Abstract
Background and Design:
Adiponectin is an adipokine secreted primarily from adipose tissue that can influence circulating plasma glucose and lipid levels through multiple mechanisms involving a variety of organs. In humans, reduced plasma adiponectin levels induced by obesity are associated with insulin resistance and type 2 diabetes, suggesting that low adiponectin levels may contribute the pathogenesis of obesity-related insulin resistance.
Methods and Results:
The objective of the present study was to investigate whether gene therapy designed to elevate circulating adiponectin levels is a viable strategy for ameliorating insulin resistance in mice fed a high-fat, high-sucrose (HFHS) diet. Electroporation-mediated gene transfer of mouse adiponectin plasmid DNA into gastrocnemius muscle resulted in elevated serum levels of globular and high-molecular weight adiponectin compared with control mice treated with empty plasmid. In comparison to HFHS-fed mice receiving empty plasmid, mice receiving adiponectin gene therapy displayed significantly decreased weight gain following 13 weeks of HFHS diet associated with reduced fat accumulation, and exhibited increased oxygen consumption and locomotor activity as measured by indirect calorimetry, suggesting increased energy expenditure in these mice. Consistent with improved whole-body metabolism, mice receiving adiponectin gene therapy also had lower blood glucose and insulin levels, improved glucose tolerance and reduced hepatic gluconeogenesis compared with control mice. Furthermore, immunoblot analysis of livers from mice receiving adiponectin gene therapy showed an increase in insulin-stimulated phosphorylation of insulin signaling proteins.
Conclusion:
Based on these data, we conclude that adiponectin gene therapy ameliorates the metabolic abnormalities caused by feeding mice a HFHS diet and may be a potential therapeutic strategy to improve obesity-mediated impairments in insulin sensitivity.
Keywords: adiponectin, gene delivery, electroporation, obesity, insulin signaling, high fat diet
Introduction
The World Health Organization (WHO) estimates that >1 billion adults worldwide are overweight and >300 million people are obese.1, 2 Obesity is a significant risk factor for the development of several diseases, including insulin resistance, type 2 diabetes (T2D), cardiovascular disease and nonalcoholic fatty liver disease.3, 4 Moreover, obesity-related diseases account for up to 7% of total health care costs worldwide.2 Importantly, obesity is closely associated with insulin resistance and T2D and is one of the most important modifiable risk factors for the prevention of T2D. Although multiple tissues are affected by adiposity, adipose tissue is a major contributor to many of the metabolic abnormalities associated with obesity. Indeed, adipose tissue is now recognized as a highly metabolic and dynamic endocrine organ that secretes several inflammatory and immune mediators known as adipokines.5 Dysregulation of adipokine secretion induced by increased fatty acid storage in the adipocyte has been strongly implicated in several obesity-related diseases.6 Based on this, it is likely that normalizing adipokine levels may be an effective therapeutic approach to lessen the metabolic complications induced by the onset of obesity.
Adiponectin is an adipose-tissue-derived bioactive peptide that exerts a plethora of direct effects on multiple tissues and has been shown to have a beneficial role in the setting of metabolic and cardiovascular disease states.7, 8 Adiponectin is a ∼30 kDa protein that circulates in the blood at high concentrations (2–10 μg ml−1 in human) and constitutes ∼0.01% of total plasma protein.9, 10, 11 In contrast to several other adipokines, adiponectin is an anti-inflammatory, anti-atherogenic and insulin-sensitizing hormone whose levels are found to be reduced in obesity.2, 10 Adiponectin can exist in the blood as a trimer, with the potential to associate into hexamers and finally into multimers of high-molecular weight (HMW) species.12 Evidence strongly suggests that HMW adiponectin oligomer is the most metabolically active multimer and, in many cases, the proportion of HMW adiponectin rather than total adiponectin levels are more closely associated with the beneficial metabolic and cardiovascular effects of adiponectin.10, 13, 14, 15 As previously mentioned, there is a strong inverse relationship between adiponectin levels and obesity and subsequently insulin sensitivity.16 Moreover, decreased adiponectin levels were seen in parallel with the progression of insulin resistance and T2D in nonhuman primates,11 suggesting that maintaining high circulating adiponectin levels may be a potential approach to prevent the development of these disorders.
Since various pharmacological treatments, including thiazolidinediones15 and statins,17 have been reported to indirectly increase circulating adiponectin levels, there is potential that therapies that directly increase circulating adiponectin levels may have greater beneficial effects in the setting of obesity. Therefore, we chose a gene therapy approach to deliver a plasmid containing adiponectin cDNA into skeletal muscle of high-fat, high-sucrose (HFHS)-fed mice in order to increase circulating levels of adiponectin. Using this approach, we show that long-term expression of adiponectin prevented weight gain and ameliorated the metabolic abnormalities induced by a HFHS diet.
Materials and methods
Animal care and diets
This investigation conforms with the guidelines of The University of Alberta Animal Policy and Welfare Committee, which adheres to the principles for biomedical research involving animals developed by the Council for International Organizations of Medical Sciences. C57/BL6 male mice (7–8 weeks of age) were purchased from Charles River Laboratories (St Constant, QC, Canada). All animals were given unrestricted access to food (6.5% fat, 49% carbohydrate and 23% protein wt/wt; 3.5 kcal g−1 Purina Rodent Chow 5008; Ralston-Purina, St Louis, MO, USA) and water unless otherwise stated. Mice were maintained on a 12:12-h light–dark cycle and at an ambient room temperature of 20–22 °C throughout the study. Immediately following gene transfer, mice were fed a HFHS diet (44.9% fat; D12451; Research Diets, New Brunswick, NJ, USA) for 13 weeks.
