Abstract
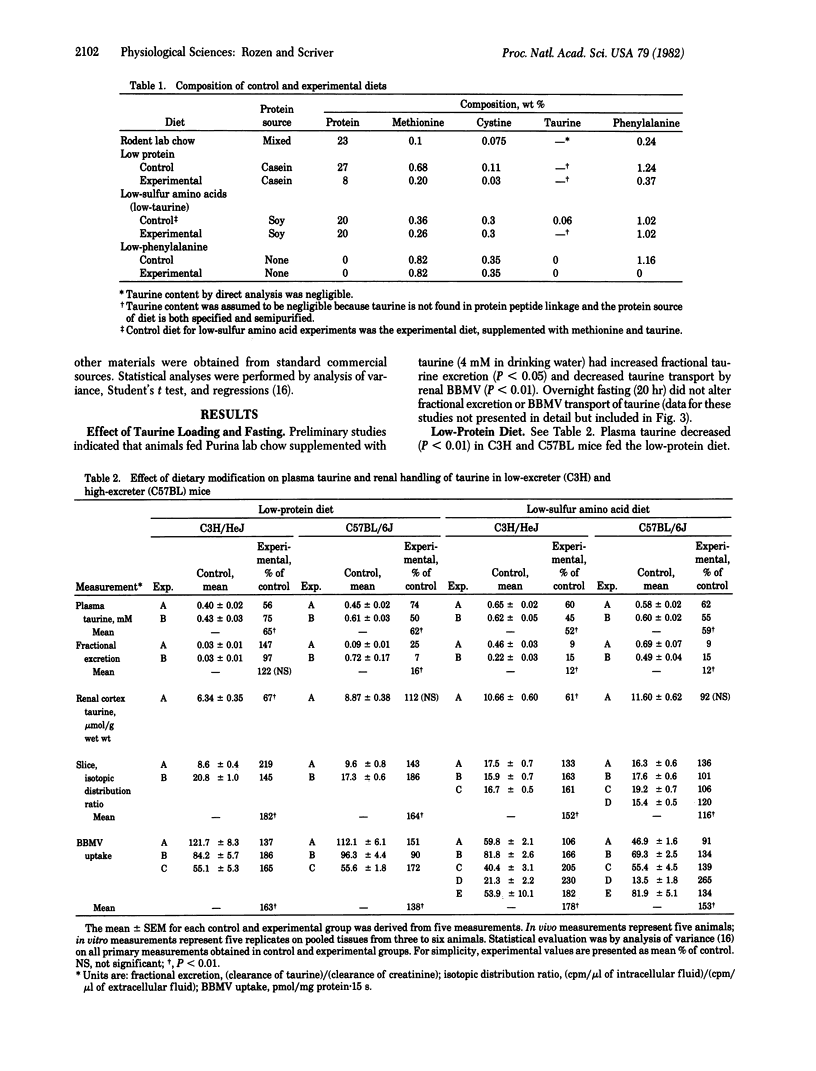
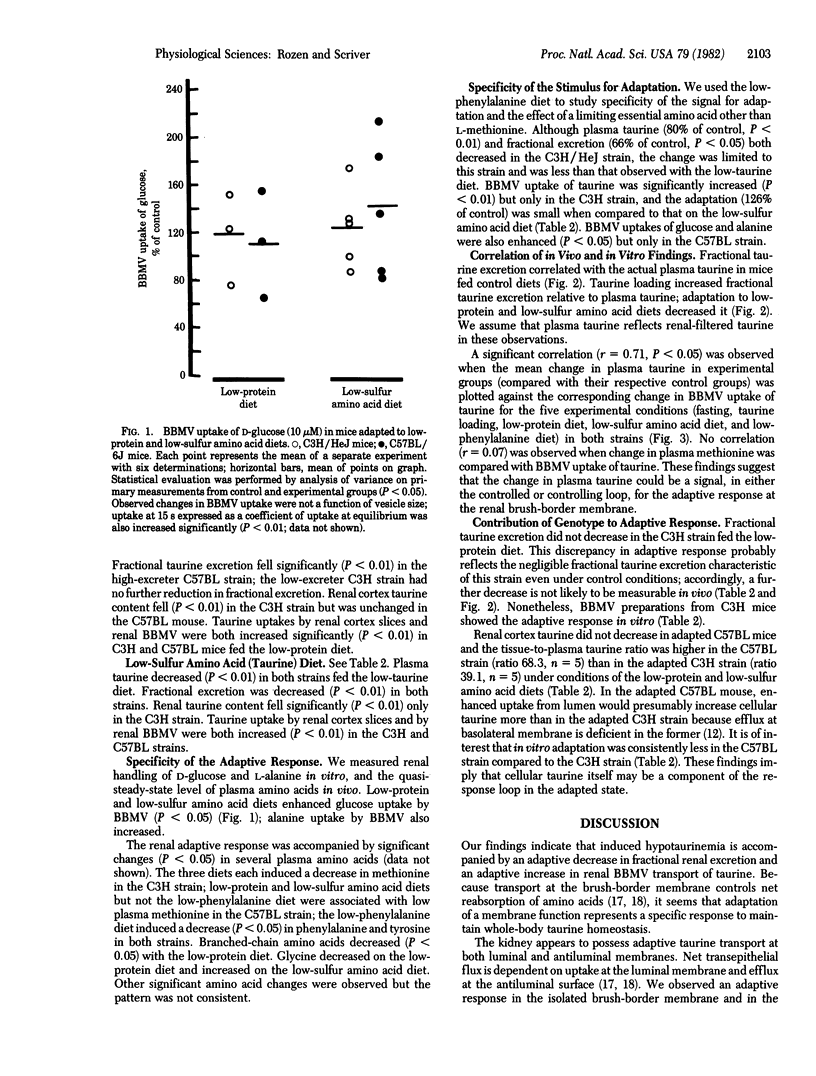
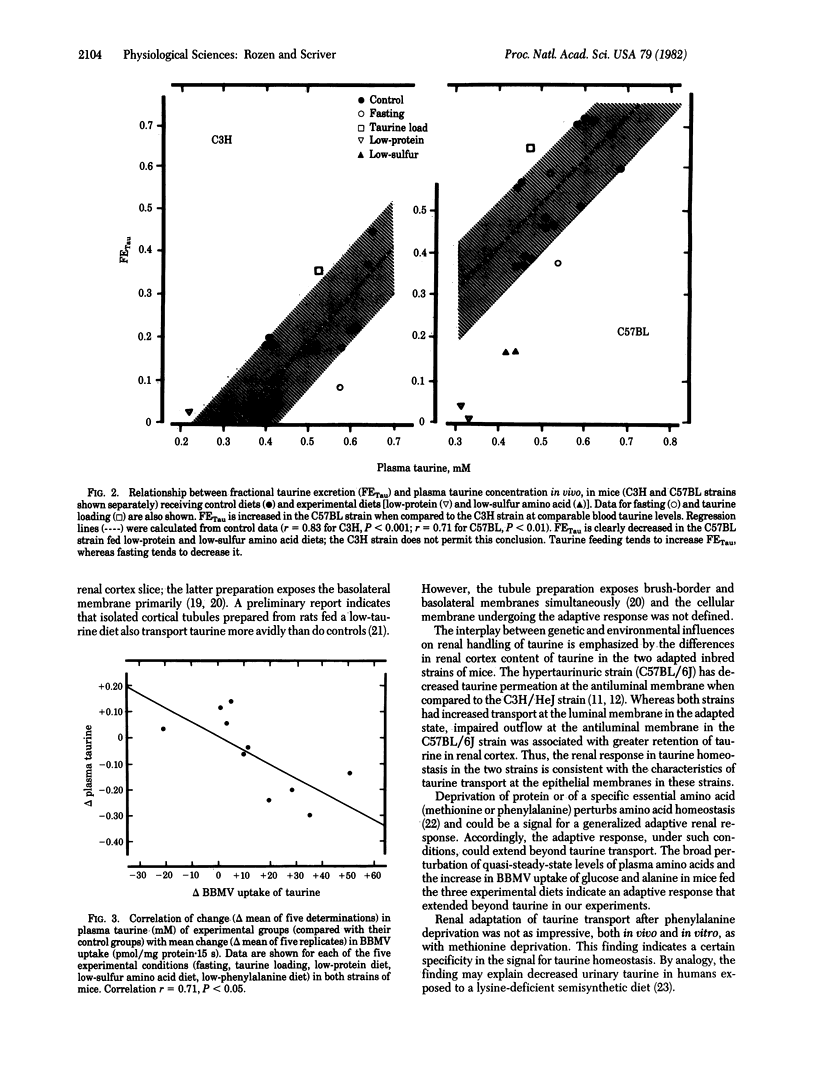
Renal adaptation apparently contributes to the homeostasis of taurine, a beta-amino compound that behaves as a conserved metabolite in the mammal. We studied two strains of inbred mice: C3H/HeJ (low-taurine excreter) and C57BL/6J (high-taurine excreter due to impaired basolateral membrane permeability to taurine). Low-protein and low-sulfur amino acid diets fed for two weeks significantly decreased plasma taurine in both strains, decreased fractional taurine excretion in vivo (particularly in the C57BL strain), and increased net uptake of taurine by renal cortex slices and isolated brush-border membrane vesicles (BBMV) in vitro in both strains. Renal adaptation was less obvious in vivo in the low-taurine excreter C3H strain, but in vitro adaptation, as observed in slices and BBMV (P less than 0.01), was greater than that observed in the C57BL strain. Renal cellular taurine content fell (P less than 0.01) only in the adapted C3H strain. The in vitro adaptive response was not confined to taurine; BBMV uptake of D-glucose and L-alanine was also enhanced in the adapted state. Specificity of the stimulus for adaptation was tested with a low-phenylalanine diet; a modest adaptation was observed in vivo and in vitro but only in the C3H strain. BBMV adaptation did not correlate with blood methionine but correlated inversely with plasma taurine (r = 0.71, P less than 0.05), implying that change in extracellular taurine may be a signal for renal adaptation in taurine homeostasis in the mammal.