Abstract
Objectives
1. To test whether alteration of the vocal fold medial surface contour can improve phonation. 2. To demonstrate that implant material properties affect vibration even when implant is deep to the vocal fold lamina propria.
Study Design
Induced phonation of excised human larynges.
Methods
Thirteen larynges were harvested within 24 hours post-mortem. Phonation threshold pressure (PTP) and flow (PTF) were measured before and after vocal fold injections using either calcium hydroxylapatite (CaHA) or hyaluronic acid (HA). Small-volume injections (median 0.0625 mL) were targeted to the infero-medial aspect of the thyroarytenoid (TA) muscle. Implant locations were assessed histologically.
Results
The effect of implantation on PTP was material-dependent. CaHA tended to increase PTP, whereas HA tended to decrease PTP (Wilcoxon test P = 0.00013 for onset). In contrast, the effect of implantation on PTF was similar, with both materials tending to decrease PTF (P = 0.16 for onset). Histology confirmed implant presence in the inferior half of the vocal fold vertical thickness.
Conclusions
Taken together, these data suggested the implants may have altered the vocal fold medial surface contour, potentially resulting in a less convergent or more rectangular glottal geometry as a means to improve phonation. An implant with a closer viscoelastic match to vocal fold cover is desirable for this purpose, as material properties can affect vibration even when the implant is not placed within the lamina propria. This result is consistent with theoretical predictions and implies greater need for surgical precision in implant placement and care in material selection.
Keywords: Vocal fold contour, phonation threshold pressure, phonation threshold flow, vocal fold injection, excised larynx
INTRODUCTION
Restoration of phonation in patients with deficient glottal function has been a focus of laryngeal surgeons for over a century. Since Brünings 1 pioneered vocal fold injection in 1911, much effort over the ensuing century has been devoted to perfecting surgical means to reposition a paralyzed vocal fold to restore glottal closure. This included the development of type 1 thyroplasty 2, arytenoid adduction 3, arytenopexy 4, as well as continued refinement of vocal fold injections. 5,6,7 It is noteworthy that the longstanding focus on glottal closure and ways to alter the adductory-abductory position of vocal folds may rest with the predominant misconception that glottal function takes place in two dimensions in the transverse plane, a perception perpetuated by routine clinical laryngoscopy that provides only a two-dimensional view. In fact, not only does vocal fold adduction-abduction take place in three dimensions, the key glottal function of phonation is generated by vocal fold vibration best appreciated in the coronal plane. Hirano’s pioneering work on characterizing the fine structure of the vocal fold lamina propria 8,9 in combination with earlier high-speed imaging studies of vocal fold vibration 10–12 established that vibration consisted of a mucosal wave that began at the inferior aspect of the vocal fold and travelled superiorly along its medial surface to the top, in the direction of phonatory air flow. The medial surface contour of the vocal fold thus has direct impact on oscillatory mechanics and acoustic output. The medial surface of the vocal fold is not, however, readily visualized in routine clinical examination and therefore may not have received as much attention from laryngeal surgeons as a two-dimensional parameter such as the size of the glottal gap as viewed superiorly.
In contrast, the medial vocal fold surface contour was recognized as a key determinant of vocal function very early in the development of theoretical models of phonatory mechanics. In 1979, Titze and Talkin13 modeled the vocal fold medial surface as quadratic and found that vocal efficiency would increase with more medial bulging. The medial vocal fold surface contour continued to carry particular significance in later analytical models of vocal fold vibration. 14,15 The glottal shape can be convergent, rectangular, or divergent with respect to the direction of air flow during phonation (Fig. 1).16 Physical models showed that a rectangular or near-rectangular prephonatory glottal shape produced the lowest threshold subglottal pressure required to sustain phonation. 17 As the models became more refined, the actual contour of the vocal fold medial surface was also characterized with increasing sophistication. 18–20 There appeared to be a growing awareness that perhaps it would be possible to surgically manipulate the contour of the medial vocal fold surface to achieve greater vocal efficiency. 21 However, there has been little empirical data in human larynges to support the clinical potential of such an approach.
Figure 1.
Glottal shape in coronal plane, defined with respect to the direction of air flow during phonation. Modified from Titze 16.
Previous studies in excised human larynges suggested injections could increase the vertical thickness of the vocal folds, and that this may produce a favorable effect on phonatory mechanics independent from and beyond that of improved glottal closure, as predicted by theory. 17,22 It was also observed that injections changed the contour of the vocal fold medial surface (Fig. 2). 23 The next logical step is to investigate whether such alterations, e.g. the change of a convergent glottal geometry to a more rectangular or less convergent one, as conceptualized in Figure 3, would in fact lead to functional gain, as predicted by biomechanical models of phonation. 15,17
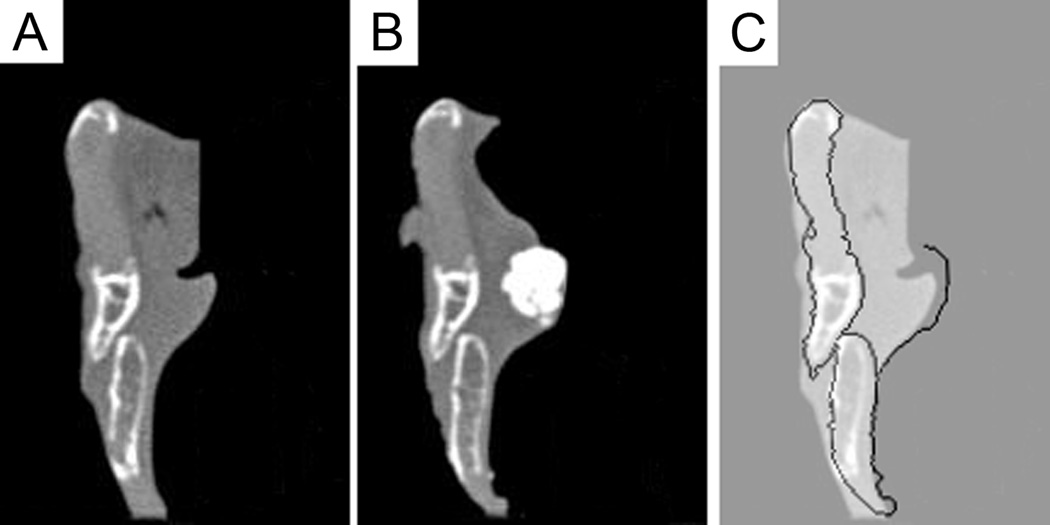
Figure 2.
