Abstract
Background
Cannabis is the most commonly abused drug of abuse and is commonly quantified during urine drug testing. We conducted a controlled drug administration studies investigating efficacy of urinary cannabinoid glucuronide metabolites for documenting recency of cannabis intake and for determining stability of urinary cannabinoids.
Methods
A liquid chromatography tandem mass spectrometry method was developed and validated quantifying Δ9-tetrahydrocannabinol (THC), 11-hydroxy-THC (11-OH-THC), 11-nor-9-carboxy-THC (THCCOOH), cannabidiol, cannabinol, THC-glucuronide and THCCOOH-glucuronide in 0.5 ml human urine via supported-liquid extraction. Chromatography was performed on an Ultra Biphenyl column with a gradient of 10 mmol/l ammonium acetate, pH 6.15 and 15% methanol in acetonitrile at 0. 4ml/min. Analytes were monitored by positive and negative mode electrospray ionization and multiple reaction monitoring mass spectrometry.
Results
Linear ranges were 0.5–50 ng/ml for THC-glucuronide, 1–100 ng/ml for THCCOOH, 11-OH-THC and cannabidiol, 2–100 ng/ml for THC and cannabinol, and 5–500 ng/ml for THCCOOH-glucuronide (R2>0.99). Mean extraction efficiencies were 34–73% with analytical recovery (bias) 80.5–118.0% and total imprecision 3.0–10.2% coefficient of variation.
Conclusion
This method simultaneously quantifies urinary cannabinoids and phase II glucuronide metabolites, and enables evaluation of urinary cannabinoid glucuronides for documenting recency of cannabis intake and cannabinoid stability. The assay is applicable for routine urine cannabinoid testing.
Keywords: cannabinoids, cannabinoid glucuronides, urine, metabolites, analytical method, LCMSMS
1. INTRODUCTION
Cannabis is the most commonly abused drug of abuse [1] and is often present in drug treatment, pain management, forensic, workplace and driving under the influence of drugs (DUID) cases. Prolonged excretion of cannabinoids during abstinence confounds interpretation of recency of cannabis intake [2–5]. Δ9-Tetrahydrocannabinol (THC), the major psychoactive cannabis component, is predominantly metabolized to 11-hydroxy-THC (11-OH-THC) and 11-nor-9-carboxy-THC (THCCOOH); 11-OH-THC and THCCOOH are extensively conjugated via glucuronidation [6]. Traditionally, cannabinoids urine testing measures total concentrations after alkaline, enzymatic and/or tandem hydrolysis [7–9]. Alkaline hydrolysis is effective for ester THCCOOH- and 11-OH-THC-glucuronide de-conjugation while cleavage of ether linked THC- and 11-OHTHC-glucuronides requires costly and time-consuming enzyme hydrolysis via E. coli β-glucuronidase [8, 9]. Direct measurement of free and glucuronide conjugated cannabinoids simplifies result interpretation, as hydrolysis variability can confound interpretation, while also affording time and cost savings compared to alkaline and/or enzyme hydrolysis. Cannabinoid glucuronides and minor cannabis components cannabidiol and cannabinol were hypothesized as useful for determining recency of cannabis intake [10–13].
Traditionally gas chromatographic-mass spectrometric analysis for total urinary cannabinoids following alkaline and/or enzyme hydrolysis is employed for routine cannabinoid analysis in drug treatment, pain management, forensic and workplace testing [7, 9,14–23]. Recent availability of THC-glucuronide and THCCOOH-glucuronide reference standards combined with liquid chromatography tandem mass spectrometry via electrospray ionization (ESI) enables direct measurement of cannabinoids and cannabinoid glucuronides [24–26]. Skopp and Potsch developed the first LCMSMS method for quantifying THCCOOH and THCCOOH-glucuronide in urine prepared via liquid-liquid extraction (LLE) prior to solid phase extraction (SPE) [24]. More recently Felli et al. reported an LCMSMS method with methanolic precipitation of urine for THCCOOH and THCCOOH-glucuronide [25]. Gronewold and Skopp reported the most complete cannabinoids analytical panel to date extracting THC, 11-OH-THC, THCCOOH, cannabidiol, cannabinol and THCCOOH-glucuronide from urine via LLE prior to LCMSMS analysis; however, THC glucuronide was not included and the method was not fully validated for urine [27]. Mareck et al. detailed the only existing method for directly measuring THC-glucuronide, extracting urine by LLE prior to LCMSMS analysis solely for THC-glucuronide [12].
We describe a validated LCMSMS method for directly and simultaneously quantifying THC, 11-OH-THC, THCCOOH, cannabidiol, cannabinol, THC-glucuronide and THCCOOH-glucuronide in human urine employing supported-liquid extraction (SLE). This method will be employed for assessing utility of urinary THC-glucuronide, THCCOOH-glucuronide, cannabidiol and cannabinol as indicators of recent cannabis intake following controlled cannabis smoking, and to conduct the most thorough investigation of cannabinoids’ and cannabinoid glucuronides’ stabilities in human urine.
2. METHODS
2.1. Reagents and supplies
Standards and deuterated internal standards were purchased from Cerilliant (Round Rock, TX); THC-glucuronide was from ElSohly Laboratories, Inc (Oxford, MS, USA). Ammonium acetate, formic acid, acetonitrile and ethyl acetate were obtained from Sigma-Aldrich (St. Louis, MO). Methanol was acquired from Fisher Scientific (Fair Lawn, NJ), and dibutylammonium acetate (0.5 mol/l) from VWR International (Radnor, PA, USA). Water was purified in house with an ELGA Purelab Ultra Analytic purifier (Siemens Water Technologies, Lowell, MA). All solvents were HPLC grade or better. 1-ml Isolute SLE+ cartridges were utilized for preparing samples (Biotage, Inc, Charlotte, NC). A Cerex System 48 positive pressure manifold (SPEware Corp, Baldwin Park, CA) was employed for specimen extraction by SLE. Bond Elut Plexa columns (6 ml/200 mg, Agilent, Wilmington DE) and Styre Screen THC columns (10 ml/60 mg, United Chemical Technologies, Bristol, PA) also were evaluated during method development. Analytical chromatography was performed on an Ultra Biphenyl HPLC column (100 × 2.1 mm; 5 µm particle size) combined with a 10 × 2.1 mm guard column of identical phase purchased from Restek Inc (Bellefonte, PA).
2.2. Instrumentation
Tandem mass spectrometry analysis was performed on an ABSciex API 3200 QTrap® triple quadrupole/linear ion trap mass spectrometer with a TurboIonSpray source (ABSciex, Foster City, CA). The high performance liquid chromatography (HPLC) system consisted of a DGU-20A3 degasser, LC-20ADxr pumps, SIL-20ACxr autosampler and a CTO-20 column oven (Shimadzu Corp, Columbia, MD). Analyst software ver. 1.5.1 was employed for acquisition and data analysis.
2.3. Calibrators, quality control (QC) and internal standards
Blank urine was evaluated with the methodology detailed in this manuscript to ensure absence of detectable cannabinoids or metabolites prior to fortification with working stock solutions to prepare calibrators and quality control samples. Primary stock solution containing THC, 11-OH-THC, THCCOOH, cannabidiol, cannabinol and THCOOH-glucuronide at 10 µg/ml was prepared in methanol. Dilutions of the stock solution (adding in THC-glucuronide) created calibrators at 0.5, 1.0, 2.0, 5.0, 10, 20, 50, 100, and 250 and 500 ng/ml when fortifying 25 µl of standard solution into 500 µl of blank human urine.
QC samples were prepared with different lot numbers of reference standard solutions than calibrators. Three mixed QC working solutions, ranging from 30–4500 ng/ml, were prepared in methanol. QC samples were prepared by adding working solutions to 0.5 ml blank urine to yield 6, 25, and 75 ng/ml THC and cannabinol, 2.5, 25, and 75 ng/ml THCCOOH, 11-OH-THC and cannabidiol, 1.5, 15, and 45 ng/ml THC-glucuronide and 7.5, 75, and 225 ng/ml THCCOOH-glucuronide (low, medium, and high QC, respectively). Primary stock solutions of THC-d3, THCCOOH-d9, 11-OH-THC-d3 and CBD-d3 were diluted in methanol producing a mixed internal standard solution of 200 ng/ml. All primary and working solutions were stored at −20°C in amber glass vials.
