Abstract
Background
Accurate pre-operative imaging in pancreatic cancer helps avoid unsuccessful surgical explorations and forewarns surgeons regarding aberrant anatomy. This review aimed to determine the role of current imaging modalities in the diagnosis and determination of resectability of pancreatic and peri-ampullary adenocarcinomas.
Methods
A systematic search of the scientific literature was carried out using EMBASE, PubMed/MEDLINE and the Cochrane Central Register of Controlled Trials for the years 1990 to 2011 to obtain access to all publications, especially randomized controlled trials, reporting on the diagnostic accuracy of ultrasonography, multi-detector computed tomography (MDCT), magnetic resonance imaging (MRI), endoscopic ultrasonography (EUS) or positron emission tomography (PET)-computed tomography (CT) and the evaluation of resectability of pancreatic and peri-ampullary adenocarcinomas.
Results
Based on 66 articles analysed in the review, MDCT and MRI/MRCP have comparable sensitivity and specificity rates for diagnosis and staging of pancreatic cancers. EUS offers the best sensitivity and specificity rates for lesions <2 cm. Improved staging has been noted when PET-CT scans are added to pre-operative evaluation.
Conclusions
MDCT with angiography or MRI/MRCP should constitute the first imaging modality in suspected pancreatic adenocarcinomas. EUS is recommended for assessing lesions not clearly detected, but suspected, on CT/MRI and in tumours considered ‘borderline resectable’ on MDCT to assess vascular involvement. PET-CT in locally advanced lesions will help rule out distant metastases.
Introduction
Carcinomas of the pancreas, although uncommon, are amongst the leading causes of cancer-related deaths around the world. According to the American Cancer Society, the relative 1-year survival is only 24%, and the overall 5-year survival rate is 5%1 and with similar outcomes reported worldwide.2–4 Complete surgical resection with chemotherapy (when indicated) offers the best outcomes in this cancer.5 However, owing to the insidious onset of the disease and the delayed presentation of patients, surgery with a curative intent may not always be possible. Owing to the morbidity associated with a surgical exploration that is likely to be unsuccessful, pre-operative determination of the extent of the disease assumes significance. As a result of the increased morbidity and technical difficulty associated with aberrant arterial anatomy, an accurate pre-operative assessment of the vascular anatomy is important.6 Over the years, various radiological imaging modalities for the pancreas have been used for the diagnosis and staging of these cancers including abdominal ultrasonography, computed tomography (CT) scans, magnetic resonance imaging (MRI), laparoscopy and endoscopic ultrasonography (EUS). The aims of this review were to define the role of pre-operative imaging in the evaluation of pancreatic adenocarcinomas by providing the evidence available to date on each of these modalities.
Methods
A systematic search of the scientific literature was carried out using EMBASE, PubMed/MEDLINE and the Cochrane Central Register of Controlled Trials for the years 1990–2011 to obtain access to all publications, especially randomized controlled trials, that reported on the diagnostic accuracy of ultrasonography, CT, MRI or EUS and evaluation of resectability of pancreatic and peri-ampullary adenocarcinomas.
The search strategy was that described by Dickersin et al.7 with the appropriate specific search terms, namely, ‘peri-ampullary cancer’, ‘pancreatic cancer’, ‘diagnosis’, ‘staging’, ‘resectability’, ‘computed tomography’, ‘CT’, ‘magnetic resonance imaging’, ‘MRI’, ‘ultrasonography’, ‘endoscopic ultrasonography’, ‘EUS’, ‘laparoscopy’, ‘positron emission tomography’, ‘PET’, ‘randomized controlled trials’, ‘review’ and ‘meta-analysis’. All available major publications from the past 21 years were retrieved.
Inclusion criteria:
Original articles on the role of CT scan/MRI or MRCP/ultrasonography (abdominal and laparoscopic)/PET-CT/EUS in the diagnosis, determination of the extent of disease and resectability of pancreatic and peri-ampullary adenocarcinoma.
Original articles comparing CT scans, MRI or MRCP, ultrasonography (abdominal and laparoscopic), PET-CT and/or EUS in the diagnosis, determination of the extent of disease and resectability of pancreatic and peri-ampullary adenocarcinoma.
Exclusion criteria:
Case reports.
Articles on the imaging of tumours other than adenocarcicoma.
From the retrieved articles, only papers reporting on ultrasonography, CT, MRI/MRCP, EUS or laparoscopy in the diagnosis, staging and the determination of resectability in patients with pancreatic and peri-ampullary cancer were included in the review.
For clarification, the term staging has been used to indicate the ability of the imaging modality to delineate the tumour, nodal and metastatic disease whereas the term resectability is mainly used to indicate technical resectability in the absence of distant metastases and vascular involvement (beyond the definition of borderline resectable disease – for definition see below).
Results
Using the above search strategy, 351 articles were identified of which only 67 articles were analysed in this review (Fig. 1). These included three articles on abdominal ultrasonography,8–10 23 on CT and CT angiography,11–33 4 on MRI/MRCP,34–37 2 on laparoscopic staging and laparosopcic ultrasonography,38,39 9 on PET and PET-CT,40–48 3 on EUS49–51 and 23 comparing the various modalities.52–74
Figure 1.
Flow chart of the search strategy employed
A recent review by Low et al.75 provides useful information in differentiating benign from malignant lesions of the pancreas and hence this aspect has not been addressed in the present review. Other important reviews published on the topic in past 11 years include the role of a standard MR in pre-operative imaging of pancreatic masses76 and the use of an MR protocol including non-contrast T1-weighted fat-suppressed and dynamic gadolinium-enhanced gradient-echo imaging in pancreatic cancer by Vachiranubhap et al.77 Bipat et al.,78 in 2005, published a sentinel meta-analysis on the role of ultrasonography, CT and MRI for the diagnosis and determination of resectability of pancreatic adenocarcinoma in which they concluded that helical CT was the preferred imaging modality.
Diagnosis and staging: imaging modalities
Abdominal ultrasonography in pancreatic cancer
Ultrasonography is amongst the most widely available and often the first investigative imaging modality used to assess patients with hepatopancreatobiliary complaints or even non-specific abdominal pain. Karlson et al.8 reported a diagnostic sensitivity of up to 90% for exocrine pancreatic tumours. Moreover, the presence of obvious hepatic metastases on ultrasonography will often prevent the need for further imaging.
However, in general, body habitus, the retroperitoneal location of the pancreas obscured often by bowel gas, as well as the operator-dependant nature of the investigation preclude the use of ultrasonography as an accurate staging modality.
Morrin et al.,10 using gray scale and colour Doppler ultrasonography of the abdomen, were able to demonstrate results similar to helical CT and CT angiography in detecting venous involvement. The role of the real-time imaging modality of contrast-enhanced ultrasonography in pancreatic tumours has been trialled since the late 1990s.9 Kitano et al.52 used coded phase inversion harmonic ultrasonography to overcome the limitations from these previous techniques and demonstrated a higher sensitivity of this technique compared with contrast-enhanced CT but similar to EUS for detecting lesions ≤2 cm.