Change in contour of the medial vocal fold edge produced by injection. Coronal CT image of a right hemilarynx before (A) and after (B) injection of calcium hydroxylapatite. (C) shows superposition of the post-injection contour onto the pre-injection image. Previously unpublished data from work described in Mau and Courey 23.
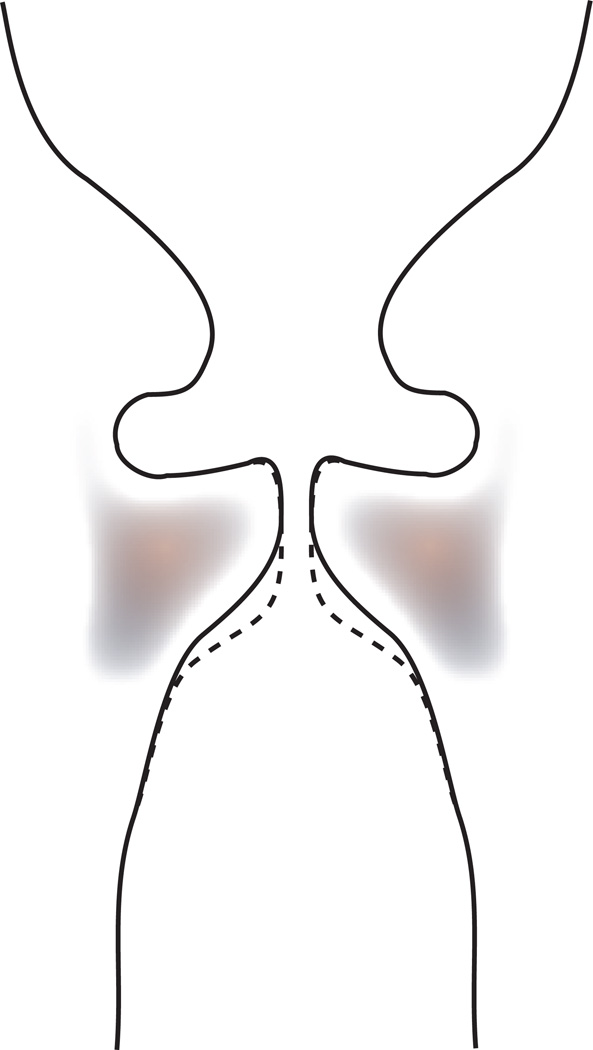
Figure 3.
Schematic of the concept of medial vocal fold contour alteration. A surgical intervention would bring the contour from the solid line to the dashed line, thereby increasing vertical thickness and creating a less convergent, more rectangular glottis.
The first objective of this study is to demonstrate that a surgical intervention intended to change the vocal fold medial surface contour can lead to improved phonatory function. The specific aim is to quantify the effect of vocal fold contour change on phonation threshold pressure (PTP) and flow (PTF) in an excised larynx phonation model. PTP and PTF are, respectively, the minimum subglottal pressure and the minimum glottal flow required to initiate or to sustain phonation. PTP and PTF directly relate to the viscous shear properties of the vocal fold cover, the mucosal wave velocity, and the prephonatory glottal geometry. 15,24,25 As such, these aerodynamic parameters have been established as good indicators of phonatory function 26,27 and have been extensively studied in the excised larynx phonation model. 28–32 In this study, targeted vocal fold injection is used to alter the vocal fold contour in excised human larynges. PTP and PTF will be determined before and after injection while the distance between the vocal processes is kept constant. Based on predictions from biomechanical models of phonation, we hypothesize that injection-induced alteration of vocal fold contour can lower the PTP and PTF.
A secondary objective of the study is to demonstrate, through the same set of experiments, that the viscoelasticity of materials introduced into the vocal fold can affect phonatory function even when they are not placed into the vocal fold lamina propria. The viscoelastic shear properties of the vocal fold cover are critical determinants of PTP and PTF. 15,24, 25 As a consequence, the viscoelastic properties of implant materials used in vocal fold injection augmentation have also received considerable attention. 33–36 It is now widely recognized that implants with viscoelastic mismatch dampen normal vocal fold vibration when introduced into the vocal fold lamina propria. Yet it is not clear to what extent the material properties may also be important when the implants are placed deep to the lamina propria. The specific aim here is to compare the PTP and PTF in human excised larynges following vocal fold injection with calcium hydroxylapatite (CaHA) paste versus hyaluronic acid (HA) gel, two materials with significant differences in viscoelastic properties.36 The hypothesis is that the material with the closer viscoelastic match to the vocal fold cover, i.e. HA gel, will produce the more favorable phonatory result.
The overall goals of this study are to further our understanding of the biomechanical basis for phonation and to clarify the consequence of phonosurgical implant placement. The long-term goal of this research is to develop novel surgical strategies to improve phonatory function.
MATERIALS AND METHODS
Larynges
Excised human larynges were procured through the Willed Body Program of the study institution from donors with no known history of laryngeal pathology. The larynges were harvested within 24 hours post-mortem. The larynges were dissected for mounting onto the excised larynx phonation apparatus as previously described.32 All phonation experiments took place within 24 hours post-mortem.37
Excised Larynx Phonation Apparatus
A bench apparatus (Fig. 4) similar to that described in Alipour and Jaiswal 31 and Chan and Titze 24 was used and has been described in detail previously.32 In brief, compressed air controlled by a pressure regulator was passed through an in-line rotameter to monitor mean air flow. AC flow was monitored by a pneumotach connected to a differential pressure transducer. The air was then heated and humidified to about 37°C and 100% humidity before exiting in a short section of PVC pipe above the bench top. A pressure tap located 5 cm below the pipe opening was connected to a water manometer to measure mean subglottal pressure, and the AC subglottal pressure was monitored by a pressure transducer mounted across the pipe from the manometer pressure tap. Acoustic signal was monitored by a condenser microphone positioned 8 cm from the glottis. Electrode plates from an electroglottograph (EGG) device placed in contact with the strap muscles monitored the EGG signal. Analog signals of AC subglottal pressure, AC flow, acoustics, and EGG were sampled at 5000 samples/second per channel by an analog-to-digital converter and recorded on a computer.