2.4. Specimen preparation approaches evaluated during method development
Cannabinoids and cannabinoid glucuronides recovery studies were conducted in triplicate during method development to evaluate potential sample preparation approaches with Bond Elut Plexa columns, Styre Screen THC columns and SLE.
0.5 ml blank urine was fortified with standard and internal standard solution followed by dilution with 3 ml 25 mmol/l dibutylammonium acetate prior to Bond Elut Plexa or Styre Screen THC extraction. Bond Elute Plexa extraction was conducted as detailed by Schwope et al. [13], except elution with 3 ml 1% glacial acetic acid in acetonitrile was followed by elution with 3 ml hexane: ethyl acetate (80:20 v/v).
Styre Screen THC columns were conditioned with 1 ml methanol and water prior to sample application. Columns were washed with 2 ml 15% acetonitrile in water (v/v) and dried for 5 min at maximum vacuum. Analytes were eluted from columns with 3 ml hexane: ethyl acetate: glacial acetic acid (49:49:2; v/v/v) followed by 3 ml of 1% glacial acetic acid in acetonitrile (v/v).
Prior to SLE extraction, 0.5 ml urine was fortified with calibrator or QC working solutions in a glass 13 × 100 mm culture tube. 0.5 ml pH 6.3 buffer consisting of 0.2 mol/l ammonium acetate and 0.025M dibutylammonium acetate buffer was added to each sample. Samples were transferred onto SLE columns and gently driven onto column phase with fine pressure control by slowly increasing pressure up to 1 l/min (achieving 21 ml/min through each column). After equilibration at ambient pressure for 5 min, analytes were eluted with 5 ml ethyl acetate into 16 × 100 mm conical tubes. Positive pressure was gradually applied up to 5 l/min (100 ml/min through each column) with fine pressure control until elution was complete. All sample extracts were completely dried at 40°C under nitrogen in a Zymark TurboVap. Samples were reconstituted in 150 µL mobile phase A:B 70:30 (v/v), vortexed 15 sec prior to centrifugation at 4°C, 4000 × g for 5 min and transferred to autosampler vials containing 200 µl glass inserts. 25 µl was injected onto the LCMSMS instrument.
2.5. LCMSMS
Chromatographic separation was performed on an Ultra Biphenyl column equipped with a guard column containing identical packing material. Gradient elution was performed with (A) 10mM ammonium acetate adjusted to pH 6.15 with formic acid and (B) 15% methanol in acetonitrile at a flow rate of 0.4 ml/min. The initial gradient conditions were 30% B, hold for 30 sec, then increased to 90% B at 6.0 min. 90% B was maintained for 7.5 min, at which time the column was re-equilibrated to 30% B over 0.75 min and held for 1.75 min (total runtime 16 min). HPLC eluent was diverted to waste for the first 2.5 min and the final 9 min of analysis. The column oven and auto-sampler were maintained at 40 and 4°C, respectively. Mass spectrometric data were collected via ESI. THC-glucuronide, THCCOOH-glucuronide, THCCOOH, 11-OH-THC and cannabidiol were acquired in negative ionization mode while THC and cannabinol were acquired in positive ionization mode. MS/MS parameter settings (Table 1) were optimized via direct infusion of individual analytes (250 ng/ml in initial mobile phase) at 10 µl/min. Optimized source parameters were: gas-1 45, gas-2 70, curtain gas 25, source temperature 650°C. Nitrogen collision gas was set at medium for all experiments. Quadrupoles one and three were set to unit resolution. Quantifier and qualifier ion transitions were monitored for each analyte and internal standard.
Table 1.
Liquid chromatography tandem mass spectrometry parameters for cannabinoids and cannabinoid glucuronides in human urine.
| Analyte | Q1 | Q3 | Dwell | Declustering | Entrance | Collision | Collision | Cell exit | Retention |
|---|---|---|---|---|---|---|---|---|---|
| mass | mass | time | potential | potential | entrance | energy | potential | time | |
| (amu) | (amu) | (msec) | (volts) | (volts) | potential | (volts) | (volts) | (min) | |
| (volts) | |||||||||
| THC | 315.3 | 193.2 | 50 | 51 | 4.5 | 16 | 29 | 4 | 6.23 |
| 315.3 | 123.2 | 50 | 51 | 4.5 | 16 | 43 | 4 | ||
| THC-d3 | 318.3 | 196.2 | 50 | 51 | 4.0 | 14 | 31 | 4 | 6.22 |
| 318.3 | 123.0 | 50 | 51 | 4.0 | 14 | 43 | 4 | ||
| 11-OH-THC | 329.1 | 268.1 | 50 | −50 | −4.5 | −14 | −46 | −2 | 5.24 |
| 329.1 | 311.2 | 50 | −50 | −4.5 | −14 | −28 | −4 | ||
| 11-OH-THC-d3 | 332.2 | 271.2 | 100 | −50 | −5.0 | −18 | −42 | −4 | 5.23 |
| 332.2 | 314.1 | 100 | −50 | −5.0 | −18 | −26 | −4 | ||
| THCCOOH | 343.1 | 245.0 | 100 | −60 | −5.0 | −16 | −40 | −2 | 4.13 |
| 343.1 | 190.9 | 100 | −60 | −5.0 | −16 | −42 | −2 | ||
| THCCOOH-d9 | 352.2 | 253.9 | 50 | −55 | −8.5 | −18 | −42 | −4 | 4.10 |
| 352.2 | 194.3 | 50 | −55 | −8.5 | −18 | −44 | −4 | ||
| Cannabidiol | 313.2 | 245.2 | 25 | −55 | −5.5 | −14 | −32 | −2 | 5.78 |
| 313.2 | 179.2 | 25 | −55 | −5.5 | −14 | −28 | −2 | ||
| Cannabidiol-d3 | 316.2 | 247.8 | 25 | −55 | −6.0 | −14 | −34 | −2 | 5.77 |
| 316.2 | 182.2 | 25 | −55 | −6.0 | −14 | −26 | −2 | ||
| Cannabinol | 311.3 | 223.2 | 100 | 46 | 4.0 | 18 | 29 | 4 | 6.18 |
| 311.3 | 178.1 | 100 | 46 | 4.0 | 18 | 79 | 4 | ||
| THC-glucuronide | 489.2 | 313.2 | 100 | −60 | −4.5 | −24 | −44 | −4 | 3.11 |
| 489.2 | 112.9 | 100 | −60 | −4.5 | −24 | −30 | −2 | ||
| THCCOOH- glucuronide |
519.3 | 343.2 | 50 | −50 | −4.5 | −24 | −32 | −4 | 3.10 |
| 519.3 | 299.2 | 50 | −50 | −4.5 | −24 | −46 | −4 |
Q1= quadrupole 1, Q3= quadrupole 3, THC= Δ9-tetrahydrocannabinol, 11-OH-THC= 11-hydroxy-THC, THCCOOH= 11-nor-9-carboxy-THC.
Bold masses depict quantification transitions.
2.6. Data analysis
Peak area ratios of analytes to corresponding internal standards were calculated for each concentration to construct daily calibration curves via linear least-squares regression with a 1/x2 weighting factor. Calibration curves were from 2–100 ng/ml for THC and CBN, 1–100 ng/ml for THCCOOH, 11-OH-THC and CBD, 0.5–50 ng/ml for THC-glucuronide and 5–500 ng/ml for THCCOOH-glucuronide.
2.7. Method validation
Specificity, sensitivity, linearity, imprecision, analytical recovery, extraction efficiency, matrix effect, stability, dilution integrity and carry-over were evaluated during method validation.
