Abstract
Background
Proper ascertainment of the history of alcohol consumption by an individual is an important component of medical diagnosis of disease and influences the implementation of appropriate treatment strategies that include prescription of medication, as well as intervention for the negative physical and social consequences of hazardous/harmful levels of alcohol consumption. Biological (biometric) diagnostic tests that provide information on current and past quantity and frequency of alcohol consumption by an individual, prior to onset of organ damage, continue to be sought.
Methods
Platelet monoamine oxidase B (MAO-B) protein was quantitated in 2 populations of subjects who had histories of different levels of alcohol consumption. Levels were assayed by immunoblotting or by ELISA. The development and evaluation of the new ELISA-based measure of platelet MAO-B protein levels is described.
Results
One subject population constituted a nontreatment-seeking, cross-sectional subject sample, and the other population was a longitudinally followed, hospitalized group of subjects. An algorithm combining measures of platelet MAO-B protein with the plasma levels of carbohydrate-deficient transferrin (CDT) and with liver enzymes (aspartate aminotransferase or γ-glutamyltransferase [GGT]) can detect hazardous/harmful alcohol use (HHAU) with the highest sensitivity and specificity in the cross-sectional nontreatment-seeking population. In the treatment-seeking population, low MAO-B protein levels at admission are associated with heavy drinking prior to admission, and these protein levels increase over a period of abstinence from alcohol.
Conclusions
The platelet MAO-B protein measurement is particularly effective for male alcohol consumers. The combined use of MAO-B protein measures together with measures of CDT and GGT does, however, improve the diagnostic utility of both markers for ascertaining HHAU in women. Furthermore, measurement of changes in platelet MAO-B protein levels during treatment for alcohol dependence may help monitor the success of the treatment program.
Keywords: Alcohol Consumption, Biomarkers, MAO Protein, Platelets
Platelet monoamine oxidase type B (MAO-B) has been evaluated extensively as a possible genetic trait (not state) marker for alcohol dependence (Wargelius et al., 2010). Many studies have demonstrated an association between low platelet MAO-B enzymatic activity and alcohol dependence and ascribed this association to a genetic predisposition with alcohol dependence (Eriksson et al., 2000; Pombo et al., 2008; Wiberg et al., 1977). Others have generated data on the association of certain personality characteristics [impulsivity, risk-taking behaviors, aggressiveness (Shih et al., 1999; von Knorring et al., 1984)], as well as depression and addiction to drugs other than alcohol, with low levels of plate-let MAO-B enzymatic activity.
The genetic association between low platelet MAO-B enzymatic activity and all of these psychiatric disorders was called into question when it was reported that individual differences in platelet MAO-B enzymatic activity could largely be explained by the subject’s smoking behavior (Anthenelli et al., 1998; Berlin and Anthenelli, 2001; Fowler et al., 1996; Norman et al., 1987; Snell et al., 2002; Whitfield et al., 2000; Yu and Boulton, 1987). Thus, the co-occurrence of high levels of smoking by individuals exhibiting numerous psychiatric disorders discredited many prior studies that did not control for smoking in establishing an association of platelet MAO-B enzymatic activity and psychiatric illness, personality type, or alcohol dependence.
We also demonstrated (Snell et al., 2002) that current smoking significantly reduces MAO-B enzymatic activity in platelets, but that smoking does not affect MAO-B protein levels. These findings were consistent with the results of others (Fowler et al., 2003) that tobacco products contain benzoquinone and naphthylamine derivatives, which are xenobiotic inhibitors of MAO. On the other hand, individuals with a current history of high levels of alcohol intake, even though they were not smokers, had reduced levels of MAO-B protein (Snell et al., 2002). We have now conducted a series of studies to evaluate whether measures of platelet MAO-B protein levels provide a sensitive and specific marker for hazardous/harmful alcohol use (HHAU) that can be used in a clinical setting. Our current studies also show that low MAO-B protein levels (measured using a newly developed ELISA-based assay) in platelets of alcohol-consuming individuals increase during a period of abstinence. Such observations further question the proposal (Oreland, 2004) that MAO-B is a stable genetic marker for alcohol-abusing individuals.
MATERIALS AND METHODS
Local ethics committees approved all study protocols, and all participants gave written informed consent. All personal information was separated from information used in this study, and material analyzed was anonymized by use of numerical identifiers.
WHO/ISBRA Collaborative Study on State and Trait Markers of Alcoholism
This study was established in 1988 by a collaborative effort of the World Health Organization (WHO) and the International Society for Biomedical Research on Alcoholism (ISBRA) to assess, in a multi-center trial (Montreal, Canada; Helsinki, Finland; São Paulo, Brazil; Sydney, Australia; Sapporo, Japan), markers of recent alcohol use (state markers) and trait (genetic)markers of predisposition to alcohol dependence (Glanz et al., 2002). Blood samples were obtained from a stratified random sample of participants where stratification was based on levels of alcohol consumption. Platelet pellets were isolated as described elsewhere and stored at −70°C. This population was extensively characterized not only for alcohol drinking and alcohol dependence but also for a large number of demographic, medical, and psychiatric variables (Glanz et al., 2002). Subjects’ alcohol consumption thresholds were classified as nonhazardous alcohol use (NHAU) or HHAU by means of established WHO criteria (40 g/d for men and 20 g/d for women) (Saunders and Lee, 2000). The volume and chronology of ethanol consumption was recorded using the timeline followback procedure (Sobell et al., 1979), and amount of ethanol consumed is calculated by taking the type of beverage as well as the volume consumed into account. The data were then converted to grams of ethanol. The total amount of ethanol (in grams) consumed over the prior 30 days was divided by 30 to arrive at average grams of ethanol consumed per day. A subset of the platelets collected during the course of this study was available for our analysis (123 men and 82 women). Serum samples from the same subjects for whom platelet MAO-B protein levels were determined were assayed for carbohydrate-deficient transferrin (%CDT TIA; BioRad, Hercules, CA) and for γ-glutamyltransferase (GGT) and aspartate aminotransferase (AST) using reflectance spectrometry (Vitros 250 Analyzer; Ortho Clinical Diagnostics, Rochester, NY). The measurement of platelet MAO-B protein for this population was achieved using quantitative immunoblotting. Table 1 gives a detailed description of the demographic and other characteristics of this population.
Table 1.