Computed tomography (CT)
CT scanners have developed tremendously over the past few decades resulting in the improved resolution and hence their diagnostic capability. The thin-cut (64 section) intravenous contrast-enhanced multi-detector CT (MDCT) is the radiological investigation of choice.79 The scans are performed in phases, as follows the non-contrast, arterial, pancreatic parenchymal and the portal venous phases. MDCT allows rapid anatomic coverage coupled with excellent spatial resolution.12
The sensitivity of CT in the detection of pancreatic cancers has improved over the years with the advent of multi-phasic scans and lies between 75–100% with a specificity of 70–100%.11,13,15,52,53,62,63,72 However, in spite of these improvements, the sensitivity of CT scan for lesions ≤2 cm is between 68–77%13,52 with an accuracy of 77%.63 However, for tumours >2 cm, the sensitivity may be as high as 98%.52
Pancreatic carcinomas on CT appear mainly hypoattenuating especially on the arterial phase. However, they have also been noted to be isoattenuating in 11% of individuals in which pancreatic or biliary duct dilation are signs that may indicate the presence of an underlying pathology.28 The pancreatic tumours noted to be hyperattenuating are mainly the neuroendocrine tumours.24 The pancreatic parenchymal and the portal venous phase appear to be similar but better than the arterial phase for delineating pancreatic adenocarcinomas.17,26 However, for vascular invasion, the sensitivity of images obtained in the portal venous phase are better than those obtained in the pancreatic or arterial phases as images obtained in the pancreatic phase demonstrated more flow artefacts and decreased attenuation in the superior mesenteric vein, compared with the artefacts revealed on images obtained in the portal venous (hepatic) phase.16
Imbriaco et al.18 found thin-section single-phase MDCT to be very accurate for the diagnosis and assessment of resectability in patients with a suspected pancreatic neoplasia owing to the optimal tumour-to-pancreas contrast and maximal pancreatic parenchymal and peri-pancreatic vascular enhancement. They also found that it allowed visualization of the entire liver and the whole upper abdomen during the portal phase for accurate identification of liver metastases and peritoneal seeding.
CT criteria for unresectable disease formerly included findings such as extrapancreatic disease involving the liver or peritoneum, and contiguous invasion of adjacent organs such as the stomach and colon, as well as involvement of peri-pancreatic vessels.27 In 1997, Howard et al.56 reported a sensitivity and specificity of 63% and 100%, and an overall accuracy of 86%. However, Lu et al.25 and Diehl et al.15 found the sensitivity rates to be 84% and 91%, respectively. Karmazonovsky et al.,20 in 2005, found that spiral CT had a sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV) and accuracy of 79%, 82%, 91%, 62% and 81%, respectively, in predicting unresectability. They also found that the sensitivity, specificity, positive predictive value, negative predictive value and accuracy of spiral CT in the diagnosis of vascular invasion were 94%, 84.2%, 94%, 84%, and 91.3%, based on their correlation with the intra-operative findings.
The increasing surgical aggression in terms of pancreatic resections that have gone hand in hand with the refinements in techniques of surgery, coupled with advancements in chemotherapy and radiotherapy have now led to the development of yet another intermediate disease stage: borderline resectable disease.21,29,31 By definition,30 borderline resectable tumours are those tumours that exhibit the following: (i) encasement of a short segment of the hepatic artery, without evidence of tumour extension to the celiac axis, that is amenable to resection and reconstruction; and (ii) tumour abutment of the superior mesenteric artery involving <180° of the circumference of the artery or short-segment occlusion of the superior mesenteric vein, portal vein or their confluence with a suitable option available for vascular reconstruction because the veins are normal above and below the area of tumour involvement. This stage strongly relies on the accurate delineation of peripancreatic vascular involvement based on CT imaging.
Brugel et al.14 confirmed that thin-slice multiplanar reconstructions obtained with multislice helical CT provided an exact depiction of the spatial relation between the tumour and the potentially invaded vessels and thus had the capability to improve the assessment of local resectability. Kaneko et al.19 retrospectively compared multidetector computed tomographic angiography (MDCTA) done pre-operatively to the actual surgical outcomes with the aim of determining the value of MDCTA in predicting resectability of pancreatic head cancers. In this previous study, MDCTA was found to have a sensitivity, specificity, positive predictive value, negative predictive values and accuracy of 100%, 71%, 85%,100% and 89% which was similar to the results reported by Fusari et al.53 and Zamboni et al.32 The additional finding of the study by Zamboni et al.32 was the lack of evidence suggesting varying results from the various generations of MDCT scanners used.
Shrikhande et al.29 identified 12 patients with borderline resectable disease in a mixed pathological cohort of pancreatic adenocarcinomas, solid pseudopapillary and neuroendocrine tumours according to the MD Anderson Cancer Centre classification.30 They correlated the MDCT images with the intra-operative findings. Eight of the 12 patients actually underwent a curative R0 resection while another 2 had microscopically positive margins (R1 resections). Based on this they determined that a grading system based on three radiological features on MDCT, viz. maximum degree of circumferential contact (CC), length of contact of the tumour with major vessels (LC) and luminal narrowing of vessels at the point of contact with the tumour (venous deformity, VD) is helpful to predict which patients with apparently borderline disease may actually be offered an up-front, potentially curative surgical resection. This grading system needs to be validated in a larger cohort of patients with pancreatic adenocarcinoma.
Similarly, Kent et al.22 have proposed a CT grading system for pancreaticobiliary tumours. This five-point scale describes the radiographical relationship of the pancreatobiliary mass to adjacent vessels, viz. portal vein, superior mesenteric vein, superior mesenteric artery and celiac trunk. An increasing grade has been shown to be associated with a higher probability of unresectability and microscopic positive resection margins.22
Zhao et al.33 performed a recent meta-analysis on the role of CT in the diagnosis and determination of vascular invasion in pancreatic and periampullary cancer. Based on the results obtained from the 18 studies selected, CT was found to have a sensitivity and specificity of 85% and 82%, respectively, in the diagnosis, and a sensitivity and specificity of 77% and 81% in the diagnosis of vascular invasion. However, the results did indicate that the addition of other imaging modalities, such as EUS, could further improve the diagnostic accuracy of vascular invasion.
Kim et al.,23 on the other hand, compared the accuracy of MDCT in the pre-operative evaluation of resectability between patients who had received neoadjuvant chemo-radiotherapy with patients who had not received any neoadjuvant treatment. They found no difference between the two groups (83% versus 81%). In this previous study, the absence of para aortic lymph node metastasis (>1 cm in short axis) was considered a feature of resectability in addition to the definition of resectability provided in the methods section of this review.
Magnetic resonance imaging (MRI) and magnetic resonance cholangio pancreatography (MRCP)
In 1997, Trede et al.64 suggested that ultrafast MRI was the most accurate staging modality when compared with percutaneous ultrasonography, dual-phase helical CT, selective visceral angiography and endoscopic retrograde cholangiopancreatography (ERCP) for pancreatic and peri-ampullary tumours.
Megibow et al.36 found that MRI had a diagnostic accuracy of 70% for pancreatic carcinomas. Megibow et al. determined that gradient-echo and T1-weighted spin-echo sequences ranked equally in the evaluation of vascular invasion, T1-weighted spin-echo sequences were preferred for assessing lymphadenopathy and T2-weighted spin-echo sequences were preferred for detecting hepatic metastases. Hanninen et al.34 noted that single-shot thick slab MRCP had superior image quality as compared with multi-section MRCP images. They also noted a significant improvement in the diagnostic accuracy of MRCP by two readers (89% and 84% vs. 72% and 69%) with the addition of T1 and T2-weighted images. This led them to infer that a comprehensive MR approach should comprise both MRCP techniques and parenchymal sequences.34
More recently, Fusari et al.53 demonstrated sensitivity, specificity, accuracy, positive and negative predictive values of 100%, 88%, 98%. 97% and 100%, respectively, for MRCP in terms of diagnosis, and sensitivity, specificity, accuracy, positive and negative predictive values of 88%, 100%, 90%, 100% and 70%, respectively, for the evaluation of resectability of pancreatic carcinomas.
Tapper et al.37 determined that MRCP had a 100% and 83%, 81% and 96%, and 87% and 95% sensitivity and accuracy rates in the determination of resectability, arterial and venous involvement in pancreatic head carcinomas. These results were similar to those of Hochwald et al.35 who determined that MRI and MRCP had a sensitivity of 100%, specificity of 83%, PPV of 94%, NPV of 100% and accuracy of 95% in determining resectability of pancreatic cancer. Resectability in this previous study was defined as surgically removable with grossly negative margins. Hochwald et al.35 noted that malignant lesions appeared as discrete, hypointense masses with respect to background pancreatic parenchyma on post-contrast T1-weighted images. Based on a comparison of pre-operatively performed MDCT and MR imaging with angiography with the intra-operative findings, Lee et al.,65 however, noted that both modalities demonstrated an equal ability in detection, prediction of vascular involvement and determination of resectability for pancreatic ductal adenocarcinoma.
