine monoclonal PRB-1, diluted 1:20–1:50; Invitrogen) for 2 hours at room temperature, washed three times with PBS, and counterstained with Hoechst 33258 dye.
Melanocyte Cultures
Primary melanocytes were cultured from the epidermis of newborn wild-type mice as described (Halaban et al., 1998). Melanocytes were grown in Ham’s F10 medium (Invitrogen) supplemented with 7% horse serum (Hyclone), 7% fetal bovine serum (Hyclone), 50 mM dbcAMP (N6, 2'-dibutytryladenosine 3':5'-cyclic monophosphate; Sigma), 1 mM Na3VO4, 2 mM L-glutamine (Invitrogen), 100 U/ml penicillin, and 100 mg/ml streptomycin (Invitrogen).
mRNA and Protein Analyses
Bmpr2 and Acvr2a mRNA were detected by RT-PCR in primary melanocytes. Total RNA was isolated using the RNeasy Mini Kit (Qiagen), and reverse transcribed into cDNA using the iScript cDNA Synthesis Kit (Bio-Rad). cDNA and primers were added to GoTaq Green master Mix (Promega) for a 40-cycle PCR reaction, and the products were run on 2% agarose gels. For Bmpr2, the primers were 5′-CAT CAA AGC CCA GAA GAG CAC AG-3′ and 5′- CAC CTG ATC CTG ATT TGC CAT C-3′, generating an expected product 120 bp. For Acvr2a, the primers were 5′- TCC AAA GGG ACG CAT TTC TGA G-3′ and 5’- GAT GCT GGC CAA TTT CTT CCT C-3′, generating an expected product 138 bp. Both primer sets spanned introns and thus specifically amplified cDNA.
Bmpr2 and Acvr2a proteins were analyzed in primary melanocytes by immunoblotting. Cell lysates were generated using 2% SDS, 50 mM Tris-HCl, pH 6.8, separated on 7.5% SDS-polyacrylamide gels, and transferred to Immobilon membranes (Millipore). Blots were blocked with 5% milk/TBST (Tris-buffered saline, 0.1% Tween-20) and probed under the same conditions with murine monoclonal antibodies to Bmpr2 (1:500; BD Biosciences) or goat polyclonal antibodies to Acvr2a (N-17, 1:250; Santa Cruz Biotechnology). Primary antibody reactions were detected using horseradish peroxidase (HRP)-conjugated anti-mouse antibodies (for the antibodies to Bmpr2; GE Healthcare) and HRP-conjugated donkey anti-goat IgG (for the antibodies to Acvr2a; Santa Cruz Biotechnology). Antibody reactions were visualized using the SuperSignal West Femto Maximum Sensitivity Substrate (for Bmpr2 immunoblots; Thermo Scientific) or the Western Lightning Chemiluminescence Reagent Plus (for Acvr2a immunoblots; PerkinElmer).
Supplementary Material
ACKNOWLEDGMENTS
We thank George E. Rogers for antibodies to trichohyalin, Yimin Ruan for technical assistance, Pierre Chambon for K14-Cre mice, and Mary McKee and Wei Quan for assistance with electron microscopy. This work was supported by grants from NIH/NIAMS to J.L.B. (AR045284 and AR055218), NIH/NICHD to J.L.B. and E.L. (HD035286), and the Cutaneous Biology Research Center via the Massachusetts General Hospital/Shiseido Co. Ltd. Agreement to J.L.B. L.L. was supported by the Ligue Nationale Contre le Cancer (Equipe labellisÈe).