Characteristics of WHO/ISBRA Study Population
| Males | Females | |
|---|---|---|
| Subjects (N) | 123 | 82 |
| Age | 39 (18–60) | 39 (18–60) |
| Total body water | 41.0 (26.8–55.5) | 30.2 (21.3–42.3) |
| Body mass index | 24.8 (13.9–38.2) | 24.0 (15.6–39.0) |
| Regular exercise (N) | 63 (57%) | 41 (54%) |
| Recruitment site | ||
| Helsinki, Finland | 7 (6%) | 0 (0%) |
| Montreal, Canada | 72 (59%) | 75 (91%) |
| Sao Paulo, Brazil | 27 (22%) | 7 (9%) |
| Sydney, Australia | 17 (14%) | 0 (0%) |
| Race (N) | ||
| White | 104 (85%) | 75 (91%) |
| Black | 5 (4%) | 4 (5%) |
| Asian | 6 (5%) | 0 (0%) |
| Other | 8 (6%) | 3 (4%) |
| Alcohol-related variables (N) | ||
| Drank alcohol during last 30 days | 107 (87%) | 57 (70%) |
| Hazardous/harmful alcohol use in last 30 days | 73 (59%) | 28 (34%) |
| Current alcohol dependence | 63 (51%) | 23 (28%) |
| Current alcohol abuse | 71 (58%) | 24 (29%) |
| Family history of alcohol dependence | 76 (64%) | 59 (74%) |
| Familial alcohol dependence | 49 (42%) | 21 (26%) |
| Ethanol intake during last 30 days (g/d) | 83.0 (0–386.0) | 34.9 (0–292.8) |
| Sobriety in subjects who drank in the last 30 days (days) | 3.1 (0–21) | 4.3 (0–21) |
| Medical history (N)a | ||
| Enlarged liver | 12 (10%) | 1 (1%) |
| Hepatitis | 10 (8%) | 6 (7%) |
| Hyperlipidemia | 15 (12%) | 2 (2%) |
| Convulsion (epilepsy) | 12 (10%) | 2 (2%) |
| Vitamin deficiency | 10 (8%) | 16 (20%) |
| Emphysema | 11 (9%) | 6 (7%) |
| Arthritis | 11 (9%) | 7 (9%) |
| High blood pressure | 24 (20%) | 8 (10%) |
| Drug use (N)b | ||
| Marijuana | 33 (27%) | 11 (13%) |
| Cocaine | 15 (12%) | 4 (5%) |
| Smoking | 86 (70%) | 38 (46%) |
| Prescription or over the counter | 69 (56%) | 68 (83%) |
| DSM-IV diagnoses (N) | ||
| Marijuana dependence | 5 (4%) | 3 (4%) |
| Cocaine dependence | 16 (13%) | 7 (9%) |
| Antisocial personality disorder | 29 (24%) | 15 (18%) |
| Conduct disorder | 13 (11%) | 7 (9%) |
| Major depression | 22 (18%) | 24 (29%) |
| Familial depression | 7 (6%) | 7 (9%) |
| Plasma/serum measures | ||
| Platelet MAO-B protein levelc | 0.90 (0.05–2.67) | 1.19 (0.08–3.43) |
| Aspartate aminotransferase (AST; U/l) | 40.6 (16.0–210.0) | 30.2 (10.0–525.0) |
| γ -glutamyltransferase (GGT; U/l) | 101 (11–2,556) | 46 (7–792) |
| Carbohydrate-deficient transferrin (%CDT) | 3.7 (1.0–11.5) | 2.6 (1.45–5.51) |
Values for continuous variables are means, followed by the range in parentheses, and values for categorical variables are the number of subjects in that category, followed by the percentage of all subjects with valid data.
Diagnosed with given medical condition at any time during life.
Use/abuse during previous 30 days.
Monoamine oxidase B (MAO-B) protein levels given in µg equivalents/µg protein derived from immunoblot measurements.
WHO/ISBRA, World Health Organization International Society for Biomedical Research on Alcoholism.
NIAAA/LCTS Study
This subject population was recruited from an ongoing clinical study of the assessment and treatment of individuals with alcohol drinking problems, performed at the National Institute on Alcohol Abuse and Alcoholism Laboratory of Clinical and Translational Studies (NIAAA/LCTS). The 87 male and 58 female treatment-seeking subjects recruited in this study enrolled in a 4-week inpatient protocol. Patients underwent a series of verbal, observational, and medical assessments. Data were used from blood samples obtained on the day of admission (day 1) and on day 8 and day 15 after admission. Platelet pellets were isolated as described elsewhere and stored at −70°C. Day 1 serum samples from the same subjects for whom platelet MAO-B protein levels were determined were assayed for GGT, alanine aminotransferase (ALT), and AST as part of a standard clinical blood chemistry panel assayed in the Department of Laboratory Medicine, NIH Clinical Center, Bethesda, MD. Additionally, plasma from 128 of these individuals (73 men and 55 women) collected on day 1 was assayed for %CDT (Helander et al., 2003). Table 2 describes the characteristics of this population.
Table 2.
Characteristics of NIAAA/LCTS Study Population
| Males | Females | |
|---|---|---|
| Subjects (N) | 87 | 58 |
| Age | 42 (21–57) | 41 (22–62) |
| Weight (kg) | 85.6 (57.7–224.3) | 69.6 (44.1–180.6) |
| Height (cm) | 176.9 (69–192) | 164 (112–177) |
| Body mass index | 29.8 (19.2–151.4) | 25.9 (15.8–57.6) |
| Race (N) | ||
| White | 60 (69%) | 44 (76%) |
| Black | 21 (24%) | 10 (17%) |
| Asian | 2 (2%) | 0 (0%) |
| Other | 3 (3%) | 2 (3%) |
| Unknown | 1 (1%) | 2 (3%) |
| Alcohol-related variables | ||
| Days abstinent | 2.7 (0–30) | 1.9 (0–22) |
| No. drinking days in last 90 days | 72.3 (17–90) | 64.6 (0.90) |
| No. nondrinking days in last 90 days | 17.4 (0–72) | 25.2 (0–90) |
| No. heavy drinking days in last 90 days | 67.7 (1–90) | 59.3 (0–90) |
| Mean alcohol intake 10 days prior to admission (g/d) | 173.1 (0–453.6) | 132.9 (0–407.4) |
| Mean alcohol intake 90 days prior days prior to admission (g/d) | 223.6 (36–1,015.5) | 173.4 (0–524.8) |
| Drug use (N) | ||
| Cocaine | 19 (24%) | 20 (36%) |
| Opiates | 6 (8%) | 5 (9%) |
| Marijuana | 27 (34%) | 14 (25%) |
| Smoking (N)a | 59 (76%) | 39 (71%) |
| DSM-IV diagnosesb (N) | ||
| Cocaine dependence | 31 (37%) | 25 (43%) |
| Cocaine abuse | 13 (16%) | 7 (12%) |
| Marijuana dependence | 35 (42%) | 12 (21%) |
| Marijuana abuse | 26 (31%) | 9 (16%) |
| Opioid dependence | 8 (10%) | 8 (14%) |
| Opioid abuse | 3 (4%) | 6 (10%) |
| Amphetamine dependence | 8 (10%) | 5 (9%) |
| Amphetamine abuse | 9 (11%) | 5 (9%) |
| Sedative/anxiolytic abuse | 8 (10%) | 3 (5%) |
| Hallucinogen dependence | 7 (8%) | 2 (3%) |
| Hallucinogen abuse | 15 (18%) | 7 (12%) |
| PCP dependence | 3 (4%) | 2 (3%) |
| PCP abuse | 1 (1%) | 1 (2%) |
| Major depression | 11 (13%) | 17 (29%) |
| Obsessive–compulsive personality disorder | 9 (11%) | 11 (19%) |
| Antisocial personality disorder | 11 (13%) | 7 (12%) |
| Posttraumatic stress disorder | 13 (16%) | 18 (31%) |
| Baseline plasma/serum measures | ||
| Platelet MAO-B protein levelc | 9.3 (0.9–25.1) | 14.4 (4.0–40.9) |
| γ -glutamyltransferase (GGT; U/l) | 128.7 (13.0–923.0) | 81.0 (11.0–574.0) |
| % CDT (diasialotransferrin/total transferrin) | 3.8 (0.8–19.6) | 3.0 (0.9–12.3) |
| RBC mean corpuscular volume (MCV; fl) | 94.7 (76.3–112.0) | 95.6 (68.8–109.0) |
| Aspartate aminotransferase (AST; U/l) | 64.9 (8.0–313.0) | 58.6 (15.0–388.0) |
| Alanine aminotransferase (ALT; U/l) | 61.3 (11.0–446.0) | 46.4 (13.0–312.0) |
| Platelet count (thousands of cells/µl)d | 247.8 (41.0–495.0) | 286.7 (163.0–452.0) |
| Lymphocyte count (thousands/µl) | 2.0 (0.6–3.8) | 2.1 (0.7–3.8) |
| Transferrin (mg/dl) | 232.6 (147.0–331.0) | 240.4 (142.0–378.0) |
Values for continuous variables are means, followed by the range in parentheses, and values for categorical variables are the number of subjects in that category, followed by the percentage of all subjects with valid data.
