Abstract
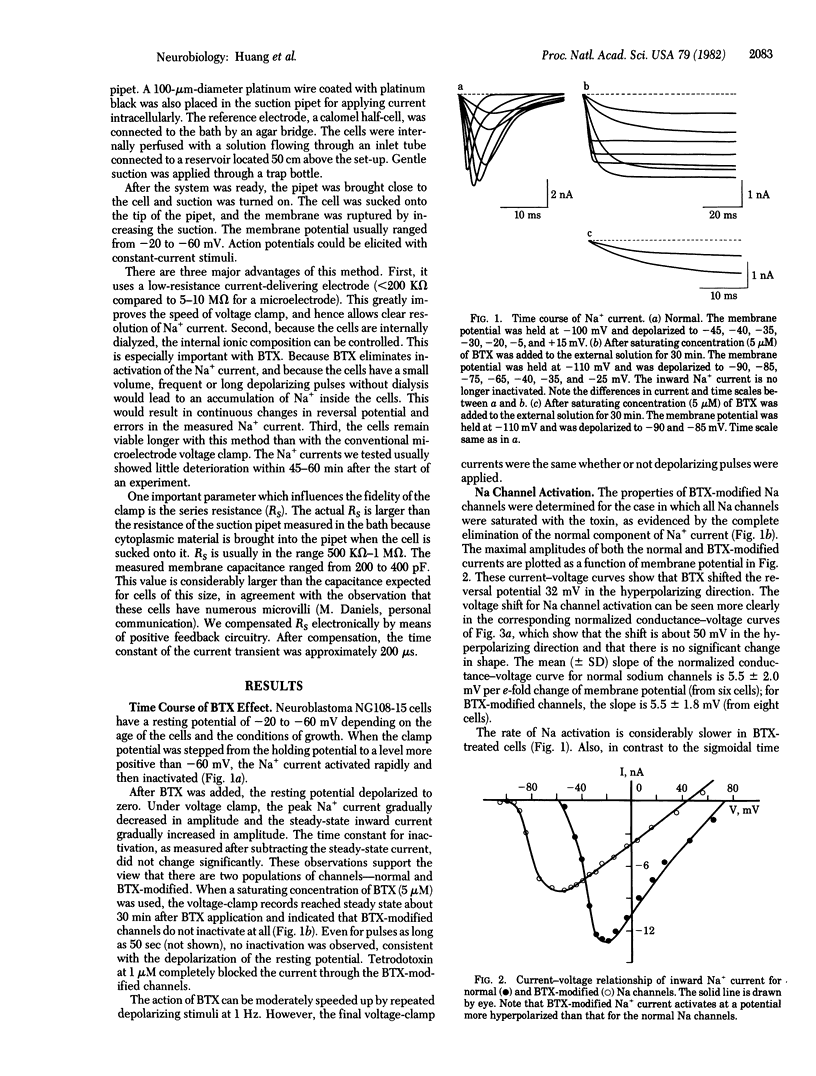
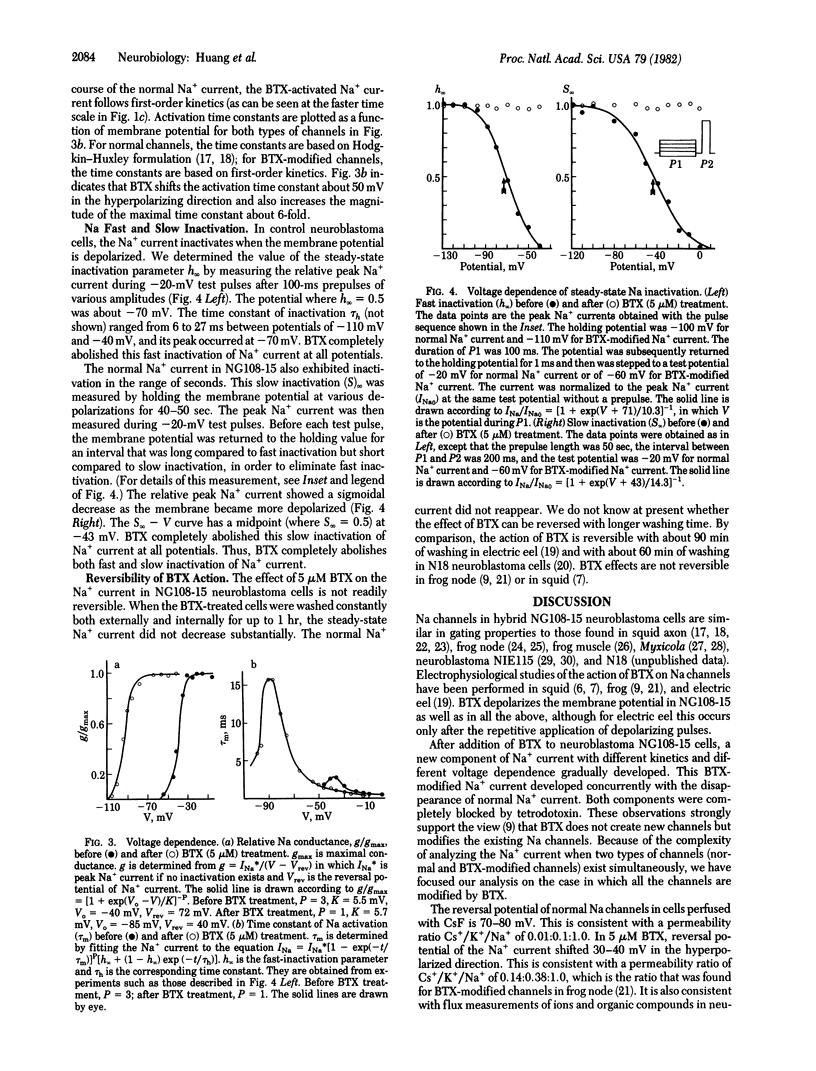
We have studied the effects of batrachotoxin (BTX) on sodium channels in hybrid mouse neuroblastoma cells NG108-15 by using the suction pipet voltage clamp method. BTX-modified sodium channels activate with first-order kinetics and, over most of the potential range, activate more slowly than normal sodium channels. The peak conductance-voltage curve and the time constant of activation-versus-voltage curve for BTX-modified sodium channels are shifted about 50 mV in the hyperpolarizing direction compared to the corresponding curves for normal sodium channels. There is no change in the slope of the conductance-voltage curve. These results suggest that BTX slows down one of the steps leading to channel opening, which consequently becomes rate-limiting. In addition, BTX eliminates both fast and slow inactivation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman W. J., Jr, Palti Y. The influence of external potassium on the inactivation of sodium currents in the giant axon of the squid, Loligo pealei. J Gen Physiol. 1969 Jun;53(6):685–703. doi: 10.1085/jgp.53.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnew W. S., Levinson S. R., Brabson J. S., Raftery M. A. Purification of the tetrodotoxin-binding component associated with the voltage-sensitive sodium channel from Electrophorus electricus electroplax membranes. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2606–2610. doi: 10.1073/pnas.75.6.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque E. X., Seyama I., Narahashi T. Characterization of batrachotoxin-induced depolarization of the squid giant axons. J Pharmacol Exp Ther. 1973 Feb;184(2):308–314. [PubMed] [Google Scholar]
- Amano T., Hamprecht B., Kemper W. High activity of choline acetyltransferase induced in neuroblastoma x glia hybrid cells. Exp Cell Res. 1974 Apr;85(2):399–408. doi: 10.1016/0014-4827(74)90142-6. [DOI] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F. Inactivation of the sodium channel. II. Gating current experiments. J Gen Physiol. 1977 Nov;70(5):567–590. doi: 10.1085/jgp.70.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels-Bernal E., Rosenberry T. L., Daly J. W. Effect of batrachotoxin on the electroplax of electric eel: evidence for voltage-dependent interaction with sodium channels. Proc Natl Acad Sci U S A. 1977 Mar;74(3):951–955. doi: 10.1073/pnas.74.