Figure 4.
Schematic of the excised larynx phonation bench apparatus.
Larynx Mounting and Data Acquisition
The larynx was mounted on the excised larynx phonation apparatus as described in detail previously.32 The larynx was stabilized to maintain neutral vocal fold length and tension, i.e. in situ length and natural tension of the vocal folds with the excised larynx in a cadaveric state. Vocal fold adduction was manipulated by arytenoid adduction sutures. Transverse screw heads placed in contact with the muscular processes further stabilized the arytenoids. Posterior glottal width, defined as the distance between the vocal processes, was controlled by placement of plastic shims of defined thicknesses (0.5, 1, 2, 3 mm) between the arytenoids. The no-shim condition with the vocal processes in contact was considered to have a nominal posterior glottal width of 0 mm.
Five phonation trials were carried out at each posterior glottal width for each larynx, before and after vocal fold injection. For each phonation trial, the subglottal pressure was gradually increased by adjusting the pressure regulator slowly until vocal fold vibration occurred (phonation onset). The subglottal pressure was then gradually decreased until phonation ceased (offset). Threshold flow and pressure were determined based on discrete changes in the signal amplitude and periodicity in the pressure and EGG signals. The determination was facilitated by custom routines written in MATLAB 7.8.0 (The MathWorks, Natick, MA) and confirmed visually via a graphical user interface code written for this purpose.
Vocal Fold Injections
Injections were performed with each larynx mounted on the excised larynx phonation apparatus, with the inter-arytenoid shims removed. Injections were carried out with a 25-gauge needle 5/8-inch in length. The point on the needle 3 mm from the tip of the bevel was marked with a permanent marker to gauge the depth of insertion. As the intent was to modify the contour of the vocal fold medial surface, the implant was targeted to the infero-medial aspect of the TA. The point of needle entry was the superior surface of the mid-membranous vocal fold, 2 mm lateral to the free edge. The needle was inserted to the 3-mm mark, and material was injected in a single bolus. Calcium hydroxylapatite paste (Radiesse, BioForm Medical, San Mateo, CA) or crosslinked hyaluronic acid gel (Juvederm Ultra, Allergan, Santa Barbara, CA) were used. Injection was carried out in increments of 0.025 mL. The minimum amount required to produce a visible change in the contour of the vocal fold vertical surface was used. Amount injected ranged from 0.025 to 0.125 mL, with a median of 0.0625 mL. The vocal fold edges were then gently palpated in an anterior-posterior direction to make sure there was no discrete convexity produced by the bolus.
Histology
Following collection of aerodynamic data, all but one of the larynges were placed into formalin. The one exception was used for further experiments unrelated to the current study. Once the tissue was fixed, the membranous vocal folds were bisected in the coronal plane, resected from the cartilaginous framework, and placed into cassettes. Vocal folds injected with CaHA were decalcified prior to sectioning. Sections from the bisected cut surface were stained with hematoxylin and eosin (H & E). For vocal folds injected with HA gel, sections were also stained with Alcian blue, which binds glycosaminoglycans (GAGs), to highlight the injected HA implant bolus. The bolus was clearly distinguishable from native GAGs as the latter were layered along the organized micro-structure of the vocal fold lamina propria. To obtain a qualitative assessment of implant position in the vertical dimension, the implant was classified to be predominantly in the superior half of the vertical thickness, the inferior half, or both.
Statistical Analysis
To determine whether injection increased or decreased PTP or PTF for each experimental condition, two-tailed t tests were computed between the two data sets of 5 trials obtained before and after injection. If the P value exceeded 0.05, the aerodynamic parameter was considered to be unchanged for that condition. To determine if the choice of implant material had an overall effect on PTP or PTF, two statistical measures were calculated. First, the number of conditions in which PTP and PTF increased or decreased was placed in two-way tables according to the material used. The Chi-square statistic was computed for the respective two-way tables to determine whether an association existed between the choice of injected material and the direction of change in PTP and PTF. Second, the magnitude of change in the respective aerodynamic parameter was analyzed with the Wilcoxon test. Each experimental condition was considered separately. All conditions involving CaHA injection were considered as one group, and all conditions involving HA gel injection were considered as another group. The two groups of differences, e.g. (post-injection PTPonset) - (pre-injection PTPonset), were compared using the Wilcoxon test. Non-parametric tests were used because of large sample variances in the aerodynamic parameters. Exact P values were computed. Statistical calculations were performed with SAS 9.2 (SAS Institute Inc., Cary, NC) and Excel 2007 (Microsoft, Redmond, WA).
RESULTS
Subjects
Aerodynamic data were collected from 15 larynges. Only fresh larynges were used, i.e. larynges were harvested and data collected within 24 hours postmortem, in order to minimize adverse effects on tissue properties due to post-mortem changes and preservative methods such as freezing. 37 Data from two larynges were excluded from analysis because no phonation could be obtained prior to injection. Demographic data on the remaining 13 donors are listed in Table 1.
Table 1.
Demographics of Laryngeal Donors.
| Larynx | Gender | Age, yr |
|---|---|---|
| A | Male | 87 |
| B | Male | 85 |
| C | Male | 71 |
| D | Female | 86 |
| E | Female | 87 |
| F | Male | 90 |
| G | Male | 62 |
| H | Female | 90 |
| I | Female | 81 |
| J | Female | 92 |
| K | Male | 66 |
| L | Female | 69 |
| M | Male | 93 |
Aerodynamic Measurements
For each larynx, PTP and PTF were measured at defined posterior glottal widths (Fig. 5) at both phonation onset and offset. Data were available for 57 conditions, i.e. 13 larynges each at 3–5 posterior glottal widths. Seven larynges were injected with CaHA and aerodynamic parameters were measured in a total of 28 conditions. Six larynges were injected with HA gel with measurements collected in a total of 29 conditions. Data were not available for all conditions because some larynges did not phonate at certain posterior glottal widths.