2.8. Specificity
Analyte peak identification criteria were relative retention time within ± 0.1 min of the lowest calibrator and qualifier/quantifier transition peak area ratios ±20% of mean calibrator transition ratios. Potential endogenous interferences were assessed by analyzing ten urine specimens from different individuals. In addition, potential interferences from commonly used drugs were evaluated by fortifying drugs into low QC samples. Final interferent concentrations were 1000 ng/ml cocaine, benzoylecgonine, norcocaine, norbenzoylecgonine, ecgonine ethyl ester, ecgonine methyl ester, ecgonine, anhydroecgonine methyl ester, cocaethylene, norcocaethylene, m-hydroxycocaine, p-hydroxycocaine, m-hydroxybenzoylecgonine, p-hydroxybenzoylecgonine, morphine, normorphine, morphine-3-beta-D-glucuronide, morphine-6-beta-D-glucuronide, codeine, norcodeine, 6-acetylmorphine, 6-acetylcodeine, buprenorphine, norbuprenorphine, methadone, 2-ethyl-5-methyl-3,3-diphenylpyrroline, 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine, hydrocodone, hydromorphone, oxycodone, noroxycodone, oxymorphone, noroxymorphone, diazepam, lorazepam, oxazepam, alprazolam, amphetamine, methamphetamine, 3,4-methylenedioxymethamphetamine, 3,4-methylenedioxyamphetamine, 4-hydroxy-3-methoxymethamphetamine, 4-hydroxy-3-methoxyamphetamine, p-methoxymethamphetamine, p-methoxyamphetamine, p-hydroxymethamphetamine, clonidine, ibuprofen, pentazocine, caffeine, diphenhydramine, chlorpheniramine, brompheniramine, aspirin, acetaminophen, phencyclidine, nitrazepam, flunitrazepam, temazepam, nordiazepam, bromazepam, clonazepam, flurazepam, pseudoephedrine, ephedrine, phenylpropanolamine, fenfluramine, phentermine, nicotine, cotinine, trans-3’-hydroxycotinine and norcotinine. No interference was noted if all analytes in the low QC sample quantified within ±20% of target concentrations with acceptable qualifier/quantifier transition ratios.
2.9. Sensitivity and linearity
Limit of detection (LOD) was evaluated in triplicate and defined as the lowest concentration producing a peak eluting within ±0.1 min of analyte retention time for the lowest calibrator, a signal-to-noise ratio of at least 3, Gaussian peak shape and qualifier/quantifier transition peak area ratios ±20% of mean calibrator transition ratios. Limit of quantification (LOQ) also was evaluated in triplicate and defined as the lowest concentration that met LOD criteria, signal-to-noise of at least 10 and measured concentration within ± 20% of target. Performance at the LOQ was confirmed in each batch of specimens.
Preliminary experiments with six sets of calibrators determined the most appropriate calibration model comparing goodness-of-fit for unweighted linear least squares, linear least squares employing 1/x and 1/x2 weighting. Calibration curves were fit by linear least squares regression with at least 6 concentrations across the linear dynamic range for each analyte. Calibrators were required to quantify within ±15% and correlation coefficients (R2) were required to exceed 0.99.
2.10. Analytical recovery and imprecision
Intra-day and inter-day analytical recovery (bias) and imprecision were determined from four replicates at three different QC concentrations across the linear dynamic range of the assay. Analytical recovery was determined by comparing the mean result for all analyses to the nominal concentration value (i.e. mean % of expected concentration). Inter-day imprecision and analytical recovery were evaluated on five different runs with four replicates in each run, analyzed on five separate days (n=20). Imprecision was expressed as % coefficient of variation (% CV) of the calculated concentrations. The guidelines detailed by Krouwer et al. [28] were employed to calculate pooled intra-day, inter-day and total imprecision.
2.11. Extraction efficiency and matrix effect
Extraction efficiency and matrix effect were evaluated via three sets of samples as described by Matuszewski et al. (n=4 for each set) [29]. In the first set, urine samples were fortified with analytes and internal standards prior to SLE. In set 2, urine samples were fortified with analytes and internal standards after SLE, and the third set contained analytes and internal standards in mobile phase. Extraction efficiency, expressed as a percentage, was calculated by dividing analyte mean peak areas of set 1 by set 2. Absolute matrix effect was calculated by dividing the mean peak area of the analyte in set 2 by the mean analyte area in set 3. The value was converted to a percentage and subtracted from 100 to represent the amount of signal suppressed by the presence of matrix. As an additional evaluation of matrix effect ten blank urine lots were fortified with low QC solution and internal standard and were processed along with calibrators prepared using a separate lot of blank urine to verify accurate quantification.
2.12. Stability
Analyte stability during extraction was evaluated by fortifying blank urine with each analyte individually at its upper limit of quantification (ULOQ) immediately prior to extraction and analysis. Stability also was evaluated with blank human urine fortified with analytes of interest at three QC concentrations (n = 3). Short-term temperature stability was evaluated for fortified human urine stored in the dark in polypropylene cryovials for 24 h at room temperature, 72 h at 4°C, 48 h on the autosampler (4°C), and after three freeze-thaw cycles at −20°C. On the day of analysis, internal standard was added to each specimen and analyzed as described. Autosampler stability was assessed by re-injecting QC specimens after 48 h and comparing calculated concentrations to values obtained against the original calibration curve.
2.13. Dilution integrity
Dilution integrity was evaluated by diluting a fortified urine sample (n=3) containing 45 ng/ml THC-glucuronide, 225 ng/ml THCCOOH-glucuronide and 75 ng/ml THC, 11-OH-THC, THCCOOH, cannabidiol and cannabinol in blank urine to achieve 1:1 and 1:4 (v/v) dilution. Internal standards were added and samples extracted as described. Dilution integrity was maintained if specimens quantified within ±20% of expected diluted concentration.
2.14. Carry-over
Carry-over was investigated in triplicate by injecting extracted blank urine samples containing internal standards immediately after samples containing target analytes at twice the ULOQ. Blank urine specimens could not meet LOD criteria to document absence of carryover.
2.15. Clinical study
Urine was collected from a healthy cannabis smoker that provided written informed consent to participate in a National Institute on Drug Abuse (NIDA) Institutional Review Board approved protocol investigating cannabinoid pharmacokinetics, in vitro cannabinoid stability and novel markers of cannabis intake following a single smoked cannabis dose. A cannabis cigarette containing 6.8% THC (w/w) was smoked ad libitum over 10 min after an overnight stay on a secure residential unit. Urine was collected ad libitum in polypropylene bottles and was stored at −20°C until analysis within 24 h.
3. RESULTS
3.1. Evaluation of potential sample preparation approaches
Bond Elut Plexa provided efficient recoveries for THCCOOH, 11-OH-THC, THCCOOH-glucuronide and THC-glucuronide (60–94% recovery), 32% cannabidiol recovery and <18% recovery for THC and cannabinol from urine. Styre Screen THC columns yielded efficient extraction of THC, 11-OH-THC, THCCOOH, cannabidiol and cannabinol but achieved <1% extraction efficiencies of THC-glucuronide and THCCOOH-glucuronide from urine (Supplementary Table 1). SLE achieved 34–73% extraction efficiencies for all our urinary cannabinoid analytes of interest (Table 2).
Table 2.
Mean extraction efficiency and matrix effect for cannabinoids and cannabinoid glucuronides extracted from urine by supported-liquid extraction
| Analyte | Extraction efficiency | Matrix effect | ||||
|---|---|---|---|---|---|---|
| (%, N = 4) |
(% of signal suppressed, N = 4) |
|||||
| Low | Mid | High | Low | Mid | High | |
| THC a | 38.4 | 33.9 | 41.1 | 28.0 | 16.8 | 4.8 |
| 11-OH-THC b | 58.6 | 50.0 | 48.0 | 5.6 | 14.9 | 17.8 |
| THCCOOH b | 71.2 | 69.8 | 67.6 | −0.8 | −1.6 | −2.4 |
| Cannabidiol b | 53.8 | 40.8 | 43.9 | −10.3 | 8.9 | −0.7 |
| Cannabinol a | 40.9 | 35.6 | 41.4 | 16.4 | 13.4 | 7.6 |
| THC-glucuronide c | 71.4 | 72.6 | 69.6 | 31.8 | 4.8 | −1.9 |
| THCCOOH-glucuronide d | 65.5 | 64.4 | 61.2 | 88.7 | 24.1 | 7.1 |
| THC-d3 | 40.8 | 35.2 | 41.5 | 27.5 | 12.0 | 3.6 |
| 11-OH-THC-d3 | 54.8 | 49.9 | 47.7 | 6.8 | 15.4 | 14.5 |
| THCCOOH-d9 | 69.9 | 67.9 | 65.1 | 4.3 | 1.8 | 1.3 |
| cannabidiol-d3 | 52.6 | 41.7 | 44.7 | −7.3 | 12.0 | −1.7 |
THC= Δ9-tetrahydrocannabinol, 11-OH-THC= 11-hydroxy-THC, THCCOOH= 11-nor-9-carboxy-THC.
THC and cannabinol low, mid and high quality control concentrations were 6, 25 and 75 ng/ml, respectively.
11-OH-THC, cannabidiol and THCCOOH low, mid and high quality control concentrations were 2.5, 25 and 75 ng/ml, respectively.