Plasmid construct
Adiponectin cDNA was subcloned into the vector containing a muscle-specific, synthetic promoter (SPc5–12) as described.18, 19 The plasmids were produced by ADViSYS, Inc. (The Woodlands, TX, USA), according to previously described methods.20
In vivo gene delivery
Mice were mildly anesthetized using isoflurane (5.0% isoflurane in 100% O2 until unconscious and anesthesia was maintained with 1.25% isoflurane in 100% O2 at a flow rate of 1 liter min−1) and 100 μg of plasmid containing either adiponectin or empty control vector in 25 μl sterile saline (control) were injected directly into each gastrocnemius muscle of the mice. Transcutaneous electric pulses were applied to the muscle using two stainless steel needle electrodes placed 1-cm apart on each side of the injection site immediately after DNA injection. All muscles were electrotransferred using the following conditions: 100 V cm−1, 24 pulses and 20 s pulse−1, 1 Hz, using a BTX ECM 830 electroporator (BTC Harvard Apparatus, Holliston, MA, USA) as previously described.21
Glucose tolerance tests and pyruvate tolerance tests
Mice were fasted for 6 h and then either administered glucose (2 g glucose per kg body weight) by oral gavage or pyruvate (2 g pyruvate per kg body weight) by intraperitoneal injection. Blood samples were collected from the tail vein for measurements of blood glucose levels at 0, 15, 30, 60 and 120 min post-injection using an ACCU-CHEK Aviva Advantage glucometer (Roche Diagnostics, Laval, QC, Canada). Data was expressed as the absolute change in blood glucose concentrations from baseline (0 min). Total area under the curve of the glucose or pyruvate response was calculated using the trapezoidal method.22
Body composition and metabolic measurements
Body composition was determined in conscious mice at the end of 13 weeks of a HFHS diet using Echo-MRI (Echo Medical Systems, Houston, TX, USA). Indirect calorimetry was performed on mice using an eight-chamber open-circuit Oxymax system of the Comprehensive Lab Animal Monitoring System (CLAMS; Columbus Instruments, Columbus, OH, USA) as we have previously described.23 All mice were individually housed and acclimatized to the metabolic cages for 24 h before hourly recordings of metabolic parameters commenced. Mice then underwent a 3-day course in the metabolic cages consisting of a 24-h fed, 24-h fasted and 24-h refed states. Mice were weighed before each 24-h trial. The respiratory exchange ratio (RER; RER=VCO2/VO2) was used to estimate the contribution of fat and carbohydrate to in vivo whole-body energy metabolism in mice. Ambulatory activity of mice was evaluated within the metabolic chambers on a relative basis using an eight-cage rack OPTO-M3 sensor system (Columbus Instruments, Columbus, OH, USA). Consecutive photobeam breaks occurring in adjacent photobeams were scored as an ambulatory movement. Cumulative ambulatory activity counts were recorded every hour throughout the light and dark cycles.
Analysis of serum insulin and adipokines
Serum insulin and leptin concentrations were determined using the enzyme-linked immunosorbent assay (ELISA) kits (Crystal Chem, Downers Grove, IL, USA). HMW adiponectin was determined by an ELISA kit (Alpco, Salem, NH, USA) capable of selective quantification of adiponectin oligomers using protease pretreatment.24
In vivo insulin signaling studies
Mice were fasted for 6 h and then injected intraperitoneally with human recombinant insulin (10 U per kg body weight). Mice were killed by cervical dislocation 10 min post-injection and liver tissue was rapidly collected, frozen in liquid nitrogen and stored at −80 °C.
Immunoblot analysis
Frozen powdered liver samples were homogenized in ice-cold lysis buffer (20 mmol/l Tris-HCl, pH 7.4, 5 mmol/l EDTA, 10 mmol/l Na4P2O7, 100 mmol/l NaF, 1% Nonidet P-40, 2 mmol/l Na3VO4, 1 mℳ phenylmethylsulfonyl fluoride, 14 μℳ leupeptin and 1 μℳ aprotinin). Lysates were centrifuged at 1000 × g for 30 min at 4 °C and supernatants were stored at −80 °C until analysis. Lysate protein was separated by SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) and transferred to nitrocellulose membranes (Biorad, Hercules, CA, USA). Phosphorylated and total proteins were identified by immunoblotting using the following primary antibodies: anti-phospho-Akt (Ser-473), anti-phospho-GSK-3β (glycogen synthase kinase 3β Ser-9), anti-phospho-AMPKα (adenosine monophosphate (AMP)-activated protein kinase α Thr-172), anti-α-tubulin (all from Cell Signaling Technology, Danvers, MA, USA) and anti-α-GAPDH (glyceraldehyde-3-phosphate dehydrogenase; Calbiochem, Billerica, MA, USA). For the immunodetection of serum adiponectin, serum samples were diluted (1:50), incubated for 1 h at room temperature in reducing sample buffer (3% sodium dodecyl sulfate, 50 mmol l−1 Tris-HCl, pH 6.8, 10% glycerol, 5% 2-mercaptoethanol and 10 mmol l−1 dithiothreitol) and subjected to SDS-PAGE under reducing/heat-denaturing conditions (samples were heated at 95 °C for 10 min) with Criterion precast Tris-HCl 4–15% gel (Bio-Rad, Hercules, CA, USA). Antibodies were visualized using the Amersham Pharmacia-enhanced chemiluminescence Western blotting detection system as previously described.25 Densitometric analysis of immunoblots was performed using ImageJ software (National Institutes of Health). Phosphorylation status of proteins were expressed as a ratio to their respective total protein levels and representative immunoblots of GAPDH were shown as a loading control to confirm equal loading of protein. Memcode reversible protein stain (Thermo Scientific Pierce, Rockford, IL, USA) for western blot membranes was used as a protein loading control for western blots of serum samples.