Use during previous 30 days.
DSM-IV criteria for clinical diagnoses during lifetime using SCID-I and SCID-II.
Monoamine oxidase B (MAO-B) protein levels given in ng/µg protein derived from ELISA measurements.
One extreme outlying value (a male with a value of 866) was excluded.
CDT, carbohydrate-deficient transferrin; NIAAA/LCTS, National Institute on Alcohol Abuse and Alcoholism Laboratory of Clinical and Translational Studies; RBC, red blood cell.
Platelet Preparation
After drawing blood into EDTA-treated collection tubes, platelet counts were performed in whole blood as part of a standard hematologic profile for all subjects (WHO/ISBRA and NIAAA/LCTS subjects). Platelet-rich plasma (PRP) was prepared from subject’s blood samples within 4 hours after blood collection, and platelet pellets were obtained from PRP by centrifugation at 3,000×g for 10 min (Corash, 1980; Glanz et al., 2002).
Quantitative Immunoblotting
For assaying samples from the WHO/ISBRA population, platelet pellets were thawed at 4°C and resuspended in 500 µl of 100 mM phosphate buffer (KiHiPO4), pH 7.4, by brief sonication. Platelets were repelleted by centrifugation at 31,500×g for 30 min at 4°C, and resuspended in 100 µl of 10 mM phosphate buffer, pH 7.4. Aliquots of each sample (4 and 8 µg, BCA assay; Thermo Scientific/Pierce, Rockford, IL) were analyzed by standard immunoblotting techniques (Snell et al., 2002). Blots were washed and incubated (1 hour, room temperature, 1:1,000) with a rabbit polyclonal antibody for MAO-B, which was produced by Rockland Immunochemicals (Gilbertville, PA), using a peptide provided by Lohocla Research Corporation, followed by anti-rabbit IgG (1:10,000; BioRad). Bands were detected using enhanced chemiluminescence (Renaissance, Dupont-NEN, Boston, MA) and exposed to Kodak X-Omat film (Sigma-Aldrich, St. Louis, MO). The optical density of immunoreactive bands was analyzed using Quantity One software (BioRad).
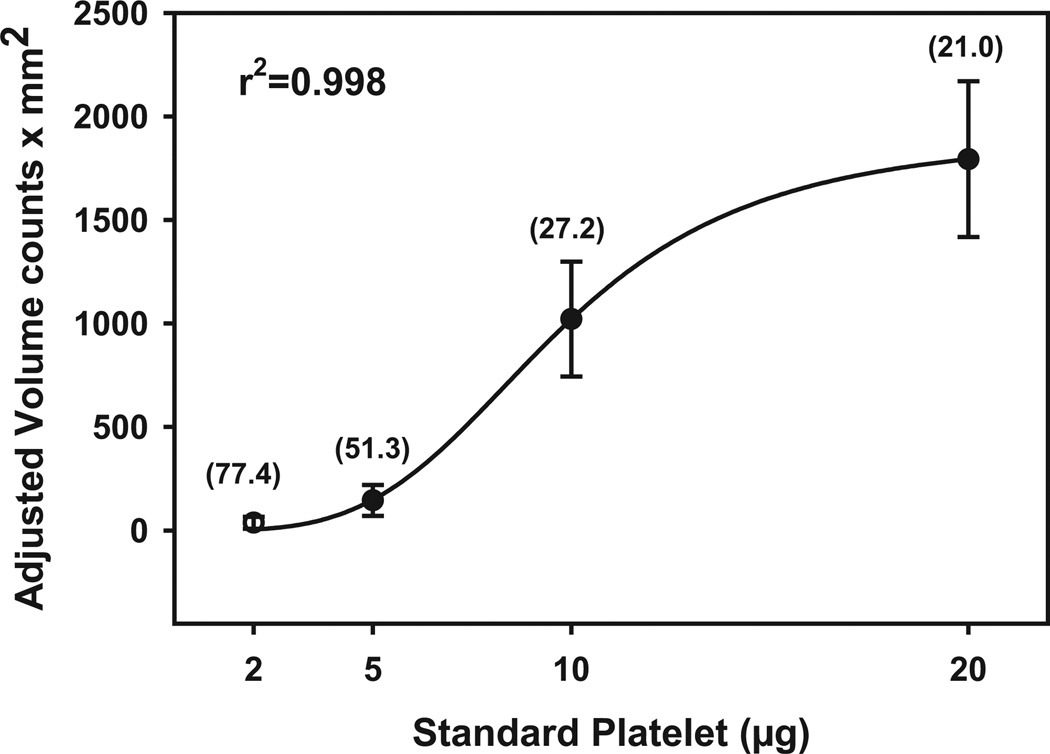
Each blot also contained increasing amounts of a standard platelet membrane preparation (5, 10, and 20 µg protein). Standard curves were established using nonlinear iterative curve fitting (Sigmaplot; SPSS Inc., Chicago, IL). Results are expressed as microgram equivalents of the MAO immunoreactivity (relative to the standard platelet preparation) per microgram of protein (mean ± SEM). Figure 1 shows a plot of the mean values for 3 standard curves obtained on 3 separate occasions, with associated coefficient of variation (CV) values at each platelet protein amount. Inter-assay variability ranged from 21% at high platelet protein amount to 77% at low platelet protein amount, while intra-assay variability, calculated from triplicates run on the same gel, ranged from 4 to 28%.
Fig. 1.
Averaged standard curves of standard platelet proteins used for quantitative immunoblotting with associated curve fit. Units on abscissa are optical density units adjusted for volume of immunoreactive bands on film. Values in parentheses are the %CV of 3 measurements at each protein amount (each measurement represents the average of triplicates from each of the 3 standard curves).
MAO-B ELISA
To assay samples from the NIAAA/LCTS population, platelet pellets were thawed in a room temperature water bath for 3 to 4 min. Platelet protein was solubilized in 200 µl of 1% (v/v) n-octylpolyoxyethylene (C8POE; Bachem, Torrance, CA) in phosphate-buffered saline containing a protease inhibitor cocktail (Sigma-Aldrich), followed by sonication and vortexing. Unsolubilized material was removed by centrifugation at 25,000×g for 30 min at 20°C. Protein concentration in the supernatant was determined by the BCA assay (Pierce), and samples were diluted to 50 µg protein/ml.
Measurements of MAO-B protein levels were taken with an ELISA kit (Mediagnost Product Code E102), which was designed through a cooperative agreement between Lohocla Research Corporation and Mediagnost, GmbH (Reutlingen, Germany). Using monoclonal antibodies provided by Lohocla Research Corporation, Mediagnost produced a “sandwich” ELISA assay that included plates and all reagents necessary for performing the ELISA assay. Solubilized platelet samples were diluted to 10 µg protein/ml in the dilution buffer, and 100 µl of this preparation was assayed in triplicate. The plate was incubated for 1.5 hours on a rotating shaker followed by washing 3 times with 300 µl of wash buffer. This was followed, in order, by incubation for 1 hour with a “detection” monoclonal antibody conjugated to biotin and incubation for 30 min with a streptavidin– horseradish peroxidase conjugate. Color was developed by addition of tetramethylbenzidine for 15 min. The reaction was stopped by addition of sulfuric acid, and the intensity of the color was read on a BioTek plate reader (Winooski, VT) set at 450 nm.
