TO THE EDITOR
The skin is subjected to continuous physical stress. Keratinocytes resist mechanical stress by tethering the tension-bearing keratin intermediate filament cytoskeleton to sites of intercellular contact known as desmosomes (Garrod and Chidgey, 2008; Green and Simpson, 2007). The plakin protein desmoplakin (DP) is an obligate desmosomal constituent necessary for keratin anchorage at cell-cell contacts. Establishing and maintaining the DP-keratin association is essential for regulating desmosomal adhesive strength in both developing epidermis and adult stratified tissue (Huen et al., 2002; Vasioukhin et al., 2001).
Desmosome assembly is a calcium-dependent process, where major cytoplasmic plaque components of the desmosome (e.g. desmoplakin, keratin) coalesce with other desmosomal proteins (i.e. cadherins, armadillo proteins) at sites of nascent junction formation (Pasdar et al., 1991). Desmosomal proteins accumulate at cell-cell borders over time to form robust intercellular junctions capable of resisting mechanical stress. More recently, it has been reported that as desmosomes mature over the course of several days, they no longer require calcium to maintain intercellular adhesion (Cirillo et al., 2010; Wallis et al., 2000).Calcium-independent desmosomes have been associated with a state of enhanced intercellular adhesive strength termed hyperadhesion (Cirillo et al., 2010; Thomason et al., 2010). It has been hypothesized that the acquisition of hyperadhesion is essential for the epidermis to resist the perpetual mechanical stress to which it is subjected (Garrod and Kimura, 2008).
The underlying signaling and structural properties that govern desmosome maturation and acquisition of hyperadhesion have only recently begun to be studied in greater detail. The data suggest that activation of PKC in hyperadhesive epithelial sheets of Madin-Darby canine kidney (MDCK) or HaCaT cells is sufficient to convert desmosomes from calcium-independent to calcium-dependent (Cirillo et al., 2010; Wallis et al., 2000). Likewise, inhibition of PKC signaling is adequate to promote calcium-independence and hyperadhesion acquisition (Wallis et al., 2000). Additionally, hyperadhesive epithelial sheets become calcium-dependent upon wounding, and PKCα has been reported to localize to the desmosome at the leading edge of wounded epithelial cell sheets (Garrod et al., 2005; Wallis et al., 2000). Furthermore, a transmission electron microscopy and crystallographic modeling study reported that the ectodomains of desmosomal cadherins in hyperadhesive cells may undergo a structural reorganization upon wounding (Garrod et al., 2005). Altogether, these findings suggest an “inside-out” signal, whereby PKC-driven modification of a desmosomal component may induce a structural rearrangement within the desmosome to facilitate the loss of hyperadhesion and the formation of calcium-dependent desmosomes. However, the events downstream of PKC signaling have yet to be elucidated.
DP-keratin anchorage is critical for maintaining intercellular adhesive strength in epithelial sheets (Huen et al., 2002). Thus, we hypothesized that regulation of the DP-keratin association might contribute to the desmosome maturation process necessary for establishing calcium-independence and robust resistance to mechanical stress. To test our prediction that enhancing the DP-keratin interaction would promote the acquisition of hyperadhesion in epithelial sheets, we took advantage of a previously engineered DP point mutation (Ser2849Gly) that enhances intermediate filament binding by 9-fold compared to wild-type DP (Meng et al., 1997). This enhanced binding results in sequestration of the mutant DP on keratin filaments, thus altering its dynamic properties and delaying its assembly into newly forming desmosomes. However, when allowed time to accumulate at cell-cell borders, the DP Ser2849Gly protein is capable of localizing to sites of cell-cell contact (Godsel et al., 2005).
These previous findings led us to posit that incorporation of the DP Ser2849Gly mutant into the junction would promote the acquisition of hyperadhesion due to the enhanced DP-keratin association at cell borders. To address this hypothesis, A431 epithelial cells inducibly expressing wild-type DP or DP Ser2849Gly (mutant DP) (Godsel et al., 2005) were grown 2–6 days past confluency and treated with low calcium growth media (DMEM, 10% FBS, 1% penicillin/streptomycin, 0.05 mM Ca2+) for 45 minutes to preserve only calcium-independent desmosomes. Intact epithelial sheets were lifted off the surface of the dish using dispase II treatment (30–45 minutes; 2.4 U/mL, Roche #04942078001) and subjected to rotational mechanical stress (e.g. 150 rpm for 5 minutes) to induce fragmentation.