Statistical analysis
Data are expressed as means±s.e.m. Comparisons between two groups were performed using unpaired Student's two-tailed t-test. Measurements of body weight, as well as glucose and pyruvate tolerance over time were analyzed by two-way analysis of variance with time and group as sources of variation and Bonferroni multiple comparison post-hoc tests were performed where appropriate. For all analyses, P values of <0.05 were considered statistically significant. All statistical analyses were carried out using GraphPad Prism software version 5.0 (GraphPad Software Inc., San Diego, CA, USA).
Results
Adiponectin gene therapy increases serum adiponectin levels in mice
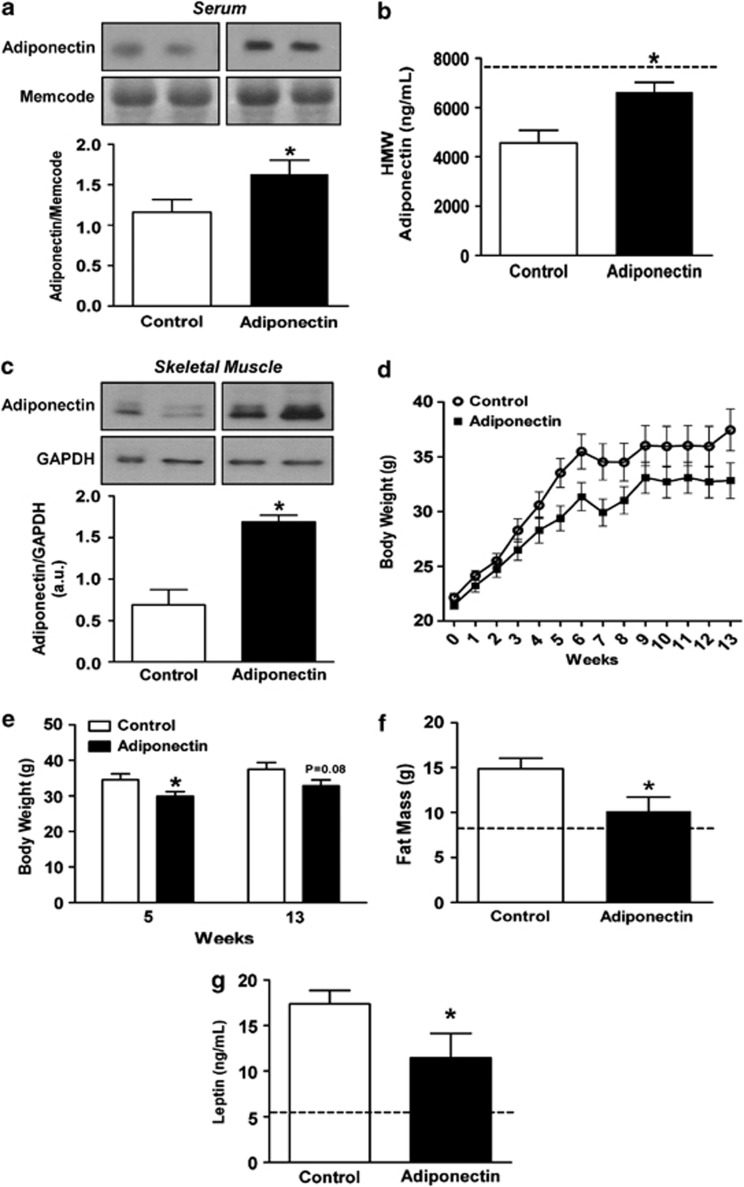
To comprehensively evaluate the metabolic effects of adiponectin gene therapy, we utilized a plasmid containing a muscle-specific synthetic promoter19 driving the expression of mouse adiponectin cDNA (pAV0243-adiponectin).26 Serum adiponectin levels were measured both by immunoblot and ELISA to detect concentrations of globular domain adiponectin and HMW adiponectin, respectively. Serum globular adiponectin (Figure 1a) and HMW adiponectin levels (Figure 1b) were significantly increased in pAV0243-adiponectin-injected mice compared with mice injected with the control plasmid at 2 weeks following skeletal muscle electroporation and the start of HFHS diet. To confirm that adiponectin gene therapy results in long-term skeletal muscle production of adiponectin, immunoblot analysis was performed on gastrocnemius muscle showing that adiponectin expression was still markedly elevated at 13 weeks post-pAV0243-adiponectin injection (Figure 1c). Together with the adiponectin levels measured in serum, these data verify that muscle-derived adiponectin was secreted into the blood.
Figure 1.