REFERENCES
- Alanko T, Saksela O. Transforming growth factor beta1 induces apoptosis in normal melanocytes but not in nevus cells grown in type I collagen gel. J Invest Dermatol. 2000;115:286–291. doi: 10.1046/j.1523-1747.2000.00045.x. [DOI] [PubMed] [Google Scholar]
- Andl T, Ahn K, Kairo A, Chu EY, Wine-Lee L, Reddy ST, Croft NJ, Cebra-Thomas JA, Metzger D, Chambon P, Lyons KM, Mishina Y, Seykora JT, Crenshaw EB, 3rd, Millar SE. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 2004;131:2257–2268. doi: 10.1242/dev.01125. [DOI] [PubMed] [Google Scholar]
- Attisano L, Wrana JL, Cheifetz S, Massague J. Novel activin receptors: distinct genes and alternative mRNA splicing generate a repertoire of serine/threonine kinase receptors. Cell. 1992;68:97–108. doi: 10.1016/0092-8674(92)90209-u. [DOI] [PubMed] [Google Scholar]
- Beppu H, Kawabata M, Hamamoto T, Chytil A, Minowa O, Noda T, Miyazono K. BMP type II receptor is required for gastrulation and early development of mouse embryos. Dev Biol. 2000;221:249–258. doi: 10.1006/dbio.2000.9670. [DOI] [PubMed] [Google Scholar]
- Beppu H, Lei H, Bloch KD, Li E. Generation of a floxed allele of the mouse BMP type II receptor gene. Genesis. 2005;41:133–137. doi: 10.1002/gene.20099. [DOI] [PubMed] [Google Scholar]
- Bilodeau ML, Greulich JD, Hullinger RL, Bertolotto C, Ballotti R, Andrisani OM. BMP-2 stimulates tyrosinase gene expression and melanogenesis in differentiated melanocytes. Pigment Cell Res. 2001;14:328–336. doi: 10.1034/j.1600-0749.2001.140504.x. [DOI] [PubMed] [Google Scholar]
- Blessing M, Nanney LB, King LE, Jones CM, Hogan BL. Transgenic mice as a model to study the role of TGF-beta-related molecules in hair follicles. Genes Dev. 1993;7:204–215. doi: 10.1101/gad.7.2.204. [DOI] [PubMed] [Google Scholar]
- Blessing M, Schirmacher P, Kaiser S. Overexpression of bone morphogenetic protein-6 (BMP-6) in the epidermis of transgenic mice: inhibition or stimulation of proliferation depending on the pattern of transgene expression and formation of psoriatic lesions. J Cell Biol. 1996;135:227–239. doi: 10.1083/jcb.135.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchkarev VA, Botchkareva NV, Roth W, Nakamura M, Chen LH, Herzog W, Lindner G, McMahon JA, Peters C, Lauster R, McMahon AP, Paus R. Noggin is a mesenchymally derived stimulator of hair-follicle induction. Nat Cell Biol. 1999;1:158–164. doi: 10.1038/11078. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Sharov AA. BMP signaling in the control of skin development and hair follicle growth. Differentiation. 2004;72:512–526. doi: 10.1111/j.1432-0436.2004.07209005.x. [DOI] [PubMed] [Google Scholar]
- Chang H, Brown CW, Matzuk MM. Genetic analysis of the mammalian transforming growth factor-beta superfamily. Endocr Rev. 2002;23:787–823. doi: 10.1210/er.2002-0003. [DOI] [PubMed] [Google Scholar]
- Delmas V, Martinozzi S, Bourgeois Y, Holzenberger M, Larue L. Cre-mediated recombination in the skin melanocyte lineage. Genesis. 2003;36:73–80. doi: 10.1002/gene.10197. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Ebisawa T, Tada K, Kitajima I, Tojo K, Sampath TK, Kawabata M, Miyazono K, Imamura T. Characterization of bone morphogenetic protein-6 signaling pathways in osteoblast differentiation. J Cell Sci. 1999;112(Pt 20):3519–3527. doi: 10.1242/jcs.112.20.3519. [DOI] [PubMed] [Google Scholar]