THC-glucuronide low, mid and high quality control concentrations were 1.5, 15 and 45 ng/ml, respectively.
THCCOOH-glucuronide low, mid and high quality control concentrations were 7.5, 75 and 225 ng/ml, respectively.
THC-d3, 11-OH-THC-d3, THCCOOH-d9 and cannabidiol-d3 concentrations were 10 ng/ml.
3.2. Specificity
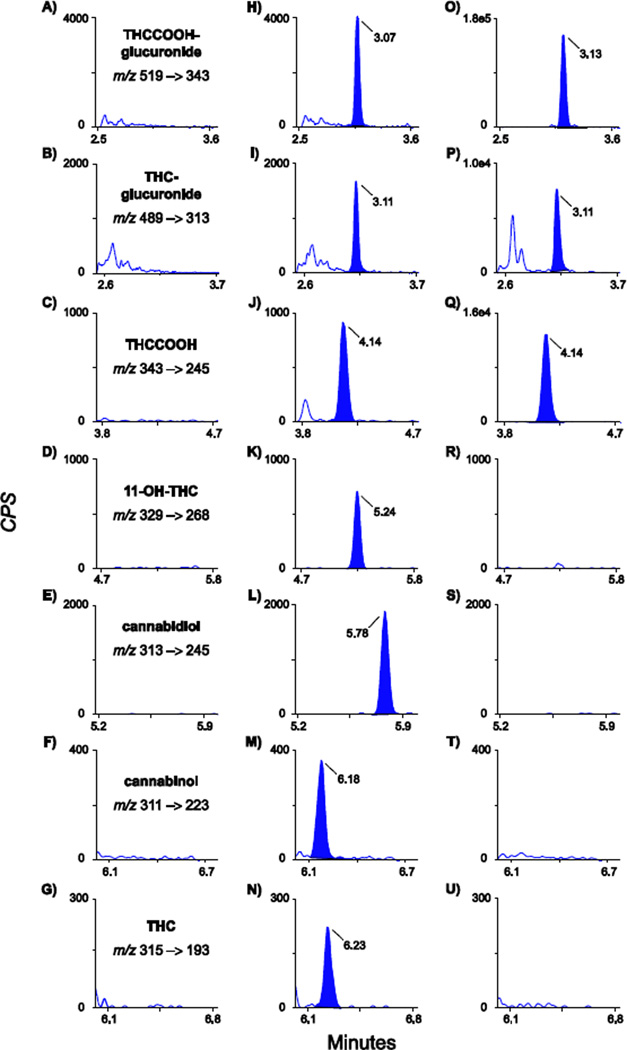
There were no interfering peaks in urine from ten cannabis-abstinent individuals. None of the 72 potential exogenous interferences fortified at 1000 ng/ml into low QC samples produced transition ratio or quantification criteria failure. Multiple reaction monitoring (MRM) ion chromatograms from a blank urine specimen, a blank urine specimen fortified with analytes at the LOQ and a urine specimen from a study participant 23 h after smoking a 6.8% (w/w) cannabis cigarette are shown in Figure 1.
Figure 1.
Multiple reaction monitoring ion chromatograms for quantification transitions: m/z 519–343 (11-nor-9-carboxy-Δ9-tetrahydrocannabinol-glucuronide: THCCOOH-glucuronide), m/z 489–313 (Δ9-tetrahydrocannabinol-glucuronide: THC-glucuronide), m/z 343–245 (11-nor-9-carboxy-Δ9-tetrahydrocannabinol: THCCOOH), m/z 329–268 (11-hydroxy- Δ9-tetrahydrocannabinol: 11-OH-THC), m/z 313–245 (cannabidiol), m/z 311-223 (cannabinol) and m/z 315–193 (Δ9-tetrahydrocannabinol: THC). Panels A–G are extracted blank human urine, panels H–N blank human urine fortified with analytes at the limit of quantification and panels O–U are from an authentic urine specimen collected 23 h after smoking a 6.8% THC (w/w) cigarette. Limits of quantification are 0.5 ng/ml for THC-glucuronide, 1 ng/ml for 11-OH-THC, THCCOOH and cannabidiol, 2 ng/ml for THC and cannabinol and 5 ng/ml for THCCOOH-glucuronide. Urine cannabinoid concentrations in panels O–Q are 213, 2.9 and 15.1 ng/ml of THCCOOH-glucuronide, THC-glucuronide and THCCOOH, respectively. Concentrations for all analytes shown in panels R–U were less than limits of quantification. Qualifier transitions are not displayed for sake of clarity, but are listed in Table 1.
3.3. Sensitivity and linearity
Initial experiments were conducted with six sets of calibration curves fit via unweighted linear least squares and linear least squares with 1/x and 1/x2 weighting factor to identify the most appropriate calibration model. Inspection of residuals indicated linear least squares with 1/x2 weighting factor produced the best fit for the calibration data. All correlation coefficients exceeded 0.99 (Table 3).
Table 3.
Cannabinoids and cannabinoid glucuronides in human urine by liquid chromatography tandem mass spectrometry: limits of detection (LOD), limits of quantification (LOQ) linear ranges and calibration results (N=6)
| Internal | LOD | LOQ | Linear range | Y-intercept | Slope | ||
|---|---|---|---|---|---|---|---|
| Analyte | Standard | (ng/ml) | (ng/ml) | (ng/ml) | Mean ± SD | Mean ± SD | R2 (range) |
| THC | THC-d3 | 1 | 2 | 2–100 | 0.006±0.018 | 0.606±0.064 | 0.995–0.998 |
| 11-OH-THC | 11-OH-THC-d3 | 0.5 | 1 | 1–100 | 0.002±0.010 | 1.142±0.064 | 0.995–0.999 |
| THCCOOH | THCCOOH-d9 | 0.5 | 1 | 1–100 | −0.002±0.010 | 0.982±0.065 | 0.998–0.999 |
| Cannabidiol | cannabidiol-d3 | 0.5 | 1 | 1–100 | 0.005±0.014 | 0.976±0.114 | 0.995–0.999 |
| Cannabinol | THC-d3 | 1 | 2 | 2–100 | −0.002±0.020 | 0.843±0.100 | 0.993–0.999 |
| THC-glucuronide | THCCOOH-d9 | 0.25 | 0.5 | 0.5–50 | −0.001±0.008 | 1.942±0.132 | 0.997–0.999 |
| THCCOOH-glucuronide | THCCOOH-d9 | 0.5 | 5 | 5–500 | 0.029±0.016 | 0.615±0.026 | 0.997–0.999 |
THC= Δ9-tetrahydrocannabinol, 11-OH-THC= 11-hydroxy-THC, THCCOOH= 11-nor-9-carboxy-THC.
Table 3 details LOD, LOQ, linearity and mean calibration results. LOD were between 0.25 and 1.0 ng/ml; LOQ were between 0.5 and 5.0 ng/ml. Assays were linear to 100 ng/ml for all analytes except 50 and 500 ng/ml for THC-glucuronide and THCCOOH-glucuronide, respectively.
3.4. Analytical recovery and imprecision
Analytical recovery and imprecision were evaluated at three concentrations across the linear dynamic range. Analytical recovery in urine ranged from 80.5–118.0% of expected concentrations for intra-day and inter-day analytical recoveries (Table 4). Pooled intra-day, inter-day and total imprecision were 2.6–10.2, 0–5.6, and 3.0–10.2% CV, respectively (Table 4).
Table 4.