Effect of muscle-specific adiponectin electroporation on serum adiponectin, body weight gain, fat mass and leptin levels following a high-fat, high-sucrose diet. (a) Immunoblot analysis of serum collected 2 weeks post-adiponectin injection using an antibody specific to globular adiponectin. Memcode protein stain was used as a marker of equal protein loading. (b) Serum levels of HMW adiponectin were measured 2 weeks following adiponectin gene therapy. (c) Immunoblot analysis of gastrocnemius muscle collected 13 weeks following gene therapy using antibody specific to globular adiponectin. Levels of adiponectin were normalized to GAPDH as a marker of equal protein loading. (d and e) Weight gain, (f) total fat mass and (g) serum leptin levels in control and pAV0243-adiponectin-injected mice following 13 weeks of a HFHS diet. n=7–9 per group, *P<0.05 vs control plasmid injected animals as determined by Student's t-test. Dashed line (b, f and g) represent the mean value for age-matched chow-fed control mice.
Adiponectin gene therapy decreased weight gain and attenuated adiposity in response to HFHS feeding
The effectiveness of adiponectin gene therapy in preventing diet-induced obesity was tested in male C57/BL6 mice fed a HFHS diet for 13 weeks.27 As we hypothesized, pAV0243-adponectin-injected mice gained less body weight compared with control mice injected with empty vector over the 13-week period of HFHS feeding (Figures 1d and e). Starting at 5 weeks post-injection and HFHS diet, pAV0243-adiponectin-injected mice exhibited significantly reduced body weight compared with control mice on a HFHS diet (Figures 1d and e). Furthermore, echo-MRI studies demonstrated that this reduction in diet-induced weight gain was associated with a significant decrease in total fat mass in these mice (Figure 1f). Additionally, serum leptin levels in pAV0243-adiponectin injected mice were significantly lower than in control mice fed a HFHS diet (Figure 1g), which is consistent with the reduced adiposity in pAV0243-adiponectin-injected mice.
Adiponectin gene therapy improves glucose clearance and suppresses hepatic gluconeogenesis in HFHS-fed mice
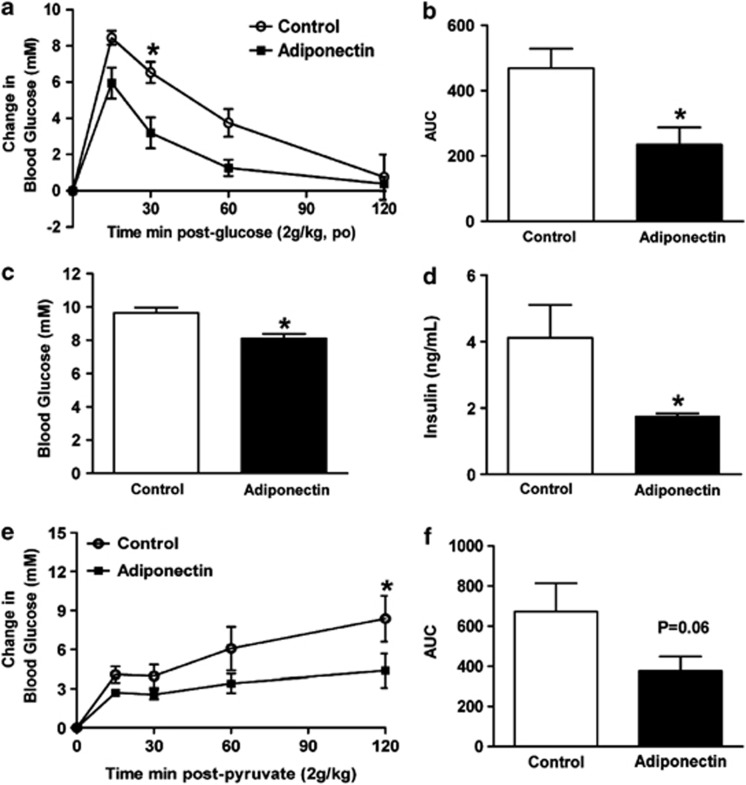
To further evaluate the effect of adiponectin gene therapy on HFHS diet-induced metabolic perturbations, we subjected pAV0243-adiponectin and pAV0243-control-vector-injected mice to oral glucose tolerance tests following 13 weeks of HFHS feeding. pAV0243-adponectin injected mice had significantly improved glucose clearance as compared with pAV0243-control-injected mice when subjected to oral glucose tolerance tests (Figures 2a and b). In agreement with improved glucose clearance, adiponectin-injected mice also had significantly reduced 6 h fasting blood glucose (Figure 2c) and insulin levels (Figure 2d). Together, these data indicate that adiponectin-injected mice subjected to a HFHS diet had improved whole-body glucose tolerance compared with control mice on a HFHS diet. Furthermore, pyruvate tolerance tests revealed reduced blood glucose levels in pAV0243-adponectin-injected mice following intraperitoneal pyruvate injection (Figures 2e and f), suggesting that increased levels of circulating adiponectin may also suppress hepatic gluconeogenesis. Although adiponectin likely has multiple mechanisms of action on different tissues to improve whole-body glucose clearance, these findings suggest that alterations in hepatic gluconeogenesis may account for some of the beneficial metabolic effects of adiponectin observed in vivo.
Figure 2.