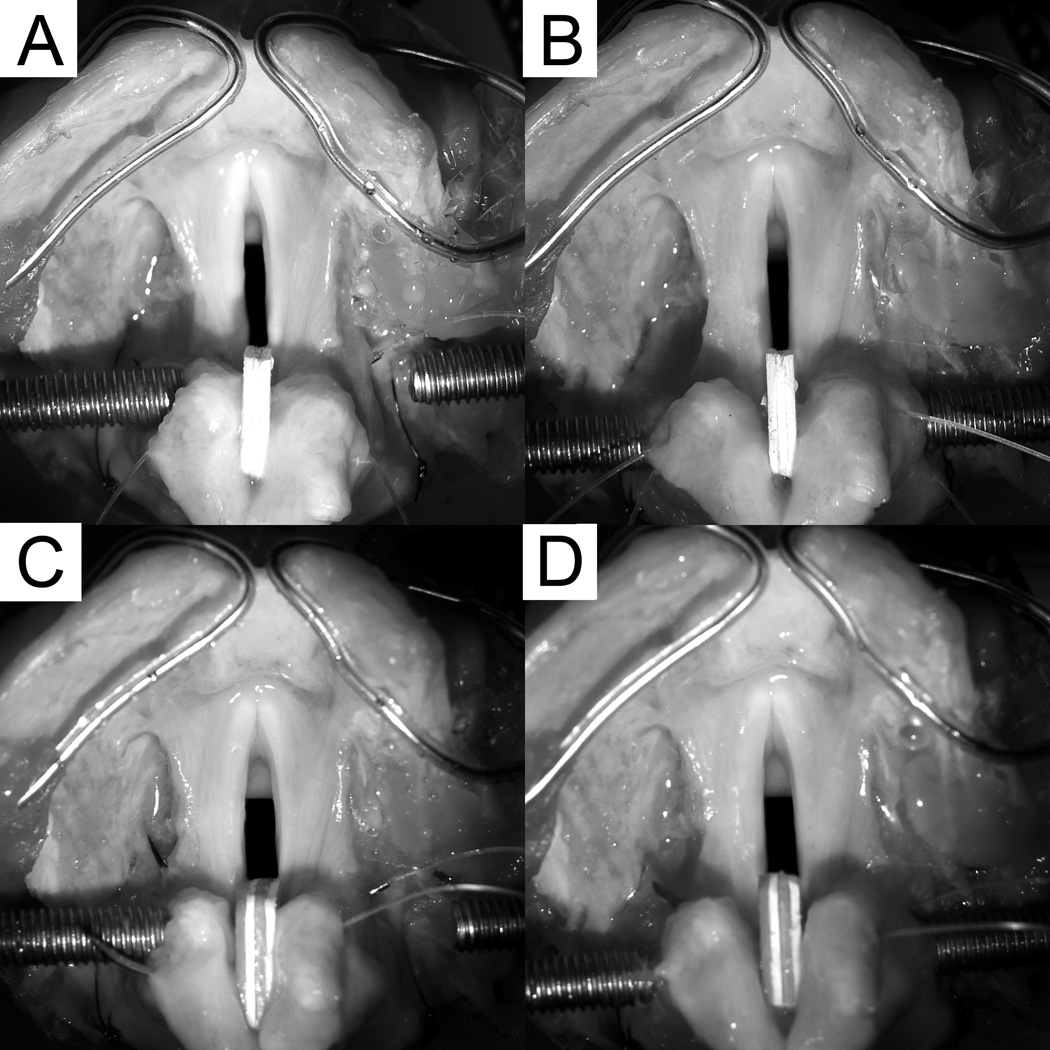
Figure 5.
Excised human larynx mounted on the induced phonation apparatus with posterior glottal width of (A) 2 mm before vocal fold injection, (B) 2 mm after injection, (C) 3 mm before injection, and (D) 3 mm after injection.
Effect of Choice of Implant Material on PTP and PTF
Figure 6 shows the individual larynx data, separated by implant material. The effect of the choice of the implant material on PTP and PTF was evaluated by several measures. Table 2 lists the percentages of conditions in which PTP and PTF increased or decreased, by material type. CaHA tended to increase PTP, whereas HA gel tended to decrease PTP. This difference between the two materials was statistically significant. Both materials tended to decrease PTF. Figure 7 shows the mean PTP and PTF before and after injection, by material type. There was a statistically significant difference between the two materials in their effect on PTP. In contrast, there was not a statistically significant difference between the two materials in their effect on PTF. Figure 8 shows the mean percentage changes in PTP and PTF.
Figure 6.
Change in PTP and PTF, individual larynx data. Each data point represents the mean value measured at different posterior glottal widths (0.5–3 mm) for a single larynx. Graphs on the left show data from larynges injected with CaHA, and graphs on the right show data from larynges injected with HA gel.
Table 2. Effect of Choice of Implant Material on PTP and PTF.
Percentages of conditions in which PTP and PTF changed as indicated in the left column.
| PTPonset | PTPoffset | PTFonset | PTFoffset | |||||
|---|---|---|---|---|---|---|---|---|
| CaHA | HA Gel | CaHA | HA Gel | CaHA | HA Gel | CaHA | HA Gel | |
| Increased | 61 % | 28 % | 50 % | 24 % | 11 % | 10 % | 18 % | 10 % |
| Decreased | 25 % | 69 % | 29 % | 62 % | 64 % | 83 % | 71 % | 86 % |
| No Change | 14 % | 3 % | 21 % | 14 % | 25 % | 7 % | 11 % | 3 % |
| P value for Chi-square | 0.0035 | 0.038 | 0.16 | 0.36 | ||||
Figure 7.
Mean PTP and PTF before and after injection, by implant material. The change produced by injection is compared between the two materials, as indicated by the P values from the Wilcoxon test.
Figure 8.
Percentage change in PTP and PTF, by implant material. Solid bars represent data for CaHA, and open bars represent data for HA gel.
Location of Injected Implant by Histology
To determine the position of the implant in the vocal fold, histological sections from the mid-membranous vocal fold were examined in 12 larynges. In all cases, the implants were located in the medial aspect of the TA muscle, deep to the vocal ligament (Fig. 9). Implant position in the vertical dimension was also assessed qualitatively. In 5 larynges, the implant was positioned predominantly in the inferior half of the vocal fold vertical thickness (e.g. Fig. 9C), whereas in the other 7, the implant was equally present in both the superior half and the inferior half (e.g. Fig. 9B).