Analytical recovery and imprecision data for cannabinoids and cannabinoid glucuronides in human urine by liquid chromatography tandem mass spectrometry
| Analytical recovery | Imprecision | |||||||
|---|---|---|---|---|---|---|---|---|
| (% of expected concentration) | (% coefficient of variation, N= 20) | |||||||
| Intra-day, N=4 | Inter-day, N=20 | |||||||
| Concentration | Pooled | |||||||
| (ng/ml) | Mean | Range | Mean | Range | Intra-day | Inter-day | Total | |
| 6 | 99.0 | 91.7–112.3 | 99.0 | 88.2–112.3 | 5.7 | 1.4 | 5.9 | |
| THC | 25 | 97.7 | 85.2–104.8 | 96.7 | 81.2–118.0 | 10.2 | 0 | 10.2 |
| 75 | 97.5 | 87.1–111.2 | 94.7 | 80.5–111.2 | 8.6 | 0 | 8.6 | |
| 2.5 | 97.9 | 85.6–108.0 | 101.3 | 85.6–108.0 | 4.7 | 0 | 4.7 | |
| 11-OH-THC | 25 | 96.5 | 94.4–98.4 | 100.4 | 94.4–111.2 | 3.0 | 2.6 | 3.9 |
| 75 | 91.5 | 89.3–93.7 | 97.3 | 89.3–103.5 | 2.6 | 3.3 | 4.2 | |
| 2.5 | 87.7 | 83.6–90.8 | 92.8 | 83.2–102.0 | 4.1 | 3.6 | 5.5 | |
| THCCOOH | 25 | 89.5 | 88.0–91.6 | 92.9 | 84.4–104.4 | 3.2 | 4.7 | 5.6 |
| 75 | 88.1 | 85.5–90.1 | 93.0 | 85.5–102.0 | 2.6 | 4.0 | 4.7 | |
| 2.5 | 97.1 | 90.0–113.6 | 98.1 | 81.6–116.8 | 7.9 | 5.6 | 9.7 | |
| Cannabidiol | 25 | 109.0 | 103.2–113.6 | 100.5 | 85.2–114.8 | 6.5 | 4.8 | 8.1 |
| 75 | 95.5 | 88.1–102.9 | 98.3 | 81.2–118.0 | 6.9 | 4.8 | 8.4 | |
| 6 | 95.3 | 82.7–108.3 | 97.9 | 82.7–115.0 | 6.9 | 3.9 | 7.9 | |
| Cannabinol | 25 | 98.9 | 91.6–107.2 | 94.8 | 81.2–114.0 | 9.6 | 0 | 9.6 |
| 75 | 93.5 | 87.6–99.5 | 93.1 | 83.1–113.5 | 8.0 | 0 | 8.0 | |
| 1.5 | 93.2 | 88.0–97.3 | 96.1 | 88.0–102.7 | 3.0 | 0 | 3.0 | |
| THC-glucuronide | 15 | 95.2 | 90.7–100.7 | 100.1 | 90.7–112.0 | 2.8 | 4.4 | 5.2 |
| 45 | 98.1 | 95.8–102.7 | 101.0 | 93.3–111.1 | 3.1 | 3.8 | 4.9 | |
| 7.5 | 85.0 | 82.9–87.9 | 88.7 | 82.0–97.5 | 3.1 | 3.7 | 4.8 | |
| THCCOOH- glucuronide |
75 | 88.3 | 83.7–93.6 | 92.4 | 83.7–101.3 | 3.0 | 3.7 | 4.7 |
| 225 | 90.8 | 87.6–92.4 | 90.7 | 83.6–98.2 | 3.2 | 1.9 | 3.7 | |
THC= Δ9-tetrahydrocannabinol, 11-OH-THC= 11-hydroxy-THC, THCCOOH= 11-nor-9-carboxy-THC.
3.5. Extraction efficiency and matrix effect
Extraction efficiencies and matrix effects for cannabinoids and cannabinoid glucuronides from urine are presented in Table 2. Mean extraction efficiencies were 33.9–72.6% (n=4). Mean matrix effects (% suppressed signal) were −10.3 to 88.7% (n=4, Table 2).
3.6. Stability, dilution integrity and carryover
There was no de-conjugation of THC-glucuronide or THCCOOH-glucuronide or conversion of cannabidiol to THC observed when individual analytes were fortified at the assay upper limits of linearity prior to extraction. Analytes at three QC concentrations in urine extracts were stable for 48 h at 4°C in the autosampler (Table 5). 11-OH-THC, THCCOOH, THC-glucuronide and THCCOOH-glucuronide were stable for 20 h at room temperature and after three freeze/thaw cycles, but significant losses were observed for THC, cannabidiol and cannabinol (Table 5). Significant losses were observed for THC and cannabinol after 72 h at 4°C; all other analytes were stable with refrigerated storage (Table 5).
Table 5.
Cannabinoids and cannabinoid glucuronides stability in human urine
| Analyte | 48 h autosampler | 16 h room temperature | 72 h 4°C | 3 Freeze/thaw cycles | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (% difference, n=3) |
(% difference, n=3) |
(% difference, n=3) |
(% difference, n=3) |
|||||||||
| Low | Mid | High | Low | Mid | High | Low | Mid | High | Low | Mid | High | |
| THC a | −13.8 | −0.3 | 3.2 | −59.9 | −70.1 | −68.2 | −38.1 | −18.7 | −18.4 | −45.8 | −41.3 | −45.2 |
| 11-OH-THC b | 2.9 | 2.1 | 1.6 | −7.6 | −5.7 | −13.9 | −7.3 | 3.2 | −3.8 | −15.1 | −13.7 | −18.0 |
| THCCOOH b | −12.4 | −6.9 | −9.8 | −11.7 | −10.5 | −15.5 | −12.3 | −10.0 | −8.3 | −12.7 | −12.0 | −11.1 |
| Cannabidiol b | 3.6 | 7.1 | −1.5 | −65.1 | −53.5 | −56.8 | −13.7 | −9.7 | −8.8 | −40.9 | −29.9 | −31.8 |
| Cannabinol a | −13.0 | −7.3 | −14.1 | −52.1 | −66.4 | −64.5 | −36.1 | −26.0 | −27.3 | −41.9 | −45.3 | −43.5 |
| THC-glucuronide c | −9.3 | −4.4 | −6.1 | −3.3 | −1.3 | −6.3 | −5.8 | −4.7 | −5.5 | −6.4 | −6.7 | −5.4 |
| THCCOOH- glucuronide d |
−13.0 | −12.2 | −14.4 | −16.5 | −14.0 | −17.9 | −11.0 | −16.3 | −15.9 | −18.7 | −17.6 | −17.3 |
THC= Δ9-tetrahydrocannabinol, 11-OH-THC= 11-hydroxy-THC, THCCOOH= 11-nor-9-carboxy-THC.
THC and cannabinol low, mid and high quality control concentrations were 6, 25 and 75 ng/ml, respectively.
11-OH-THC, cannabidiol and THCCOOH low, mid and high quality control concentrations were 2.5, 25 and 75 ng/ml, respectively.
THC-glucuronide low, mid and high quality control concentrations were 1.5, 15 and 45 ng/ml, respectively.
THCCOOH-glucuronide low, mid and high quality control concentrations were 7.5, 75 and 225 ng/ml, respectively.
Dilution integrity was acceptable (within ±20% of expected diluted concentration) for THC, 11-OH-THC, THCCOOH, cannabidiol, cannabinol, THC-glucuronide and THCCOOH-glucuronide after diluting 1:1 with blank urine. Dilution integrity was also acceptable for THCCOOH, THC-glucuronide and THCCOOH-glucuronide after diluting 1:3 with blank urine. Samples fortified with 75, 45 and 225 ng/ml of THCCOOH, THC-glucuronide and THCCOOH-glucuronide, respectively, prior to 1:3 dilution quantified within ±19.3% of theoretical diluted concentrations. Mean measured concentrations were 83.7, 96.3 and 82.3% of theoretical diluted THCCOOH, THC-glucuronide and THCCOOH-glucuronide concentrations, respectively. Dilution integrity was not acceptable for THC, 11-OH-THC, cannabidiol and cannabinol. Mean measured concentrations were 77.2, 61.5, 69.2, 77.2% of theoretical diluted THC, 11-OH-THC, cannabidiol and cannabinol concentrations, respectively.
There was no evidence of carryover for cannabinoids and cannabinoid glucuronides. Negative specimens injected after samples containing twice the ULOQ did not contain analyte peaks satisfying assay LOD criteria (n=3).
3.7. Proof of method
The method was applied to measurement of cannabinoids and cannabinoid glucuronides in urine specimens collected after a participant smoked a 6.8% THC cigarette (Fig. 1 and Table 6). THC, 11-OH-THC, cannabidiol and cannabinol did not exceed assay LOQ in any urine specimen. THCCOOH concentrations ranged from <1 to 29.4 ng/ml, THC-glucuronide from 0.8 to 34.4 ng/ml and THCCOOH-glucuronide from 36.3 to 670 ng/ml (Table 6).
Table 6.