Effect of pAV0243-adiponectin or control plasmid on glucose tolerance in male C57/BL6 mice fed a HFHS diet for 13 weeks. Adiponectin treatment significantly improved (a) glucose tolerance and (b) area under the curve (AUC) in HFHS-fed mice. pAV0243-adiponectin-injected HFHS mice had decreased fasting (c) blood glucose and (d) serum insulin levels and (e and f) improved intraperitoneal pyruvate tolerance test. n=6 per group, *P<0.05 vs control-plasmid-injected animals as determined by two-way analysis of variance (a and e) with a Bonferroni post-hoc test or Student's t-test (b–d and f).
Adiponectin gene therapy improves hepatic insulin signaling in HFHS-fed mice
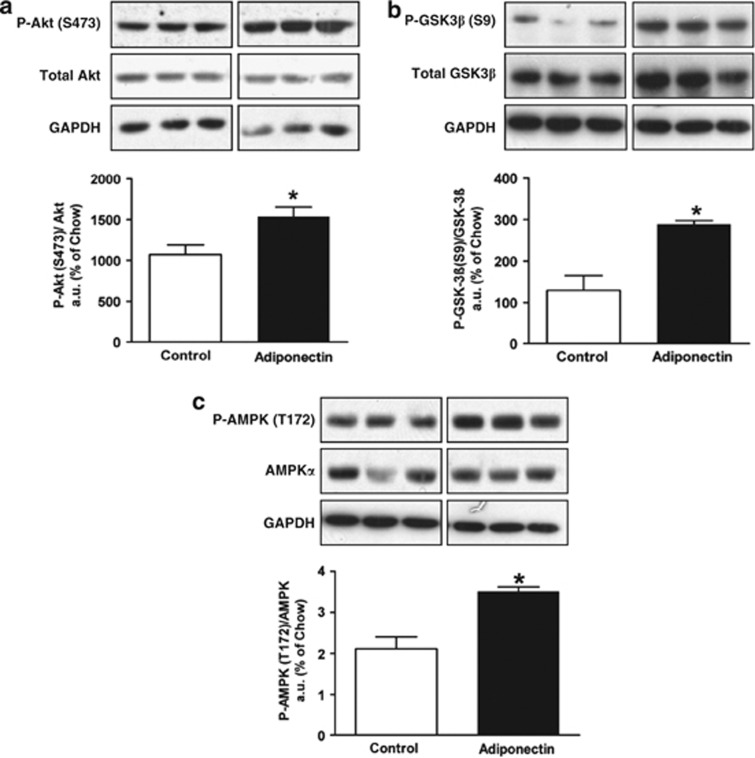
To further understand how elevated levels of circulating adiponectin may contribute to improved hepatic control of glucose homeostasis in HFHS-fed mice, we evaluated hepatic insulin signaling in these mice. Consistent with improved glucose disposal and insulin sensitivity, insulin-stimulated phosphorylation of Akt (Ser-473, Figure 3a) and GSK-3β (Ser-9, Figure 3b) was significantly enhanced in HFHS-fed pAV0243-adponectin-injected mice compared with control mice injected with control vector. Moreover, as adiponectin has been shown to activate AMPK in mice,6 we also investigated the phosphorylation of AMPK at threonine-172, which is indicative of its activation. As expected, pAV0243-adiponectin-injected mice had increased AMPK phosphorylation in the liver indicating enhanced AMPK activity in these mice compared with controls (Figure 3c).
Figure 3.
Immunoblot analysis of liver tissues after insulin stimulation from HFHS-fed mice injected with pAV0243-adiponectin or control plasmid. Phosphorylation of (a) Akt (Ser-473), (b) GSK-3β (Ser-9) and (c) AMPK (Thr-172). n=5 per group. Immunoblots were quantified by densitometry and normalized against total protein levels of Akt (a), GSK-3β (b) and AMPKα (c). *P<0.05 vs control-plasmid-injected animals as determined by Student's t-test. Representative immunoblots of GAPDH are shown for each as a loading control.
Metabolic analysis of mice receiving adiponectin gene therapy
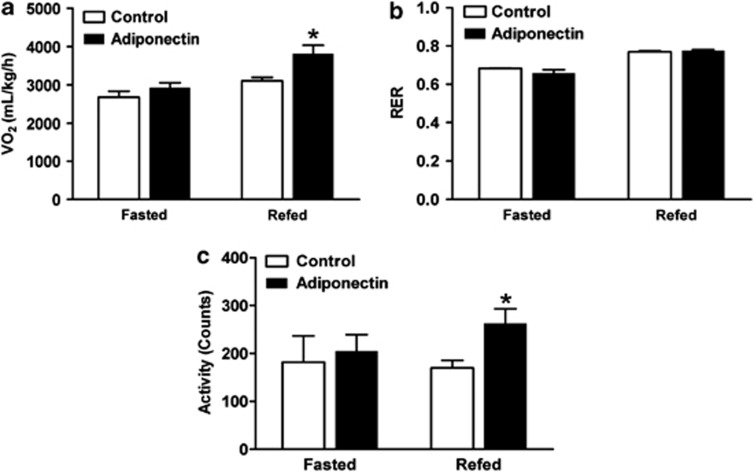
To further characterize the effects of adiponectin gene therapy on in vivo whole-body energy metabolism, control- and adiponectin-injected mice were subjected to indirect calorimetry. There was no difference in RER (RER=VCO2/VO2) between control and adiponectin-injected mice indicating that the type of substrate utilized was not altered as a result of adiponectin gene therapy (Figure 4b). Although oxygen consumption (VO2) was similar between groups of mice during the fasted state (Figure 4a), adiponectin-injected mice had significantly higher VO2 than control-vector–injected mice during the refed state (Figure 4a), suggesting increased energy expenditure in adiponectin-injected mice. In agreement with this, adiponectin-injected mice were significantly more active as compared with control-injected mice during the refed state following a 24-h fast (Figure 4c). Taken together, these data suggest that increased locomotor activity and metabolic rate contributes to the leaner phenotype of HFHS-fed adiponectin-injected mice as compared with HFHS-fed control mice.