Figure 9.
Location of injected implant in coronal sections of vocal folds. (A) Photograph of the cut surface at the mid-section of a vocal fold containing bolus of CaHA, after fixation and prior to decalcification and embedding. Ruler in the image contains markings in millimeters. (B) H & E-stained section taken from the same vocal fold. The CaHA implant has dissected into fibers of the TA muscle in its medial aspect. The implant is clearly deep to the vocal ligament. (C) Alcian blue-stained section containing bolus of HA gel in a different vocal fold. The scale bar equals 1 mm for (B) and (C).
DISCUSSION
The contour of the vocal fold medial surface has long been considered a key determinant of vibratory dynamics in analytical and computer models of phonation but has not been a specific target of surgical intervention. This study aimed to provide preliminary data to validate the concept of altering vocal fold contour to improve phonatory function. The experiment was designed with an intervention meant to modify the vocal fold medial surface contour. A small amount of injectate was targeted to the inferior aspect of the medial TA muscle, just lateral to the lamina propria. The results showed that when a material with a closer viscoelastic match to the human vocal fold cover was used, injection tended to lower the PTP and PTF, consistent with the hypothesis that alteration of the medial surface contour can improve phonatory function. Nonetheless, this interpretation is somewhat limited by the inability to completely decouple two simultaneous effects of injection in the current experimental design: the change in medial surface contour, and the reduction in prephonatory glottal width or area. We attempted to minimize the latter by using the minimum injectate volume required to bring about visible augmentation of the vocal fold medial surface in the vertical direction, without a noticeable change in the glottal width (Fig. 5). The volume used (median of 0.0625 mL) was substantially less than what is required to medialize a vocal fold through a medial injection (0.14–0.23 mL).23 Despite this effort, it is possible that some degree of prephonatory glottal area reduction might have accompanied the medial surface contour change, so that the effect due to the change in the vertical dimension could not be assessed independently from that due to the change in the transverse dimension, i.e. glottal area. This concern is partially offset by prior excised larynx phonation data showing that PTP is relatively insensitive to changes in glottal area,29,32 which implies that the observed reduction in PTP was likely due to contour change rather than glottal area reduction. We believe the results are promising and motivate further studies to test the hypothesis regarding the exact role of the vocal fold medial surface contour. A physical model of phonation in which the anatomical variables in the vertical and transverse dimensions can be independently controlled may be suitable for such experiments. 17,38
Interpretation of data relevant to the secondary objective regarding implant material properties was more straightforward. Implant placement into the medial TA muscle produced an effect on PTP that was material-dependent. In principle, several geometric and tissue viscoelastic properties altered by the implant can affect the direction of change in PTP. An increase in the vocal fold vertical thickness or a reduction in the overall glottal width and area lowers the PTP. 15,24 In terms of glottal geometry (vertical contour), a rectangular or near-rectangular glottis is expected to produce the lowest threshold pressure.15,17,39,40 In terms of the effect on vocal fold tissue properties, both implant materials in this study are significantly stiffer and more viscous than human vocal fold cover. In the human phonatory range of 100–250 Hz, the elastic shear modulus G’ and dynamic viscosity η’ of CaHA paste are several times higher than those of the HA gel used in this study, which are in turn higher than those of the human vocal fold cover. 36 Intended only as a means to alter glottal geometry in the excised larynx, neither implant material was expected to produce a favorable tissue effect on vibratory mechanics. If geometric factors were unchanged by injection, both materials should have increased PTP.
The observed reduction in PTP, then, must come from favorable geometric effects of implant placement. The implant in the medial aspect of the TA muscle had the potential to increase the vocal fold vertical thickness as suggested in previous work. 22 In addition, in all 12 larynges examined, the implant was present in the inferior half of the vertical thickness of the vocal fold, which would be required to produce a more rectangular glottal shape (Figure 3; compare Figure 9 to Figure 1). This was suggestive but not confirmatory of actual change, since the pre-injection glottal shape was not characterized. In cases where PTP increased, the unfavorable effects due to material properties presumably exceeded favorable geometric effects. In cases where PTP decreased, the favorable geometric effects probably outweighed any negative material effects. Since injection with one material, HA gel, did show the tendency to lower PTP, the data were suggestive of a favorable geometric effect.
In contrast with PTP, PTF decreased in most cases irrespective of the implant material. Like PTP, PTF is predicted to decrease as a convergent glottis becomes less convergent or more rectangular. 25 The fact that PTF decreased while PTP increased in many conditions in this experiment could reflect the differential sensitivity of these two aerodynamic parameters to factors that affect phonation, as observed previously.29,32 PTF appears to correlate with glottal width 29 and glottal area 32, whereas PTP is relatively insensitive to either.29,32 The results here suggest that PTF may be more sensitive to the geometry of the glottal channel, while PTP may be more sensitive to tissue properties.
The lack of morphologic analysis of the vocal fold medial surface contour before injection is a major limitation of this study with regards to its first objective. This was due to the necessity of obtaining aerodynamic measurements on fresh excised larynges as soon as they became available, which made obtaining pre-injection imaging such as CT scan logistically difficult. The contour difference before and after injection may be analyzable in a parallel series of excised larynges in the future, since implant placement in the medial TA muscle appeared to be reproducible.