Miller et al.73 have provided specific scenarios in which MRCP may add to the CT findings in the diagnosis and staging of tumours. In case of patients suspected to have pancreatic tumours on CT which would appear as hypoattenuating lesions on the arterial phase but who have signs such as duct dilation or focal pancreatic atrophy, MRCP may be useful in such ‘non-contour deforming’ lesions. The contrast resolution of MRI facilitates detection of such small tumours on gadolinium-enhanced fat-suppressed images. Similarly, in patients with a ‘double duct’ sign, Miller et al.73 suggested that MRCP may be useful in differentiating a malignant from a benign aetiology. They also suggested a complementary role for MRCP in the characterization of suspected liver metastases and the detection of omental and nodal lesions.
In patients with borderline resectable disease who received down-staging chemotherapy, Donahue et al.74 studied the use of CT and MRI signs pre-operatively. They noted that features suggestive of vascular involvement persisted even after chemotherapy. In spite of these features, patients underwent exploration with a curative intent based on other characteristics such as a reduction in tumour markers, a reduction in the size of the tumour on imaging and good functional status. In 83% of patients in whom a complete resection could be achieved, fibrosis, and not tumour, was the cause for the observed ‘involvement’ noted on preoperative imaging. Thus they concluded that CT and MRI had a low sensitivity (71%) and specificity (58%) in predicting vascular involvement and resectability in the post-chemotherapy setting.
Endoscopic ultrasonography (EUS)
Studies reporting the diagnostic ability of EUS in the past were fraught with the bias that even benign lesions were included. Hence care should be exerted when interpreting earlier results of EUS.80
The three types of echoendoscopes available today include: radial echoendoscopes that provide axial images that correspond to the familiar computed tomography (CT) or magnetic resonance imaging (MRI) slices, and provide images from 2700 or 3600, depending on the manufacturer; curvilinear echoendoscopes that provide an image parallel to the shaft of the endoscope and allow for the visualization and directing of fine-needle aspiration (FNA), fine-needle injection (FNI), stent placement and other interventional EUS procedures; and finally the catheter-based specialty probes that are high-frequency probes that are passed through either a forward-viewing endoscope or a side-viewing duodenoscope to provide direct visualization of a specific area or lesion (i.e. submucosal mass, intraductal lesions or discrete gastric lesion).50
EUS has emerged as a useful, albeit invasive, modality in the diagnosis of pancreatic tumours with sensitivities and accuracy approaching 100% and specificity >95% even for lesions <2 cm.52,62,63,72 In ampullary tumours, EUS has been found to be more sensitive and specific as compared with CT with a statistically significant (P < 0.05) strength of tumour and nodal agreement with the final pathology in a cohort of 27 patients (7.4% – T1; 48.1% – T2; 44.4% – T3 and 63% – N0 and 37% – N1 disease).71
Tadic et al.51 published their experience of EUS-FNA for lesions <3 cm in all portions of the pancreas and were able to demonstrate a sensitivity, specificity, PPV, NPV and diagnostic accuracy of 68%, 100%, 100%, 73% and 83%, respectively. Similarly, Fisher et al.49 analysed their results of EUS-FNA for lesions <5 cm predominantly in the head of pancreas (73%) and found sensitivity, specificity, PPV, NPV and accuracy of 94.3%, 100%, 100%, 72.2% and 95%, respectively. The value of EUS as a medium to obtain histological evidence is increasing and must be sought in centres where the expertise for EUS is available.
While Cannon et al.54 found that the presence of an endobiliary stent affected staging accuracy of EUS for ampullary lesions, Chen et al.,55 in a recent study, found that stents did not affect the staging accuracy.
Positron emission tomography (PET) scan
Pancreatic adenocarcinomas are 18F-Fluorodeoxyglucose (FDG)-avid tumours and hence FDG PET is a useful investigation for the detection of these lesions.
A recent meta-analysis69 on the role of PET-CT for the detection of pancreatic carcinoma demonstrated a pooled sensitivity of 90.1% for PET-CT as compared with EUS (81.2%) and a pooled specificity of 80.1% as compared with 92.3% for EUS. These results are similar to the findings of two previously published reviews of literature42,47 on the role of PET-CT in pancreatic cancer detection that had noted a sensitivity of 90% and 95% and specificity from 82% and 100%, respectively.
While FDG PET may help differentiate pancreatic adenomas from carcinomas by the lack of avidity to adenomas,70 the differentiation from chronic active pancreatitis is difficult owing to FDG uptake in the presence of inflammation.40 FDG uptake has been noted in up to 13% of patients with chronic pancreatitis in the absence of acute inflammation48 but may increase to as high as 100% in autoimmune chronic pancreatitis.46 However, the avid uptake of FDG in the salivary glands has been suggested as useful clues associated with autoimmune pancreatitis that may help differentiate it from pancreatic cancer.45 Delayed image acquisition may help to differentiate chronic pancreatitis from pancreatic cancer.43 Similarly, in the future, the development of tracers for PET measuring cellular proliferation may help to reduce the false-positive uptake noted in chronic pancreatitis.40
For staging of pancreatic cancer, PET-CT does not provide any benefit in terms of local tumour and regional lymph nodal disease spread.41,44 In terms of loco-regional staging, PET/CT has been shown to be similar to CT (accuracy rate of 84–85%).67 For assessing recurrent or progressive disease, PET-CT has been shown to be more sensitive (90%) than CT (80%). The complimentary role of PET-CT and CT was best demonstrated by Farma et al.68 who demonstrated an improvement in the sensitivity by 30% by the addition of PET-CT to CT in the initial staging of pancreatic cancer.
Laparoscopic staging and laparoscopic ultrasonography
The role of diagnostic laparoscopy and laparoscopic ultrasonography in the diagnosis and staging of peri-ampullary and pancreatic cancer was aimed at detecting missed occult metastatic lesions in the liver and peritoneal cavity.81 Thus the use of such a strategy would seem most prudent in patients with locally advanced cancers noted on imaging. For this indication, Shoup et al.39 were able to demonstrate metastasis in up to 37% of these patients causing them to be unresectable.
A recent meta-analysis38 addressed the role of staging laparoscopy and laparoscopic ultrasonography for peri-ampullary and pancreatic cancers. Twenty-two studies were included in the analysis of which only six studies included patients with locally advanced disease on CT scan. Staging laparoscopy and laparoscopic ultrasonography was found to have an overall sensitivity of 64% and specificity of 99% with an improvement in the resection rate from 61% to 80%.
Comparative studies
Table 1 summarizes some of the studies comparing the efficacy of CT, EUS and MRI/MRCP in the diagnosis, staging and determination of respectability of pancreatic and peri-ampullary cancers.54–61
Table 1.