Figure 4.
Whole-body energy metabolism in pAV0243-adiponectin-injected and control-plasmid-injected mice following 13 weeks of HFHS diet. (a) Oxygen consumption (VO2), (b) respiratory exchange ratio (RER=VCO2/VO2) and (c) total locomotor activity were simultaneously measured in HFHS-fed mice during the fasted and refed state by indirect calorimetry using a CLAMS apparatus. n=6 per group, *P<0.05 vs, control-plasmid-injected animals during the fasted or refed states as determined by Student's t-test.
Discussion
Several studies have investigated the relationship between circulating adiponectin levels and diabetes.28 Low concentrations of plasma adiponectin levels correlate strongly with reduced insulin sensitivity and individuals with high concentrations of adiponectin were less likely to develop T2D than those with low concentrations.29, 30 Overall, these clinical studies suggest that the concentration of plasma adiponectin independently predicts the progression of diabetes7, 29, 30, 31 and that adiponectin deficiency may contribute to the development of insulin resistance/T2D.32 Indeed, mice lacking adiponectin have severe hepatic insulin resistance33 and administration of adiponectin has been shown to reduce adipose tissue mass and improve insulin sensitivity in animal models of diabetes.7, 34 Therefore, adiponectin gene therapy to increase circulating adiponectin levels may prevent the pathological effects of insulin resistance/T2D.35, 36, 37 Recent work has shown that adiponectin gene therapy using adeno-associated virus vectors improved insulin sensitivity in diet-induced obese rats38 by increasing AMPK activity as well as suppressing hepatic gluconeogenesis.39 Although the benefits of adiponectin gene therapy are clear, several drawbacks to viral vectors have been well-documented,40, 41 suggesting that viral approaches to gene therapy have inherent risks associated with them and often preclude repetitive treatments.
In the current study, we have achieved long-term adiponectin production after injection of naked plasmid DNA of adiponectin into the skeletal muscle. We used a myogenic-restricted gene promoter to achieve high expression of adiponectin in the skeletal muscle and thus provide physiologically relevant circulating levels of adiponectin.18, 19 Herein, we show that adiponectin gene therapy results in a reduction in diet-induced weight gain, which corroborates with a previous report showing that continuous infusion of globular adiponectin led to similar reductions in body weight in mice fed a high-fat diet for 32 weeks.42 Although all of the mechanisms by which weight gain is reduced in adiponectin-treated mice are likely not known, we have shown that adiponectin gene therapy results in increased locomotor activity and energy expenditure. These data are consistent with earlier reports indicating adiponectin decreases body weight by increasing energy expenditure.34 Although the current study and others have shown that treatment with adiponectin decreases body weight and fat content,7, 42 increasing evidence also suggest that adiponectin may also act via the hypothalamus to increase energy expenditure,34 suggesting some of the observed effects of adiponectin gene therapy in the present study could be centrally mediated. Regardless of the precise mechanisms, the finding in our study that chronic and stable adiponectin gene therapy can reduce weight gain is especially important given the fact that obesity is one of the most frequent causes of insulin resistance in genetically predisposed individuals.29
In addition to reducing weight gain, increased circulating adiponectin levels resulted in a significant reduction in fasting glucose and insulin levels and improved peripheral insulin sensitivity. Consistent with this, adiponectin gene therapy also increased insulin-stimulated phosphorylation of Akt and GSK-3β in the liver. Although several organs contribute to improved insulin sensitivity, adiponectin-treated mice also had lower glucose levels during pyruvate tolerance test, which is suggestive of suppressed hepatic gluconeogenesis. Although the pyruvate tolerance test is not the gold standard and we did not directly measure hepatic glucose production by in vivo techniques such as infusion of a radiolabeled gluconeogenic precursor, our findings are consistent with an earlier report in which administration of adiponectin decreased gene expression of the hepatic gluconeogenic enzyme phosphoenolpyruvate carboxykinase.38 Taken together, our data indicate that adiponectin gene therapy may exert its anti-diabetic actions via multiple mechanisms, including enhanced hepatic insulin sensitivity and inhibition of hepatic gluconeogenesis.
Adiponectin has also been reported to activate AMPK, which is the ‘metabolic master switch' controlling pathways of hepatic ketogenesis, cholesterol synthesis and triglyceride synthesis.43 Several studies attribute the beneficial metabolic effects of adiponectin to activation of AMPK.44, 45, 46 In agreement with this, our data show that adiponectin gene therapy also results in the activation of AMPK in the liver, suggesting that some of the metabolic improvements of adiponectin gene therapy may be mediated through AMPK and its downstream target molecules.