Previous studies in excised human larynges 41 as well as rheologic properties of phonosurgical materials 34–36 suggested that injection of most currently available materials into the vocal fold lamina propria would adversely affect vibration. This is widely attributed to unfavorable viscoelastic match between the material and the vocal fold cover. By extension, the common assumption is that injection away from the cover should circumvent the viscoelastic mismatch problem. This is supported by experiments that showed minimal adverse effect from injection into the medial aspect of the TA muscle. 41 The present study shows that phonatory aerodynamics can be adversely affected even when the injected bolus resides entirely within the TA muscle, and that this effect may vary according to viscoelastic properties of the material. This finding challenges the conventional view that avoidance of superficial injection into the cover may be sufficient to preserve the normal vibratory properties of the vocal folds. Our result is consistent with analytical modeling of vocal fold vibration based on the body-cover model. 14,42 The depth of tissue that participates in vibration depends on the fundamental frequency (pitch) and the amplitude of vibration (loudness). In loud phonation at low pitch, the medial portion of the TA muscle could become part of the effective vibratory mass. 42 The introduction of an implant into this region therefore is expected to have some impact on vibration. The magnitude of the impact may depend on how much the material properties of the implant deviate from TA muscle properties. Qualitatively, a relatively stiff implant in the medial portion of the TA muscle is expected to reduce the effective mass of tissue available for vibration and reduce the vibratory amplitude. Quantitatively, the impact of introducing an implant whose density and viscoelastic properties differ from those of the TA muscle could be appreciated in a finite element model. 14 The implication for clinical vocal fold injection medialization is that most currently available materials that are significantly stiffer than the human vocal fold cover should be placed not only into the muscle but in the lateral aspect of the muscle, where it is less likely to interfere with vibration.
It was not the intent of this study to compare the two specific implant materials for their suitability for vocal fold injections. The two materials were chosen because they are in clinical use 43,44, can be obtained in standardized commercial preparations, and have known differences in viscoelastic properties. 36 The hypothesis of the research could have been tested with any other pair of materials that differed in viscoelastic properties. The suitability of implant materials for clinical use must be assessed in vivo, where host response, implant stability, and tissue integration can be assessed over time. It was also not our intent to advocate implant placement into the medial aspect of the TA muscle as a means to improve vocal function. The low-volume targeted injection was used as a means to produce a desired change in glottal geometry. The objective of the study was to validate the concept of altering vocal fold contour as a potential surgical intervention, not the demonstration of the utility of a particular procedure. The results clearly showed that the phonatory consequence, at least as measured by threshold aerodynamic parameters, can be variable as implemented in this experiment.
Vocal fold injection may not be the best method to alter medial vocal fold surface contour. It was originally conceived as a means to improve glottic closure in unilateral vocal fold paralysis. For that indication, it is reasonably well established that lateral deposition of the implant away from the medial surface is preferred to avoid negative impact on vibration. In this study, injection into the medial TA muscle was utilized as a means to alter the vertical contour of the glottal channel despite the potential downside of unfavorable viscoelastic match. Lateral injection was deliberately not used for this purpose because laterally injected boluses have been shown to adopt irregular shapes in the paraglottic space. 22 Medially injected boluses, in contrast, tend to be more geometrically uniform, possibly due to their smaller volumes and proximity to the vocal ligament, which acts as a geometric constraint. 22,23 Medial injection was therefore used in this study for the experimental purpose of altering vocal fold medial surface contour with relative reproducibility. However, future work should be devoted to develop new surgical techniques designed to alter the medial surface contour with more precision and minimal negative impact on the vibratory properties of the vocal fold cover.
A condition that may particularly benefit from surgical alteration of medial surface contour is glottic insufficiency due to vocal fold atrophy. The most common scenario is that of the aging voice. A number of anatomical and physiological changes in the larynx accompany aging, including reduced pulmonary function, impaired respiratory coordination for phonation 45, decrease in motor activity and control 46, and thinning of the vocal fold lamina propria 47. An element directly associated with glottal aerodynamics is the loss of muscle bulk, which reduces the vocal fold contact area and leads to decreased acoustic intensity and increased vocal effort. The resultant difficulty in communication constitutes a growing but under-recognized problem as the population gains in longevity. 48 In fact, since most larynges in this experiment came from donors of advanced age, the results may be particularly applicable to this population. A second scenario, much less common, is vocal fold atrophy secondary to acquired denervation, which entails not only reduction in muscle bulk but also loss of tone. In either case, decrease in TA muscle bulk results in lateral pulling of the mid-membranous vocal folds, commonly appreciated as bowing on laryngoscopy. 49 The thickness of the vocal folds in contact in the vertical dimension is also reduced, which is less obvious but nevertheless important. Current medialization procedures such as implant thyroplasty and injection augmentation are designed to improve glottic closure as viewed in two-dimensional laryngoscopy. While they likely also increase the vertical thickness in contact, they do not specifically address the contour of the vocal fold medial surface. Further increase in phonatory efficiency is conceivably attainable with procedures that target optimization of the glottal channel geometry to maximize acoustic output.
CONCLUSION
Alteration of the vocal fold medial surface contour may be a means to improve phonatory function. Specifically, biomechanical models of phonation predict that an increase in the vertical thickness of the vocal folds and a more rectangular glottal geometry should lower the threshold pressure and flow required to initiate phonation. These geometric changes may occur to some extent with current vocal fold medialization techniques, even though the intent of the existing procedures is to minimize the glottic gap and not alteration of the vocal fold medial surface contour. The concept of contour optimization may be particularly useful in cases of vocal fold atrophy, in which contour deficiency exists in both vertical and transverse dimensions. Future work investigating the effect of vocal fold contour changes should utilize techniques that modify the vertical contour without simultaneously reducing glottal area, in order to remove the latter as a confounding variable.
If implant placement is used as a means to alter vocal fold contour, its location in the vocal fold in terms of depth relative to the vocal fold lamina propria requires careful consideration. A material with viscoelastic properties significantly different from those of the cover may affect vibration even when it is entirely contained within the TA muscle. This effect is possibly under-appreciated. As phonation increases in loudness or decreases in frequency, the depth of vibration involves more of the medial TA muscle. Any implant within the zone of tissue movement can impact vibration. Materials without a good viscoelastic match should be placed in the lateral aspect of the TA muscle. While this is consistent with current clinical practice, it highlights the importance of the precision of implant placement as well as the choice of materials.
ACKNOWLEDGMENTS
The first author thanks Mark Courey, MD for inspiration to pursue his clinical subspecialty and the present line of inquiry; Gaelyn Garrett, MD, and Peter Roland, MD for sponsoring his membership to the Triological Society; and his wife and children for making it all possible. We also thank Elhum Naseri and Mindy Du for assistance with data collection and Karen Pawlowski, PhD, and Paula Timmons for generating the histology slides.