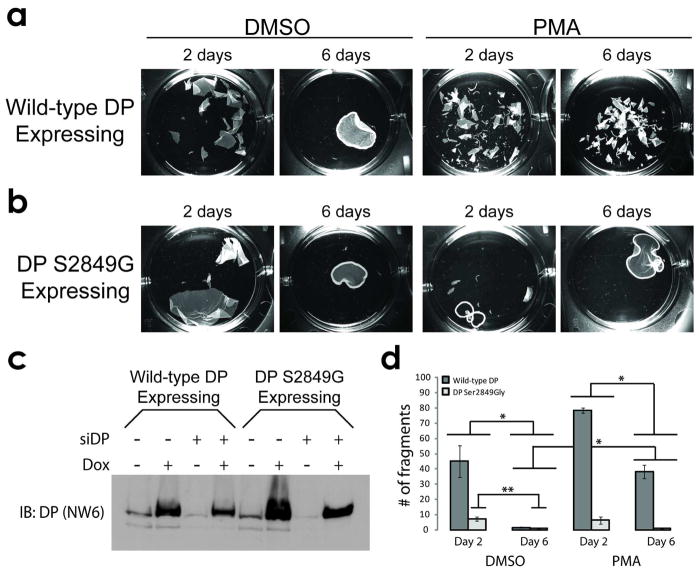
We observed that 6-day old sheets acquired a state of calcium-independence and robustly resisted fragmentation following the application of mechanical stress, whereas 2-day old sheets did not exhibit this level of stress resistance (Fig 1a, d). Pharmacologic stimulation of PKC signaling (15 minute treatment with 1 μM phorbol 12-myristate 13-acetate (PMA)) prior to incubation of sheets in low calcium media induced greater fragmentation for both 2- and 6-day sheets (Fig 1a, d). We observed similar findings for sheets of normal human epidermal keratinocytes (NHEKs) and SCC12f cells (SFig 1).
Figure 1. DP Ser2849Gly promotes hyperadhesion.
(A, left) DMSO-treated A431 epithelial sheets resist mechanical stress following 6 days at confluency, but not at 2 days. (A, right) PKC stimulation is sufficient to weaken the adhesive strength of day 2 and day 6 sheets. (B, left) A431 sheets expressing DP Ser2849Gly exhibit enhanced stress resistance at day 2, and (B, right) do not respond to PMA-induced fragmentation. (C) Western blot of A431 monolayers collected in urea sample buffer at day 6 and probed for DP (NW6) showing doxycycline-induced expression of exogenous DP in the absence of endogenous DP (siDP lanes). (D) Quantification of fragments under each condition in A and B. Graph represents three independent experiments, performed in triplicate. Bars = mean +/− SEM. *p<0.01 (Bonferroni-corrected two factor ANOVA with replication, α=0.0125) for the interaction between the indicated groups of data. **p<0.02 (Bonferroni-corrected single factor ANOVA, α=0.0125) between the indicated data sets.
As hypothesized, mutant DP expressing appreciably resisted fragmentation compared to wild-type DP expressing sheets (Fig 1b, d). Additionally, mutant DP expressing sheets were no longer responsive to the PMA-induced reversion of hyperadhesion (Fig 1b, d) indicating that DP Ser2849 can control the response of hyperadhesive epithelial sheets to PKC signaling. Furthermore, mutant DP expressing sheets exhibited reduced fragmentation at 6-days compared to 2-days (Fig 1d), suggesting that robust keratin anchorage over the course of desmosome maturation may be a driving factor in governing the acquisition of desmosome hyperadhesion. Parallel results were observed in SCC12f cells constitutively expressing wild-type DP or mutant DP (SFig 1b). Importantly, these results were obtained in the background of endogenous DP depletion using siRNA oligos targeting the DSP 3′ UTR region, which ensured the inducibly-expressed DP transgene would be the predominant form of DP expressed during the assay (Fig 1c).
Altogether, our data indicates that DP Ser2849 is a key mediator in the acquisition of desmosomal hyperadhesion. As DP Ser2849 resides within a PKC consensus sequence, the data raises the possibility that PKC-mediated phosphorylation of DP Ser2849 may serve as a molecular mechanism to tune the adhesive strength of epithelia during wound healing and morphogenesis (Fig 2). Given the recent report of hyperadhesion protecting epithelial sheets from pemphigus-induced loss of adhesion (Cirillo et al., 2010), identifying underlying mechanisms that regulate the acquisition of hyperadhesion may lead to novel therapeutic strategies for treating skin blistering disorders.
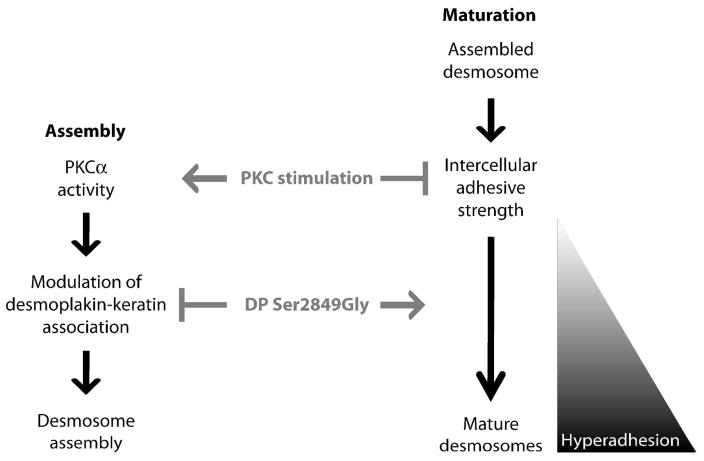
Figure 2. Model depicting the roles of PKC signaling and DP-keratin association in regulating desmosome assembly and maturation.