Studies comparing the efficacy of CT, EUS and MRI/MRCP in the evaluation of pancreatic and periampullary cancers54–61
| Author | Year | Factor analysed | Modality | Sensitivity | Specificity | Accuracy | Comments |
|---|---|---|---|---|---|---|---|
| Periampullary cancer | |||||||
| Howard TJ et al. | 1997 | Diagnosis and resectability | Helical CT | 63% | 100% | 85% | EUS better for tumour detection but CT better for determining tumour resectability |
| EUS | 75% | 77% | 76% | ||||
| Rivadeneira et al. | 2003 | Diagnosis | Helical CT | 68% | 50% | 67% | Linear array EUS consistently superior to helical CT in preoperative local staging of periampullary malignancies |
| Linear array EUS | 100% | 75% | 98% | ||||
| Nodal staging | Helical CT | 33% | 92% | 68% | |||
| Linear array EUS | 61% | 100% | 84% | ||||
| Vascular involvement | Helical CT | 45% | 100% | 88% | |||
| Linear array EUS | 100% | 100% | 100% | ||||
| Shoup et al. | 2000 | Diagnosis | Axial / Helical CT | 82% | 66% | CT – initial study of choice in patients with suspected periampullary tumours | |
| EUS | 97% | 33% | |||||
| Nodal staging | Axial / Helical CT | 42% | 73% | EUS – superior for tumour detection and predicting vascular invasion | |||
| EUS | 21% | 80% | |||||
| Vascular invasion | Axial / Helical CT | 80% | 87% | ||||
| EUS | 20% | 100% | |||||
| Ampullary Tumours | |||||||
| Cannon et al. | 1999 | Tumour staging | Helical CT | – | – | 24% | EUS – superior to CT and MRI in assessing T stage but not N stage of ampullary lesions |
| EUS | – | – | 78% | ||||
| MRI | – | – | 46% | ||||
| Nodal staging | Helical CT | – | – | 59% | |||
| EUS | – | – | 68% | ||||
| MRI | – | – | 77% | ||||
| Chen et al. | 2009 | Detection | CT | – | – | 28% | EUS was superior to CT and was equivalent to MRI for tumour detection and T and N staging of ampullary tumours |
| EUS | – | – | 98% | ||||
| MRI | – | – | 81% | ||||
| Tumour staging | CT | – | – | 26% | |||
| EUS | – | – | 73% | ||||
| MRI | – | – | 54% | ||||
| Nodal staging | CT | – | – | 44% | |||
| EUS | – | – | 67% | ||||
| MRI | – | – | 77% | ||||
| Periampullary and Pancreatic Cancer | |||||||
| Midwinter et al. | 1999 | Venous involvement (SMV / PV) | Spiral CT | 56% | 100% | – | EUS is an important additional investigation to CT |
| EUS | 81% | 86% | – | ||||
| Nodal staging | Spiral CT | 33% | 86% | – | |||
| EUS | 44% | 93% | – | ||||
| Arterial involvement (SMA) | Spiral CT | 50% | 100% | – | |||
| EUS | 17% | 67% | |||||
| Pancreatic Cancer | |||||||
| Rosch et al. | 1991 | Diagnosis | CT | 77% | 53% | – | EUS superior to CT in pancreatic tumour diagnosis |
| EUS | 99% | 100% | – | ||||
| Soriano et al. | 2004 | Locoregional extension | Helical CT | 66% | 100% | 74% | Helical CT and EUS – most useful individual imaging techniques in the staging of pancreatic cancer |
| EUS | 44% | 100% | 62% | ||||
| MRI | 53% | 100% | 68% | ||||
| Nodal staging | Helical CT | 37% | 795 | 62% | In potentially resectable tumours – sequential approach: initially helical CT followed by confirmatory EUS – most reliable and cost effective | ||
| EUS | 36% | 87% | 65% | ||||
| MRI | 15% | 93% | 61% | ||||
| Vascular invasion | Helical CT | 67% | 94% | 83% | |||
| EUS | 42% | 97% | 76% | ||||
| MRI | 59% | 84% | 74% | ||||
| Distant Metastases | Helical CT | 55% | 96% | 88% | |||
| EUS | 0% | 100% | 855 | ||||
| MRI | 30% | 95% | 83% | ||||
Abbreviations: PV, Portal Vein; SMA, Superior Mesenteric artery; SMV, Superior mesenteric vein.
Legmann et al.63 compared the accuracy of CT vs. EUS in determining the T and N stage of the disease based on the size of the lesion. The overall accuracy for T-staging was not different between the two modalities (90% for EUS vs. 86% for CT). For T stage lesions less than 15 mm, CT had a lower accuracy as compared with EUS (66% vs. 90%). However, for lesions larger than 35 mm, CT had a better accuracy than EUS (100% vs. 86%). For lymph nodal staging, EUS did perform marginally better than CT (86% vs. 77%). These findings were similar to the findings of Shoup et al.60 who also found EUS to have a higher accuracy compared with CT for peri-ampullary tumours <2 cm in size (90% vs. 70%).
Dewitt et al.62 compared CT and EUS in a prospective, observational cohort study in order to determine the accuracy of the two modalities in staging tumours as well as determining resectability. Surgically resected pancreatic cancer with negative microscopic histological margins was considered resectable. Of the 80 patients with pancreatic cancer, EUS had a sensitivity of 98% as compared with 86% for CT. EUS also had a significantly better accuracy in terms of determining the T stage of the tumour. While the two modalities were similar in terms of nodal staging, CT was better able to identify patients amenable to a resection, although the difference was not significant. Based on their findings they concluded that although EUS was superior to CT in terms of tumour detection and determination of T stage, the two modalities were comparable in terms of nodal staging and determination of resectability.
Park et al.66 found that gadolinium-enhanced dynamic three-dimensional–gradient echo MRI with MRCP showed superior tumour conspicuity and similar diagnostic performance compared with MDCT in evaluating the resectability of pancreatic cancer.
Discussion
While abdominal ultrasonography may be the first investigation in patients with upper gastrointestinal and biliary complaints (which could arise as a result of an underlying pancreatic malignancy), CT and MRI/MRCP constitute the most commonly performed primary investigations performed for the diagnosis and staging of pancreatic and peri-ampullary cancers. The choice between CT or MRI/MRCP is more often determined by the availability of the individual modality as well as the technical expertise in reporting them at the individual centres.
The major advantage of CT in comparison with EUS is its ability to provide an assessment of the entire abdominal cavity thus providing more information than EUS on distant metastases.
In interpreting the results from the earlier studies, it should be remembered that these studies were comparing EUS with older generation CT scanners.
EUS appears to be most important in the assessment of lesions not clearly detected, but suspected, on CT/MRI, as well as in ampullary tumours.71 It thus serves as a useful complementary investigation to CT82 or even MRI/MRCP.
The role of CT angiography in confirming vascular anatomy/involvement has been recently reviewed by one of the authors6 and has been strongly recommended in the pre-operative assessment of patients deemed to be suitable for a pancreatoduodenectomy.
Specific indications where PET-CT may aid in the decision making for tumours of the pancreas include: borderline resectable disease, locally advanced disease and/or resectable disease with suspected metastases.
Mayo et al.83 recently conducted a statewide review of all patients with surgically managed pancreatic cancer from 1996 to 2003 using data from the Oregon State Cancer Registry and found that the use of a CT scan formed the corner stone of staging and determination of respectability. They also noted that the use of laparoscopy, as a staging modality, was restricted to those patients suspected to have metastatic disease not identifiable on imaging.
Although a cost-analysis in the United States indicated that the routine use of laparoscopic staging did not add significantly to the overall expense of treatment,84 the routine use of staging laparoscopy in peripancreatic cancer was not found to be beneficial.85 The role of staging laparoscopy and laparoscopic ultrasonography definitely have a complimentary role to radiological imaging in patients with locally advanced disease by helping to reduce the number of non-therapeutic laparotomies.38
Figure 2 provides the authors' algorithm to the pre-operative evaluation of patients with pancreatic and peri-ampullary cancer.
Figure 2.