Laboratory equipment was funded by the Department of Otolaryngology-Head and Neck Surgery, UT Southwestern Medical Center. The participation of JM was supported by the UT Southwestern Summer Medical Student Research Program. This work was supported by NIDCD grants R01 DC006101S1, R01 DC006101, and R01 DC005788.
Footnotes
This manuscript has been accepted as a Triological Thesis and has been accepted for oral presentation at the 2012 Triological Society Annual Meeting.
Financial Disclosure: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute On Deafness And Other Communication Disorders or the National Institutes of Health.
Conflict of Interest: None.
REFERENCES
- 1.Brunings W. Uber eine neue Behandlungsmethode der Rekurrenslahmung. Verhandl Dtsch Laryngol. 1911;18:93–151. [Google Scholar]
- 2.Isshiki N, Morita H, Okamura H, Hiramoto M. Thyroplasty as a new phonosurgical technique. Acta Otolaryngol. 1974;78:451–457. doi: 10.3109/00016487409126379. [DOI] [PubMed] [Google Scholar]
- 3.Isshiki N, Tanabe M, Sawada M. Arytenoid adduction for unilateral vocal cord paralysis. Arch Otolaryngol. 1978;104:555–558. doi: 10.1001/archotol.1978.00790100009002. [DOI] [PubMed] [Google Scholar]
- 4.Zeitels SM, Hochman I, Hillman RE. Adduction arytenopexy: a new procedure for paralytic dysphonia with implications for implant medialization. Ann Otol Rhinol Laryngol Suppl. 1998;173:2–24. [PubMed] [Google Scholar]
- 5.Sulica L, Rosen CA, Postma GNet al. Current practice in injection augmentation of the vocal folds: indications, treatment principles, techniques, and complications. Laryngoscope. 2010;120:319–325. doi: 10.1002/lary.20737. [DOI] [PubMed] [Google Scholar]
- 6.Ford CN. Laryngeal Injection Techniques. In: Ford CN, Bless DM, editors. Phonosurgery: assessment and surgical management of voice disorders. New York: Raven Press; 1991. [Google Scholar]
- 7.Ford CN, Bless DM, Loftus JM. Role of injectable collagen in the treatment of glottic insufficiency: a study of 119 patients. Ann Otol Rhinol Laryngol. 1992;101:237–247. doi: 10.1177/000348949210100307. [DOI] [PubMed] [Google Scholar]
- 8.Hirano M. Morphological structure of the vocal cord as a vibrator and its variations. Folia Phoniatr (Basel) 1974;26:89–94. doi: 10.1159/000263771. [DOI] [PubMed] [Google Scholar]
- 9.Hirano M. Phonosurgery: Basic and Clinical Investigations. Official Report of the 76th Annual Convention of the Oto-Rhino-Laryngological Society of Japan. 1975 [Google Scholar]
- 10.Farnsworth DW. High speed motion pictures of human vocal cords. Bell Laboratories Record. 1940;18:203–208. [Google Scholar]
- 11.Smith S. Remarks on the physiology of the vibrations of the vocal cords. Folia Phoniatr (Basel) 1954;6:166–178. doi: 10.1159/000262656. [DOI] [PubMed] [Google Scholar]
- 12.Timcke R, Von Leden H, Moore P. Laryngeal vibrations: measurements of the glottic wave. I. The normal vibratory cycle. AMA Arch Otolaryngol. 1958;68:1–19. doi: 10.1001/archotol.1958.00730020005001. [DOI] [PubMed] [Google Scholar]
- 13.Titze IR, Talkin DT. A theoretical study of the effects of various laryngeal configurations on the acoustics of phonation. J Acoust Soc Am. 1979;66:60–74. doi: 10.1121/1.382973. [DOI] [PubMed] [Google Scholar]
- 14.Alipour F, Scherer RC. Vocal fold bulging effects on phonation using a biophysical computer model. J Voice. 2000;14:470–483. doi: 10.1016/s0892-1997(00)80004-1. [DOI] [PubMed] [Google Scholar]
- 15.Titze IR. The physics of small-amplitude oscillation of the vocal folds. J Acoust Soc Am. 1988;83:1536–1552. doi: 10.1121/1.395910. [DOI] [PubMed] [Google Scholar]
- 16.Titze IR. Principles of Voice Production. Iowa City, IA: National Center for Voice and Speech; 2000. pp. 102–108. [Google Scholar]
- 17.Chan RW, Titze IR, Titze MR. Further studies of phonation threshold pressure in a physical model of the vocal fold mucosa. J Acoust Soc Am. 1997;101:3722–3727. doi: 10.1121/1.418331. [DOI] [PubMed] [Google Scholar]
- 18.Berry DA, Clark MJ, Montequin DW, Titze IR. Characterization of the medial surface of the vocal folds. Ann Otol Rhinol Laryngol. 2001;110:470–477. doi: 10.1177/000348940111000514. [DOI] [PubMed] [Google Scholar]
- 19.Doellinger M, Berry DA. Visualization and quantification of the medial surface dynamics of an excised human vocal fold during phonation. J Voice. 2006;20:401–413. doi: 10.1016/j.jvoice.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Sidlof P, Svec JG, Horacek J, Vesely J, Klepacek I, Havlik R. Geometry of human vocal folds and glottal channel for mathematical and biomechanical modeling of voice production. J Biomech. 2008;41:985–995. doi: 10.1016/j.jbiomech.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 21.Scherer RC. Physiology of phonation: a review of basic mechanics. In: Ford CN, Bless DM, editors. Phonosurgery: Assessment and Surgical Management of Voice Disorders. New York: Raven Press; 1991. pp. 77–94. [Google Scholar]
- 22.Mau T, Brewer JM, Gatzert ST, Courey MS. Three-dimensional conformation of the injected bolus in vocal fold injections in a cadaver model. Otolaryngol Head Neck Surg. 2011;144:552–557. doi: 10.1177/0194599810395107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mau T, Courey MS. Influence of gender and injection site on vocal fold augmentation. Otolaryngol Head Neck Surg. 2008;138:221–225. doi: 10.1016/j.otohns.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 24.Chan RW, Titze IR. Dependence of phonation threshold pressure on vocal tract acoustics and vocal fold tissue mechanics. J Acoust Soc Am. 2006;119:2351–2362. doi: 10.1121/1.2173516. [DOI] [PubMed] [Google Scholar]