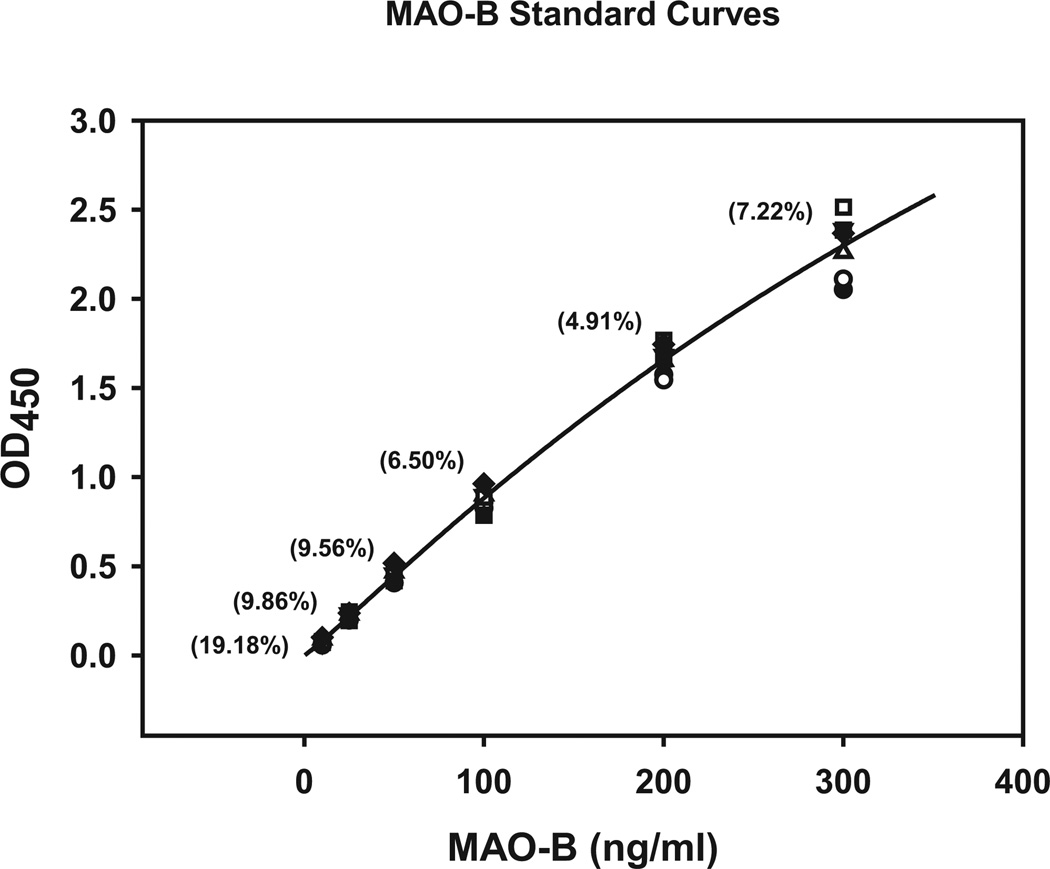
Using FDA/ICH guidelines (http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070107.pdf; http://www.bioforum.org.il/Uploads/Editor/karen/q2_r1_step4.pdf), the MAO-B ELISA was evaluated for sensitivity, linearity, precision, and accuracy with recombinant MAO-B (10 to 300 ng/ml). Intra-assay variability (triplicates, %CV) was determined to be from 5 to 10% using recombinant MAO-B and from 1 to 10% using platelet protein. Inter-assay variability, determined as described elsewhere, was determined to be 5 to 19% using recombinant MAO-B standards (Fig. 2) and 2 to 15% using platelet protein. The working range of the assay is 0.3 to 2.5 µg platelet protein with a lower limit of quantitation of 0.2 µg platelet protein (Fig. 3). MAO-B recovery was established by adding recombinant MAO-B at known concentrations to platelet-poor plasma. Adding 20 to 200 ng/ml recombinant MAO-B yielded an average recovery of 95% (range 93 to 99%).
Fig. 2.
Averaged standard curves of recombinant monoamine oxidase B (MAO-B) used for ELISA measures of MAO-B in platelets. Units on the abscissa are the optical densities (OD) read at 450 nm using a BioTek LX800 plate reader. Values in parentheses are the %CV of the 3 measurements at each recombinant MAO-B protein concentration (each measurement is the average of triplicates in each standard curve).
Fig. 3.
Representative plot of the working concentrations of platelet protein established for the monoamine oxidase B (MAO-B) ELISA.
On each ELISA plate, in addition to samples, a standard curve was generated for recombinant human MAO-B (0 to 300 ng/ml; 100 µl each in triplicate). Based on the standard curves, sample data are expressed as nanogram MAO-B protein per microgram total protein (ng/µg protein) (mean ± SEM).
Statistical Analysis
Initially, we examined the capacity of MAO-B protein levels to predict the categorization of subjects’ alcohol consumption as NHAU and HHAU separately for men and women from the WHO-ISBRA population and without taking into account any other characteristics of the subjects. For this simplistic model, we used a traditional receiver operating characteristic (ROC) analysis to establish the optimal cutoffs for values of MAO-B protein to minimize the error rate when distinguishing individuals with HHAU from those with NHAU.
We also ascertained whether any of the other variables on which we had information should be considered as a moderator or confounder of the predictive utility of platelet MAO-B protein for categorizing HHAU versus NHAU. We examined the association of HHAU and MAO-B protein levels with the covariates in Table 1, excluding the drinking-related variables and the plasma/serum-related variables. Potential moderators (Baron and Kenny, 1986) of the relationship between MAO-B and HHAU were evaluated using 2 tests. Initially, each potential moderator (Table 1) was examined independently for association with MAO-B levels using linear regression. Second, the interaction of MAO-B and the potential moderator was evaluated when predicting HHAU using logistic regression. Variables with a significant interaction effect with MAO-B when predicting HHAU (p < 0.05) were further examined by stratifying the population by the moderator and testing for an association between MAO-B and HHAU within strata.
Our additional analytical objective was to identify a parsimonious model for predicting HHAU in the WHO/ISBRA population from MAO-B protein values and values obtained from measures of other biomarkers and for predicting MAO-B protein levels at admission in the NIAAA/LCTS population. To do this, we used a forward selection (entrance criterion p < 0.05) method to build a multivariate model. The final multivariate logistic model for the WHO/ISBRA population was further characterized using an ROC analysis. Area under the ROC curves (AUC) in all cases was calculated using the trapezoidal rule, and AUC values were compared using a contrast matrix (DeLong et al., 1988). In the NIAAA/LCTS population, we examined the effect of time since admission into the inpatient treatment program (i.e., days of monitored abstinence) on MAO-B protein levels using a mixed linear regression model that accounted for within-subject correlations. We also identified variables from Table 2 that influence the rate of change in MAO-B protein levels over time by building a multivariate model that included interaction effects of variables with time. Using a forward selection process, variables and their interaction with time were retained if their interaction effect was statistically significant (p < 0.05). Use of other standard statistical comparisons between groups is noted in the text, tables and figure legends. All analyses were executed using either the MIXED or LOGISTIC procedures in SAS Statistical Software version 9.3 (SAS Institute, Inc., Cary, NC).
RESULTS
WHO/ISBRA Study Subjects
Our earlier study (Snell et al., 2002) established that men and women differ in baseline platelet MAO-B protein levels. Therefore, platelet MAO-B protein data were compared, using quantitative immunoblot procedures, separately in men and women (Table 3). In this current sample, female subjects had significantly higher MAO-B protein levels than male subjects after accounting for HHAU (p = 0.0133). In addition, HHAU subjects had significantly lower MAO-B protein levels than NHAU subjects after accounting for gender (p = 0.0002). The difference between HHAU and NHAU subjects was retained across genders (interaction p-value = 0.70).
Table 3.
Platelet MAO-B Protein Levels in the WHO-ISBRA Study Population
| Gender | Drinking status |
Number of subjects |
MAO-B Protein Levels | |
|---|---|---|---|---|
| Mean | Standard error | |||
| Males | NHAU | 50 | 1.10 | 0.07 |
| HHAU | 73 | 0.76 | 0.06 | |
| Females | NHAU | 54 | 1.29 | 0.11 |
| HHAU | 28 | 1.01 | 0.09 | |
Protein levels are reported in microgram equivalents per microgram protein.