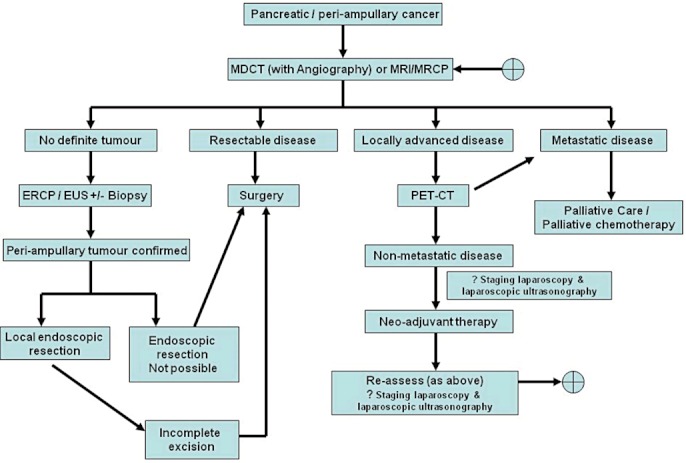
Algorithm outlining the role of the individual imaging modalities in the management of pancreatic and peri-ampullary cancers. MDCT, multi-detector computed tomography; MRI, magnetic resonance imaging; MRCP, magnetic resonance cholangiopancreatography; PET-CT, positron emission tomography-computed tomography; EUS, endoscopic ultrasonography; ERCP, endoscopic retrograde cholangiopancreatography
It is pertinent to note that in spite of pre-operative imaging suggesting the disease to be resectable, it is possible that the resultant exploration may not lead to a complete (R0) resection. Borderline resectable tumours (defined above) constitute an important class of such patients. While it has been shown that such tumours which appear borderline resectable on pre-operative imaging may be amenable to a complete resection,29 the value of such resections in terms of overall survival need to be better defined. Offering these patients neoadjuvant chemo-radiotherapy has been suggested to be a viable option.21,30 In spite of increasing surgical aggression and the availability of neoadjuvant treatment algorithms, certain features noted on pre-operative imaging have been linked to poor outcomes even in the event of an apparently curative resection in non-metastatic pancreatic cancer. Some authors have used techniques such as correlation of imaging with the surgical pathology86,87 to demonstrate this aspect. These include:
Features of local unresectability, including peri-pancreatic infiltration and a tumour size >3 cm.88
Venous involvement – where the length of invasion is >3 cm.89
Arterial involvement on pre-operative imaging.90
With the increasing use of neoadjuvant chemo- and chemoradiotherapy protocols in patients with borderline pancreatic tumours, further studies are needed to determine the imaging modalities that can best guide surgical decision making after neoadjuvant therapy especially when offering these patients the option of an exploration with the intent of cure.
Conclusion
MDCT with angiography usually constitutes the first imaging modality in suspected cancers of the pancreas. MRI and MRCP alternatively may be used in centres where these facilities are readily available. The role of EUS appears complementary to the conventional CT and MRI/MRCP. It is of most benefit in the assessment of lesions not clearly detected on CT. PET-CT serves as a useful complementary investigation in patients with locally advanced disease to rule out metastases outside the abdomen. Staging laparoscopy and laparoscopic ultrasonography may help in the re-staging of locally advanced/borderline resectable lesions treated with neo-adjuvant therapy.
Conflicts of interest
None declared.
References
- 1.Cancer Facts and Figures. American Cancer Society. 2009. Available at http://www.cancer.org/acs/groups/content/@nho/documents/document/500809webpdf.pdf (last accessed 8 June 2012)
- 2.Cancer Research UK. Available at http://info.cancerresearchuk.org/cancerstats/types/pancreas/incidence/ (last accessed 8 June 2012)
- 3.Chen KX, Wang PP, Zhang SW, Li LD, Lu FZ, Hao XS. Regional variations in mortality rates of pancreatic cancer in China: results from 1990–1992 national mortality survey. World J Gastroenterol. 2003;9:2557–2560. doi: 10.3748/wjg.v9.i11.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seino T, Nakadaira H, Endoh K, Yamamoto M. Changes in pancreatic cancer mortality, period patterns, and birth cohort patterns in Japan: analysis of mortality data in the period 1968–2002. Environ Health Prev Med. 2008;13:234–242. doi: 10.1007/s12199-008-0036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paez D, Labonte M, Lenz H. Pancreatic Cancer: medical management (novel chemotherapeutics) Gastroenterol Clin North Am. 2012;41:189–209. doi: 10.1016/j.gtc.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Shukla PJ, Barreto SG, Kulkarni A, Nagarajan G, Fingerhut A. Vascular anomalies encountered during pancreatoduodenectomy: do they influence outcomes? Ann Surg Oncol. 2010;17:186–193. doi: 10.1245/s10434-009-0757-1. [DOI] [PubMed] [Google Scholar]
- 7.Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ. 1994;309:1286–1291. doi: 10.1136/bmj.309.6964.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karlson BM, Ekbom A, Lindgren PG, Kallskog V, Rastad J. Abdominal US for diagnosis of pancreatic tumor: prospective cohort analysis. Radiology. 1999;213:107–111. doi: 10.1148/radiology.213.1.r99oc25107. [DOI] [PubMed] [Google Scholar]
- 9.Koito K, Namieno T, Nagakawa T, Morita K. Inflammatory pancreatic masses: differentiation from ductal carcinomas with contrast-enhanced sonography using carbon dioxide microbubbles. AJR Am J Roentgenol. 1997;169:1263–1267. doi: 10.2214/ajr.169.5.9353439. [DOI] [PubMed] [Google Scholar]
- 10.Morrin MM, Kruskal JB, Raptopoulos V, Weisinger K, Farrell RJ, Steer ML, et al. State-of-the-art ultrasonography is as accurate as helical computed tomography and computed tomographic angiography for detecting unresectable periampullary cancer. J Ultrasound Med. 2001;20:481–490. doi: 10.7863/jum.2001.20.5.481. [DOI] [PubMed] [Google Scholar]
- 11.Bluemke DA, Cameron JL, Hruban RH, Pitt HA, Siegelman SS, Soyer P, et al. Potentially resectable pancreatic adenocarcinoma: spiral CT assessment with surgical and pathologic correlation. Radiology. 1995;197:381–385. doi: 10.1148/radiology.197.2.7480681. [DOI] [PubMed] [Google Scholar]
- 12.Brennan DD, Zamboni GA, Raptopoulos VD, Kruskal JB. Comprehensive preoperative assessment of pancreatic adenocarcinoma with 64-section volumetric CT. Radiographics. 2007;27:1653–1666. doi: 10.1148/rg.276075034. [DOI] [PubMed] [Google Scholar]
- 13.Bronstein YL, Loyer EM, Kaur H, Choi H, David C, DuBrow RA, et al. Detection of small pancreatic tumors with multiphasic helical CT. AJR Am J Roentgenol. 2004;182:619–623. doi: 10.2214/ajr.182.3.1820619. [DOI] [PubMed] [Google Scholar]
- 14.Brugel M, Rummeny EJ, Dobritz M. Vascular invasion in pancreatic cancer: value of multislice helical CT. Abdom Imaging. 2004;29:239–245. doi: 10.1007/s00261-003-0102-2. [DOI] [PubMed] [Google Scholar]
- 15.Diehl SJ, Lehmann KJ, Sadick M, Lachmann R, Georgi M. Pancreatic cancer: value of dual-phase helical CT in assessing resectability. Radiology. 1998;206:373–378. doi: 10.1148/radiology.206.2.9457188. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher JG, Wiersema MJ, Farrell MA, Fidler JL, Burgart LJ, Koyama T, et al. Pancreatic malignancy: value of arterial, pancreatic, and hepatic phase imaging with multi-detector row CT. Radiology. 2003;229:81–90. doi: 10.1148/radiol.2291020582. [DOI] [PubMed] [Google Scholar]