In conclusion, we show that muscle-specific electrotransfer of adiponectin transgene results in long-term weight reduction and insulin-sensitizing effects in a mouse model of obesity and T2D. These effects appear to be mediated through diverse metabolic pathways, including activation of Akt and AMPK in the liver. As electroporation and muscle gene delivery has already reached clinical trials and is a viable strategy to treat long-term diseases,21, 47 our findings suggest an alternative therapeutic modality that may be considered in the treatment of obesity and T2D.
Acknowledgments
We thank Dr Ruxandra Draghia-Akli M and ADViSYS, Inc. for providing the vector containing the muscle-specific synthetic promoter (SPc5-12). We also acknowledge the expert technical assistance of Sandra Kelly, Carrie-Lynn Soltys, Donna Beker and Brandi Sidlick. This research was supported by grants from the Canadian Institutes of Health Research and the Canadian Diabetes Association (CIHR and CDA to JRBD). ADK was supported by postdoctoral fellowships from the Alberta Heritage Foundation for Medical Research (AHFMR) and the Heart and Stroke Foundation of Canada. JRBD is an AHFMR Senior Scholar.
The authors declare no conflict of interest.
References
- Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- Phillips L, Prins J. The link between abdominal obesity and the metabolic syndrome. Curr Hypertens Rep. 2008;10:156–164. doi: 10.1007/s11906-008-0029-7. [DOI] [PubMed] [Google Scholar]
- Fracanzani AL, Valenti L, Bugianesi E, Vanni E, Grieco A, Miele L, et al. Risk of nonalcoholic steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease and low visceral adiposity. J Hepatol. 2011;54:1244–1249. doi: 10.1016/j.jhep.2010.09.037. [DOI] [PubMed] [Google Scholar]
- Isomaa B, Almgren P, Tuomi T, Forsén B, Lahti K, Nissén M, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316:129–139. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- Brochu-Gaudreau K, Rehfeldt C, Blouin R, Bordignon V, Murphy BD, Palin MF. Adiponectin action from head to toe. Endocrine. 2010;37:11–32. doi: 10.1007/s12020-009-9278-8. [DOI] [PubMed] [Google Scholar]
- Whitehead JP, Richards AA, Hickman IJ, Macdonald GA, Prins JB. Adiponectin—a key adipokine in the metabolic syndrome. Diabetes Obes Metab. 2006;8:264–280. doi: 10.1111/j.1463-1326.2005.00510.x. [DOI] [PubMed] [Google Scholar]
- Badman MK, Flier JS. The adipocyte as an active participant in energy balance and metabolism. Gastroenterology. 2007;132:2103–2115. doi: 10.1053/j.gastro.2007.03.058. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lam KS, Yau MH, Xu A. Post-translational modifications of adiponectin: mechanisms and functional implications. Biochem J. 2008;409:623–633. doi: 10.1042/BJ20071492. [DOI] [PubMed] [Google Scholar]
- Hirose H, Yamamoto Y, Seino-Yoshihara Y, Kawabe H, Saito I. Serum high-molecular-weight adiponectin as a marker for the evaluation and care of subjects with metabolic syndrome and related disorders. J Atheroscler Thromb. 2010;17:1201–1211. doi: 10.5551/jat.6106. [DOI] [PubMed] [Google Scholar]
- Verkest KR, Rand JS, Fleeman LM, Morton JM, Richards AA, Rose FJ, et al. Distinct adiponectin profiles might contribute to differences in susceptibility to type 2 diabetes in dogs and humans. Domest Anim Endocrinol. 2011;41:67–73. doi: 10.1016/j.domaniend.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Li P, Shibata R, Unno K, Shimano M, Furukawa M, Ohashi T, et al. Evidence for the importance of adiponectin in the cardioprotective effects of pioglitazone. Hypertension. 2010;55:69–75. doi: 10.1161/HYPERTENSIONAHA.109.141655. [DOI] [PubMed] [Google Scholar]
- Tapan S, Tascilar E, Abaci A, Sonmez A, Kilic S, Erbil MK, et al. Decreased plasma apelin levels in pubertal obese children. J Pediatr Endocrinol Metab. 2010;23:1039–1046. doi: 10.1515/jpem.2010.165. [DOI] [PubMed] [Google Scholar]
- von Eynatten M, Liu D, Bluemm A, Schuster T, Baumann M, Lutz J, et al. Changes in adiponectin multimer distribution in response to atorvastatin treatment in patients with type 2 diabetes. Clin Endocrinol (Oxf) 2009;71:27–32. doi: 10.1111/j.1365-2265.2008.03412.x. [DOI] [PubMed] [Google Scholar]
- Draghia-Akli R, Fiorotto ML, Hill LA, Malone PB, Deaver DR, Schwartz RJ. Myogenic expression of an injectable protease-resistant growth hormone-releasing hormone augments long-term growth in pigs. Nat Biotechnol. 1999;17:1179–1183. doi: 10.1038/70718. [DOI] [PubMed] [Google Scholar]
- Li X, Eastman EM, Schwartz RJ, Draghia-Akli R. Synthetic muscle promoters: activities exceeding naturally occurring regulatory sequences. Nat Biotechnol. 1999;17:241–245. doi: 10.1038/6981. [DOI] [PubMed] [Google Scholar]
- Hebel H, Attra H, Khan A, Draghia-Akli R. Successful parallel development and integration of a plasmid-based biologic, container/closure system and electrokinetic delivery device. Vaccine. 2006;24:4607–4614. doi: 10.1016/j.vaccine.2005.08.049. [DOI] [PubMed] [Google Scholar]