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneski D. A., Catterall W. A. Covalent labeling of protein components of the sodium channel with a photoactivable derivative of scorpion toxin. Proc Natl Acad Sci U S A. 1980 Jan;77(1):639–643. doi: 10.1073/pnas.77.1.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D. T., Hille B. Kinetic and pharmacological properties of the sodium channel of frog skeletal muscle. J Gen Physiol. 1976 Mar;67(3):309–323. doi: 10.1085/jgp.67.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A. Activation of the action potential Na+ ionophore of cultured neuroblastoma cells by veratridine and batrachotoxin. J Biol Chem. 1975 Jun 10;250(11):4053–4059. [PubMed] [Google Scholar]
- Catterall W. A. Cooperative activation of action potential Na+ ionophore by neurotoxins. Proc Natl Acad Sci U S A. 1975 May;72(5):1782–1786. doi: 10.1073/pnas.72.5.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A. Membrane potential-dependent binding of scorpion toxin to the action potential Na+ ionophore. Studies with a toxin derivative prepared by lactoperoxidase-catalyzed iodination. J Biol Chem. 1977 Dec 10;252(23):8660–8668. [PubMed] [Google Scholar]
- Catterall W. A., Morrow C. S., Daly J. W., Brown G. B. Binding of batrachotoxinin A 20-alpha-benzoate to a receptor site associated with sodium channels in synaptic nerve ending particles. J Biol Chem. 1981 Sep 10;256(17):8922–8927. [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Slow changes in membrane permeability and long-lasting action potentials in axons perfused with fluoride solutions. J Physiol. 1970 Dec;211(3):707–728. doi: 10.1113/jphysiol.1970.sp009300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE F. A., FRANKENHAEUSER B. Sodium currents in the myelinated nerve fibre of Xenopus laevis investigated with the voltage clamp technique. J Physiol. 1959 Oct;148:188–200. doi: 10.1113/jphysiol.1959.sp006281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. Quantitative description of sodium currents in myelinated nerve fibres of Xenopus laevis. J Physiol. 1960 Jun;151:491–501. doi: 10.1113/jphysiol.1960.sp006455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L., Schauf C. L. Quantitative description of sodium and potassium currents and computed action potentials in Myxicola giant axons. J Gen Physiol. 1973 Mar;61(3):361–384. doi: 10.1085/jgp.61.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L. Y., Catterall W. A., Ehrenstein G. Comparison of ionic selectivity of batrachotoxin-activated channels with different tetrodotoxin dissociation constants. J Gen Physiol. 