- Fietz MJ, McLaughlan CJ, Campbell MT, Rogers GE. Analysis of the sheep trichohyalin gene: potential structural and calcium-binding roles of trichohyalin in the hair follicle. J Cell Biol. 1993;121:855–865. doi: 10.1083/jcb.121.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii M, Takeda K, Imamura T, Aoki H, Sampath TK, Enomoto S, Kawabata M, Kato M, Ichijo H, Miyazono K. Roles of bone morphogenetic protein type I receptors and Smad proteins in osteoblast and chondroblast differentiation. Mol Biol Cell. 1999;10:3801–3813. doi: 10.1091/mbc.10.11.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha U, Mecklenburg L, Cowin P, Kan L, O'Guin WM, D'Vizio D, Pestell RG, Paus R, Kessler JA. Bone morphogenetic protein signaling regulates postnatal hair follicle differentiation and cycling. Am J Pathol. 2004;165:729–740. doi: 10.1016/S0002-9440(10)63336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaban R, Cheng E, Zhang Y, Mandigo CE, Miglarese MR. Release of cell cycle constraints in mouse melanocytes by overexpressed mutant E2F1E132, but not by deletion of p16INK4A or p21WAF1/CIP1. Oncogene. 1998;16:2489–2501. doi: 10.1038/sj.onc.1201773. [DOI] [PubMed] [Google Scholar]
- Han R, Baden HP, Brissette JL, Weiner L. Redefining the skin's pigmentary system with a novel tyrosinase assay. Pigment Cell Res. 2002;15:290–297. doi: 10.1034/j.1600-0749.2002.02027.x. [DOI] [PubMed] [Google Scholar]
- He W, Li AG, Wang D, Han S, Zheng B, Goumans MJ, Ten Dijke P, Wang XJ. Overexpression of Smad7 results in severe pathological alterations in multiple epithelial tissues. Embo J. 2002;21:2580–2590. doi: 10.1093/emboj/21.11.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron BJ, Liddell RA, Parker A, Grant S, Kinne J, Fisher JK, Siracusa LD. A mutation in stratifin is responsible for the repeated epilation (Er) phenotype in mice. Nat Genet. 2005;37:1210–1212. doi: 10.1038/ng1652. [DOI] [PubMed] [Google Scholar]
- Hoodless PA, Haerry T, Abdollah S, Stapleton M, O'Connor MB, Attisano L, Wrana JL. MADR1, a MAD-related protein that functions in BMP2 signaling pathways. Cell. 1996;85:489–500. doi: 10.1016/s0092-8674(00)81250-7. [DOI] [PubMed] [Google Scholar]
- Hsu MY, Rovinsky SA, Lai CY, Qasem S, Liu X, How J, Engelhardt JF, Murphy GF. Aggressive melanoma cells escape from BMP7-mediated autocrine growth inhibition through coordinated Noggin upregulation. Lab Invest. 2008;88:842–855. doi: 10.1038/labinvest.2008.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsicker PR. Repeated epilation, Er. Mouse News Lett. 1960;23:58–59. [Google Scholar]
- Jin EJ, Erickson CA, Takada S, Burrus LW. Wnt and BMP signaling govern lineage segregation of melanocytes in the avian embryo. Dev Biol. 2001;233:22–37. doi: 10.1006/dbio.2001.0222. [DOI] [PubMed] [Google Scholar]
- Klopcic B, Maass T, Meyer E, Lehr HA, Metzger D, Chambon P, Mann A, Blessing M. TGF-beta superfamily signaling is essential for tooth and hair morphogenesis and differentiation. Eur J Cell Biol. 2007;86:781–799. doi: 10.1016/j.ejcb.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Kobielak K, Pasolli HA, Alonso L, Polak L, Fuchs E. Defining BMP functions in the hair follicle by conditional ablation of BMP receptor IA. J Cell Biol. 2003;163:609–623. doi: 10.1083/jcb.200309042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobielak K, Stokes N, de la Cruz J, Polak L, Fuchs E. Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling. Proc Natl Acad Sci U S A. 2007;104:10063–10068. doi: 10.1073/pnas.0703004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig BB, Cook JS, Wolsing DH, Ting J, Tiesman JP, Correa PE, Olson CA, Pecquet AL, Ventura F, Grant RA, Chen GX, Wrana JL, Massague J, Rosenbaum JS. Characterization and cloning of a receptor for BMP-2 and BMP-4 from NIH 3T3 cells. Mol Cell Biol. 1994;14:5961–5974. doi: 10.1128/mcb.14.9.