Monoamine oxidase B (MAO-B) protein levels are significantly higher in women than in men, after accounting for HHAU (p = 0.013). MAO-B protein levels are significantly lower in subjects with HHAU, compared to those with NHAU, after accounting for gender (p = 0.0002).
HHAU, hazardous/harmful alcohol use; NHAU, nonhazardous alcohol use; WHO/ISBRA, World Health Organization International Society for Biomedical Research on Alcoholism.
ROC analysis was used to identify the optimal cutoff for MAO-B protein levels for distinguishing HHAU based on minimizing the error rate in a hypothetical population split evenly between subjects with HHAU and NHAU (i.e., finding the balance between sensitivity and specificity that would result in the fewest number of subjects being misclassified). In male subjects, the optimal cutoff was 0.94 µg equivalents/µg protein with a sensitivity of 71% and a specificity of 68%. The optimal cutoff MAO-B level to identify HHAU in female subjects was 1.55 µg equivalents/µg protein with a sensitivity of 30% and a specificity of 93%.
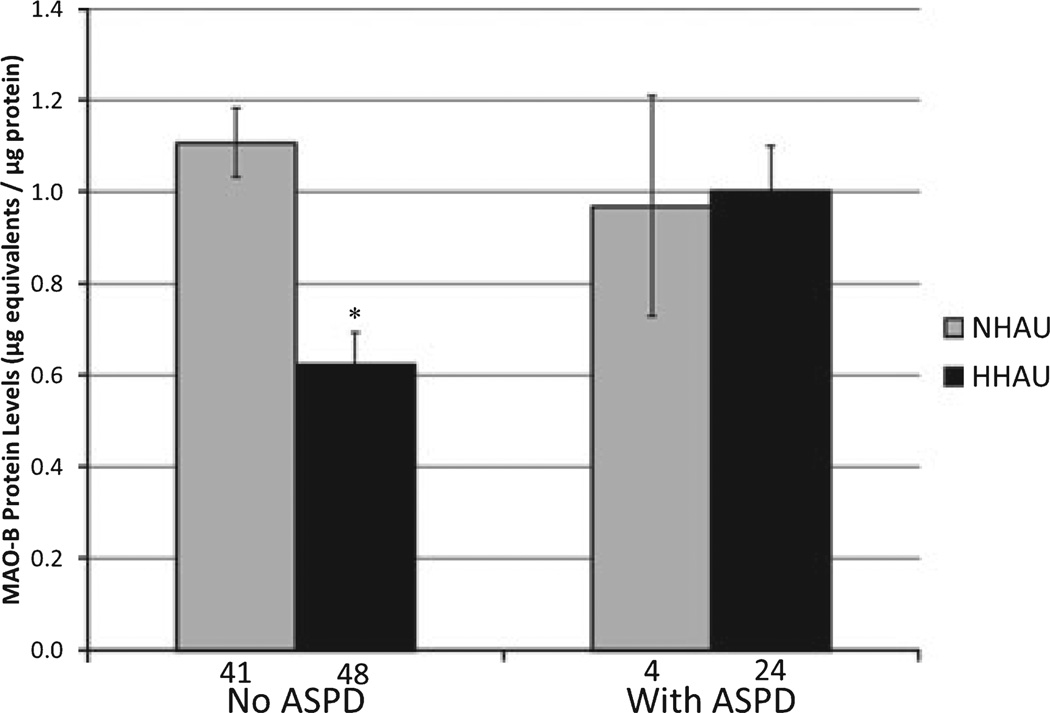
In the WHO/ISBRA men, but not in women, race was a confounder because it was associated with both HHAU and MAO-B protein levels. When data from the 6 Asian male subjects were eliminated from further analysis, HHAU and race were no longer associated. Elimination of data from these subjects did not measurably affect the MAO-B cutoff values obtained from ROC analysis, as described elsewhere. The regression analysis of the remaining male subjects revealed that antisocial personality disorder (ASPD) met criteria for moderator status, that is, was not associated with MAO-B protein levels but had a significant interaction effect with MAO-B protein levels when predicting HHAU (Table 4A). ASPD moderates the association between HHAU and MAO-B levels by eliminating the association between the two in ASPD-diagnosed men, while the predictive power of MAO-B protein levels in men without ASPD is retained (Fig. 4). Note that there were only 4 of 28 men with ASPD who were not also hazardous/harmful alcohol users, suggesting a considerable comorbidity between ASPD and HHAU in our WHO/ISBRA subject population. Including ASPD and its interaction with MAO-B protein levels in our predictive model of HHAU improved the predictive power of MAO-B in men (AUC: MAO-B only = 0.71, MAO-B and ASPD =0.79; p = 0.07, combined vs.MAO-B only).
Table 4.
Logistic Regression for WHO/ISBRA Population
| (A) Predicting HHAU in men (>40 g/d) | ||||||
|---|---|---|---|---|---|---|
| Univariate association with | Interaction effect with MAO-B when predicting | |||||
| Potential moderators | MAO-B p-value |
HHAU p-value |
HHAU p-value |
|||
| Antisocial personality disorder | 0.1834 | 0.0049 | 0.0356 | |||
| Univariate association with | Final multivariate model predicting HHAU | |||||
| MAO-B p-value |
HHAU p-value |
Odds ratio | p-Value | |||
| MAO-B protein (dichotomized) | – | <0.0001 | 0.13 | 0.0003 | ||
| %CDT | 0.0341 | 0.0017 | 1.71 | 0.0160 | ||
| Ln (AST) | 0.4716 | 0.0001 | 14.02 | 0.0046 | ||
| ROC | ||||||
| AUC ± standard error | 95% Wald confidence limits | |||||
| MAO-B only (dichotomized) | 0.72 ± 0.05 | 0.63 | 0.81 | |||
| %CDT only | 0.74 ± 0.05 | 0.64 | 0.84 | |||
| AST only | 0.77 ± 0.05 | 0.67 | 0.87 | |||
| MAO-B and %CDT | 0.83 ± 0.04 | 0.75 | 0.91 | |||
| MAO-B and AST | 0.84 ± 0.04 | 0.77 | 0.92 | |||
| %CDT and AST | 0.81 ± 0.04 | 0.72 | 0.89 | |||
| MAO-B, %CDT, and AST | 0.87a ± 0.04 | 0.80 | 0.94 | |||
| (B) Predicting HHAU in women (>20 g/d) | ||||||
|---|---|---|---|---|---|---|
| Univariate association with | Final multivariate model predicting HHAU | |||||
| MAO-B p-value |
HHAU p-value |
Odds ratio | p-Value | |||
| MAO-B protein (dichotomized) | – | 0.0318 | 0.20 | 0.0499 | ||
| %CDT | 0.5434 | 0.0112 | 2.12 | 0.0467 | ||
| ln (GGT) | 0.4552 | 0.0063 | 2.63 | 0.0198 | ||
| ROC | ||||||
| AUC ± standard error | 95% Wald confidence limits | |||||
| MAO-B only (dichotomized | 0.61 ± 0.04 | 0.53 | 0.79 | |||
| %CDT only | 0.65 ± 0.06 | 0.52 | 0.77 | |||
| GGT only | 0.66 ± 0.07 | 0.53 | 0.79 | |||
| MAO-B and %CDT | 0.71 ± 0.06 | 0.60 | 0.83 | |||
| MAO-B and GGT | 0.74 ± 0.04 | 0.62 | 0.86 | |||
| %CDT and AST | 0.72 ± 0.06 | 0.59 | 0.84 | |||
| MAO-B, %CDT, and AST | 0.76b,c,d ± 0.06 | 0.64 | 0.88 | |||
Significantly different from each marker alone (p < 0.05) and significant (p < 0.0001) with regard to distinguishing HHAU better than by chance.