- 17.Graf O, Boland GW, Warshaw AL, Fernandez-del-Castillo C, Hahn PF, Mueller PR. Arterial versus portal venous helical CT for revealing pancreatic adenocarcinoma: conspicuity of tumor and critical vascular anatomy. AJR Am J Roentgenol. 1997;169:119–123. doi: 10.2214/ajr.169.1.9207510. [DOI] [PubMed] [Google Scholar]
- 18.Imbriaco M, Megibow AJ, Ragozzino A, Liuzzi R, Mainenti P, Bortone S, et al. Value of the single-phase technique in MDCT assessment of pancreatic tumors. AJR Am J Roentgenol. 2005;184:1111–1117. doi: 10.2214/ajr.184.4.01841111. [DOI] [PubMed] [Google Scholar]
- 19.Kaneko OF, Lee DM, Wong J, Kadell BM, Reber HA, Lu DS, et al. Performance of multidetector computed tomographic angiography in determining surgical resectability of pancreatic head adenocarcinoma. J Comput Assist Tomogr. 2010;34:732–738. doi: 10.1097/RCT.0b013e3181e5d6f7. [DOI] [PubMed] [Google Scholar]
- 20.Karmazanovsky G, Fedorov V, Kubyshkin V, Kotchatkov A. Pancreatic head cancer: accuracy of CT in determination of resectability. Abdom Imaging. 2005;30:488–500. doi: 10.1007/s00261-004-0279-z. [DOI] [PubMed] [Google Scholar]
- 21.Katz MH, Pisters PW, Evans DB, Sun CC, Lee JE, Fleming JB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. 2008;206:833–846. doi: 10.1016/j.jamcollsurg.2007.12.020. discussion 46–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kent TS, Raptopoulos V, Callery MP, Gautam S, Vollmer CM. Escalating computed tomography angiogram (CTA) grade predicts unresectability and margin status for pancreaticobiliary neoplasms. HPB. 2010;12:115–122. doi: 10.1111/j.1477-2574.2009.00145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim YE, Park MS, Hong HS, Kang CM, Choi JY, Lim JS, et al. Effects of neoadjuvant combined chemotherapy and radiation therapy on the CT evaluation of resectability and staging in patients with pancreatic head cancer. Radiology. 2009;250:758–765. doi: 10.1148/radiol.2502080501. [DOI] [PubMed] [Google Scholar]
- 24.Kinney T. Evidence-based imaging of pancreatic malignancies. Surg Clin North Am. 2010;90:235–249. doi: 10.1016/j.suc.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Lu DS, Reber HA, Krasny RM, Kadell BM, Sayre J. Local staging of pancreatic cancer: criteria for unresectability of major vessels as revealed by pancreatic-phase, thin-section helical CT. AJR Am J Roentgenol. 1997;168:1439–1443. doi: 10.2214/ajr.168.6.9168704. [DOI] [PubMed] [Google Scholar]
- 26.McNulty NJ, Francis IR, Platt JF, Cohan RH, Korobkin M, Gebremariam A. Multi–detector row helical CT of the pancreas: effect of contrast-enhanced multiphasic imaging on enhancement of the pancreas, peripancreatic vasculature, and pancreatic adenocarcinoma. Radiology. 2001;220:97–102. doi: 10.1148/radiology.220.1.r01jl1897. [DOI] [PubMed] [Google Scholar]
- 27.O'Malley ME, Boland GW, Wood BJ, Fernandez-del Castillo C, Warshaw AL, Mueller PR. Adenocarcinoma of the head of the pancreas: determination of surgical unresectability with thin-section pancreatic-phase helical CT. AJR Am J Roentgenol. 1999;173:1513–1518. doi: 10.2214/ajr.173.6.10584794. [DOI] [PubMed] [Google Scholar]
- 28.Prokesch RW, Chow LC, Beaulieu CF, Bammer R, Jeffrey RB., Jr Isoattenuating pancreatic adenocarcinoma at multi-detector row CT: secondary signs. Radiology. 2002;224:764–768. doi: 10.1148/radiol.2243011284. [DOI] [PubMed] [Google Scholar]
- 29.Shrikhande SV, Arya S, Barreto SG, Ingle S, D'Souza MA, Hawaldar R, et al. Borderline resectable pancreatic tumors: is there a need for further refinement of this stage? Hepatobiliary Pancreat Dis Int. 2011;10:319–324. doi: 10.1016/s1499-3872(11)60053-2. [DOI] [PubMed] [Google Scholar]
- 30.Varadhachary GR, Tamm EP, Abbruzzese JL, Xiong HQ, Crane CH, Wang H, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13:1035–1046. doi: 10.1245/ASO.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Varadhachary GR, Tamm EP, Crane C, Evans DB, Wolff RA. Borderline resectable pancreatic cancer. Curr Treat Options Gastroenterol. 2005;8:377–384. doi: 10.1007/s11938-005-0040-x. [DOI] [PubMed] [Google Scholar]
- 32.Zamboni GA, Kruskal JB, Vollmer CM, Baptista J, Callery MP, Raptopoulos VD. Pancreatic adenocarcinoma: value of multidetector CT angiography in preoperative evaluation. Radiology. 2007;245:770–778. doi: 10.1148/radiol.2453061795. [DOI] [PubMed] [Google Scholar]
- 33.Zhao WY, Luo M, Sun YW, Xu Q, Chen W, Zhao G, et al. Computed tomography in diagnosing vascular invasion in pancreatic and periampullary cancers: a systematic review and meta-analysis. Hepatobiliary Pancreat Dis Int. 2009;8:457–464. [PubMed] [Google Scholar]
- 34.Hanninen EL, Pech M, Jonas S, Ricke J, Thelen A, Langrehr J, et al. Magnetic resonance imaging including magnetic resonance cholangiopancreatography for tumor localization and therapy planning in malignant hilar obstructions. Acta Radiol. 2005;46:462–470. doi: 10.1080/02841850510021625. [DOI] [PubMed] [Google Scholar]
- 35.Hochwald SN, Rofsky NM, Dobryansky M, Shamamian P, Marcus SG. Magnetic resonance imaging with magnetic resonance cholangiopancreatography accurately predicts resectability of pancreatic carcinoma. J Gastrointest Surg. 1999;3:506–511. doi: 10.1016/s1091-255x(99)80104-8. [DOI] [PubMed] [Google Scholar]
- 36.Megibow AJ, Zhou XH, Rotterdam H, Francis IR, Zerhouni EA, Balfe DM, et al. Pancreatic adenocarcinoma: CT versus MR imaging in the evaluation of resectability–report of the Radiology Diagnostic Oncology Group. Radiology. 1995;195:327–332. doi: 10.1148/radiology.195.2.7724748. [DOI] [PubMed] [Google Scholar]
- 37.Tapper EB, Martin D, Adsay NV, Kooby D, Kalb B, Sarmiento JM. An MRI-driven practice: a new perspective on MRI for the evaluation of adenocarcinoma of the head of the pancreas. J Gastrointest Surg. 2010;14:1292–1297. doi: 10.1007/s11605-010-1221-z. [DOI] [PubMed] [Google Scholar]
- 38.Hariharan D, Constantinides VA, Froeling FE, Tekkis PP, Kocher HM. The role of laparoscopy and laparoscopic ultrasound in the preoperative staging of pancreatico-biliary cancers–a meta-analysis. Eur J Surg Oncol. 2010;36:941–948. doi: 10.1016/j.ejso.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 39.Shoup M, Winston C, Brennan MF, Bassman D, Conlon KC. Is there a role for staging laparoscopy in patients with locally advanced, unresectable pancreatic adenocarcinoma? J Gastrointest Surg. 2004;8:1068–1071. doi: 10.1016/j.gassur.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 40.Buck AC, Schirrmeister HH, Guhlmann CA, Diederichs CG, Shen C, Buchmann I, et al. Ki-67 immunostaining in pancreatic cancer and chronic active pancreatitis: does in vivo FDG uptake correlate with proliferative activity? J Nucl Med. 2001;42:721–725. [PubMed] [Google Scholar]