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulessa H, Turk G, Hogan BL. Inhibition of Bmp signaling affects growth and differentiation in the anagen hair follicle. Embo J. 2000;19:6664–6674. doi: 10.1093/emboj/19.24.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavery K, Swain P, Falb D, Alaoui-Ismaili MH. BMP-2/4 and BMP-6/7 differentially utilize cell surface receptors to induce osteoblastic differentiation of human bone marrow-derived mesenchymal stem cells. J Biol Chem. 2008;283:20948–20958. doi: 10.1074/jbc.M800850200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AG, Koster MI, Wang XJ. Roles of TGFbeta signaling in epidermal/appendage development. Cytokine Growth Factor Rev. 2003;14:99–111. doi: 10.1016/s1359-6101(03)00005-4. [DOI] [PubMed] [Google Scholar]
- Li M, Chiba H, Warot X, Messaddeq N, Gerard C, Chambon P, Metzger D. RXR-alpha ablation in skin keratinocytes results in alopecia and epidermal alterations. Development. 2001;128:675–688. doi: 10.1242/dev.128.5.675. [DOI] [PubMed] [Google Scholar]
- Li Q, Lu Q, Estepa G, Verma IM. Identification of 14-3-3sigma mutation causing cutaneous abnormality in repeated-epilation mutant mouse. Proc Natl Acad Sci USA. 2005;102:15977–15982. doi: 10.1073/pnas.0508310102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Ventura F, Doody J, Massague J. Human type II receptor for bone morphogenic proteins (BMPs): extension of the two-kinase receptor model to the BMPs. Mol Cell Biol. 1995;15:3479–3486. doi: 10.1128/mcb.15.7.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Liu J, Wu T, Plikus M, Jiang TX, Bi Q, Liu YH, Muller-Rover S, Peters H, Sundberg JP, Maxson R, Maas RL, Chuong CM. 'Cyclic alopecia' in Msx2 mutants: defects in hair cycling and hair shaft differentiation. Development. 2003;130:379–389. doi: 10.1242/dev.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias-Silva M, Hoodless PA, Tang SJ, Buchwald M, Wrana JL. Specific activation of Smad1 signaling pathways by the BMP7 type I receptor, ALK2. J Biol Chem. 1998;273:25628–25636. doi: 10.1074/jbc.273.40.25628. [DOI] [PubMed] [Google Scholar]
- Mathews LS, Vale WW. Expression cloning of an activin receptor, a predicted transmembrane serine kinase. Cell. 1991;65:973–982. doi: 10.1016/0092-8674(91)90549-e. [DOI] [PubMed] [Google Scholar]
- Mathews LS, Vale WW, Kintner CR. Cloning of a second type of activin receptor and functional characterization in Xenopus embryos. Science. 1992;255:1702–1705. doi: 10.1126/science.1313188. [DOI] [PubMed] [Google Scholar]
- Milner Y, Sudnik J, Filippi M, Kizoulis M, Kashgarian M, Stenn K. Exogen, shedding phase of the hair growth cycle: characterization of a mouse model. J Invest Dermatol. 2002;119:639–644. doi: 10.1046/j.1523-1747.2002.01842.x. [DOI] [PubMed] [Google Scholar]
- Ming Kwan K, Li AG, Wang XJ, Wurst W, Behringer RR. Essential roles of BMPR-IA signaling in differentiation and growth of hair follicles and in skin tumorigenesis. Genesis. 2004;39:10–25. doi: 10.1002/gene.20021. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Matzuk MM, Gerstmayer B, Bosio A, Lauster R, Miyachi Y, Werner S, Paus R. Control of pelage hair follicle development and cycling by complex interactions between follistatin and activin. Faseb J. 2003;17:497–499. doi: 10.1096/fj.02-0247fje. [DOI] [PubMed] [Google Scholar]
- Nishitoh H, Ichijo H, Kimura M, Matsumoto T, Makishima F, Yamaguchi A, Yamashita H, Enomoto S, Miyazono K. Identification of type I and type II serine/threonine kinase receptors for growth/differentiation factor-5. J Biol Chem. 1996;271:21345–21352. doi: 10.1074/jbc.271.35.21345. [DOI] [PubMed] [Google Scholar]