1979 Jun;73(6):839–854. doi: 10.1085/jgp.73.6.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L. Y., Ehrenstein G., Catterall W. A. Interaction between batrachotoxin and yohimbine. Biophys J. 1978 Aug;23(2):219–231. doi: 10.1016/S0006-3495(78)85444-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C. Y. Tetrodotoxin, saxitoxin and their significance in the study of excitation phenomena. Pharmacol Rev. 1966 Jun;18(2):997–1049. [PubMed] [Google Scholar]
- Khodorov B. I., Revenko S. V. Further analysis of the mechanisms of action of batrachotoxin on the membrane of myelinated nerve. Neuroscience. 1979;4(9):1315–1330. doi: 10.1016/0306-4522(79)90159-3. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Akaike N., Brown A. M. Properties of internally perfused, voltage-clamped, isolated nerve cell bodies. J Gen Physiol. 1978 May;71(5):489–507. doi: 10.1085/jgp.71.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Akaike N., Brown A. M. The suction pipette method for internal perfusion and voltage clamp of small excitable cells. J Neurosci Methods. 1980 Feb;2(1):51–78. doi: 10.1016/0165-0270(80)90045-x. [DOI] [PubMed] [Google Scholar]
- Minna J., Glazer D., Nirenberg M. Genetic dissection of neural properties using somatic cell hybrids. Nat New Biol. 1972 Feb 23;235(60):225–231. doi: 10.1038/newbio235225a0. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Spector I. Ionic currents in cultured mouse neuroblastoma cells under voltage-clamp conditions. J Physiol. 1978 May;278:265–286. doi: 10.1113/jphysiol.1978.sp012303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NARAHASHI T., MOORE J. W., SCOTT W. R. TETRODOTOXIN BLOCKAGE OF SODIUM CONDUCTANCE INCREASE IN LOBSTER GIANT AXONS. J Gen Physiol. 1964 May;47:965–974. doi: 10.1085/jgp.47.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T., Albuquerque E. X., Deguchi T. Effects of batrachotoxin on membrane potential and conductance of squid giant axons. J Gen Physiol. 1971 Jul;58(1):54–70. doi: 10.1085/jgp.58.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxford G. S., Wu C. H., Narahashi T. Removal of sodium channel inactivation in squid giant axons by n-bromoacetamide. J Gen Physiol. 1978 Mar;71(3):227–247. doi: 10.1085/jgp.71.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B. Slow inactivation of the sodium conductance in squid giant axons. Pronase resistance. J Physiol. 1978 Oct;283:1–21. doi: 10.1113/jphysiol.1978.sp012485. [DOI] [PMC free article] [PubMed] [Google Scholar]