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Booth A. G., Kenny A. J. A rapid method for the preparation of microvilli from rabbit kidney. Biochem J. 1974 Sep;142(3):575–581. doi: 10.1042/bj1420575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesney R. W., Scriver C. R., Mohyuddin F. Localization of the membrane defect in transepithelial transport of taurine by parallel studies in vivo and in vitro in hypertaurinuric mice. J Clin Invest. 1976 Jan;57(1):183–193. doi: 10.1172/JCI108258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzler W. H., Silbernagl S. Renal tubular reabsorption of taurine, gamma-aminobutyric acid (GABA) and beta-alanine studied by continuous microperfusion. Pflugers Arch. 1976 Dec 28;367(2):123–128. doi: 10.1007/BF00585147. [DOI] [PubMed] [Google Scholar]
- Gaull G. E., Rassin D. K., Räihä N. C., Heinonen K. Milk protein quantity and quality in low-birth-weight infants. III. Effects on sulfur amino acids in plasma and urine. J Pediatr. 1977 Mar;90(3):348–355. doi: 10.1016/s0022-3476(77)80692-6. [DOI] [PubMed] [Google Scholar]
- Nutzenadel W., Scriver C. R. Uptake and metabolism of beta-alanine and L-carnosine by rat tissues in vitro: role in nutrition. Am J Physiol. 1976 Mar;230(3):643–651. doi: 10.1152/ajplegacy.1976.230.3.643. [DOI] [PubMed] [Google Scholar]
- PORTMAN O. W., MANN G. V. The disposition of taurine-S35 and taurocholate-S35 in the rat: dietary influences. J Biol Chem. 1955 Apr;213(2):733–743. [PubMed] [Google Scholar]