- Nohno T, Ishikawa T, Saito T, Hosokawa K, Noji S, Wolsing DH, Rosenbaum JS. Identification of a human type II receptor for bone morphogenetic protein-4 that forms differential heteromeric complexes with bone morphogenetic protein type I receptors. J Biol Chem. 1995;270:22522–22526. doi: 10.1074/jbc.270.38.22522. [DOI] [PubMed] [Google Scholar]
- Owens P, Bazzi H, Engelking E, Han G, Christiano AM, Wang XJ. Smad4-dependent desmoglein-4 expression contributes to hair follicle integrity. Dev Biol. 2008;322:156–166. doi: 10.1016/j.ydbio.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panteleyev AA, Botchkareva NV, Sundberg JP, Christiano AM, Paus R. The role of the hairless (hr) gene in the regulation of hair follicle catagen transformation. Am J Pathol. 1999;155:159–171. doi: 10.1016/S0002-9440(10)65110-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piscione TD, Yager TD, Gupta IR, Grinfeld B, Pei Y, Attisano L, Wrana JL, Rosenblum ND. BMP-2 and OP-1 exert direct and opposite effects on renal branching morphogenesis. Am J Physiol. 1997;273:F961, F975. doi: 10.1152/ajprenal.1997.273.6.F961. [DOI] [PubMed] [Google Scholar]
- Plikus M, Wang WP, Liu J, Wang X, Jiang TX, Chuong CM. Morpho-regulation of ectodermal organs: integument pathology and phenotypic variations in K14-Noggin engineered mice through modulation of bone morphogenic protein pathway. Am J Pathol. 2004;164:1099–1114. doi: 10.1016/S0002-9440(10)63197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, Mayer JA, de la Cruz D, Baker RE, Maini PK, Maxson R, Chuong CM. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451:340–344. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao W, Li AG, Owens P, Xu X, Wang XJ, Deng CX. Hair follicle defects and squamous cell carcinoma formation in Smad4 conditional knockout mouse skin. Oncogene. 2006;25:207–217. doi: 10.1038/sj.onc.1209029. [DOI] [PubMed] [Google Scholar]
- Qiu W, Li X, Tang H, Huang AS, Panteleyev AA, Owens DM, Su GH. Conditional activin receptor type 1B (Acvr1b) knockout mice reveal hair loss abnormality. J Invest Dermatol. 2011;131:1067–1076. doi: 10.1038/jid.2010.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig BL, Imamura T, Okadome T, Cox GN, Yamashita H, ten Dijke P, Heldin CH, Miyazono K. Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc Natl Acad Sci U S A. 1995;92:7632–7636. doi: 10.1073/pnas.92.17.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmierer B, Hill CS. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8:970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- Schneider MR, Schmidt-Ullrich R, Paus R. The hair follicle as a dynamic miniorgan. Curr Biol. 2009;19:R132–R142. doi: 10.1016/j.cub.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Sellheyer K, Bickenbach J, Rothnagel J, Bundman B, Longley M, Kreig T, Roche N, Roberts A, Roof D. Inhibition of skin development by overexpression of transforming growth factor beta-1 in the epidermis of transgenic mice. Proc Natl Acad Sci USA. 1993;90:5237–5241. doi: 10.1073/pnas.90.11.