- Wang XD, Tang JG, Xie XL, Yang JC, Li S, Ji JG, et al. A comprehensive study of optimal conditions for naked plasmid DNA transfer into skeletal muscle by electroporation. J Gene Med. 2005;7:1235–1245. doi: 10.1002/jgm.765. [DOI] [PubMed] [Google Scholar]
- Tai MM. A mathematical model for the determination of total area under glucose tolerance and other metabolic curves. Diabetes Care. 1994;17:152–154. doi: 10.2337/diacare.17.2.152. [DOI] [PubMed] [Google Scholar]
- Koonen DP, Sung MM, Kao CK, Dolinsky VW, Koves TR, Ilkayeva O, et al. Alterations in skeletal muscle fatty acid handling predisposes middle-aged mice to diet-induced insulin resistance. Diabetes. 2010;59:1366–1375. doi: 10.2337/db09-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebinuma H, Miyazaki O, Yago H, Hara K, Yamauchi T, Kadowaki T. A novel ELISA system for selective measurement of human adiponectin multimers by using proteases. Clin Chim Acta. 2006;372:47–53. doi: 10.1016/j.cca.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Chan AY, Soltys CL, Young ME, Proud CG, Dyck JR. Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J Biol Chem. 2004;279:32771–32779. doi: 10.1074/jbc.M403528200. [DOI] [PubMed] [Google Scholar]
- Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004;10:1384–1389. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden CH, Fisler JS. Comparisons of diets used in animal models of high-fat feeding. Cell Metab. 2008;7:277. doi: 10.1016/j.cmet.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2009;302:179–188. doi: 10.1001/jama.2009.976. [DOI] [PubMed] [Google Scholar]
- Lindsay RS, Funahashi T, Hanson RL, Matsuzawa Y, Tanaka S, Tataranni PA, et al. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet. 2002;360:57–58. doi: 10.1016/S0140-6736(02)09335-2. [DOI] [PubMed] [Google Scholar]
- Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- Snehalatha C, Mukesh B, Simon M, Viswanathan V, Haffner SM, Ramachandran A. Plasma adiponectin is an independent predictor of type 2 diabetes in Asian indians. Diabetes Care. 2003;26:3226–3229. doi: 10.2337/diacare.26.12.3226. [DOI] [PubMed] [Google Scholar]
- Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem. 2006;281:2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, et al. Adiponectin acts in the brain to decrease body weight. Nat Med. 2004;10:524–529. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- Fukushima M, Hattori Y, Tsukada H, Koga K, Kajiwara E, Kawano K, et al. Adiponectin gene therapy of streptozotocin-induced diabetic mice using hydrodynamic injection. J Gene Med. 2007;9:976–985. doi: 10.1002/jgm.1104. [DOI] [PubMed] [Google Scholar]
- Nan MH, Park JS, Myung CS. Construction of adiponectin-encoding plasmid DNA and gene therapy of non-obese type 2 diabetes mellitus. J Drug Target. 2010;18:67–77. doi: 10.3109/10611860903225719. [DOI] [PubMed] [Google Scholar]
- Park JH, Lee M, Kim SW. Non-viral adiponectin gene therapy into obese type 2 diabetic mice ameliorates insulin resistance. J Control Release. 2006;114:118–125. doi: 10.1016/j.jconrel.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Shklyaev S, Aslanidi G, Tennant M, Prima V, Kohlbrenner E, Kroutov V, et al. Sustained peripheral expression of transgene adiponectin offsets the development of diet-induced obesity in rats. Proc Natl Acad Sci USA. 2003;100:14217–14222. doi: 10.1073/pnas.2333912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh H, Nguyen MT, Trujillo M, Imamura T, Usui I, Scherer PE, et al. Adenovirus-mediated adiponectin expression augments skeletal muscle insulin sensitivity in male Wistar rats. Diabetes. 2005;54:1304–1313. doi: 10.2337/diabetes.54.5.1304. [DOI] [PubMed] [Google Scholar]
- Gregorevic P, Blankinship MJ, Chamberlain JS. Viral vectors for gene transfer to striated muscle. Curr Opin Mol Ther. 2004;6:491–498. [PubMed] [Google Scholar]
- Braun S. Muscular gene transfer using nonviral vectors. Curr Gene Ther. 2008;8:391–405. doi: 10.2174/156652308786070998. [DOI] [PubMed] [Google Scholar]
- Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci. 2001;98:2005–2010. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg GR, Kemp BE. AMPK in Health and Disease. Physiol Rev. 2009;89:1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW, et al. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59:554–563. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullerton MD, Steinberg GR. SIRT1 takes a backseat to AMPK in the regulation of insulin sensitivity by resveratrol. Diabetes. 2010;59:551–553. doi: 10.2337/db09-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, et al. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature. 2010;464:1313–1319. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]
- Bloquel C, Fabre E, Bureau MF, Scherman D. Plasmid DNA electrotransfer for intracellular and secreted proteins expression: new methodological developments and applications. J Gene Med. 2004;6 (Suppl 1:S11–S23. doi: 10.1002/jgm.508. [DOI] [PubMed] [Google Scholar]