Cannabinoids and cannabinoid glucuronides concentrations in urine collected from a participant after smoking a single 6.8% Δ9-tetrahydrocannabinol (w/w) cigarette ad libitum over 10 minutes a
| THCCOOH b | THC-glucuronide | THCCOOH-glucuronide | |
|---|---|---|---|
| Time since smoking (h) | (ng/ml) | ||
| −7.5 | <LLOQ c | 0.8 | 36.3 |
| 1.6 | 15.4 | 34.4 | 245.0 |
| 6.4 | 29.4 | 12.5 | 670.0 |
| 12.6 | 4.4 | 3.0 | 184.0 |
| 14.3 | 7.0 | 4.0 | 232.0 |
| 20.0 | 5.0 | 2.3 | 108.0 |
| 23.2 | 15.1 | 2.9 | 213.0 |
| 24.0 | <LLOQ c | 0.5 | 37.2 |
| 25.8 | 1.9 | 1.2 | 78.3 |
| 27.5 | 6.3 | 2.7 | 216.0 |
| 30.3 | 1.7 | 2.8 | 176.0 |
Δ9-tetrahydrocannabinol (THC), 11-hydroxy-THC, cannabidiol and cannabinol did not exceed 2, 1, 1 and 2 ng/ml, respectively, in any urine specimen.
11-nor-9-carboxy-THC (THCCOOH).
THCCOOH concentration less than the lowest limit of quantification, 1 ng/ml.
4. DISCUSSION
A validated and sensitive LCMSMS method for simultaneously quantifying cannabinoids and cannabinoid glucuronides in urine is necessary to support our Institutional Review Board approved clinical protocol investigating urinary cannabinoid pharmacokinetics, in vitro cannabinoid stability and novel markers of cannabis intake following a single smoked cannabis dose. We previously reported prolonged urinary excretion of THC, 11-OH-THC and THCCOOH in chronic cannabis smokers for up to 24 days [2,3]. The current method will be employed to evaluate cannabinoid glucuronide pharmacokinetics and to determine urinary detection windows following controlled cannabis smoking. We will investigate THCCOOH free:glucuronide ratios over time during abstinence in daily and less than daily cannabis smokers, which could assist determining recency of cannabis intake. We also will determine if urinary THC-glucuronide, cannabidiol and/or cannabinol are useful markers of recent cannabis smoking.
Glucuronide conjugates are unstable, undergoing hydrolysis to the unconjugated form during storage [24,30], which could confound interpretation of cannabis results. We will apply this method for stability studies of cannabinoids and cannabinoid glucuronides under various in vitro storage conditions for up to one year in authentic urine specimens collected from participants following controlled smoked cannabis. Only 2 studies directly evaluated THCCOOH-glucuronide stability in authentic urine specimens [25,31]. Skopp and Potsch reported stability of THCCOOH and THCCOOH-glucuronide for 2 weeks at −20°C, minimal conversion during storage for 5 days at 4°C, but >25% loss of THCCOOH-glucuronide in 12 of 38 specimens after 5 days at room temperature [31]. Felli et al. reported that THCCOOH and THCCOOH-glucuronide were stable for 7 days at 4°C and for 120 days at −20°C [25]. Stability studies with authentic specimens collected after cannabis smoking only evaluated stability of THCCOOH and THCCOOH-glucuronide; no stability studies of other cannabinoids and THC-glucuronide in authentic specimens after cannabis smoking exist.
Results from our stability studies with fortified urine at room temperature, 4°C and after three freeze/thaw cycles highlight the importance of method validation to assist interpretation of analytical results in drug treatment, pain management, forensic, workplace and driving under the influence of drugs testing. We observed 30–70% THC, cannabidiol and cannabinol losses after 16 h at room temperature and three freeze/thaw cycles; 18–38% losses of THC and cannabinol also occurred after 72 h at 4°C, while all other analytes were stable. We observed losses of THC, cannabidiol and cannabinol without simultaneous increases in other analyte concentrations suggesting that conversion of THC, cannabidiol or cannabinol to another of our target analytes is not occurring during our stability studies. During validation of a GCMS method for total THC, 11-OH-THC and THCCOOH, we observed that fortified free THC concentrations remained within 20% of original concentrations in glass tubes for 24 h at room temperature, 72 h at 4°C and after three freeze/thaw cycles [9]. Grauwiler et al. observed minimal decreases in total THC, cannabidiol and cannabinol after 5 months at −70°C in silanized glass vials [32], possibly suggesting that storage in glass or silanized glass limits THC adsorption during storage. Storage of biological specimens in glass tubes presents safety concerns precluding routine use of glass tubes for specimen storage. Adsorption of free THC to plastic tubes during storage was previously reported [33] and may produce false negative free THC results; however, THC-glucuronide predominates in urine relative to free THC [8]. THC-glucuronide was stable under all conditions that we evaluated during method validation. Our stability results indicate that false negative THC, cannabidiol and cannabinol urine test results could occur for specimens stored in polypropylene containers for <16 h at room temperature and <72 h at 4°C or after repeated freeze/thaw cycles, indicating that these analytes are likely not effective urinary biomarkers for identifying recency of cannabis intake. Our preliminary stability results with fortified samples during this method validation highlight the importance of conducting more clinically relevant stability studies with authentic specimens collected from participants after smoking cannabis.
Dilution integrity was acceptable for all analytes when high QCs were diluted 1:1. We observed acceptable dilution integrity for THCCOOH, THC-glucuronide and THCCOOH-glucuronide but unacceptable losses (greater than ±20%) were observed for THC, 11-OH-THC, cannabidiol and cannabidiol in urine after 1:3 dilution. The mechanism for THC, 11-OH-THC, cannabidiol and cannabinol losses during dilution integrity experiments is unknown. We do not suspect pipetting error since THCCOOH, THC-glucuronide and THCCOOH-glucuronide fulfilled dilution integrity requirements. It is possible that losses result from adsorption to the tubes or precipitant material in the sample. Dilution integrity for THC, 11-OH-THC, cannabidiol and cannabinol are unlikely to be clinically relevant since urinary concentrations of these analytes are expected to be less than our assay’s ULOQ. Unconjugated THC and 11-OH-THC urinary concentrations were less than 5 and 10 ng/ml, respectively, 0.08–8 h after smoking a single 3.6% THC cigarette (n=8) [8]. Cannabidiol and cannabinol are minor components of cannabis [6] and urinary concentrations are unlikely to exceed our assay ULOQ after cannabis smoking; however, ingestion of oral cannabidiol or Sativex that contains a 1:1 ratio of THC and CBD and administered by the oral mucosal route might produce urinary concentrations exceeding cannabidiol’s ULOQ after 1:1 dilution.
Cannabinoids are extensively metabolized via phase I and II mechanisms with glucuronidated forms predominating relative to free cannabinoids in urine [34–37]. Prior to cannabinoid glucuronide reference standards becoming available, hydrolysis prior to GCMS or LCMS analysis was required to quantify total cannabinoids [8,9]. Incomplete or variable hydrolysis can confound interpretation of results. Two LCMSMS-ESI methods reported direct measurement of THCCOOH-glucuronide and THCCOOH in 0.5 ml urine with LOQs of 6 [24] and 10 ng/ml [25]. Gronewold and Skopp recently reported cannabinoids via LCMSMS-ESI with a 1 ml specimen volume achieving LOQ’s of 0.6, 0.5, 5, 0.4, 0.6 and 41 ng/ml for THC, 11-OH-THC, THCCOOH, cannabidiol, cannabinol and THCCOOH-glucuronide in urine, respectively, although only whole blood analysis was extensively validated not urine [27]. Mareck et al. reported the only validated LCMSMS-ESI method for THC-glucuronide although no other cannabinoids were included, extracting 2 ml urine by LLE achieving an LOQ of 0.3 ng/ml [12]; stability was not rigorously investigated [12]. The current method is the first method capable of measuring THC, 11-OH-THC, THCCOOH, cannabidiol, cannabinol, while also directly quantifying THC-glucuronide and THCCOOH-glucuronide, and achieving similar or better LOQ’s than previous reports for less comprehensive cannabinoid analyte panels.