(Left, Assembly) Stimulation of PKC signaling promotes desmosome assembly, in part through regulation of the DP-keratin interaction in a Ser2849-dependent manner (Godsel 2005, Bass-Zubek 2008). (Right, Maturation) Once assembled, desmosomes provide intercellular adhesive strength, which increases over time as desmosomes mature and ultimately reach a state of hyperadhesion. PKC stimulation induces the reversion of hyperadhesion, but not in epithelial sheets expressing the DP Ser2849Gly point mutant, which is unresponsive to PKC stimulation and promotes hyperadhesion.
Supplementary Material
Acknowledgments
This work was supported by an American Heart Association fellowship (0810061Z) and NIH training grant to (T32 CA009560) to R.P.H. and NIH R37 AR043380 to K.J.G. K.J.G. is supported in part by the Joseph L. Mayberry Endowment.
Abbreviations
- DP
Desmoplakin
- PKC
Protein kinase C
- Ser
Serine
- Gly
Glycine
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Cirillo N, Lanza A, Prime SS. Induction of hyper-adhesion attenuates autoimmune-induced keratinocyte cell-cell detachment and processing of adhesion molecules via mechanisms that involve PKC. Exp Cell Res. 2010;316:580–592. doi: 10.1016/j.yexcr.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Garrod D, Chidgey M. Desmosome structure, composition and function. Biochim Biophys Acta. 2008;1778:572–587. doi: 10.1016/j.bbamem.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Garrod D, Kimura TE. Hyper-adhesion: a new concept in cell-cell adhesion. Biochem Soc Trans. 2008;36:195–201. doi: 10.1042/BST0360195. [DOI] [PubMed] [Google Scholar]
- Garrod DR, Berika MY, Bardsley WF, Holmes D, Tabernero L. Hyper-adhesion in desmosomes: its regulation in wound healing and possible relationship to cadherin crystal structure. J Cell Sci. 2005;118:5743–5754. doi: 10.1242/jcs.02700. [DOI] [PubMed] [Google Scholar]
- Godsel LM, Hsieh SN, Amargo EV, Bass AE, Pascoe-McGillicuddy LT, Huen AC, et al. Desmoplakin assembly dynamics in four dimensions: multiple phases differentially regulated by intermediate filaments and actin. J Cell Biol. 2005;171:1045–1059. doi: 10.1083/jcb.200510038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KJ, Simpson CL. Desmosomes: new perspectives on a classic. J Invest Dermatol. 2007;127:2499–2515. doi: 10.1038/sj.jid.5701015. [DOI] [PubMed] [Google Scholar]
- Huen AC, Park JK, Godsel LM, Chen X, Bannon LJ, Amargo EV, et al. Intermediate filament-membrane attachments function synergistically with actin-dependent contacts to regulate intercellular adhesive strength. J Cell Biol. 2002;159:1005–1017. doi: 10.1083/jcb.200206098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng JJ, Bornslaeger EA, Green KJ, Steinert PM, Ip W. Two-hybrid analysis reveals fundamental differences in direct interactions between desmoplakin and cell type-specific intermediate filaments. J Biol Chem. 1997;272:21495–21503. doi: 10.1074/jbc.272.34.21495. [DOI] [PubMed] [Google Scholar]
- Pasdar M, Krzeminski KA, Nelson WJ. Regulation of desmosome assembly in MDCK epithelial cells: coordination of membrane core and cytoplasmic plaque domain assembly at the plasma membrane. J Cell Biol. 1991;113:645–655. doi: 10.1083/jcb.113.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason HA, Scothern A, McHarg S, Garrod DR. Desmosomes: adhesive strength and signalling in health and disease. Biochem J. 2010;429:419–433. doi: 10.1042/BJ20100567. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Bowers E, Bauer C, Degenstein L, Fuchs E. Desmoplakin is essential in epidermal sheet formation. Nat Cell Biol. 2001;3:1076–1085. doi: 10.1038/ncb1201-1076. [DOI] [PubMed] [Google Scholar]
- Wallis S, Lloyd S, Wise I, Ireland G, Fleming TP, Garrod D. The alpha isoform of protein kinase C is involved in signaling the response of desmosomes to wounding in cultured epithelial cells. Mol Biol Cell. 2000;11:1077–1092. doi: 10.1091/mbc.11.3.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.