Significantly different from monoamine oxidase B (MAO-B) alone p = 0.003.
Different from %CDT alone p = 0.061.
Different from GGT alone p = 0.079 and significant (p < 0.0007) with regard to distinguishing HHAU better than by chance.
HHAU, hazardous/harmful alcohol use; ROC, receiver operating characteristic; AUC, area under the ROC curve; CDT, carbohydrate-deficient transferrin; AST, aspartate aminotransferase; GGT, γ-glutamyltransferase; WHO/ISBRA, World Health Organization International Society for Biomedical Research on Alcoholism.
Fig. 4.
Platelet monoamine oxidase B (MAO-B) protein levels associated with hazardous/harmful alcohol use (HHAU) and nonhazardous alcohol use (NHAU) in subjects with and without antisocial personality disorder (ASPD). MAO-B platelet protein levels associated with HHAU or NHAU are illustrated for male WHO/ISBRA subjects with or without a lifetime diagnosis of ASPD. Note that 1 ASPD subject (Table 1) was Asian, and was excluded from this analysis. Data represent least squares means from a 2-way ANOVA. *p < 0.0001, HHAU compared to NHAU (t-statistic for individual contrasts).
%CDT and the liver enzymes GGT and AST have been evaluated extensively as markers of alcohol use and abuse (Chen et al., 2003; Conigrave et al., 1995, 2002, 2003). Therefore, we assessed the ability of these clinical biomarkers individually and in combination with MAO-B protein to predict HHAU in men of the WHO/ISBRA cohort. To do this, we built a multivariate logistic regression model using a forward selection method. The final multivariate model contained MAO-B protein level (dichotomized), %CDT, and AST level (log-transformed). GGT did not enter the multivariate model in men. The dichotomized version of the MAO-B protein measure in men was established using the optimal cutoff from the ROC analysis performed earlier (0.94 µg equivalents/µg protein). Levels equal to or below this value were assigned to the “low” category, and levels above this value were assigned to the “high” category. Neither %CDT nor AST had a significant interaction effect with the dichotomized MAO-B protein levels, indicating that they act independently and in an additive fashion to predict HHAU. Comparing the ROC curves, it can be seen that the combination of 3 variables (MAO-B protein, %CDT, and AST) is significantly better than any variable independently, but not better than any pair (Table 4A).
In female subjects of the WHO/ISBRA cohort, none of the variables we tested (Table 1) were either a confounder or moderator of the association between MAO-B and HHAU. In the multivariate model of biomarkers, using data obtained with women, MAO-B protein levels (dichotomized), GGT (log-transformed), and %CDT entered the model (Table 4B). AST did not enter the multivariate model in women. The dichotomized version of MAO-B protein level was again based on the previously established cutoff (by ROC) of 1.55 µg equivalents/µg protein (NHAU vs. HHAU) in women. The results of the ROC analyses of the combination of MAO-B protein, %CDT, and GGT in women to discriminate HHAU from NHAU indicated that the combination of all 3 of these variables was significantly better than the use of MAO-B protein levels alone, but not significantly better than %CDT, GGT, or any of the pairwise combinations of these variables (Table 4B).
NIAAA/LCTS Study Subjects
ELISA-based measurements of platelet MAO-B were taken in the cohort recruited from a heavy drinking, treatment-seeking population at the NIAAA/LCTS. At admission, the daily average number of grams of ethanol consumed in the last 10 days by men was significantly different from the threshold of 40 g per day for hazardous/harmful drinking (172.3 g/day ± 12.9; p-value <0.0001). Likewise in women, the daily average number of grams of ethanol consumed over the last 10 days was significantly different from the threshold of 20 g per day for hazardous/harmful drinking in women (132.9 g/d ± 13.6; p-value < 0.0001).
We initially assessed which patient characteristics, including drinking history and plasma measurements, were associated with levels of MAO-B protein on the day of admission to the inpatient program (day 1) by building a multivariate linear model. In men, number of days of heavy drinking in the last 90 days had the strongest univariate association with MAO-B protein levels on day 1 (p < 0.0001). The association with MAO-B levels was negative, that is, more days of heavy drinking were associated with lower MAO-B levels and accounted for 19% of the total variation in MAO-B. The final multivariate model also included several other patient characteristics. Platelet counts on day 1, a history of posttraumatic stress disorder (PTSD), and a history of marijuana abuse and dependence were all associated with higher levels of MAO-B protein. Being Caucasian, cocaine use in the 30 days prior to admission, and a history of opioid abuse were associated with lower levels of MAO-B protein. In women, number of days abstinent prior to admission (if more than 10 days abstinent, values were winsorized to 10 days) was the strongest univariate predictor of MAO-B protein levels at admission, had a positive association with MAO-B protein levels, and explained 19% of the total variance (p = 0.0007). In addition to the number of days abstinent prior to admission, the final multivariate model included age, which was also positively associated with MAO-B protein levels.
In both men and women, MAO-B protein levels increased over time in the inpatient program (i.e., monitored abstinence) (Table 5). The findings that MAO-B protein levels change over a period of abstinence, and that drinking-related variables were associated with MAO-B protein level at admission, suggest that a recent history of alcohol consumption does influence MAO-B levels and that the effect is at least partially reversible. However, we also wished to determine whether there are other patient characteristics that affect the magnitude and direction of change in MAO-B protein levels during the period of alcohol abstinence. We therefore built a multivariate mixed linear regression model specifically looking for patient characteristics that had a significant interaction with time (i.e., number of days in inpatient program). For men, the final multivariate model included time interactions with ALT (negative interaction; p = 0.005), AST (positive interaction; p < 0.001), and mean corpuscular volume (MCV) (negative interaction; p < 0.001) levels. A positive interaction between AST and time indicates that higher AST levels at admission are associated with a more pronounced increase in MAO-B protein levels after beginning abstinence. Likewise, a negative interaction indicates that lower levels of ALT and MCV are associated with a larger increase in MAO-B protein levels over time. It is important to note that AST was the first interaction to enter the model and only after that interaction was accounted for did ALT and MCV have significant interactions with time. This is particularly relevant because all 3 values are positively correlated with each other, yet the interaction effect for AST differs in direction from that of ALT and MCV. This indicates that a prior history of alcohol use and damage to organs because of alcohol use do alter the rate of change in MAO-B protein levels during alcohol abstinence.
Table 5.
Platelet MAO-B Protein Levels in NIAAA/LCTS Study Population
| Gender | Number of days in treatment |
Number of subjects |
MAO-B Protein Levels | ||
|---|---|---|---|---|---|
| Mean | Standard error |
p-Value* | |||
| Males | Day 1 | 85 | 9.4 | 0.6 | 0.0003 |
| Day 8 | 85 | 11.2 | 0.8 | ||
| Day 15 | 83 | 12.2 | 0.9 | ||
| Females | Day 1 | 58 | 14.4 | 1.0 | <0.0001 |
| Day 8 | 57 | 18.2 | 1.1 | ||
| Day 15 | 57 | 19.3 | 1.2 | ||
Values are ng MAO-B/µg protein.
p-Value represents a significant linear association between time (in days) and MAO-B protein levels.
NIAAA/LCTS, National Institute on Alcohol Abuse and Alcoholism Laboratory of Clinical and Translational Studies; MAO-B, monoamine oxidase B.
In women, opioid dependence (positive interaction; p = 0.018) and AST (positive interaction; p = 0.027) both interacted with the effect of time abstinent on MAO-B protein levels. As in men, higher AST levels at admission are associated with a greater increase in MAO-B protein levels during abstinence.