- 41.Diederichs CG, Staib L, Vogel J, Glasbrenner B, Glatting G, Brambs HJ, et al. Values and limitations of 18F-fluorodeoxyglucose-positron-emission tomography with preoperative evaluation of patients with pancreatic masses. Pancreas. 2000;20:109–116. doi: 10.1097/00006676-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 42.Gambhir SS, Czernin J, Schwimmer J, Silverman DH, Coleman RE, Phelps ME. A tabulated summary of the FDG PET literature. J Nucl Med. 2001;42:1S–93S. [PubMed] [Google Scholar]
- 43.Imdahl A, Nitzsche E, Krautmann F, Hogerle S, Boos S, Einert A, et al. Evaluation of positron emission tomography with 2-[18F]fluoro-2-deoxy-D-glucose for the differentiation of chronic pancreatitis and pancreatic cancer. Br J Surg. 1999;86:194–199. doi: 10.1046/j.1365-2168.1999.01016.x. [DOI] [PubMed] [Google Scholar]
- 44.Kauhanen SP, Komar G, Seppanen MP, Dean KI, Minn HR, Kajander SA, et al. A prospective diagnostic accuracy study of 18F-fluorodeoxyglucose positron emission tomography/computed tomography, multidetector row computed tomography, and magnetic resonance imaging in primary diagnosis and staging of pancreatic cancer. Ann Surg. 2009;250:957–963. doi: 10.1097/SLA.0b013e3181b2fafa. [DOI] [PubMed] [Google Scholar]
- 45.Lee TY, Kim MH, Park do H, Seo DW, Lee SK, Kim JS, et al. Utility of 18F-FDG PET/CT for differentiation of autoimmune pancreatitis with atypical pancreatic imaging findings from pancreatic cancer. AJR Am J Roentgenol. 2009;193:343–348. doi: 10.2214/AJR.08.2297. [DOI] [PubMed] [Google Scholar]
- 46.Ozaki Y, Oguchi K, Hamano H, Arakura N, Muraki T, Kiyosawa K, et al. Differentiation of autoimmune pancreatitis from suspected pancreatic cancer by fluorine-18 fluorodeoxyglucose positron emission tomography. J Gastroenterol. 2008;43:144–151. doi: 10.1007/s00535-007-2132-y. [DOI] [PubMed] [Google Scholar]
- 47.Pakzad F, Groves AM, Ell PJ. The role of positron emission tomography in the management of pancreatic cancer. Semin Nucl Med. 2006;36:248–256. doi: 10.1053/j.semnuclmed.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 48.van Kouwen MC, Jansen JB, van Goor H, de Castro S, Oyen WJ, Drenth JP. FDG-PET is able to detect pancreatic carcinoma in chronic pancreatitis. Eur J Nucl Med Mol Imaging. 2005;32:399–404. doi: 10.1007/s00259-004-1689-4. [DOI] [PubMed] [Google Scholar]
- 49.Fisher L, Segarajasingam DS, Stewart C, Deboer WB, Yusoff IF. Endoscopic ultrasound guided fine needle aspiration of solid pancreatic lesions: performance and outcomes. J Gastroenterol Hepatol. 2009;24:90–96. doi: 10.1111/j.1440-1746.2008.05569.x. [DOI] [PubMed] [Google Scholar]
- 50.Owens DJ, Savides TJ. Endoscopic ultrasound staging and novel therapeutics for pancreatic cancer. Surg Oncol Clin N Am. 2010;19:255–266. doi: 10.1016/j.soc.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 51.Tadic M, Kujundzic M, Stoos-Veic T, Kaic G, Vukelic-Markovic M. Role of repeated endoscopic ultrasound-guided fine needle aspiration in small solid pancreatic masses with previous indeterminate and negative cytological findings. Dig Dis. 2008;26:377–382. doi: 10.1159/000177025. [DOI] [PubMed] [Google Scholar]
- 52.Kitano M, Kudo M, Maekawa K, Suetomi Y, Sakamoto H, Fukuta N, et al. Dynamic imaging of pancreatic diseases by contrast enhanced coded phase inversion harmonic ultrasonography. Gut. 2004;53:854–859. doi: 10.1136/gut.2003.029934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fusari M, Maurea S, Imbriaco M, Mollica C, Avitabile G, Soscia F, et al. Comparison between multislice CT and MR imaging in the diagnostic evaluation of patients with pancreatic masses. Radiol Med. 2010;115:453–466. doi: 10.1007/s11547-010-0490-7. [DOI] [PubMed] [Google Scholar]
- 54.Cannon ME, Carpenter SL, Elta GH, Nostrant TT, Kochman ML, Ginsberg GG, et al. EUS compared with CT, magnetic resonance imaging, and angiography and the influence of biliary stenting on staging accuracy of ampullary neoplasms. Gastrointest Endosc. 1999;50:27–33. doi: 10.1016/s0016-5107(99)70340-8. [DOI] [PubMed] [Google Scholar]
- 55.Chen CH, Yang CC, Yeh YH, Chou DA, Nien CK. Reappraisal of endosonography of ampullary tumors: correlation with transabdominal sonography, CT, and MRI. J Clin Ultrasound. 2009;37:18–25. doi: 10.1002/jcu.20523. [DOI] [PubMed] [Google Scholar]
- 56.Howard TJ, Chin AC, Streib EW, Kopecky KK, Wiebke EA. Value of helical computed tomography, angiography, and endoscopic ultrasound in determining resectability of periampullary carcinoma. Am J Surg. 1997;174:237–241. doi: 10.1016/s0002-9610(97)00132-3. [DOI] [PubMed] [Google Scholar]
- 57.Midwinter MJ, Beveridge CJ, Wilsdon JB, Bennett MK, Baudouin CJ, Charnley RM. Correlation between spiral computed tomography, endoscopic ultrasonography and findings at operation in pancreatic and ampullary tumours. Br J Surg. 1999;86:189–193. doi: 10.1046/j.1365-2168.1999.01042.x. [DOI] [PubMed] [Google Scholar]
- 58.Rivadeneira DE, Pochapin M, Grobmyer SR, Lieberman MD, Christos PJ, Jacobson I, et al. Comparison of linear array endoscopic ultrasound and helical computed tomography for the staging of periampullary malignancies. Ann Surg Oncol. 2003;10:890–897. doi: 10.1245/aso.2003.03.555. [DOI] [PubMed] [Google Scholar]
- 59.Rosch T, Lorenz R, Braig C, Feuerbach S, Siewert JR, Schusdziarra V, et al. Endoscopic ultrasound in pancreatic tumor diagnosis. Gastrointest Endosc. 1991;37:347–352. doi: 10.1016/s0016-5107(91)70729-3. [DOI] [PubMed] [Google Scholar]
- 60.Shoup M, Hodul P, Aranha GV, Choe D, Olson M, Leya J, et al. Defining a role for endoscopic ultrasound in staging periampullary tumors. Am J Surg. 2000;179:453–456. doi: 10.1016/s0002-9610(00)00379-2. [DOI] [PubMed] [Google Scholar]
- 61.Soriano A, Castells A, Ayuso C, Ayuso JR, de Caralt MT, Gines MA, et al. Preoperative staging and tumor resectability assessment of pancreatic cancer: prospective study comparing endoscopic ultrasonography, helical computed tomography, magnetic resonance imaging, and angiography. Am J Gastroenterol. 2004;99:492–501. doi: 10.1111/j.1572-0241.2004.04087.x. [DOI] [PubMed] [Google Scholar]
- 62.DeWitt J, Devereaux B, Chriswell M, McGreevy K, Howard T, Imperiale TF, et al. Comparison of endoscopic ultrasonography and multidetector computed tomography for detecting and staging pancreatic cancer. Ann Intern Med. 2004;141:753–763. doi: 10.7326/0003-4819-141-10-200411160-00006. [DOI] [PubMed] [Google Scholar]
- 63.Legmann P, Vignaux O, Dousset B, Baraza AJ, Palazzo L, Dumontier I, et al. Pancreatic tumors: comparison of dual-phase helical CT and endoscopic sonography. AJR Am J Roentgenol. 1998;170:1315–1322. doi: 10.2214/ajr.170.5.9574609. [DOI] [PubMed] [Google Scholar]