- 25.Jiang JJ, Tao C. The minimum glottal airflow to initiate vocal fold oscillation. J Acoust Soc Am. 2007;121:2873–2881. doi: 10.1121/1.2710961. [DOI] [PubMed] [Google Scholar]
- 26.Jiang J, O'Mara T, Conley D, Hanson D. Phonation threshold pressure measurements during phonation by airflow interruption. Laryngoscope. 1999;109:425–432. doi: 10.1097/00005537-199903000-00016. [DOI] [PubMed] [Google Scholar]
- 27.Zhuang P, Sprecher AJ, Hoffman MRet al. Phonation threshold flow measurements in normal and pathological phonation. Laryngoscope. 2009;119:811–815. doi: 10.1002/lary.20165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang J, Verdolini K, Aquino B, Ng J, Hanson D. Effects of dehydration on phonation in excised canine larynges. Ann Otol Rhinol Laryngol. 2000;109:568–575. doi: 10.1177/000348940010900607. [DOI] [PubMed] [Google Scholar]
- 29.Hottinger DG, Tao C, Jiang JJ. Comparing phonation threshold flow and pressure by abducting excised larynges. Laryngoscope. 2007;117:1695–1699. doi: 10.1097/MLG.0b013e3180959e38. [DOI] [PubMed] [Google Scholar]
- 30.Regner MF, Tao C, Zhuang P, Jiang JJ. Onset and offset phonation threshold flow in excised canine larynges. Laryngoscope. 2008;118:1313–1317. doi: 10.1097/MLG.0b013e31816e2ec7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alipour F, Jaiswal S. Phonatory characteristics of excised pig, sheep, and cow larynges. J Acoust Soc Am. 2008;123:4572–4581. doi: 10.1121/1.2908289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mau T, Muhlestein J, Callahan S, Weinheimer KT, Chan RW. Phonation threshold pressure and flow in excised human larynges. Laryngoscope. 2011;121:1743–1751. doi: 10.1002/lary.21880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan RW, Titze IR. Viscosities of implantable biomaterials in vocal fold augmentation surgery. Laryngoscope. 1998;108:725–731. doi: 10.1097/00005537-199805000-00019. [DOI] [PubMed] [Google Scholar]
- 34.Klemuk SA, Titze IR. Viscoelastic properties of three vocal-fold injectable biomaterials at low audio frequencies. Laryngoscope. 2004;114:1597–1603. doi: 10.1097/00005537-200409000-00018. [DOI] [PubMed] [Google Scholar]
- 35.Caton T, Thibeault SL, Klemuk S, Smith ME. Viscoelasticity of hyaluronan and nonhyaluronan based vocal fold injectables: implications for mucosal versus muscle use. Laryngoscope. 2007;117:516–521. doi: 10.1097/MLG.0b013e31802e9291. [DOI] [PubMed] [Google Scholar]
- 36.Kimura M, Mau T, Chan RW. Viscoelastic properties of phonosurgical biomaterials at phonatory frequencies. Laryngoscope. 2010;120:764–768. doi: 10.1002/lary.20816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan RW, Titze IR. Effect of postmortem changes and freezing on the viscoelastic properties of vocal fold tissues. Ann Biomed Eng. 2003;31:482–491. doi: 10.1114/1.1561287. [DOI] [PubMed] [Google Scholar]
- 38.Titze IR, Schmidt SS, Titze MR. Phonation threshold pressure in a physical model of the vocal fold mucosa. J Acoust Soc Am. 1995;97:3080–3084. doi: 10.1121/1.411870. [DOI] [PubMed] [Google Scholar]
- 39.Lucero JC. Optimal glottal configuration for ease of phonation. J Voice. 1998;12:151–158. doi: 10.1016/s0892-1997(98)80034-9. [DOI] [PubMed] [Google Scholar]
- 40.Lucero JC. The minimum lung pressure to sustain vocal fold oscillation. J Acoust Soc Am. 1995;98:779–784. doi: 10.1121/1.414354. [DOI] [PubMed] [Google Scholar]
- 41.Courey MS. Homologous collagen substances for vocal fold augmentation. Laryngoscope. 2001;111:747–758. doi: 10.1097/00005537-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 42.Titze IR. Principles of Voice Production. Iowa City, IA: National Center for Voice and Speech; 2000. pp. 227–231. [Google Scholar]
- 43.Carroll TL, Rosen CA. Long-term results of calcium hydroxylapatite for vocal fold augmentation. Laryngoscope. 2011;121:313–319. doi: 10.1002/lary.21258. [DOI] [PubMed] [Google Scholar]
- 44.Song PC, Sung CK, Franco RA., Jr Voice outcomes after endoscopic injection laryngoplasty with hyaluronic acid stabilized gel. Laryngoscope. 2010;120(Suppl 4):S199. doi: 10.1002/lary.21666. [DOI] [PubMed] [Google Scholar]
- 45.Nagai H, Ota F, Konopacki R, Connor NP. Discoordination of laryngeal and respiratory movements in aged rats. Am J Otolaryngol. 2005;26:377–382. doi: 10.1016/j.amjoto.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 46.Connor NP, Suzuki T, Lee K, Sewall GK, Heisey DM. Neuromuscular junction changes in aged rat thyroarytenoid muscle. Ann Otol Rhinol Laryngol. 2002;111:579–586. doi: 10.1177/000348940211100703. [DOI] [PubMed] [Google Scholar]
- 47.Ximenes Filho JA, Tsuji DH, do Nascimento PH, Sennes LU. Histologic changes in human vocal folds correlated with aging: a histomorphometric study. Ann Otol Rhinol Laryngol. 2003;112:894–898. doi: 10.1177/000348940311201012. [DOI] [PubMed] [Google Scholar]
- 48.Turley R, Cohen S. Impact of voice and swallowing problems in the elderly. Otolaryngol Head Neck Surg. 2009;140:33–36. doi: 10.1016/j.otohns.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 49.Titze IR. Principles of Voice Production. Iowa City, IA: National Center for Voice and Speech; 2000. pp. 200–202. [Google Scholar]