DISCUSSION
In an attempt to uncover a utilitarian marker for monitoring a history of hazardous/harmful alcohol consumption, we ascertained, in the subjects from the WHO/ISBRA study, that levels of MAO-B protein are lower in platelets of individuals who drink alcohol at hazardous/harmful levels compared to individuals drinking below this threshold. Similarly, in the longitudinal (NIAAA) study, heavier alcohol drinking prior to admission was associated with lower MAO-B protein levels upon admission to treatment. The data in this article provide support for our initial findings (Snell et al., 2002) by use of 2 larger populations and also characterize a novel ELISA assay for MAO-B protein. The first of the current study populations (WHO/ISBRA) was drawn primarily from nonalcoholism treatment-seeking subjects who were recruited from the general population and demonstrated various levels of alcohol consumption (Tabakoff and Helander, 2002). In this “general population” sample, measures of platelet MAO-B protein, in men, provided sensitivity and specificity for identifying HHAU that was comparable to %CDT, GGT, and AST measurements. It was interesting to find that ASPD was a moderator of the ability of MAO-B protein levels to discriminate between HHAU and NHAU in men, that is, ASPD subjects with HHAU had MAO-B protein levels that prevented distinguishing them from NHAU subjects. As we selected subjects to fill cells determined by levels of alcohol consumption, the larger than expected number of ASPD subjects in our population (Table 1) may reflect the known association of drug use, including alcohol, and ASPD in our oversampled, high-drinking subjects (Goldstein et al., 2007). If one can identify and control for ASPD subjects, most of whom drink at HHAU levels or above, then the AUC for ROC analysis of MAO-B protein as an indicator of HHAU in men in the WHO/ISBRA sample is 0.80. In women, in this general population sample, measures of MAO-B protein levels in themselves produced a significant, but modest ability to distinguish between HHAU and NHAU, with no influence of ASPD.
Our analysis did not identify smoking (in the past 30 days) as a confounder or moderator of the ability of MAO-B protein to predict HHAU in either men or women. These findings support our earlier work (Snell et al., 2002) showing that platelet MAO-B protein levels were not significantly different among nonsmokers, ex-smokers (not smoking in the past 30 days), and current smokers (smoking at least 1 cigarette in the past 30 days). In contrast, Launay and colleagues (2009) reported higher platelet MAO-B protein levels in current and ex-smokers, compared with nonsmokers. However, in their study, subjects were classified only by smoking status, while other factors (e.g., alcohol drinking, ASPD) that affect MAO-B protein levels and/or associations of MAO-B protein levels with phenotype, and may have influenced their results, were not determined.
Other possible confounds of the predictive value of the level of platelet MAO-B protein for HHAU could include dietary factors, that is, Zellner and colleagues (2011) recently reported that eating a high-protein diet for 3 weeks, with controlled intake of food and beverages, resulted in a reduced level of platelet MAO-B protein. It is unlikely that many individuals would consume such highly regulated diets; however, information about dietary intake should be considered when using platelet MAO-B protein levels as a predictor of HHAU.
One of the promising ways to overcome low sensitivity or specificity of a marker is to combine information from 1 marker with that of an uncorrelated marker of the same condition. Our logistic regression analysis suggested that the best combination of markers, overall, may be to combine the measure of MAO-B protein, %CDT, and AST in men, and MAO-B protein, %CDT, and GGT in women (Table 4). Depending on the perceived benefit of maximizing the sensitivity or specificity of the biological markers, such AUC results predict successful use of the MAO-B, %CDT, and liver enzyme combination in screening or in diagnosis.
Both the immunoblotting and ELISA assays used in our studies were performed with platelets obtained using a standardized method that we have previously described (Glanz et al., 2002). This standardization is a necessary prerequisite for obtaining reliable results. The procedure is relatively simple and can be easily reproduced in a clinical laboratory. The lower variance obtained with the ELISA suggests that the ELISA will provide a clinically feasible methodology to measure platelet MAO-B protein levels as a biomarker for HHAU.
Using this ELISA, the data obtained from the measurement of MAO-B protein levels on the day of admission of subjects to the NIAAA/LCTS longitudinal study were compatible with the results of the cross-sectional, general population study, in that recent heavy drinking was the most influential factor with regard to lower levels of MAO-B protein at admission. Based on our results in this population, if one wishes to use the level of MAO-B protein as an indicator of recent heavy alcohol consumption, the sensitivity and specificity of this “marker” in men would be increased if one can account for a lifetime history of PTSD, as well as a history of marijuana and opiate abuse/dependence and cocaine use. It has previously been found that platelet MAO activity was lower in subjects who were dependent on alcohol, cocaine, or a combination of alcohol, cocaine, and marijuana (Faraj et al., 1994). Our findings, however, indicate that after accounting for differences in recent alcohol drinking, a history of marijuana abuse or dependence by itself is associated with higher MAO-B protein levels. Interestingly, these factors did not influence MAO-B protein levels in women on admission to treatment, thus questioning a supposition that cocaine, opiate, or marijuana use/dependence can, in and of themselves, lower MAO-B protein levels in human platelets.
The advantage of the longitudinal study is that one can determine whether MAO-B protein levels change when subjects abstain from alcohol drinking. MAO-B protein levels can then serve as a marker of abstinence during treatment. Our results showed that MAO-B protein levels increased significantly over the 2-week inpatient (monitored alcohol abstinence) treatment period. Interestingly, the factors most prominently associated with this increase were plasma markers of long-term heavy alcohol use (AST, ALT, MCV in men and AST in women). A high ratio of AST/ALT has been suggested to specifically reflect liver damage (Nyblom et al., 2004), and this ratio at admission was also found to be positively associated with the increase in MAO-B protein levels over time in men (p = 0.0001). One may speculate that long-term heavy alcohol consumption, as evidenced by the behavior of a number of possible markers, as well as self-report, is associated with the lowest levels of MAO-B protein at admission to treatment, leading to a greater magnitude of change as MAO-B protein levels revert to “normal” during abstinence.
Overall, our studies show that platelet MAO-B protein can distinguish between HHAU and NHAU in men and women, and combining this new marker with currently used measures of %CDT and liver enzymes can significantly improve health services screening or a physician’s diagnostic capacity to identify chronic consumers of high levels of alcohol. Our studies also show that monitoring platelet MAO-B protein levels may aid in determining the success of alcoholism treatment.
ACKNOWLEDGMENTS
Funding for Lohocla Research Corporation: SBIR grants R44AA014531 and R43AA017374 and the Banbury Fund. Mediagnost thanks Nadine Wachendorfer for her excellent technical assistance. †This work is in part a result of the work of WHO/ISBRA Investigators: K.M. Conigrave, M. Dongier, H. Edenberg, C.J.P. Eriksson, M.L.O.S. Formigoni, B.F. Grant, A. Helander, P.L. Hoffman, K. Kiianmaa, T. Koyama, L. Legault, T.-K. Li, T. Mathuen, M.G. Monteiro, T. Saito, M. Salaspuro, J.B. Saunders, B. Tabakoff, S. Tufik, J.B. Whitfield, and F.M.Wurst.