- 64.Trede M, Rumstadt B, Wendl K, Gaa J, Tesdal K, Lehmann KJ, et al. Ultrafast magnetic resonance imaging improves the staging of pancreatic tumors. Ann Surg. 1997;226:393–405. doi: 10.1097/00000658-199710000-00001. discussion -7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee JK, Kim AY, Kim PN, Lee MG, Ha HK. Prediction of vascular involvement and resectability by multidetector-row CT versus MR imaging with MR angiography in patients who underwent surgery for resection of pancreatic ductal adenocarcinoma. Eur J Radiol. 2010;73:310–316. doi: 10.1016/j.ejrad.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 66.Park HS, Lee JM, Choi HK, Hong SH, Han JK, Choi BI. Preoperative evaluation of pancreatic cancer: comparison of gadolinium-enhanced dynamic MRI with MR cholangiopancreatography versus MDCT. J Magn Reson Imaging. 2009;30:586–595. doi: 10.1002/jmri.21889. [DOI] [PubMed] [Google Scholar]
- 67.Casneuf V, Delrue L, Kelles A, Van Damme N, Van Huysse J, Berrevoet F, et al. Is combined 18F-fluorodeoxyglucose-positron emission tomography/computed tomography superior to positron emission tomography or computed tomography alone for diagnosis, staging and restaging of pancreatic lesions? Acta Gastroenterol Belg. 2007;70:331–338. [PubMed] [Google Scholar]
- 68.Farma JM, Santillan AA, Melis M, Walters J, Belinc D, Chen DT, et al. PET/CT fusion scan enhances CT staging in patients with pancreatic neoplasms. Ann Surg Oncol. 2008;15:2465–2471. doi: 10.1245/s10434-008-9992-0. [DOI] [PubMed] [Google Scholar]
- 69.Tang S, Huang G, Liu J, Liu T, Treven L, Song S, et al. Usefulness of (18)F-FDG PET, combined FDG-PET/CT and EUS in diagnosing primary pancreatic carcinoma: a meta-analysis. Eur J Radiol. 2011;78:142–150. doi: 10.1016/j.ejrad.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 70.Sperti C, Bissoli S, Pasquali C, Frison L, Liessi G, Chierichetti F, et al. 18-fluorodeoxyglucose positron emission tomography enhances computed tomography diagnosis of malignant intraductal papillary mucinous neoplasms of the pancreas. Ann Surg. 2007;246:932–937. doi: 10.1097/SLA.0b013e31815c2a29. discussion 7–9. [DOI] [PubMed] [Google Scholar]
- 71.Artifon EL, Couto D, Jr, Sakai P, da Silveira EB. Prospective evaluation of EUS versus CT scan for staging of ampullary cancer. Gastrointest Endosc. 2009;70:290–296. doi: 10.1016/j.gie.2008.11.045. [DOI] [PubMed] [Google Scholar]
- 72.Agarwal B, Abu-Hamda E, Molke KL, Correa AM, Ho L. Endoscopic ultrasound-guided fine needle aspiration and multidetector spiral CT in the diagnosis of pancreatic cancer. Am J Gastroenterol. 2004;99:844–850. doi: 10.1111/j.1572-0241.2004.04177.x. [DOI] [PubMed] [Google Scholar]
- 73.Miller FH, Rini NJ, Keppke AL. MRI of adenocarcinoma of the pancreas. AJR Am J Roentgenol. 2006;187:W365–W374. doi: 10.2214/AJR.05.0875. [DOI] [PubMed] [Google Scholar]
- 74.Donahue TR, Isacoff WH, Hines OJ, Tomlinson JS, Farrell JJ, Bhat YM, et al. Downstaging chemotherapy and alteration in the classic computed tomography/magnetic resonance imaging signs of vascular involvement in patients with pancreaticobiliary malignant tumors: influence on patient selection for surgery. Arch Surg. 2011;146:836–843. doi: 10.1001/archsurg.2011.152. [DOI] [PubMed] [Google Scholar]
- 75.Low G, Panu A, Millo N, Leen E. Multimodality imaging of neoplastic and nonneoplastic solid lesions of the pancreas. Radiographics. 2011;31:993–1015. doi: 10.1148/rg.314105731. [DOI] [PubMed] [Google Scholar]
- 76.Martin DR, Semelka RC. MR imaging of pancreatic masses. Magn Reson Imaging Clin N Am. 2000;8:787–812. [PubMed] [Google Scholar]
- 77.Vachiranubhap B, Kim YH, Balci NC, Semelka RC. Magnetic resonance imaging of adenocarcinoma of the pancreas. Top Magn Reson Imaging. 2009;20:3–9. doi: 10.1097/RMR.0b013e3181b48392. [DOI] [PubMed] [Google Scholar]
- 78.Bipat S, Phoa SS, van Delden OM, Bossuyt PM, Gouma DJ, Lameris JS, et al. Ultrasonography, computed tomography and magnetic resonance imaging for diagnosis and determining resectability of pancreatic adenocarcinoma: a meta-analysis. J Comput Assist Tomogr. 2005;29:438–445. doi: 10.1097/01.rct.0000164513.23407.b3. [DOI] [PubMed] [Google Scholar]
- 79.Khan I, Conlon K. Pancreatic adenocarcinoma. In: Garden O, editor. Hepatobiliary and Pancreatic Surgery. 4th edn. Oxford: Elsevier; 2009. pp. 283–298. [Google Scholar]
- 80.Iglesias Garcia J, Larino Noia J, Dominguez Munoz JE. Endoscopic ultrasound in the diagnosis and staging of pancreatic cancer. Rev Esp Enferm Dig. 2009;101:631–638. doi: 10.4321/s1130-01082009000900006. [DOI] [PubMed] [Google Scholar]
- 81.Barreto S, Shukla P, Shrikhande S. Periampullary carcinoma. In: Shrikhande S, Friess H, Buechler M, editors. Surgery of Pancreatic Tumors. New Delhi: BI publications; 2007. pp. 206–215. [Google Scholar]
- 82.Goldberg J, Rosenblat J, Khatri G, Schwender B, Kaushik N, Katz D, et al. Complementary roles of CT and endoscopic ultrasound in evaluating a pancreatic mass. AJR Am J Roentgenol. 2010;194:984–992. doi: 10.2214/AJR.08.2034. [DOI] [PubMed] [Google Scholar]
- 83.Mayo SC, Austin DF, Sheppard BC, Mori M, Shipley DK, Billingsley KG. Evolving preoperative evaluation of patients with pancreatic cancer: does laparoscopy have a role in the current era? J Am Coll Surg. 2009;208:87–95. doi: 10.1016/j.jamcollsurg.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 84.Enestvedt CK, Mayo SC, Diggs BS, Mori M, Austin DA, Shipley DK, et al. Diagnostic laparoscopy for patients with potentially resectable pancreatic adenocarcinoma: is it cost-effective in the current era? J Gastrointest Surg. 2008;12:1177–1184. doi: 10.1007/s11605-008-0514-y. [DOI] [PubMed] [Google Scholar]
- 85.Nieveen van Dijkum EJ, Romijn MG, Terwee CB, de Wit LT, van der Meulen JH, Lameris HS, et al. Laparoscopic staging and subsequent palliation in patients with peripancreatic carcinoma. Ann Surg. 2003;237:66–73. doi: 10.1097/00000658-200301000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aziz AM, Said T, Poovathumkadavil A, Almulla A. Using multidetector CT in predicting resectability of pancreatic head tumors: surgical and pathologic correlation. J Egypt Natl Canc Inst. 2010;22:233–239. [PubMed] [Google Scholar]
- 87.Olivie D, Lepanto L, Billiard JS, Audet P, Lavallee JM. Predicting resectability of pancreatic head cancer with multi-detector CT. Surgical and pathologic correlation. JOP. 2007;8:753–758. [PubMed] [Google Scholar]
- 88.Phoa SS, Tilleman EH, van Delden OM, Bossuyt PM, Gouma DJ, Lameris JS. Value of CT criteria in predicting survival in patients with potentially resectable pancreatic head carcinoma. J Surg Oncol. 2005;91:33–40. doi: 10.1002/jso.20270. [DOI] [PubMed] [Google Scholar]
- 89.Kaneoka Y, Yamaguchi A, Isogai M. Portal or superior mesenteric vein resection for pancreatic head adenocarcinoma: prognostic value of the length of venous resection. Surgery. 2009;145:417–425. doi: 10.1016/j.surg.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 90.Shrikhande SV, Barreto SG. Extended pancreatic resections and lymphadenectomy: an appraisal of the current evidence. World J Gastrointest Surg. 2010;2:39–46. doi: 10.4240/wjgs.v2.i2.39. [DOI] [PMC free article] [PubMed] [Google Scholar]