We investigated three sample preparation approaches for cannabinoids and cannabinoid glucuronides in urine finding that SLE afforded the best extraction efficiencies for our analyte panel. It is difficult to develop a rapid and simple sample preparation strategy providing efficient recoveries of drugs with the diverse physicochemical properties of cannabinoids included in our method. Styre Screen polymeric mixed mode anion exchange SPE columns did not efficiently recover polar analytes THC-glucuronide and THCCOOH-glucuronide from urine. Bond Elut Plexa reverse phase SPE columns did not efficiently extract nonpolar analytes, THC and cannabinol from urine. Our previous report for cannabinoids and cannabinoids in whole blood found recoveries of the same analyte panel ranging from 50–94% using Bond Elut Plexa columns [13]. The mechanism for poor THC and cannabinol recovery from urine is unclear, but we did observe that fortification of urine specimens with blank plasma improved recoveries for these two analytes (data not shown) similar to reports by Skopp et al. that addition of albumin increased cannabinoid SPE recoveries in aqueous samples [38]. Addition of blank plasma or albumin to urine specimens could confound cannabinoid testing results and we did not pursue these approaches for improving SPE extraction efficiency. SLE is a rapid, simple and cost-effective approach for extracting cannabinoids and cannabinoid glucuronides from human urine achieving required sensitivities and 34–73% extraction efficiencies for our entire analyte panel. Our observed extraction efficiencies of 68–71 and 61–65% for THCCOOH and THCCOOH-glucuronide, respectively, are similar to Skopp and Potsch’s reported 87 and 52% extraction efficiencies for THCCOOH and THCCOOH-glucuronide, respectively, from urine by SPE, but our method also included five additional cannabinoid analytes [24]. Mareck et al. reported 90% THC-glucuronide extraction efficiency from urine by LLE specifically targeting THC-glucuronide as the only analyte compared to our 70–73% SLE extraction efficiency, while efficiently extracting an extensive cannabinoid panel [12]. Overall our cannabinoid and cannabinoid glucuronide SLE extraction efficiencies were similar to other methods that were optimized for less diverse analyte panels than included in our method and achieved our desired analyte sensitivities.
Deuterated THC-glucuronide, THCCOOH-glucuronide and cannabinol were not commercially available during method development. THC-d3 was selected as an internal standard for cannabinol because they co-elute, sharing similar recovery and matrix effect. None of our deuterated internal standards were well matched with THC-glucuronide and THCCOOH-glucuronide extraction efficiency and matrix effect. THCCOOH-d9 eluted nearest to THC-glucuronide and THCCOOH-glucuronide and was chosen as the internal standard for both analytes. Although THCCOOH-d9 is not an ideal internal standard for THC-glucuronide and THCCOOH-glucuronide, all QCs met performance criteria and quantitated within ±20% of expected concentrations. Low QCs prepared in 10 different urine pools were quantified with calibrators prepared in a separate urine pool to evaluate potential matrix effects on calculated concentrations. All low QC measured concentrations were within ±20% of expected concentration. Despite acceptable performance during our method validation, it is possible that an individual’s urine may produce different matrix effect than we observed during method validation contributing to inaccurate THC-glucuronide, THCCOOH-glucuronide or cannabinol results. Additional deuterated internal standards are currently under development; cannabinol-d3 recently became commercially available. Deuterated cannabinol, THC-glucuronide and THCCOOH-glucuronide inclusion as internal standards could improve precision and decrease bias for these analytes.
This method was a robust, sensitive and specific LCMSMS method for simultaneous direct quantification of THC, 11-OH-THC, THCCOOH, cannabidiol, cannabinol, THC-glucuronide and THCCOOH-glucuronide in human urine. SLE provided a fast and simple sample preparation approach while achieving efficient recoveries for the most extensive panel of cannabinoids and cannabinoid glucuronides in any urine method to date. Our current method achieved adequate sensitivity, specificity, analytical recovery, imprecision and linear ranges which indicate applicability of this method for clinical, drug treatment, forensic, workplace and driving under the influence of drugs (DUID) testing. Instability at room temperature, 4°C and after freeze/thaw cycles indicate that THC, cannabidiol and cannabinol likely will not be useful indicators for determining time of last cannabis intake. Prolonged urinary cannabinoids excretion in abstinent chronic cannabis smokers’ urine complicates identifying recent cannabis intake in drug treatment, pain management, forensic and workplace testing. Direct quantification of THC-glucuronide and THCCOOH-glucuronide in urine could assist in determining recent cannabis intake. Previously, we developed mathematical models for distinguishing recent use from prolonged excretion of cannabinoids in urine [39–41]. These models require analysis of two urine specimens collected at different times to determine if new cannabis use occurred [39–41]. Our method directly measuring cannabinoids and cannabinoid glucuronides in urine could afford a simpler and more cost-effective approach for identifying recent cannabis intake in a single urine specimen. This method will enable our clinical studies investigating urinary cannabinoid pharmacokinetics, in vitro cannabinoid stability and novel markers of cannabis intake following a single smoked cannabis dose. This method is applicable for routine cannabinoids analysis in drug treatment, pain management, forensic and workplace drug testing.
Supplementary Material
ACKNOWLEDGMENT
The authors recognize David M. Schwope’s technical advice during method development. This research was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Results from the 2009 National Survey on Drug Use and Health: National Findings In: Office of Applied Studies, editor. NSDUH Series H-38A. Rockville, MD: Department of Health and Human Services (DHHS); 2010. Substance Abuse and Mental Health Services Administration. [Google Scholar]
- 2.Lowe R, Abraham T, Darwin W, Herning R, Cadet J, Huestis M. Extended urinary delta9-tetrahydrocannabinol excretion in chronic cannabis users precludes use as a biomarker of new drug exposure. Drug Alcohol Depend. 2009;105:24–32. doi: 10.1016/j.drugalcdep.2009.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodwin RS, Darwin WD, Chiang CN, Shih M, Li S-H, Huestis MA. Urinary elimination of 11-nor-9-carboxy-Δ9-tetrahydrocannabinol in cannabis users during continuously monitored abstinence. J Anal Toxicol. 2008;32:562–566. doi: 10.1093/jat/32.8.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karschner E, Schwilke E, Lowe R, et al. Do delta9-tetrahydrocannabinol concentrations indicate recent use in chronic cannabis users? Addiction. 2009;104:2041–2048. doi: 10.1111/j.1360-0443.2009.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karschner E, Schwilke E, Lowe R, et al. Implications of plasma delta9-tetrahydrocannabinol, 11-hydroxy-THC, and 11-nor-9-carboxy-THC concentrations in chronic cannabis smokers. J Anal Toxicol. 2009;33:469–477. doi: 10.1093/jat/33.8.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huestis MA. Pharmacokinetics and metabolism of the plant cannabinoids, delta9-tetrahydrocannabinol, cannabidiol and cannabinol. In: Pertwee RG, editor. Handbook of Experimental Pharmacology. Volume 168. New York: Springer; 2005. pp. 657–690. [DOI] [PubMed] [Google Scholar]
- 7.Kemp PM, Abukhalaf IK, Manno JE, Manno BR, Alford DD, Abusada GA. Cannabinoids in humans. I. Analysis of delta9-tetrahydrocannabinol and six metabolites in plasma and urine using GC-MS. J Anal Toxicol. 1995;19:285–291. doi: 10.1093/jat/19.5.285. [DOI] [PubMed] [Google Scholar]
- 8.Kemp PM, Abukhalaf IK, Manno JE, et al. Cannabinoids in humans. II. The influence of three methods of hydrolysis on the concentration of THC and two metabolites in urine. J Anal Toxicol. 1995;19:292–298. doi: 10.1093/jat/19.5.292. [DOI] [PubMed] [Google Scholar]