REFERENCES
- Anthenelli RM, Tipp J, Li TK, Magnes L, Schuckit MA, Rice J, Daw W, Nurnberger JI., Jr Platelet monoamine oxidase activity in subgroups of alcoholics and controls: results from the Collaborative Study on the Genetics of Alcoholism. Alcohol Clin Exp Res. 1998;22:598–604. doi: 10.1111/j.1530-0277.1998.tb04298.x. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Berlin I, Anthenelli RM. Monoamine oxidases and tobacco smoking. Int J Neuropsychopharmacol. 2001;4:33–42. doi: 10.1017/S1461145701002188. [DOI] [PubMed] [Google Scholar]
- Chen J, Conigrave KM, Macaskill P, Whitfield JB, Irwig L. Combining carbohydrate-deficient transferrin and gamma-glutamyltransferase to increase diagnostic accuracy for problem drinking. Alcohol Alcohol. 2003;38:574–582. doi: 10.1093/alcalc/agg113. [DOI] [PubMed] [Google Scholar]
- Conigrave KM, Davies P, Haber P, Whitfield JB. Traditional markers of excessive alcohol use. Addiction. 2003;98(Suppl 2):31–43. doi: 10.1046/j.1359-6357.2003.00581.x. [DOI] [PubMed] [Google Scholar]
- Conigrave KM, Degenhardt LJ, Whitfield JB, Saunders JB, Helander A, Tabakoff B. CDT, GGT, and AST as markers of alcohol use: the WHO/ISBRA collaborative project. Alcohol Clin Exp Res. 2002;26:332–339. [PubMed] [Google Scholar]
- Conigrave KM, Saunders JB, Whitfield JB. Diagnostic tests for alcohol consumption. Alcohol Alcohol. 1995;30:13–26. [PubMed] [Google Scholar]
- Corash L. Platelet heterogeneity: relevance to the use of platelets to study psychiatric disorders. Schizophr Bull. 1980;6:254–258. doi: 10.1093/schbul/6.2.254. [DOI] [PubMed] [Google Scholar]
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- Eriksson M, Berggren U, Blennow K, Fahlke C, Mansson JE, Balldin J. Alcoholics with the dopamine receptor DRD2 A1 allele have lower platelet monoamine oxidase-B activity than those with the A2 allele: a preliminary study. Alcohol Alcohol. 2000;35:493–498. doi: 10.1093/alcalc/35.5.493. [DOI] [PubMed] [Google Scholar]
- Faraj BA, Davis DC, Camp VM, Mooney AJ, III, Holloway T, Barika G. Platelet monoamine oxidase activity in alcoholics, alcoholics with drug dependence, and cocaine addicts. Alcohol Clin Exp Res. 1994;18:1114–1120. doi: 10.1111/j.1530-0277.1994.tb00090.x. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Logan J, Wang GJ, Volkow ND. Monoamine oxidase and cigarette smoking. Neurotoxicology. 2003;24:75–82. doi: 10.1016/s0161-813x(02)00109-2. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, MacGregor R, Alexoff D, Shea C, Schlyer D, Wolf AP, Warner D, Zezulkova I, Cilento R. Inhibition of monoamine oxidase B in the brains of smokers. Nature. 1996;379:733–736. doi: 10.1038/379733a0. [DOI] [PubMed] [Google Scholar]
- Glanz J, Grant B, Monteiro M, Tabakoff B. WHO/ISBRA study on state and trait markers of alcohol use and dependence: analysis of demographic, behavioral, physiologic, and drinking variables that contribute to dependence and seeking treatment. International society on biomedical research on alcoholism. Alcohol Clin Exp Res. 2002;26:1047–1061. [PubMed] [Google Scholar]
- Goldstein RB, Dawson DA, Saha TD, Ruan WJ, Compton WM, Grant BF. Antisocial behavioral syndromes and DSM-IV alcohol use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Clin Exp Res. 2007;31:814–828. doi: 10.1111/j.1530-0277.2007.00364.x. [DOI] [PubMed] [Google Scholar]
- Helander A, Husa A, Jeppsson JO. Improved HPLC method for carbohydrate-deficient transferrin in serum. Clin Chem. 2003;49:1881–1890. doi: 10.1373/clinchem.2003.023341. [DOI] [PubMed] [Google Scholar]
- Launay JM, Del Pino M, Chironi G, Callebert J, Peoc’h K, Megnien JL, Mallet J, Simon A, Rendu F. Smoking induces long-lasting effects through a monoamine-oxidase epigenetic regulation. PLoS One. 2009;4:e7959. doi: 10.1371/journal.pone.0007959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman TR, Chamberlain KG, French MA. Platelet monoamine oxidase: low activity in cigarette smokers. Psychiatry Res. 1987;20:199–205. doi: 10.1016/0165-1781(87)90079-5. [DOI] [PubMed] [Google Scholar]
- Nyblom H, Berggren U, Balldin J, Olsson R. High AST/ALT ratio may indicate advanced alcoholic liver disease rather than heavy drinking. Alcohol Alcohol. 2004;39:336–339. doi: 10.1093/alcalc/agh074. [DOI] [PubMed] [Google Scholar]
- Oreland L. Platelet monoamine oxidase, personality and alcoholism: the rise, fall and resurrection. Neurotoxiciology. 2004;25:79–89. doi: 10.1016/S0161-813X(03)00115-3. [DOI] [PubMed] [Google Scholar]
- Pombo S, Levy P, Bicho M, Ismail F, Cardoso JM. Neuropsychological function and platelet monoamine oxidase activity levels in type I alcoholic patients. Alcohol Alcohol. 2008;43:423–430. doi: 10.1093/alcalc/agn021. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Lee NK. Hazardous alcohol use: its delineation as a subthreshold disorder, and approaches to its diagnosis and management. Compr Psychiatry. 2000;41(2) Suppl 1:95–103. doi: 10.1016/s0010-440x(00)80015-2. [DOI] [PubMed] [Google Scholar]
- Shih JC, Chen K, Ridd MJ. Monoamine oxidase: from genes to behavior. Annu Rev Neurosci. 1999;22:197–217. doi: 10.1146/annurev.neuro.22.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell LD, Glanz J, Tabakoff B. Relationships between effects of smoking, gender, and alcohol dependence on platelet monoamine oxidase-B: activity, affinity labeling, and protein measurements. Alcohol Clin Exp Res. 2002;26:1105–1113. [PubMed] [Google Scholar]
- Sobell LC, Maisto SA, Sobell MB, Cooper AM. Reliability of alcohol abusers’ self-reports of drinking behavior. Behav Res Ther. 1979;17:157–160. doi: 10.1016/0005-7967(79)90025-1. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Helander A. WHO/ISBRA study on state and trait markers in alcoholism. Alcohol Clin Exp Res. 2002;25:99S–103S. doi: 10.1097/00000374-200105051-00018. [DOI] [PubMed] [Google Scholar]
- von Knorring L, Oreland L, Winblad B. Personality traits related to monoamine oxidase activity in platelets. Psychiatry Res. 1984;12:11–26. doi: 10.1016/0165-1781(84)90134-3. [DOI] [PubMed] [Google Scholar]
- Wargelius HL, Fahlke C, Suomi SJ, Oreland L, Higley JD. Platelet monoamine oxidase activity predicts alcohol sensitivity and voluntary alcohol intake in rhesus monkeys. Ups J Med Sci. 2010;115:49–55. doi: 10.3109/03009731003605813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield JB, Pang D, Bucholz KK, Madden PA, Heath AC, Statham DJ, Martin NG. Monoamine oxidase: associations with alcohol dependence, smoking and other measures of psychopathology. Psychol Med. 2000;30:443–454. doi: 10.1017/s0033291799001798. [DOI] [PubMed] [Google Scholar]
- Wiberg A, Gottfries CG, Oreland L. Low platelet monoamine oxidase activity in human alcoholics. Med Biol. 1977;55:181–186. [PubMed] [Google Scholar]
- Yu PH, Boulton AA. Irreversible inhibition of monoamine oxidase by some components of cigarette smoke. Life Sci. 1987;41:675–682. doi: 10.1016/0024-3205(87)90446-2. [DOI] [PubMed] [Google Scholar]
- Zellner M, Babeluk R, Jakobsen LH, Gerner C, Umlauf E, Volf I, Roth E, Kondrup J. A proteomics study reveals a predominant change in MaoB expression in platelets of healthy volunteers after high protein meat diet: relationship to the methylation cycle. J Neural Transm. 2011;118:653–662. doi: 10.1007/s00702-011-0617-6. [DOI] [PubMed] [Google Scholar]