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharov AA, Fessing M, Atoyan R, Sharova TY, Haskell-Luevano C, Weiner L, Funa K, Brissette JL, Gilchrest BA, Botchkarev VA. Bone morphogenetic protein (BMP) signaling controls hair pigmentation by means of cross-talk with the melanocortin receptor-1 pathway. Proc Natl Acad Sci USA. 2005;102:93–98. doi: 10.1073/pnas.0408455102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharov AA, Sharova TY, Mardaryev AN, Tommasi di Vignano A, Atoyan R, Weiner L, Yang S, Brissette JL, Dotto GP, Botchkarev VA. Bone morphogenetic protein signaling regulates the size of hair follicles and modulates the expression of cell cycle-associated genes. Proc Natl Acad Sci USA. 2006;103:18166–18171. doi: 10.1073/pnas.0608899103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Oh SP, Schrewe H, Nomura M, Lei H, Okano M, Gridley T, Li E. The type II activin receptors are essential for egg cylinder growth, gastrulation, and rostral head development in mice. Dev Biol. 1999;213:157–169. doi: 10.1006/dbio.1999.9370. [DOI] [PubMed] [Google Scholar]
- Stove C, Vanrobaeys F, Devreese B, Van Beeumen J, Mareel M, Bracke M. Melanoma cells secrete follistatin, an antagonist of activin-mediated growth inhibition. Oncogene. 2004;23:5330–5339. doi: 10.1038/sj.onc.1207699. [DOI] [PubMed] [Google Scholar]
- Sundberg JP, Hogan ME. Hair types and subtypes in the laboratory mouse. In: Sundberg JP, editor. Handbook of mouse mutations with skin and hair abnormalities: animal models and biomedical tools. Boca Raton, FL: CRC Press; 1994. pp. 57–68. [Google Scholar]
- ten Dijke P, Yamashita H, Sampath TK, Reddi AH, Estevez M, Riddle DL, Ichijo H, Heldin CH, Miyazono K. Identification of type I receptors for osteogenic protein-1 and bone morphogenetic protein-4. J Biol Chem. 1994;269:16985–16988. [PubMed] [Google Scholar]
- Weiner L, Green H. Basonuclin as a cell marker in the formation and cycling of the murine hair follicle. Differentiation. 1998;63:263–272. doi: 10.1046/j.1432-0436.1998.6350263.x. [DOI] [PubMed] [Google Scholar]
- Xin Y, Lu Q, Li Q. 14-3-3sigma is required for club hair retention. J Invest Dermatol. 2010;130:1934–1936. doi: 10.1038/jid.2010.56. [DOI] [PubMed] [Google Scholar]
- Yaar M, Wu C, Park HY, Panova I, Schutz G, Gilchrest BA. Bone morphogenetic protein-4, a novel modulator of melanogenesis. J Biol Chem. 2006;281:25307–25314. doi: 10.1074/jbc.M600580200. [DOI] [PubMed] [Google Scholar]
- Yamashita H, ten Dijke P, Huylebroeck D, Sampath TK, Andries M, Smith JC, Heldin CH, Miyazono K. Osteogenic protein-1 binds to activin type II receptors and induces certain activin-like effects. J Cell Biol. 1995;130:217–226. doi: 10.1083/jcb.130.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Mao C, Teng Y, Li W, Zhang J, Cheng X, Li X, Han X, Xia Z, Deng H, Yang X. Targeted disruption of Smad4 in mouse epidermis results in failure of hair follicle cycling and formation of skin tumors. Cancer Res. 2005;65:8671–8678. doi: 10.1158/0008-5472.CAN-05-0800. [DOI] [PubMed] [Google Scholar]
- Yu PB, Beppu H, Kawai N, Li E, Bloch KD. Bone morphogenetic protein (BMP) type II receptor deletion reveals BMP ligand-specific gain of signaling in pulmonary artery smooth muscle cells. J Biol Chem. 2005;280:24443–24450. doi: 10.1074/jbc.M502825200. [DOI] [PubMed] [Google Scholar]
- Yuhki M, Yamada M, Kawano M, Iwasato T, Itohara S, Yoshida H, Ogawa M, Mishina Y. BMPR1A signaling is necessary for hair follicle cycling and hair shaft differentiation in mice. Development. 2004;131:1825–1833. doi: 10.1242/dev.01079. [DOI] [PubMed] [Google Scholar]
- Zhang J, He XC, Tong WG, Johnson T, Wiedemann LM, Mishina Y, Feng JQ, Li L. Bone morphogenetic protein signaling inhibits hair follicle anagen induction by restricting epithelial stem/progenitor cell activation and expansion. Stem Cells. 2006;24:2826–2839. doi: 10.1634/stemcells.2005-0544. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.