- 9.Abraham TT, Lowe RH, Pirnay SO, Darwin WD, Huestis MA. Simultaneous GC-EI-MS determination of delta9-tetrahydrocannabinol, 11-hydroxy-delta9-tetrahydrocannabinol, and 11-nor-9-carboxy-delta9-tetrahydrocannabinol in human urine following tandem enzyme-alkaline hydrolysis. J Anal Toxicol. 2007;31:477–485. doi: 10.1093/jat/31.8.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwope DM, Karschner EL, Gorelick DA, Huestis MA. Identification of recent cannabis use: whole-blood and plasma free and glucuronidated cannabinoid pharmacokinetics following controlled smoked cannabis administration. Clin Chem. 2011;57:1406–1414. doi: 10.1373/clinchem.2011.171777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skopp G, Pötsch L, Ganßmann B, et al. Freie und glucuronidierte cannabinoide im urin – untersuchungen zur einschätzung des konsumverhaltens. Rechtsmedizin. 1999;10:21–28. [Google Scholar]
- 12.Mareck U, Haenelt N, Geyer H, et al. Temporal indication of cannabis use by means of THC glucuronide determination. Drug Test Anal. 2009;1:505–510. doi: 10.1002/dta.106. [DOI] [PubMed] [Google Scholar]
- 13.Schwope DM, Scheidweiler KB, Huestis MA. Direct quantification of cannabinoids and cannabinoid glucuronides in whole blood by liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2011;401:1273–1283. doi: 10.1007/s00216-011-5197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boldis O, Kocsis G, Gachalyi A, Furesz J. Gas chromatography-mass spectrometry-single ion monitoring measurement of 11-nor-delta9-tetrahydrocannabinol-carboxylic acid in urine. J Chromatogr Sci. 2003;41:190–194. doi: 10.1093/chromsci/41.4.190. [DOI] [PubMed] [Google Scholar]
- 15.Whitter PD, Cary PL, Leaton JI, Johnson JE. Automated extraction for the analysis of 11-nor-delta9-tetrahydrocannabinol-9-carboxylic acid (THCCOOH) in urine using a six-head probe hamilton microlab 2200 system and gas chromatography-mass spectrometry. J Anal Toxicol. 1999;23:286–289. doi: 10.1093/jat/23.4.286. [DOI] [PubMed] [Google Scholar]
- 16.Paul BD, Mell LD, Jr, Mitchell JM, McKinley RM, Irving J. Detection and quantitation of urinary 11-nor-delta9-tetrahydrocannabinol-9-carboxylic acid, a metabolite of tetrahydrocannabinol by capillary gas chromatography and electron impact mass fragmentography. J Anal Toxicol. 1987;11:1–5. doi: 10.1093/jat/11.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Singh J, Johnson L. Solid-phase extraction of THC metabolite from urine using the Empore disk cartridge prior to analysis by GC-MS. J Anal Toxicol. 1997;21:384–387. doi: 10.1093/jat/21.5.384. [DOI] [PubMed] [Google Scholar]
- 18.Feng S, ElSohly MA, Salamone S, Salem MY. Simultaneous analysis of delta9-THC and its major metabolites in urine, plasma, and meconium by GC-MS using an immunoaffinity extraction procedure. J Anal Toxicol. 2000;24:395–402. doi: 10.1093/jat/24.6.395. [DOI] [PubMed] [Google Scholar]
- 19.Crockett DK, Nelson G, Dimson P, Urry FM. Solid-phase extraction of 11-nordelta9-tetrahydrocannabinol-9-carboxylic acid from workplace urine drug-testing specimens with the cerex PolyCrom-THC column. J Anal Toxicol. 2000;24:245–249. doi: 10.1093/jat/24.4.245. [DOI] [PubMed] [Google Scholar]
- 20.Langen MCJ, de Bijl GA, Egberts ACG. Automated extraction of 11-nor-delta9-tetrahydrocannabinol carboxylic acid from urine samples using the ASPEC XL solid-phase extraction system. J Anal Toxicol. 2000;24:433–437. doi: 10.1093/jat/24.6.433. [DOI] [PubMed] [Google Scholar]
- 21.Stout PR, Horn CK, Klette KL. Solid-phase extraction and GC-MS analysis of THC-COOH method optimized for a high-throughput forensic drug-testing laboratory. J Anal Toxicol. 2001;25:550–554. doi: 10.1093/jat/25.7.550. [DOI] [PubMed] [Google Scholar]
- 22.Jamerson MH, Welton RM, Morris-Kukoski CL, Klette KL. Rapid quantification of urinary 11-nor-delta9-tetrahydrocannabinol-9-carboxylic acid using fast gas chromatography-mass spectrometry. J Anal Toxicol. 2005;29:664–668. doi: 10.1093/jat/29.7.664. [DOI] [PubMed] [Google Scholar]
- 23.Moon JY, Kim JY, Moon MH, Chung BC, In MK, Choi MH. Validated gas chromatographic-mass spectrometric analysis of urinary cannabinoids purified with a calcium-hardened beta-cyclodextrin polymer. J Chromatogr A. 2008;1204:87–92. doi: 10.1016/j.chroma.2008.07.083. [DOI] [PubMed] [Google Scholar]
- 24.Skopp G, Potsch L. Stability of 11-nor-delta(9)-carboxy-tetrahydrocannabinol glucuronide in plasma and urine assessed by liquid chromatography-tandem mass spectrometry. Clin Chem. 2002;48:301–306. [PubMed] [Google Scholar]
- 25.Felli M, Martello S, Chiarotti M. LC-MS-MS method for simultaneous determination of THCCOOH and THCCOOH-glucuronide in urine: Application to workplace confirmation tests. Forensic Sci Int. 2011;204:67–73. doi: 10.1016/j.forsciint.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Mareck U, Haenelt N, Geyer H, et al. Temporal indication of cannabis use by means of THC glucuronide determination. Drug Test Anal. 2009;1:505–510. doi: 10.1002/dta.106. [DOI] [PubMed] [Google Scholar]
- 27.Gronewold A, Skopp G. A preliminary investigation on the distribution of cannabinoids in man. Forensic Sci Int. 2011;210:e7–e11. doi: 10.1016/j.forsciint.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Krouwer JS, Rabinowitz R. How to improve estimates of imprecision. Clin Chem. 1984;30:290–292. [PubMed] [Google Scholar]
- 29.Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem. 2003;75:3019–3030. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- 30.Shipkova M, Armstrong VW, Oellerich M, Wieland E. Acyl glucuronide drug metabolites: toxicological and analytical implications. Ther Drug Monit. 2003;25:1–16. doi: 10.1097/00007691-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Skopp G, Potsch L. An investigation of the stability of free and glucuronidated 11-nor-delta9-tetrahydrocannabinol-9-carboxylic acid in authentic urine samples. J Anal Toxicol. 2004;28:35–40. doi: 10.1093/jat/28.1.35. [DOI] [PubMed] [Google Scholar]
- 32.Grauwiler SB, Scholer A, Drewe J. Development of a LC/MS/MS method for the analysis of cannabinoids in human EDTA-plasma and urine after small doses of Cannabis sativa extracts. Journal of Chromatography B; 2007;850:515–522. doi: 10.1016/j.jchromb.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 33.Christophersen AS. Tetrahydrocannabinol stability in whole blood: plastic versus glass containers. J Anal Toxicol. 1986;10:129–131. doi: 10.1093/jat/10.4.129. [DOI] [PubMed] [Google Scholar]
- 34.Halldin MM, Anderson LKR, Widman M, Hollister LE. Further urinary metabolites of delta9-tetrahydrocannabinol in man. Arzneimittelforchung. 1982;32:1135–1138. [PubMed] [Google Scholar]
- 35.Halldin MM, Carlsson S, Kanter SL, Widman M, Agurell S. Urinary metabolites of delta9-tetrahydrocannabinol in man. Arzneimittelforchung. 1982;32:764–768. [PubMed] [Google Scholar]
- 36.Williams PL, Moffat AC. Identification in human urine of delta9-tetrahydrocannabinol- 11-oic glucuronide: a tetrahydrocannabinol metabolite. J Pharm Pharmacol. 1980;32:445–448. doi: 10.1111/j.2042-7158.1980.tb12966.x. [DOI] [PubMed] [Google Scholar]
- 37.Wall ME, Sadler BM, Brine D, Taylor H, Perez-Reyes M. Metabolism, disposition, and kinetics of delta9-tetrahydrocannabinol in men and women. Clin Pharmacol Ther. 1983;34:352–363. doi: 10.1038/clpt.1983.179. [DOI] [PubMed] [Google Scholar]
- 38.Skopp G, Potsch L, Mauden M, Richter B. Partition coefficient, blood to plasma ratio, protein binding and short-term stability of 11-nor-delta9-carboxy tetrahydrocannabinol glucuronide. Forensic Sci Int. 2002;126:17–23. doi: 10.1016/s0379-0738(02)00023-3. [DOI] [PubMed] [Google Scholar]
- 39.Huestis MA, Cone EJ. Differentiating new marijuana use from residual drug excretion in occasional marijuana users. J Anal Toxicol. 1998;22:445–454. doi: 10.1093/jat/22.6.445. [DOI] [PubMed] [Google Scholar]
- 40.Smith ML, Barnes AJ, Huestis MA. Identifying new cannabis use with urine creatinine-normalized THCCOOH concentrations and time intervals between specimen collections. J Anal Toxicol. 2009;33:185–189. doi: 10.1093/jat/33.4.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwilke EW, Gullberg RG, Darwin WD, et al. Differentiating new cannabis use from residual urinary cannabinoid excretion in chronic, daily cannabis users. Addiction. 2011;106:499–506. doi: 10.1111/j.1360-0443.2010.03228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.