- Rozen R., Tenenhouse H. S., Scriver C. R. Taurine transport in renal brush-border-membrane vesicles. Biochem J. 1979 Apr 15;180(1):245–248. doi: 10.1042/bj1800245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer J. A., Barfuss D. W. Membrane mechanisms for transepithelial amino acid absorption and secretion. Am J Physiol. 1980 May;238(5):F335–F346. doi: 10.1152/ajprenal.1980.238.5.F335. [DOI] [PubMed] [Google Scholar]
- Scriver C. R., Chesney R. W., McInnes R. R. Genetic aspects of renal tubular transport: diversity and topology of carriers. Kidney Int. 1976 Feb;9(2):149–171. doi: 10.1038/ki.1976.18. [DOI] [PubMed] [Google Scholar]
- Sturman J. A. Taurine pool sizes in the rat: effects of vitamin B-6 deficiency and high taurine diet. J Nutr. 1973 Nov;103(11):1566–1580. doi: 10.1093/jn/103.11.1566. [DOI] [PubMed] [Google Scholar]
- Tachiki K. H., Baxter C. F. Taurine levels in brain tissues: a need for re-evaluation. J Neurochem. 1979 Nov;33(5):1125–1129. doi: 10.1111/j.1471-4159.1979.tb05251.x. [DOI] [PubMed] [Google Scholar]
- Tenenhouse H. S., Scriver C. R. The defect in transcellular transport of phosphate in the nephron is located in brush-border membranes in X-linked hypophosphatemia (Hyp mouse model). Can J Biochem. 1978 Jun;56(6):640–646. doi: 10.1139/o78-096. [DOI] [PubMed] [Google Scholar]
- Wedeen R. P., Weiner B. The distribution of p-aminohippuric acid in rat kidney slices. I. Tubular localization. Kidney Int. 1973 Apr;3(4):205–213. doi: 10.1038/ki.1973.33. [DOI] [PubMed] [Google Scholar]



